Effects of Mn Deficiency on Hepatic Oxidative Stress, Lipid Metabolism, Inflammatory Response, and Transcriptomic Profile in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Treatments
2.3. Histopathological Observation
2.4. Transcriptome Sequencing and Transcriptomic Analysis
2.5. Liver ROS Assessment
2.6. Oxidative Stress Assessment in the Liver
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Western Blotting Analysis
2.9. Statistical Analysis
3. Results
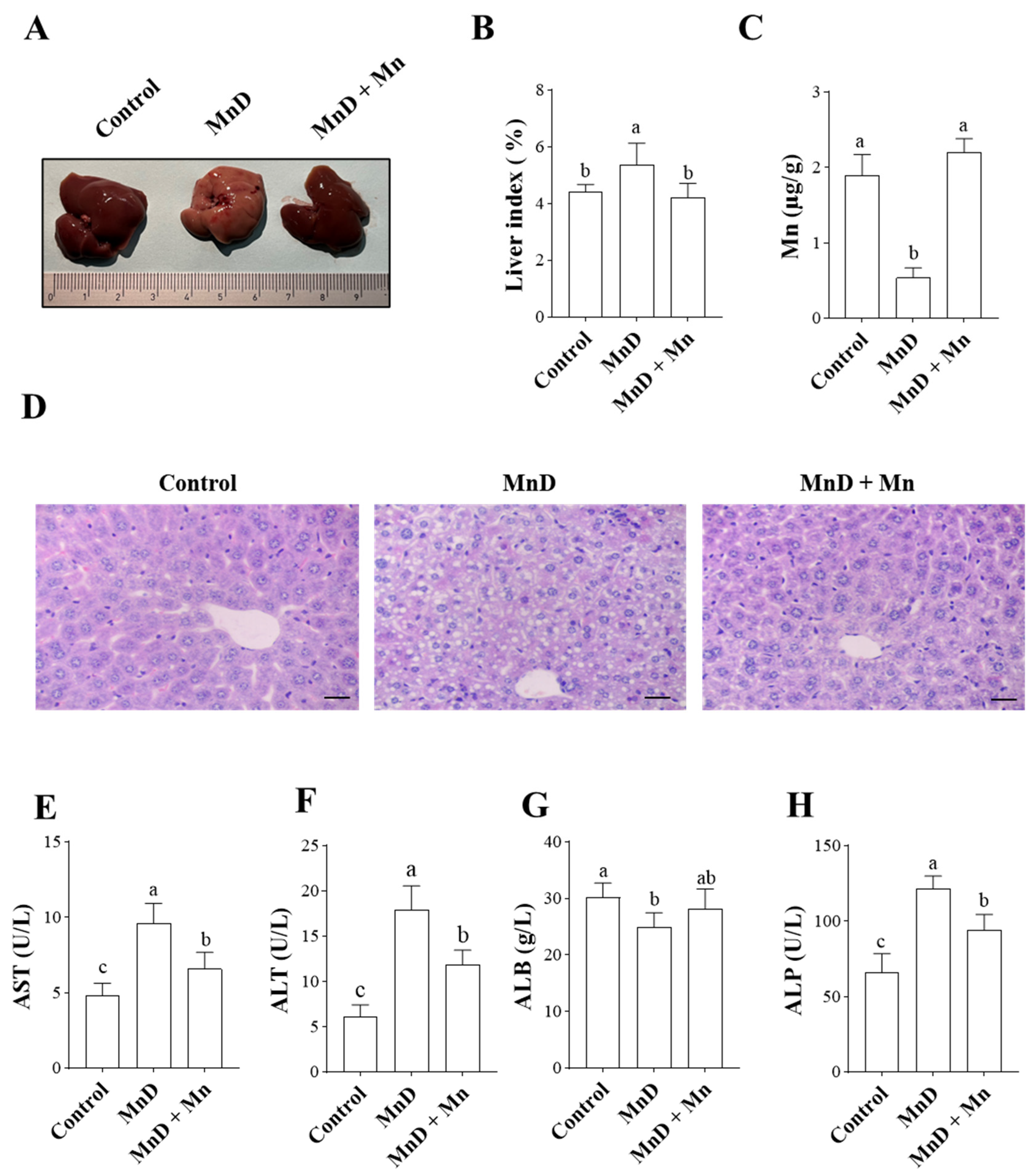
3.1. Effects of Mn Deficiency on Hepatic Histopathology and Liver Function in Mice
3.2. Effects of Mn Deficiency on Oxidative Stress in the Mice
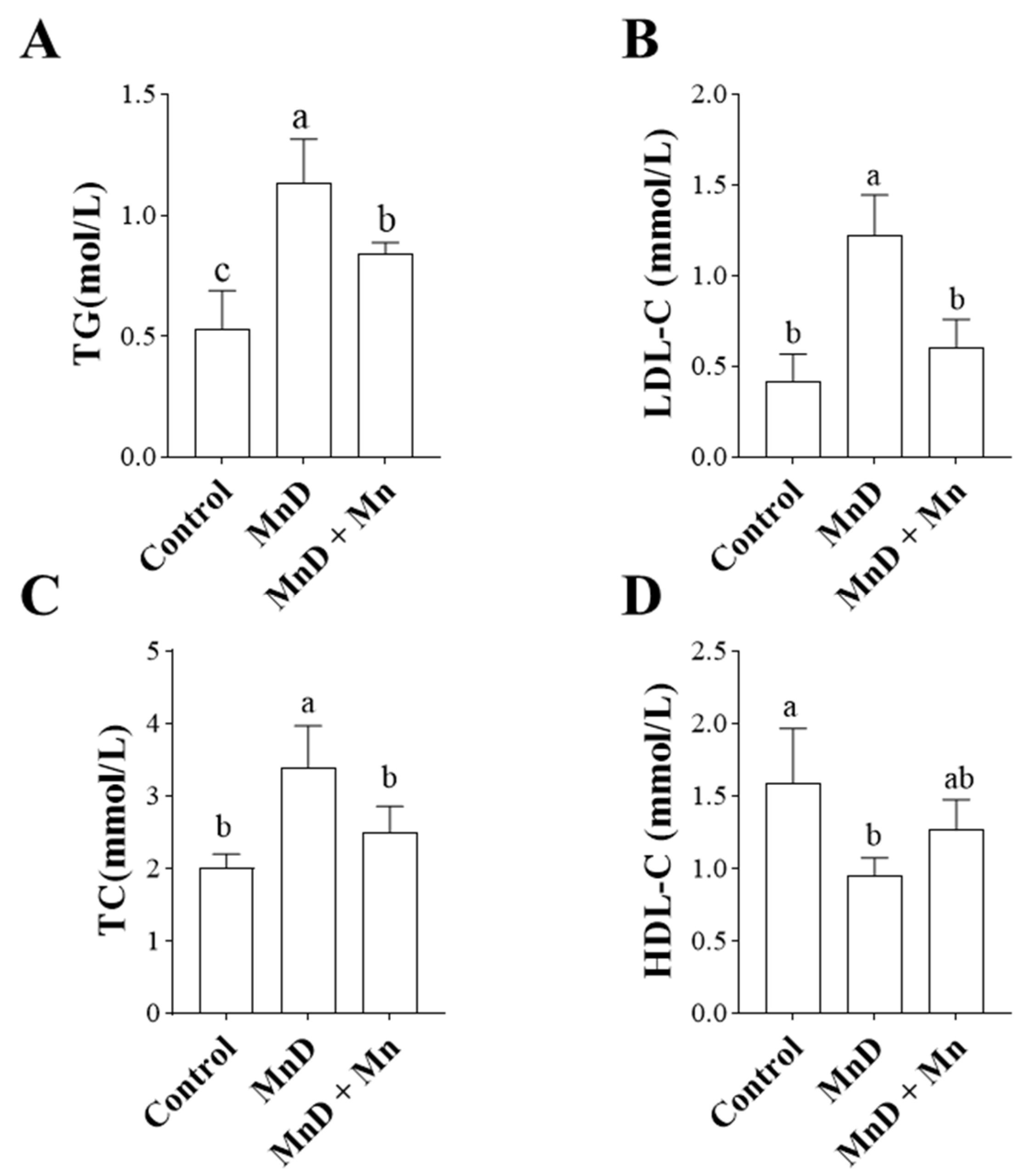
3.3. Effects of Mn Deficiency on Lipid Metabolism in the Mice
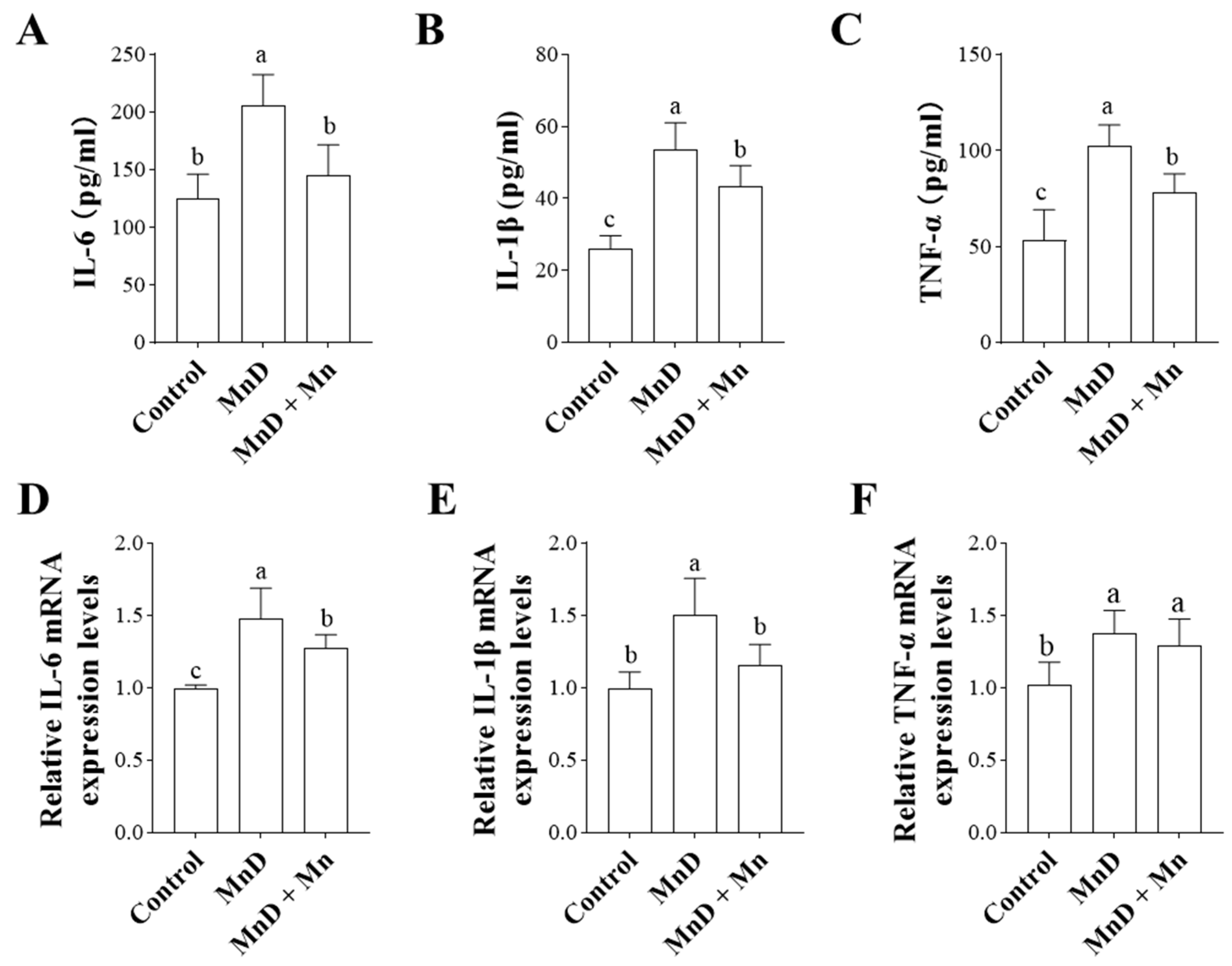
3.4. Effects of Mn Deficiency on Serum and Hepatic Inflammatory Cytokines in Mice
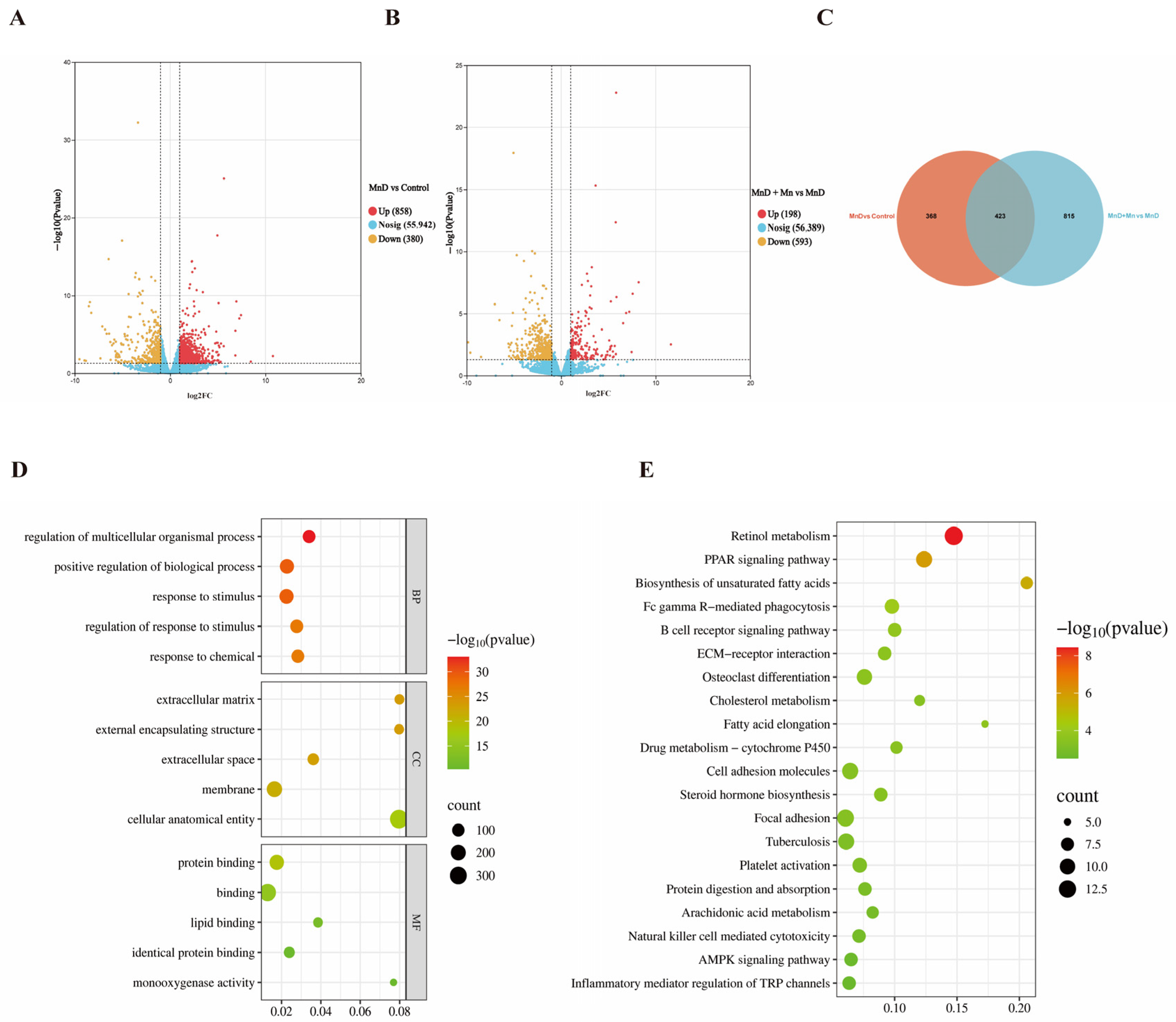
3.5. Transcriptome Analysis of the Effects of Mn Deficiency on the Mouse Liver
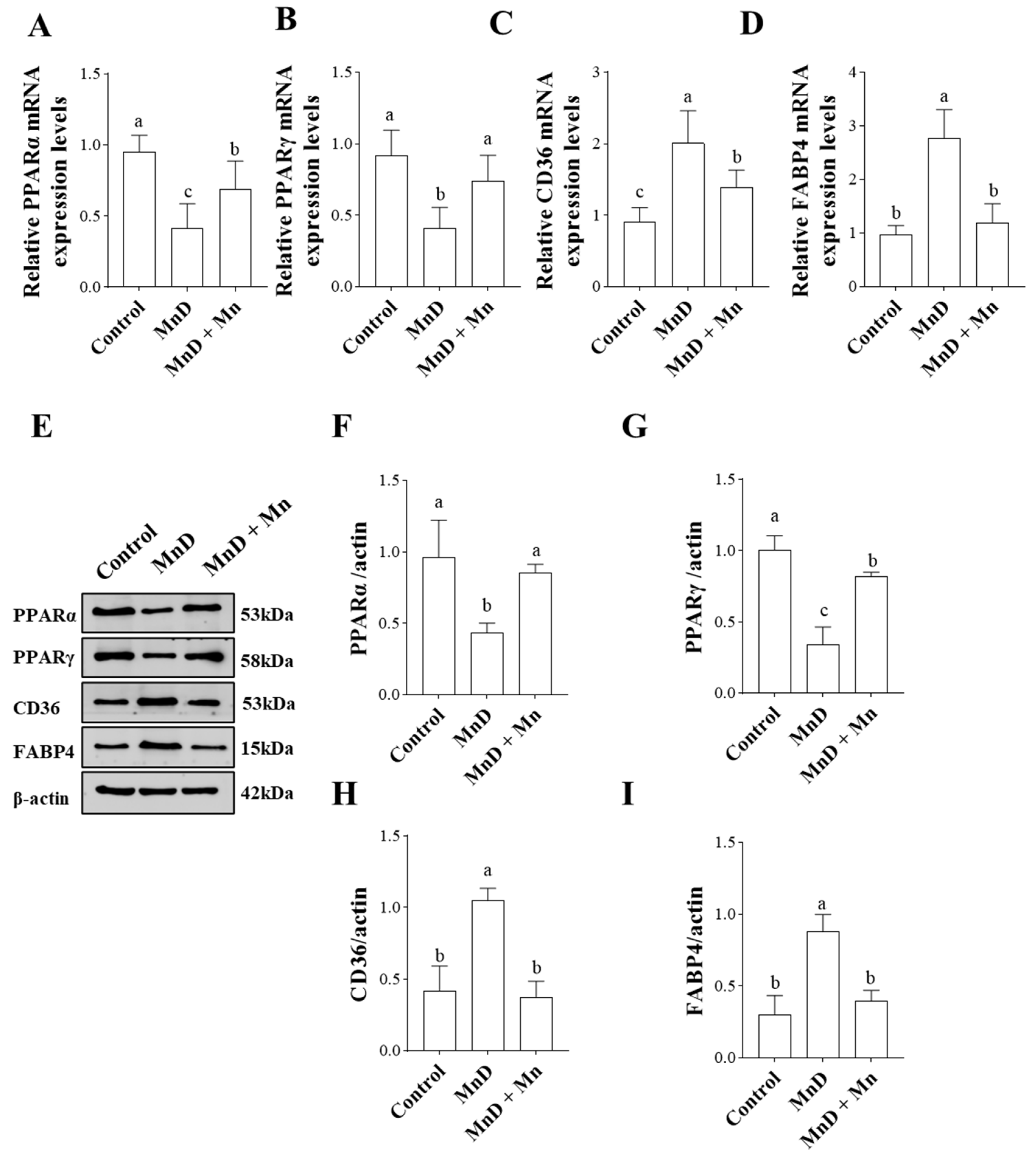
3.6. Effects of Mn Deficiency on Molecules Related to the PPAR Signaling Pathway in the Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Lin, W.; Vann, D.R.; Doulias, P.; Wang, T.; Landesberg, G.; Li, X.; Ricciotti, E.; Scalia, R.; He, M.; Hand, N.J.; et al. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Investig. 