Systematic Review of the Effects of Plant-Based Foods on Metabolic Outcomes in Adults with MASLD and Comorbidities Such as Obesity, Metabolic Syndrome, and Type 2 Diabetes
Abstract
1. Introduction
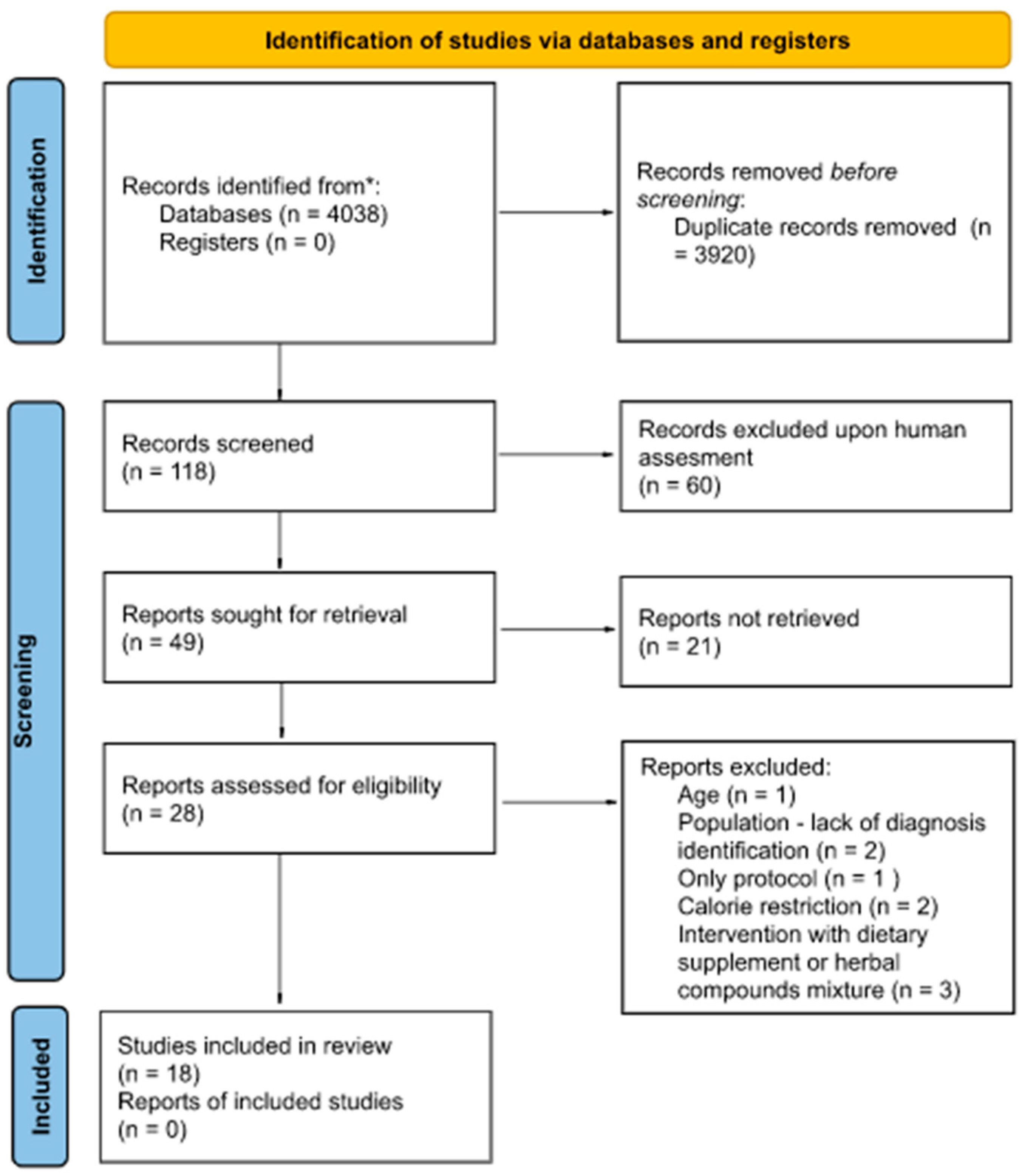
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Selection Criteria
2.4. Data Extraction
- (1)
- Anthropometric measures: These include age, body weight (BW), body mass index (BMI), waist circumference (WC), body fat percentage (BF%), and blood pressure (diastolic (DBP) and systolic (SBP)).
- (2)
- Glucose metabolism outcomes: These include fasting glucose, fasting insulin, the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), and glycated hemoglobin (HbA1c).
- (3)
- Lipid profile: This includes triglycerides (TRGs), total cholesterol (TC), Low-Density Lipoprotein Cholesterol (LDL-C), and High-Density Lipoprotein Cholesterol (HDL-C).
- (4)
- Inflammatory markers: These include High-Sensitivity C-Reactive Protein (hs-CRP) and Lipopolysaccharide (LPS).
- (5)
- Liver function outcomes: These include hepatic enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP).
- (6)
- Hepatic steatosis measures: These include the Controlled Attenuation Parameter (CAP), the hepatic inflammation index (HIS) and Fatty Liver Index (FLI), the grade of fatty liver (FL), and liver fibrosis determined by Fibroscan.
2.5. Data Synthesis and Analysis
2.6. Risk of Bias Assessment
3. Results
3.1. Study Characteristics
3.2. Influence of Dietary Interventions with Plant-Based Foods on Metabolic Outcomes in MASLD Patients
3.2.1. Anthropometric Outcomes
3.2.2. Glucose and Lipid Metabolism and Inflammatory Outcomes
3.2.3. Liver Function Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes. Facts 2024, 17, 374–444. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.-Y.; Zheng, M.-H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.; Lukic, S.; Mijac, D.; Marjanovic-Haljilji, M.; Vojnovic, M.; Bogdanovic, J.; Glisic, T.; Filipovic, N.; Al Kiswani, J.; Djokovic, A.; et al. The New Therapeutic Approaches in the Treatment of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 13219. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ding, X.; Chen, L.; Huang, Q.; He, L. Visceral adiposity index as a predictor of metabolic dysfunction-associated steatotic liver disease: A cross-sectional study. BMC Gastroenterol. 2025, 25, 1–9. [Google Scholar] [CrossRef]
- Latif, S.; Ahsan, T. Prevalence of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD) in Persons with Obesity and Type 2 Diabetes Mellitus: A Cross-sectional Study. Euroasian J. Hepato-Gastroenterol. 2024, 14, 129–133. [Google Scholar] [CrossRef]
- Qi, X.; Li, J.; Caussy, C.; Teng, G.-J.; Loomba, R. Epidemiology, screening, and co-management of type 2 diabetes mellitus and metabolic dysfunction–associated steatotic liver disease. Hepatology 2024. [Google Scholar] [CrossRef]
- Bril, F.; Berg, G.; Barchuk, M.; Nogueira, J.P. Practical Approaches to Managing Dyslipidemia in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease. J. Lipid Atheroscler. 2025, 14, 5–29. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. ‘Wegovy (Semaglutide) Injection: Highlights of Prescribing Information’, Prescribing Information, Label 215256s024. August 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/215256s024lbl.pdf (accessed on 15 September 2025).
- Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Mateos, D.; Casares, M.; Ugarriza, L.; Gómez, C.; Sureda, A.; Tur, J.A. Two-Year Mediterranean Diet Intervention Improves Hepatic Health in MASLD Patients. Foods 2025, 14, 1736. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Panagiotakos, D.B.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism 2017, 68, 119–132. [Google Scholar] [CrossRef]
- Glass, L.M.; Dickson, R.C.; Anderson, J.C.; Suriawinata, A.A.; Putra, J.; Berk, B.S.; Toor, A. Total Body Weight Loss of ≥10 % Is Associated with Improved Hepatic Fibrosis in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2014, 60, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz-Del-Campo, N.; Castelnuovo, G.; Rosso, C.; Caviglia, G.; Dileo, E.; Guariglia, M.; Armandi, A.; Poggiolini, I.; Saba, F.; Olivero, A.; et al. Response to a 6-month personalized dietary intervention in patients with metabolic dysfunction-associated steatotic liver disease. Dig. Liver Dis. 2024, 56, S55. [Google Scholar] [CrossRef]
- Fischer, R.; Hassan, M.; Muthyala, R.; Husain, S.; Razavi, P. Dietary Strategies in Metabolic-Associated Steatotic Liver Disease: Effects of Mediterranean, Ketogenic, and Plant-Based Diets on Liver Fibrosis Progression. Am. J. Clin. Med. Re 2025, 5, 983–995. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, P.; Liu, Y.; Zhang, Z.; Wu, X.; Weng, M.; Cao, S.; Wang, Y.; Zeng, C.; Yang, R.; et al. A comprehensive approach to lifestyle intervention based on a calorie-restricted diet ameliorates liver fat in overweight/obese patients with NAFLD: A multicenter randomized controlled trial in China. Nutr. J. 2024, 23, 1–14. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Grillo, A.; Colosimo, S.; Zarpellon, F.; Pozzi, G.; Furlan, D.; Amodeo, G.; Bertoli, S. Diet and physical exercise as key players to tackle MASLD through improvement of insulin resistance and metabolic flexibility. Front. Nutr. 2024, 11, 1426551. [Google Scholar] [CrossRef]
- Moon, H.; Kim, Y.; Lee, J.K.; Lee, H.A.; Kim, H.Y. Effects of Intermittent Calorie Restriction in Nondiabetic Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Gastroenterol. Hepatol. 2025, 23, 114–123.e13. [Google Scholar] [CrossRef]
- Jurek, J.M.; Zablocka-Sowinska, K.; Mestres, H.C.; Gutiérrez, L.R.; Camaron, J.; Auguet, T. The Impact of Dietary Interventions on Metabolic Outcomes in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Comorbid Conditions, Including Obesity and Type 2 Diabetes. Nutrients 2025, 17, 1257. [Google Scholar] [CrossRef]
- Li, H.-Y.; Gan, R.-Y.; Shang, A.; Mao, Q.-Q.; Sun, Q.-C.; Wu, D.-T.; Geng, F.; He, X.-Q.; Li, H.-B. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021. [Google Scholar] [CrossRef]
- Senturk, B.G.; Gurses, B.; Soyturk, C.; Copur, S.; Incir, S.; Siriopol, D.; Hasbal, N.B.; Akyildiz, M.; van Raalte, D.H.; Kanbay, M. Effects of plant-based diet on metabolic parameters, liver and kidney steatosis: A prospective interventional open-label study. Br. J. Nutr. 2025, 133, 289–298. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Yi, D.; Qiu, F.; Wu, L.; Tang, Y.; Wang, N. Mediterranean diet affects the metabolic outcome of metabolic dysfunction-associated fatty liver disease. Front. Nutr. 2023, 10, 1225946. [Google Scholar] [CrossRef]
- Handu, D.; Stote, K.; Piemonte, T. Evaluating Bioactive-Substance-Based Interventions for Adults with MASLD: Results from a Systematic Scoping Review. Nutrients 2025, 17, 453. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef]
- Alami, F.; Alizadeh, M.; Shateri, K. The effect of a fruit-rich diet on liver biomarkers, insulin resistance, and lipid profile in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Scand. J. Gastroenterol. 2022, 57, 1238–1249. [Google Scholar] [CrossRef]
- Notarnicola, M.; Tutino, V.; De Nunzio, V.; Cisternino, A.M.; Cofano, M.; Donghia, R.; Giannuzzi, V.; Zappimbulso, M.; Milella, R.A.; Giannelli, G.; et al. Daily Orange Consumption Reduces Hepatic Steatosis Prevalence in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: Exploratory Outcomes of a Randomized Clinical Trial. Nutrients 2024, 16, 3191. [Google Scholar] [CrossRef]
- Dorosti, M.; Heidarloo, A.J.; Bakhshimoghaddam, F.; Alizadeh, M. Whole-grain consumption and its effects on hepatic steatosis and liver enzymes in patients with non-alcoholic fatty liver disease: A randomised controlled clinical trial. Br. J. Nutr. 2020, 123, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Maciejewska-Markiewicz, D.; Palma, J.; Mielko, K.A.; Qasem, B.; Kozłowska-Petriczko, K.; Ufnal, M.; Sokolowska, K.E.; Hawryłkowicz, V.; Załęska, P.; et al. Precision Nutrition in NAFLD: Effects of a High-Fiber Intervention on the Serum Metabolome of NAFD Patients—A Pilot Study. Nutrients 2022, 14, 5355. [Google Scholar] [CrossRef] [PubMed]