2017, 127, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Garrick, M.D.; Singleton, S.T.; Vargas, F.; Kuo, H.; Zhao, L.; Knopfel, M.; Davidson, T.; Costa, M.; Paradkar, P.; Roth, J.A.; et al. DMT1: Which metals does it transport? Biol. Res. 2006, 39, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Aschner, M. Manganese: Its Role in Disease and Health. Met Ions Life Sci. 2019, 19, 253–266. [Google Scholar]
- Xin, Y.; Gao, H.; Wang, J.; Qiang, Y.; Imam, M.U.; Li, Y.; Wang, J.; Zhang, R.; Zhang, H.; Yu, Y.; et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 2017, 3, 17025. [Google Scholar] [CrossRef]
- Roth, J.A. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol. Res. 2006, 39, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Guilarte, T.R. Manganese and Parkinson’s disease: A critical review and new findings. Environ. Health. Perspect. 2010, 118, 1071–1080. [Google Scholar] [CrossRef]
- Ibrahim, W.H.; Habib, H.M.; Kamal, H.; St Clair, D.K.; Chow, C.K. Mitochondrial superoxide mediates labile iron level: Evidence from Mn-SOD-transgenic mice and heterozygous knockout mice and isolated rat liver mitochondria. Free. Radic. Biol. Med. 2013, 65, 143–149. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Aspects. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pentieva, K.; et al. Scientific opinion on the tolerable upper intake level for manganese. Efsa J. 2023, 21, e8413. [Google Scholar]
- Winslow, J.W.W.; Limesand, K.H.; Zhao, N. The Functions of ZIP8, ZIP14, and ZnT10 in the Regulation of Systemic Manganese Homeostasis. Int. J. Mol. Sci. 2020, 21, 3304. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Aring, L.; Das, N.K.; Solanki, S.; Inohara, N.; Iwase, S.; Samuelson, L.C.; Shah, Y.M.; Seo, Y.A. Impact of dietary manganese on experimental colitis in mice. Faseb. J. 2020, 34, 2929–2943. [Google Scholar] [CrossRef] [PubMed]
- Freeland-Graves, J.H.; Mousa, T.Y.; Kim, S. International variability in diet and requirements of manganese: Causes and consequences. J. Trace Elem. Med. Biol. 2016, 38, 24–32. [Google Scholar] [CrossRef]
- Egan, S.K.; Tao, S.S.H.; Pennington, J.A.T.; Bolger, P.M. US Food and Drug Administration’s Total Diet Study: Intake of nutritional and toxic elements, 1991–1996. Food Addit Contam. 2002, 19, 103–125. [Google Scholar] [CrossRef]
- Hallfrisch, J.; Powell, A.; Carafelli, C.; Reiser, S.; Prather, E.S. Mineral balances of men and women consuming high fiber diets with complex or simple carbohydrate. J. Nutr. 1987, 117, 48–55. [Google Scholar] [CrossRef]
- Kim, E.; Bae, Y.; Kim, S.; Choi, M. Estimation of manganese daily intake among adults in Korea. Nutr. Res. Pract. 2008, 2, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, D.W.; Baek, Y.J.; Moon, S.; Jung, H.J.; Song, Y.J.; Paik, H. Dietary supplement use and its effect on nutrient intake in Korean adult population in the Korea National Health and Nutrition Examination Survey IV (2007-2009) data. Eur. J. Clin. Nutr. 2014, 68, 804–810. [Google Scholar] [CrossRef]
- Xue, Q.; Chen, H. Association of blood manganese levels with non-alcoholic fatty liver disease in NHANES 2017-2020: A retrospective cross-sectional study. Metabol Open. 2025, 26, 100358. [Google Scholar] [CrossRef]