- Fateh, H.L.; Rashid, S.A.; Muhammad, S.S.; Al-Jaf, S.H.; Ali, A.M. Comparing effects of beetroot juice and Mediterranean diet on liver enzymes and sonographic appearance in patients with non-alcoholic fatty liver disease: A randomized control trials. Front. Nutr. 2023, 10, 1181706. [Google Scholar] [CrossRef]
- Hosseinabadi, S.; Rafraf, M.; Asghari, S.; Asghari-Jafarabadi, M.; Vojouhi, S. Effect of green coffee extract supplementation on serum adiponectin concentration and lipid profile in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Complement. Ther. Med. 2020, 49, 102290. [Google Scholar] [CrossRef]
- Hosseinabadi, S.; Rafraf, M.; Mahmoodzadeh, A.; Asghari-Jafarabadi, M.; Asghari, S. Effects of green coffee extract supplementation on glycemic indexes, leptin, and obesity values in patients with non-alcoholic fatty liver disease. J. Herb. Med. 2020, 22, 100340. [Google Scholar] [CrossRef]
- Izadi, F.; Farrokhzad, A.; Tamizifar, B.; Tarrahi, M.J.; Entezari, M.H. Effect of sour tea supplementation on liver enzymes, lipid profile, blood pressure, and antioxidant status in patients with non-alcoholic fatty liver disease: A double-blind randomized controlled clinical trial. Phytotherapy Res. 2021, 35, 477–485. [Google Scholar] [CrossRef]
- Namdar, A.B.; Omidvar, D.; Amerizadeh, F.; Kabiri, M.; Namdar, H.B.; Ravanshad, S. The efficacy of flaxseed oil on non-alcoholic fatty liver disease: A randomised controlled trial. J. Herb. Med. 2024, 48. [Google Scholar] [CrossRef]
- Khodadadi, N.; Sadeghi, A.; Poustchi, H.; Abbasi, B.; Nilghaz, M.; Melekoglu, E.; Yari, Z.; Hekmatdoost, A. Effectiveness of flaxseed consumption and fasting mimicking diet on anthropometric measures, biochemical parameters, and hepatic features in patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A randomized controlled clinical trial. Nutr. Diabetes 2024, 14, 1–9. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Liao, W.; Xia, J.-Y.; Hu, Q.; Zhao, Q.; Zhang, R.; Sun, G.; Yang, L.; Li, L. Flaxseed powder supplementation in non-alcoholic fatty liver disease: A randomized controlled clinical trial. Food Funct. 2025, 16, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Maleki Sedgi, F.; Hosseiniazar, M.M.; Alizadeh, M. The effects of replacing ghee with rapeseed oil on liver steatosis and enzymes, lipid profile, insulin resistance and anthropometric measurements in patients with non-alcoholic fatty liver disease: A randomised controlled clinical trial. Br. J. Nutr. 2024, 131, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, S.M.; Samadi, M.; Davarpanah, H.; Babajafari, S.; Clark, C.C.T.; Ghaemfar, Z.; Rezaiyan, M.; Mosallanezhad, A.; Shafiee, M.; Rostami, H. EXPRESSION OF CONCERN: The effect of Spirulina sauce, as a functional food, on cardiometabolic risk factors, oxidative stress biomarkers, glycemic profile, and liver enzymes in nonalcoholic fatty liver disease patients: A randomized double-blinded clinical trial. Food Sci. Nutr. 2022, 10, 317–328. [Google Scholar] [CrossRef]
- Rafie, R.; Hosseini, S.A.; Hajiani, E.; Malehi, A.S.; Mard, S.A. Effect of Ginger Powder Supplementation in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Clin. Exp. Gastroenterol. 2020, 13, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Azar, M.R.M.H.; Alizadeh, M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: A double-blind randomised controlled clinical trial. Br. J. Nutr. 2020, 124, 450–456. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Azar, M.R.M.H.; Alizadeh, M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement. Ther. Med. 2020, 51, 102428. [Google Scholar] [CrossRef]
- Soleimani, D.; Paknahad, Z.; Rouhani, M.H. Therapeutic Effects of Garlic on Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Diabetes, Metab. Syndr. Obes. Targets Ther. 2020, 13, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Orliacq, J.; Pérez-Cornago, A.; A Parry, S.; Kelly, R.K.; A Koutoukidis, D.; Carter, J.L. Associations between types and sources of dietary carbohydrates and liver fat: A UK Biobank study. BMC Med. 2023, 21, 1–12. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B.; et al. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Tian, X.; Wang, H.; Gan, P.; Zhang, Q. Inappropriate Diet Exacerbates Metabolic Dysfunction-Associated Steatotic Liver Disease via Abdominal Obesity. Nutrients 2024, 16, 4208. [Google Scholar] [CrossRef]
- Noreen, S.; Hashmi, B.; Tufail, T.; Ikram, A.; Arshad, M.T.; Gnedeka, K.T. Synergistic Beneficial Effects of Flaxseed (Linum usitatissimum L.) Oil and Olive (Olea europaea L.) Oil Against Metabolic Dysfunction Associated Fatty Liver and Its Complications. Food Sci. Nutr. 2025, 13, e4638. [Google Scholar] [CrossRef]
- Panjeshahin, A.; Mollahosseini, M.; Panbehkar-Jouybari, M.; Kaviani, M.; Mirzavandi, F.; Hosseinzadeh, M. Effects of garlic supplementation on liver enzymes: A systematic review and meta-analysis of randomized controlled trials. Phytotherapy Res. 2020, 34, 1947–1955. [Google Scholar] [CrossRef]