- Schophaus, S.; Creasy, K.T.; Koop, P.; Clusmann, J.; Jaeger, J.; Punnuru, V.; Koch, A.; Trautwein, C.; Loomba, R.; Luedde, T.; et al. Machine learning uncovers manganese as a key nutrient associated with reduced risk of steatotic liver disease. Liver Int. 2024, 44, 2807–2821. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Y.; Feng, Y.; Yang, X.; Lin, J.; Wang, T.; Wei, C.; Ma, X.; Yang, R.; Cao, D.; et al. Fructose overconsumption impairs hepatic manganese homeostasis and ammonia disposal. Nat. Commun. 2023, 14, 7934. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.T.; Hurley, L.S. Ultrastructural effects of manganese deficiency in liver, heart, kidney, and pancreas of mice. Lab. Invest. 1973, 29, 732–736. [Google Scholar] [PubMed]
- Wei, T.; Wang, Q.; Chen, T.; Zhou, Z.; Li, S.; Li, Z.; Zhang, D. The possible association of mitochondrial fusion and fission in copper deficiency-induced oxidative damage and mitochondrial dysfunction of the heart. J. Trace Elem. Med. Biol. 2024, 85, 127483. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, R.; Liu, Y.; Kuang, S.; Jiang, J.; Wu, P.; Zhao, J.; Zhang, Y.; Tang, L.; Tang, W.; et al. Manganese deficiency or excess caused the depression of intestinal immunity, induction of inflammation and dysfunction of the intestinal physical barrier, as regulated by NF-kappaB, TOR and Nrf2 signalling, in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 46, 406–416. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Borrello, S.; De Leo, M.E.; Galeotti, T. Transcriptional regulation of MnSOD by manganese in the liver of manganese-deficient mice and during rat development. Biochem Int. 1992, 28, 595–601. [Google Scholar]
- Hughey, C.C.; Puchalska, P.; Crawford, P.A. Integrating the contributions of mitochondrial oxidative metabolism to lipotoxicity and inflammation in NAFLD pathogenesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2022, 1867, 159209. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Fan, C.; Ling-Hu, A.; Sun, D.; Gao, W.; Zhang, C.; Duan, X.; Li, H.; Tian, W.; Yu, Q.; Ke, Z. Nobiletin Ameliorates Hepatic Lipid Deposition, Oxidative Stress, and Inflammation by Mechanisms That Involve the Nrf2/NF-kappaB Axis in Nonalcoholic Fatty Liver Disease. J. Agric. Food. Chem. 2023, 71, 20105–20117. [Google Scholar] [CrossRef]
- Ye, J.; Lv, L.; Wu, W.; Li, Y.; Shi, D.; Fang, D.; Guo, F.; Jiang, H.; Yan, R.; Ye, W.; et al. Butyrate Protects Mice Against Methio-nine-Choline-Deficient Diet-Induced Non-alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuat-ing Inflammation and Reducing Endotoxin Levels. Front. Microbiol. 2018, 9, 1967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borges, C.C.; Bringhenti, I.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Vitamin D deficiency aggravates the liver me-tabolism and inflammation in ovariectomized mice. Biomed. Pharmacother. 