- Delgado, E.M.M.; Quiroz-Aldave, J.E.; Durand-Vásquez, M.d.C.; Aldave-Pita, L.N.; Fuentes-Mendoza, J.M.; Concepción-Urteaga, L.A.; Paz-Ibarra, J.; Concepción-Zavaleta, M.J. Immunomodulatory effect of allium sativum in type 2 diabetes mellitus. World J. Exp. Med. 2025, 15, 103481. [Google Scholar] [CrossRef]
- Yari, Z.; Rahimlou, M.; Poustchi, H.; Hekmatdoost, A. Flaxseed supplementation improves anthropometric measurements, metabolic, and inflammatory biomarkers in overweight and obese adults. Int. J. Vitam. Nutr. Res. 2022, 92, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, H.; Wan, M.; Lu, Y.; Xu, D.; Yang, X.; Yang, L.; Sun, G. Comparisons of the effects of different flaxseed products consumption on lipid profiles, inflammatory cytokines and anthropometric indices in patients with dyslipidemia related diseases: Systematic review and a dose–response meta-analysis of randomized controlled trials. Nutr. Metab. 2021, 18, 1–23. [Google Scholar] [CrossRef]
- Pourreza, S.; Azar, P.S.; Sanaie, S.; Noshadi, N.; Jalali, S.; Niazkar, H.R.; Karimi, A.; Vajdi, M.; Bin Emran, T. Therapeutic Effects and Mechanisms of Action of Garlic (Allium sativum) on Nonalcoholic Fatty Liver Disease: A Comprehensive Systematic Literature Review. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dobrijević, D.; Pastor, K.; Nastić, N.; Özogul, F.; Krulj, J.; Kokić, B.; Bartkiene, E.; Rocha, J.M.; Kojić, J. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules 2023, 28, 4824. [Google Scholar] [CrossRef] [PubMed]
- Amirpoor, A.; Zavar, R.; Amerizadeh, A.; Asgary, S.; Moradi, S.; Farzaei, M.H.; Masoumi, G.; Sadeghi, M. Effect of Beetroot Consumption on Serum Lipid Profile: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 100887. [Google Scholar] [CrossRef]
- Serban, C.; Sahebkar, A.; Ursoniu, S.; Andrica, F.; Banach, M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1119–1127. [Google Scholar] [CrossRef]
- Faienza, M.F.; Cognetti, E.; Farella, I.; Antonioli, A.; Tini, S.; Antoniotti, V.; Prodam, F. Dietary fructose: From uric acid to a metabolic switch in pediatric metabolic dysfunction-associated steatotic liver disease. Crit. Rev. Food Sci. Nutr. 2025, 65, 4583–4598. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K.; Shaibi, G.Q. The relationship between excessive dietary fructose consumption and paediatric fatty liver disease. Pediatr. Obes. 2021, 16, e12759. [Google Scholar] [CrossRef]
| Ref. | Name of Plant-Based Food | Form/Examples | Dose | Duration | Additional Comments |
|---|---|---|---|---|---|
| Alami et al., 2022 [27] | A fruit-rich diet (FRD) | Whole fruit, including colored fruits, dried fruits, and other fruits | At least 4 servings of fruits daily | 24 weeks | N/A |
| Notarnicola et al., 2024 [28] | Oranges | Whole “Naveline” oranges | 400 g a day | 4 weeks | Biological oranges purchased from a BioFarm in Cosenza (Calabria Region, Italy). |
| Dorosti et al., 2020 [29] | Whole-grain products (WGPs) | High-fiber buns (HFBs) baked with fiber obtained from flour (rye) and vital fiber (plantain, psyllium, and apple) | The fiber content was 6.6 ± 0.11 g/roll | 8 weeks | Composition of the high-fiber rolls included rye flour type 2000 BIO, vital fiber (20% plantain, 80% psyllium) BIO, apple fiber BIO, ground milk thistle BIO, natural leaven from the fermentation of rye flour type 2000, and yeast. The nutritional content was fat, 2.38 ± 0.11 g/roll; protein, 20.4 ± 0.47 g/roll; and water, 63.7 ± 0.77 g/roll. |
| Stachowska et al., 2022 [30] | Whole grains including whole wheat, brown rice, oatmeal, whole corn, popcorn, quinoa, barley, buckwheat, bulgur, millet, wild rice, sorghum, amaranth, teff, and triticale | At least half of daily cereal servings obtained from whole-grain cereals | 12 weeks | Participants in both groups were asked to eat two to three servings of low-fat dairy products, five servings of fruits and vegetables, and two servings of lean meat, poultry, or fish on a daily basis, as recommended in the 2012 Dietary Guidelines for Americans. | |
| Fateh et al., 2023 [31] | Beetroot | Concentrated beetroot juice (BJ) | 250 mL of concentrated BJ daily | 12 weeks | A 100 mL serving of beetroot juice comprises 95 Kcal energy, 22.6 g carbohydrates, 0.70 g proteins, 0.16 g total lipids, 0.91 g total dietary fiber, and 12 g total sugars. Vitamin C and total flavonoids are within a range of 1.73–7.85 g, 10.75–20.36 mg, and 2.02–2.36 mg (per 100 g). |
| Hosseinabadi et al., 2020 [32] | Green coffee | Green coffee extract (GCE) | A GCE capsule (200 mg) was equal to 1200 mg green coffee bean and 100 mg of CGA obtained from extract | 8 weeks | The hydroalcoholic extract of green coffee beans contained 50% CGA and low levels of caffeine (2%). |
| Hosseinabadi et al., 2020 [33] | |||||