2018, 107, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jouihan, H.A.; Cooksey, R.C.; Jones, D.; Kim, H.J.; Winge, D.R.; McClain, D.A. Manganese supplementation protects against diet-induced diabetes in wild type mice by enhancing insulin secretion. Endocrinology. 2013, 154, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.B.; Sulsky, S.I. Risk assessment of an essential element: Manganese. J. Toxicol. Env. Health Part a. 2010, 73, 128–155. [Google Scholar]
- Briggs, K.; Tomar, V.; Ollberding, N.; Haberman, Y.; Bourgonje, A.R.; Hu, S.; Chaaban, L.; Sunuwar, L.; Weersma, R.K.; Denson, L.A.; et al. Crohn’s Disease-Associated Pathogenic Mutation in the Manganese Transporter ZIP8 Shifts the Ileal and Rectal Mucosal Microbiota Implicating Aberrant Bile Acid Metabolism. Inflamm. Bowel Dis. 2024, 30, 1379–1388. [Google Scholar]
- Zhang, S.; Wu, L.; Zhang, J.; Wang, X.; Yang, X.; Xin, Y.; Chen, L.; Li, J.; Niu, P. Multi-omics analysis reveals Mn exposure affects ferroptosis pathway in zebrafish brain. Ecotoxicol. Environ. Saf. 2023, 253, 114616. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity. 2018, 48, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Deng, C.; Shui, L.; Yin, H.; Liu, B. Copper Deficiency Induces Oxidative Stress in Liver of Mice by Blocking the Nrf(2) Pathway. Biol. Trace Elem. Res. 2024, 202, 1603–1611. [Google Scholar] [CrossRef]
- Wang, B.; Klaren, W.D.; Wels, B.R.; Simmons, D.L.; Olivier, A.K.; Wang, K.; Robertson, L.W.; Ludewig, G. Dietary Manganese Modulates PCB126 Toxicity, Metal Status, and MnSOD in the Rat. Toxicol. Sci. 2016, 150, 15–26. [Google Scholar] [CrossRef]