| Izadi et al., 2021 [34] | Sour tea | Sour tea was made with Hibiscus sabdariffa L plant which was obtained from a local market | Sour tea in the form of a 450 mg capsule containing at least 250 mg of anthocyanin | 8 weeks | Sour tea was rich in antioxidants (% of weight), primarily anthocyanins (25.46), and anthocyanidins (11.62). It also contained quercetin (7.62), cyanidin (4.78), and unique compounds like hibiscin (4.14), gossypicyanin (3.72), sabdaritrin (3.05), and hibiscitrin (0.98), all contributing to its health-promoting properties. High cellulose content (40.89) reflects its natural fiber composition. The Hibiscus sabdariffa L plant used to make tea was obtained from a local market. |
| Namdar et al., 2024 [35] | Flaxseed | Flaxseed oil | Two capsules containing flaxseed oil with total dose prescribed of 1 g two times a day | 8 weeks | N/A [35]. |
| Khodadadi et al., 2024 [36] | Flaxseed powder | A portion of 30 g of flaxseed powder per day | 12 weeks | ||
| Tian et al., 2025 [37] | Golden flaxseed powder | A serving of 30 g flaxseed powder daily before meals | 12 weeks | The flaxseed was golden flaxseed purchased from Canmar Foods Ltd. (Regina, SK, Canada) and contained the following per 100 g: protein—20.0 g; fat—48.7 g (saturated fatty acid—3.3 g; polyunsaturated fatty acid—40.0 g [omega-3 polyunsaturated fatty acid—33.3 g; omega-6 polyunsaturated fatty acid—6.7 g]; monounsaturated fatty acid—5.3 g; trans fatty acid—0 g); carbohydrate—20.0 g (dietary fiber—20.0 g; sugar—0 g); cholesterol—0 g; sodium—66.7 mg; lignan—1.6 g. | |
| Maleki Sedgi et al., 2024 [38] | Vegetable oil–rapeseed oil | Rapeseed oil | Three to eight servings of rapeseed oil daily, as part of diet | 12 weeks | Regular consumers of ghee consuming from three to eight servings of ghee daily were asked to replace the ghee with rapeseed oil in the same amount. |
| Mazloomi et al., 2022 [39] | Spirulina | Spirulina sauce | One sachet (20 mg) of sauce containing 2 g spirulina per day | 8 weeks | Spirulina sauce including spirulina (10%), oil, lemon juice, vinegar, salt, gum, spices, and water. |
| Rafie et al., 2020 [40] | Ginger | Ginger rhizome powder | Three capsules of 500 mg ginger in a powdered form | 12 weeks | Ginger powder supplement used in this study is a ready-made product. |
| Sangouni et al., 2020 [41] | Garlic | Garlic powder | Four tablets of powdered garlic daily | 15 weeks | Each enteric-coated tablet contained 400 mg garlic powder including 1.5 mg Allicin. |
| Sangouni et al., 2020 [42] | |||||
| Soleimani et al., [43] | 12 weeks |
| Ref. | Country | Population | Number of Participants at the Baseline | Age (Mean ± SD) at the Baseline | Dietary Intervention with Plant-Based Food or Its Extract | Type of Analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Duration | Intervention Group | Control/Placebo | ITT, PP, or AT | |||||
| Alami et al., 2022 [27] | Iran | MASLD | TOTAL: 80; I: 40; C: 40 | I: 47.39 ± 10.29; C: 45.11 ± 9.28 | FRD | 24 weeks | FRD group received at least 4 servings of fruits daily | CONTROL: less than 2 servings daily | PP |
| Notarnicola et al., 2024 [28] | Italy | MASLD | TOTAL: 62; I: 31; C: 31 | I: 51.8 ± 10.3; C: 50.1 ± 9.8 | Whole oranges | 4 weeks | 400 g of whole Navelina variety oranges per day | CONTROL: 400 g of non-citrus fruits daily | ITT |
| Dorosti et al., 2020 [29] | Iran | MASLD | TOTAL: 112; I: 56; C: 56 | I: 43.1 ± 8.9; C: 42.4 ± 8.6 | WGPs | 12 weeks | At least half of daily cereal servings must be from whole-grain cereals | CONTROL: at least half of daily cereal servings must be from usual grain cereals | PP |
| Stachowska et al., 2022 [30] | Poland | MASLD | TREATMENT 1—TOTAL: 40 (INTERVENTION ONLY) | MEDIAN: 51.1 (29–68) | HFBs | 8 weeks | Replace normal bread in the diet with HFBs divided between two meals a day (2 rolls every day) | NO CONTROL—DATA COMPARED WITH BASELINE | PP |
| Fateh et al., 2023 [31] | Iraq | MASLD | TOTAL: 180; I: 45; C: 45 | I: 44.91 ± 15.24; C: 44.04 ± 13.2 | BJ | 12 weeks | A 250 mL serving of concentrated BJ given in the morning 30 min before breakfast daily | PLACEBO: A 250 mL glass of water containing red carmoisine food color and a small quantity of a sweetener daily | PP |
| Hosseinabadi et al., 2020 [32] | Iran | MASLD | TOTAL: 48; I: 24; C: 24 | I: 41.14 ± 7.87; C: 41.13 ± 8.47 | GCE | 12 weeks | A daily dose of 400 mg GCE (2 × 300 mg; n = 24) | PLACEBO: placebo capsule similar to GCE tablet in terms of dosage, color, and size containing 200 mg starch | PP |
| Hosseinabadi et al., 2020 [33] | PP | ||||||||
| Izadi et al., 2021 [34] | Iran | MASLD | TOTAL: 70; I: 35; C: 35 | I: 43.3 ± 10.2; C: 42.8 ± 10.6 | Sour tea | 8 weeks | One capsule of sour tea powder (450 mg capsule containing at least 250 mg of anthocyanin) daily | PLACEBO: one placebo capsule (pure microcrystalline cellulose) | PP |
| Namdar et al., 2024 [35] | Iran | MASLD | TOTAL: 60; I: 30; C: 30 | I: 42.23 ± 9.97; C: 38.07 ± 10.40 | Flaxseed oil | 8 weeks | The dose of capsules containing flaxseed oil was prescribed as 1 g two times a day | PLACEBO: one capsule two times a day | PP |