- Dong, P.; Jin, C.; Lian, C.; Wang, L.; Wang, Z. Enhanced Extracellular Matrix Degradation in Growth Plate Contributes to Manganese Deficiency-Induced Tibial Dyschondroplasia in Broiler Chicks. Biol. Trace Elem. Res. 2022, 200, 3326–3335. [Google Scholar] [CrossRef]
- Choi, E.; Rajendiran, T.M.; Soni, T.; Park, J.; Aring, L.; Muraleedharan, C.K.; Garcia-Hernandez, V.; Kamada, N.; Samuelson, L.C.; Nusrat, A.; et al. The manganese transporter SLC39A8 links alkaline ceramidase 1 to inflammatory bowel disease. Nat. Commun. 2024, 15, 4775. [Google Scholar] [CrossRef]
- Saibil, S.D.; St Paul, M.; Laister, R.C.; Garcia-Batres, C.R.; Israni-Winger, K.; Elford, A.R.; Grimshaw, N.; Robert-Tissot, C.; Roy, D.G.; Jones, R.G.; et al. Activation of Peroxisome Proliferator-Activated Receptors alpha and delta Synergizes with Inflammatory Signals to Enhance Adoptive Cell Therapy. Cancer Res. 2019, 79, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhan, J.; Wang, S.; Chen, L.; Zhu, Q.; Nie, R.; Zhou, X.; Zheng, W.; Luo, X.; Wang, B.; et al. Chrysanthemum morifolium attenuates metabolic and alcohol-associated liver disease via gut microbiota and PPARalpha/gamma activation. Phytomedicine 2024, 130, 155774. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]

| Formula | g/Kg |
|---|---|
| Casein, low Cu & Fe | 200.0 |
| DL-Methionine | 3.0 |
| Sucrose | 549.99 |
| Corn starch | 150 |
| Corn oil | 50 |
| Mineral mix, Mn-deficient (81062) | 35 |
| Vitamin mix, AIN-76A (40077) | 10 |