| Khodadadi et al., 2024 [36] | Iran | MASLD | TOTAL: 50 I: 25; C: 25 | I: 45.07 ± 11.01; C: 45.55 ± 11.59 | Flaxseed powder | 12 weeks | A portion of 30 g of flaxseed powder per day | CONTROL: received dietary modification recommendations | ITT |
| Tian et al., 2025 [37] | China | MASLD with obesity | TOTAL: 54; I: 27; C: 27 | I: 35.44 ± 10.85; C: 36.32 ± 10.00 | Golden flaxseed powder | 12 weeks | A serving of 30 g flaxseed powder daily before lunch or dinner along with health education | CONTROL: only health education | PP |
| Maleki Sedgi et al., 2024 [38] | Iran | MASLD | TOTAL: 60; I: 30; C: 30 | Mean total age 42 (SD 9.6) years | Rapeseed oil | 12 weeks | Substitute ghee with rapeseed oil in the same amount with a healthy diet | CONTROL: continued the consumption of ghee and was instructed to adhere to a healthy diet | PP |
| Mazloomi et al., 2022 [39] | Iran | MASLD | TOTAL: 46; I: 23; C: 23 | I: 38.87 ± 14.61; C: 35.78 ± 11.14 | Spirulina sauce | 8 weeks | Spirulina sauce group consumed one sachet (20 mg) of sauce containing 2 g spirulina per day | PLACEBO: one sachet (20 mg) of placebo sauce per day; the placebo sauce was similar in terms of fat, carbohydrate, salt, flavorings, and packaging to the spirulina sauce; to normalize the sensory properties, the color of the sauce, natural dark green chlorophyll was used | ITT |
| Rafie et al., 2020 [40] | Iran | MASLD | TOTAL: 50; I: 25; C: 25 | I: 50.04 ± 10.26; C: 47.95 ± 9.24 | Ginger powder | 12 weeks | Three capsules containing 500 mg of ginger powder daily | PLACEBO: 3 capsules daily, each containing 500 mg of wheat flour | PP |
| Soleimani et al., 2020 [43] | Iran | MASLD with T2DM (26 patients) and MS (50 patients) | TOTAL: 110; I: 55; C: 55 | I:45.6 ± 11.3; C:42.9 ± 12.21 | Garlic powder | 15 weeks | Four tablets of powdered garlic daily (each tablet contained 400 mg garlic powder) | PLACEBO: four tablets of placebo containing 400 mg starch | PP |
| Sangouni et al., 2020 [42] | Iran | MASLD with MS (51 patients) | TOTAL: 110; I: 55; C: 55 | I: 46.4 ± 11.3; C: 44.1 ± 11.8 | Garlic powder | 15 weeks | Four tablets of powdered garlic daily (each tablet contained 400 mg garlic powder) | PLACEBO: placebo tablets in the form of enteric-coated tablets containing 400 mg microcrystalline cellulose | PP |
| Sangouni et al., 2020 [41] | Iran | MASLD | TOTAL: 90; I: 45; C: 45 | I: 45.2 ± 12.4; C:44.2 ± 11.1 | Garlic powder | 12 weeks | Four tablets of powdered garlic daily (each tablet contained 400 mg garlic powder) | PLACEBO: four tablets of placebo containing 400 mg starch | PP |
| Dietary Intervention with Plant-Based Food | Anthropometric Outcomes After Intervention with Plant-Based Foods | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | BMI | WC | BF | DBP | SBP | ||||||||||||||
| B | PI | p | B | PI | p | B | PI | p | B | PI | p | B | PI | p | B | PI | p | ||
| Ref. | |||||||||||||||||||
| Alami et al., 2022 [27] | FRD | 79.4 ± 9.9 | 86.4 ± 9.5 | p < 0.001 | 28.37 ± 2.09 | 31.40 ± 2.61 | p < 0.001 | 109.7 ± 11.3 | 113.5 ± 10.7 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Notarnicola et al., 2024 [28] | Whole oranges | 91.98 ± 9.96 | 91.26 ± 9.53 | p = 0.26 | 32.07 ± 4.25 | 31.95 ± 4.28 | p = 0.57 | 108.32 ± 12.72 | 107.32 ± 12.13 | p = 0.05 | 29.59 ± 15.94 | 28.99 ± 13.36 | p = 0.14 | N/A | N/A | N/A | N/A | N/A | N/A |
| Dorosti et al., 2020 [29] | WGPs | 87.7 ± 12.0 | 84.22 ± 11.8 | p < 0.001 | 32.5 ± 4.1 | 32.0 ± 4.2 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Stachowska et al., 2022 [30] | HFBs | 85.2 (59–113.9) | N/A | 28.9 (22.8–35.2) | N/A | N/A | N/A | N/A | 27.4 (11.7–43.6) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Fateh et al., 2023 [31] | BJ | N/A | N/A | N/A | 29.19 ± 3.94 | 27.75 ± 3.74 | p < 0.001 | 91.34 ± 4.53 | 89.91 ± 4.77 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hosseinabadi et al., 2020 [32] | GCE | 85.95 ± 11.76 | 84.30 ± 12.17 | p < 0.001 | 30.14 ± 2.60 | 29.54 ± 2.59 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hosseinabadi et al., 2020 [33] | 85.95 ± 11.76 | 84.30 ± 12.17 | p < 0.001 | 30.14 ± 2.60 | 29.54 ± 2.59 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Izadi et al., 2021 [34] | Sour tea | 87.72 ± 15.7 | 86.38 ± 16.1 | p < 0.001 | 31.65 ± 4.8 | 31.24 ± 4.8 | p = 0.001 | 110.28 ± 13.08 | 109.3 ± 12.4 | p = 0.012 | N/A | N/A | N/A | 8.03 ± 1.8 | 7.4 ± 1.01 | p = 0.02 | 12.75 ± 1.7 | 11.1 ± 0.60 | p < 0.001 |
| Namdar et al., 2024 [35] | Flaxseed oil | 85.93 ± 13.17 | 84.08 ± 9.50 | p = 0.089 | 29.14 ± 3.50 | 28.59 ± 2.97 | p = 0.094 | 98.23 ± 8.18 | 97.03 ± 6.70 | p = 0.372 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Khodadadi et al., 2024 [36] | Flaxseed powder | 84.66 ± 15.34 | 78.06 ± 12.74 | p < 0.001 | 30.37 ± 4.41 | 28.05 ± 3.89 | p < 0.001 | 100.08 ± 8.63 | 90.58 ± 19.32 | p = 0.028 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Tian et al., 2025 [37] | Golden flaxseed powder | 90.5 ± 12.1 | 88.1 ± 10.6 | NS | 31.33 (29.50, 34.40) | 30.67 (28.72, 32.62) | NS | N/A | N/A | N/A | 38.02 ± 5.10 | 31.62 ± 3.62 | p < 0.05 | N/A | N/A | N/A | N/A | N/A | N/A |
| Maleki Sedgi et al., 2024 [38] | Rapeseed oil | 81.1 ± 8.5 | 76.8 ± 9.1 | p < 0.001 | 28.1 ± 1.7 | 26.6 ± 1.8 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Mazloomi et al., 2022 [39] | Spirulina sauce | 69.34 ± 10.09 | 68.54 ± 9.10 | p = 0.06 | 24.82 ± 2.87 | 24.60 ± 2.88 | p = 0.07 | 93.78 ± 6.29 | 93.10 ± 5.11 | p = 0.26 | N/A | N/A | N/A | 87.60 ± 8.02 | 85.05 ± 6.07 | p = 0.17 | 130.04 ± 7.05 | 126.95 ± 7.12 | p = 0.14 |
| Rafie et al., 2020 [40] | Ginger powder | 88.61 ± 11.50 | 86.34 ± 10.85 | p < 0.001 | 31.78 ± 3.71 | 30.96 ± 3.41 | p < 0.001 | 105.15 ± 7.26 | 103.84 ± 7.25 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Soleimani et al., [43] | Garlic powder | 82.6 ± 14.3 | 80.4 ± 14 | p = 0.001 | 30.7 ± 5.2 | N/A | N/A | N/A | N/A | N/A | 27.7 ± 8.1 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sangouni et al., 2020 [42] | Garlic powder | I Group: 82.4 ± 14 | N/A | 30.7 ± 5.3 | N/A | N/A | 95.2 ± 10.7 | N/A | N/A | N/A | N/A | N/A | 8.9 ± 1.1 | −4 ± 0.84 ^ | N/A | 13.3 ± 1.2 | −6.74 ± 1.25 ^ | N/A | |
| Sangouni et al., 2020 [41] | Garlic powder | 89.8 ± 11.9 | 89.2 ± 11.8 | N/A | 30.2 ± 3.1 | 30.0 ± 3.1 | N/A | 105.6 ± 9.8 | 104.0 ± 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Change in Anthropometric Outcomes Following Interventions with Plant-Based Foods in MASLD | ||||||
|---|---|---|---|---|---|---|
| Compared Plant-Based Foods | BW | BMI | WC | BF | DBP | SBP |
| FRD [27] | p < 0.001 * | p < 0.001 * | p < 0.001 * | N/A | N/A | N/A |
| Whole oranges [28] | p = 0.43 | p = 0.17 | p = 0.91 | p = 0.97 | N/A | N/A |
| WGPs [29] | p = 0.34 | p = 0.65 | p = 0.10 | N/A | N/A | N/A |
| HFBs [30] | p = 0.35 | p = 0.057 | N/A | p = 0.18 | N/A | N/A |
| BJ [31] | N/A | p = 0.191 | p = 0.008 | N/A | N/A | N/A |
| GCE [32,44] | p < 0.001 | p < 0.001 | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | ||||
| Sour tea [34] | p < 0.05 | p < 0.05 | p < 0.05 | N/A | p < 0.05 | p < 0.05 |
| Flaxseed oil [35] | p = 0.052 | p = 0.662 | p = 0.175 | N/A | N/A | N/A |
| Flaxseed powder [36] | p = 0.058 | p = 0.058 | p = 0.219 | N/A | N/A | N/A |
| Golden flaxseed powder [37] | p < 0.05 | p < 0.05 | N/A | p < 0.05 | N/A | N/A |
| Rapeseed oil [38] | p < 0.001 | p < 0.001 | N/A | N/A | N/A | N/A |
| Spirulina sauce [39] | p = 0.16 | p = 0.57 | p = 0.35 | N/A | p = 0.06 | p = 0.68 |
| Ginger powder [40] | p = 0.773 | p = 0.544 | p = 0.221 | N/A | N/A | N/A |
| Garlic powder [41,42,43] | p = 0.010 | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | p < 0.001 | p < 0.001 | |
| p = 0.86 | p = 0.12 | p = 0.001 | p < 0.001 | N/A | N/A | |
| p = 0.86 | p = 0.12 | p = 0.001 | p < 0.001 | N/A | N/A | |
| Dietary Intervention with Plant-Based Food | Glucose Metabolism Outcomes After Intervention with Plant-Based Foods | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Insulin | HOMA-IR | HbA1c | ||||||||||
| B | PI | p | B | PI | p | B | PI | p | B | PI | p | ||
| Ref. | |||||||||||||
| Alami et al., 2022 [27] | FRD | 96.9 ± 9.4 | 115.5 ± 30.0 | p < 0.001 | 14.0 ± 5.7 | 26.6 ± 15.9 | p < 0.001 | 3.32 ± 1.41 | 7.36 ± 4.37 | p < 0.001 | N/A | N/A | N/A |
| Notarnicola et al., 2024 [28] | Whole oranges | 101.00 ± 21.62 | 99.59 ± 26.83 | p = 0.72 | 16.08 ± 8.55 | 16.42 ± 9.06 | p = 0.36 | 4.10 ± 2.50 | 4.19 ± 2.94 | p = 0.99 | N/A | N/A | N/A |
| Dorosti et al., 2020 [29] | WGPs | 88.1 ± 10.2 | 86.9 ± 8.2 | NS | 17.0 ± 9.3 | 14.9 ± 7.9 | p < 0.05 | 3.5 ± 0.3 | 3.2 ± 0.3 | p < 0.05 | N/A | N/A | N/A |
| Stachowska et al., 2022 [30] | HFBs | 96.1 (76.3–272.6) | N/A | 18.5 (4.3–129) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Fateh et al., 2023 [31] | BJ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hosseinabadi et al., 2020 [32] | GCE | 104.65 ± 9.09 | 92.15 ± 11.40 | p < 0.001 | 10.22 ± 4.04 | 10.41 ± 4.29 | p = 0.871 | 2.65 ± 1.09 | 2.42 ± 1.20 | p = 0.463 | N/A | N/A | N/A |
| Hosseinabadi et al., 2020 [33] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Izadi et al., 2021 [34] | Sour tea | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Namdar et al., 2024 [35] | Flaxseed oil | 98.67 ± 19.35 | 98.53 ± 22.44 | p = 0.776 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Khodadadi et al., 2024 [36] | Flaxseed powder | 104.00 ± 8.88 | 97.87 ± 8.02 | p < 0.001 | 10.99 ± 8.87 | 6.63 ± 5.77 | p < 0.001 | 2.80 ± 2.16 | 1.58 ± 1.32 | p < 0.001 | N/A | N/A | N/A |
| Tian et al., 2025 [37] | Golden flaxseed powder | 5.06 ± 0.56 | 5.10 ± 0.49 | NS | 113.36 (92.70, 136.60) | 106.86 (95.30, 146.40) | NS | N/A | N/A | N/A | N/A | N/A | N/A |
| Maleki Sedgi et al., 2024 [38] | Rapeseed oil | 98.7 ± 9.6 | 89.2 ± 9.3 | p < 0.001 | 13.2 ± 6.8 | 10.1 ± 5.3 | p = 0.002 | 3.2 ± 1.7 | 2.3± 1.4 | p = 0.001 | N/A | N/A | N/A |
| Mazloomi et al., 2022 [39] | Spirulina sauce | 91.43 ± 7.71 | 87.20 ± 7.80 | p = 0.18 | 8.30 ± 3.27 | 7.57 ± 2.36 | p = 0.29 | 1.90 ± 0.82 | 1.63 ± 0.56 | p = 0.10 | N/A | N/A | N/A |