| Choline bitartrate | 2.0 |
| Ethoxyquin, antioxidant | 0.01 |
| Gene | Forward (5′ → 3′) | Reverse (3′ → 5′) |
|---|---|---|
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| PPARα | GAAGCCTACCTGAAGAAC | CGGATTGTTGCTAGTCTT |
| PPARγ | CATCAGGCTTCCACTATG | GACAGTTAAGATCACACCTAT |
| CD36 | AGCACTGAAGAATCTGAAG | AACATCACTACTCCAACAC |
| FABP4 | GATCATCAGCGTAGAAGG | TTCACTTTCCTGTCATCTG |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Tang, S.; Wang, S.; Sun, C.; Chen, B.; Cai, B.; Yin, H. Effects of Mn Deficiency on Hepatic Oxidative Stress, Lipid Metabolism, Inflammatory Response, and Transcriptomic Profile in Mice. Nutrients 2025, 17, 3030. https://doi.org/10.3390/nu17193030
Hu Y, Tang S, Wang S, Sun C, Chen B, Cai B, Yin H. Effects of Mn Deficiency on Hepatic Oxidative Stress, Lipid Metabolism, Inflammatory Response, and Transcriptomic Profile in Mice. Nutrients. 2025; 17(19):3030. https://doi.org/10.3390/nu17193030
Chicago/Turabian StyleHu, Yaodong, Shi Tang, Silu Wang, Caiyun Sun, Binlong Chen, Binjian Cai, and Heng Yin. 2025. "Effects of Mn Deficiency on Hepatic Oxidative Stress, Lipid Metabolism, Inflammatory Response, and Transcriptomic Profile in Mice" Nutrients 17, no. 19: 3030. https://doi.org/10.3390/nu17193030
APA StyleHu, Y., Tang, S., Wang, S., Sun, C., Chen, B., Cai, B., & Yin, H. (2025). Effects of Mn Deficiency on Hepatic Oxidative Stress, Lipid Metabolism, Inflammatory Response, and Transcriptomic Profile in Mice. Nutrients, 17(19), 3030. https://doi.org/10.3390/nu17193030