| Rafie et al., 2020 [40] | Ginger powder | 107.52 ± 10.64 | 99.34 ± 12.57 | p = 0.007 | 13.38 ± 2.75 | 12.42 ± 2.53 | p = 0.017 | 3.72± 0.76 | 3.07 ± 0.80 | p = 0.001 | N/A | N/A | N/A |
| Soleimani et al. [43] | Garlic powder | 124.2 ± 37 | 115.8 ± 39.3 | p = 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | 6.27 ± 1.5 | 6.04 ± 1.6 | p = 0.028 |
| Sangouni et al., 2020 [42] | Garlic powder | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sangouni et al., 2020 [41] | Garlic powder | 90.1 ± 7.8 | 86.9 ± 8.2 | N/A | 8.5 ± 2.7 | 5.6 ± 2.5 | N/A | 1.88 ± 0.6 | 1.21 ± 0.5 | N/A | N/A | N/A | N/A |
| Dietary Intervention with Plant-Based Food | Lipid Metabolism Outcomes After Intervention with Plant-Based Foods | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triglycerides | Cholesterol | LDL-C | HDL-C | ||||||||||
| B | PI | p | B | PI | p | B | PI | p | B | PI | p | ||
| Alami et al., 2022 [27] | FRD | 183.2 ± 100.8 | 248.6 ± 125.0 | p < 0.001 | 174.6 ± 35.5 | 206.1 ± 40.5 | p < 0.001 | 99.9 ± 29.4 | 126.9 ± 32.3 | p < 0.001 | 50.4 ± 11.1 | 41.4 ± 8.9 | p < 0.001 |
| Notarnicola et al., 2024 [28] | Whole oranges | 132.10 ± 53.32 | 123.06 ± 53.55 | p = 0.72 | 202.29 ± 40.25 | 193.39 ± 40.83 | p = 0.28 | 132.11 ± 37.64 | 130.94 ± 35.58 | p = 0.47 | 47.10 ± 13.15 | 47.53 ± 10.74 | p = 0.72 |
| Dorosti et al., 2020 [29] | WGPs | 167.9± 13.6 | 156.7 ± 11.6 | NS | 192.4 ± 40.9 | 174.1 ± 37.3 | p < 0.001 | 114.4 ± 4.5 | 101.5 ± 4.2 | p < 0.05 | 41.2 ± 7.0 | 43.0 ± 5.9 | p < 0.05 |
| Stachowska et al., 2022 [30] | HFBs | 150.5 (50.9–452) | N/A | 178.2 (98–340.2) | N/A | 114.4 (47.9–258.3) | N/A | 44.9 (25.8–77.5) | N/A | ||||
| Fateh et al., 2023 [31] | BJ | 233.5 ± 14.1 | 217.6 ± 11.6 | p < 0.001 | 228.9 ± 6.2 | 210.5 ± 3.7 | p < 0.001 | 140.2 ± 9.2 | 132.2 ± 5.8 | p < 0.001 | 29.8 ± 3.9 | 36.6 ± 4.2 | p < 0.001 |
| Hosseinabadi et al., 2020 [32] | GCE | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hosseinabadi et al., 2020 [33] | 187.04 ± 106.04 | 159.14 ± 74.96 | p = 0.10 | 231.66 ± 43.70 | 218.33± 38.52 | p = 0.04 | 138.25 ± 29.55 | 127.92 ±30.87 | p = 0.06 | 55.04 ± 10.03 | 58.47 ± 8.71 | p = 0.08 | |
| Izadi et al., 2021 [34] | Sour tea | 165.9 ± 74.5 | 146.8 ± 61.2 | p = 0.008 | 197.2 ± 45.4 | 188.6 ± 45.1 | p = 0.03 | 122.6 ± 18.9 | 112.9 ± 20.7 | p = 0.008 | 41.6 ± 8.5 | 43.2 ± 9.4 | p = 0.130 |
| Namdar et al., 2024 [35] | Flaxseed oil | 207.27 ± 86.38 | 188.77 ± 61.54 | p = 0.371 | 203.17 ± 41.64 | 215.83 ± 32.97 | p = 0.175 | 120.3 ± 38.94 | 110.83 ± 29.20 | p = 0.066 | 40.96 ± 11.79 | 45.57 ± 9.06 | p = 0.003 |
| Khodadadi et al., 2024 [36] | Flaxseed powder | 206.17 ± 82.01 | 144.83 ± 59.76 | p < 0.001 | 203.42 ± 35.00 | 171.70 ± 26.49 | p < 0.001 | 125.93 ± 27.12 | 103.28 ± 22.62 | p < 0.001 | 36.25 ± 13.82 | 39.45 ± 12.31 | p = 0.072 |
| Tian et al., 2025 [37] | Golden flaxseed powder | 2.11 ± 0.74 | 1.71 ± 0.63 | p < 0.05 | 5.22 ± 0.80 | 4.75 ± 0.78 | p < 0.05 | 3.43 ± 0.76 | 3.20 ± 0.98 | NS | 1.01 ± 0.09 | 1.12 ± 0.17 | p < 0.05 |
| Maleki Sedgi et al., 2024 [38] | Rapeseed oil | N/A | N/A | N/A | 184.4 ±50 | 167.2 ± 40.8 | p < 0.001 | 106.6 ± 99.7 | 99.7 ± 28.4 | p = 0.008 | N/A | N/A | N/A |
| Mazloomi et al., 2022 [39] | Spirulina sauce | 165.30 ± 41.20 | 138.65 ± 41.70 | p = 0.03 | 202.48 ± 45 | 186.75 ± 49.86 | p = 0.14 | 126.96 ± 45.18 | 116.60 ± 41.76 | p = 0.11 | 42.43 ± 8.29 | 46.40 ± 11.64 | p = 0.02 |
| Rafie et al., 2020 [40] | Ginger powder | 200.60 ± 48.56 | 196.43 ± 46.24 | p = 0.503 | 220.82 ±45.95 | 196.13 ±36.23 | p = 0.006 | 136.59 ± 45.70 | 113.56 ± 37.90 | p = 0.010 | 43.69 ± 7.43 | 44.73 ± 6.54 | p = 0.341 |
| Sangouni et al., 2020 [41] | Garlic powder | 169.2 ± 67.5 | 148.8 ± 74.7 | p = 0.002 | 184.2 ± 32.5 | 171.4 ± 31.9 | p = 0.005 | 111.5 ± 28.1 | 99.1 ± 27.5 | p = 0.002 | 40.5 ± 8.8 | 42.7 ± 10.22 | p = 0.06 |
| Sangouni et al., 2020 [42] | Garlic powder | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [41] | Garlic powder | 2.1 ± 0.9 | 1.7 ± 0.7 | N/A | 5.3 ± 1.0 | 5.0 ± 0.9 | N/A | 3.2 ± 0.7 | 2.9 ± 0.6 | N/A | 1.1 ± 0.2 | 1.2 ± 0.2 | N/A |
| Dietary Intervention with Plant-Based Food | Inflammatory Outcomes After Intervention with Plant-Based Foods | ||||||
|---|---|---|---|---|---|---|---|
| hs-CRP | LPS (pg/mL) | ||||||
| B | PI | p | B | PI | p | ||
| Alami et al., 2022 [27] | FRD | N/A | N/A | N/A | N/A | N/A | N/A |
| Notarnicola et al., 2024 [28] | Whole oranges | 0.34 ± 0.43 | 0.30 ± 0.41 | p = 0.58 | N/A | N/A | N/A |
| Dorosti et al., 2020 [29] | WGPs | N/A | N/A | N/A | N/A | N/A | N/A |
| Stachowska et al., 2022 [30] | HFBs | N/A | N/A | N/A | 153 (0–481) | N/A | |
| Fateh et al., 2023 [31] | BJ | N/A | N/A | N/A | N/A | N/A | N/A |
| Hosseinabadi et al., 2020 [32] | GCE | N/A | N/A | N/A | N/A | N/A | N/A |
| Hosseinabadi et al., 2020 [33] | N/A | N/A | N/A | N/A | N/A | N/A | |
| Izadi et al., 2021 [34] | Sour tea | N/A | N/A | N/A | N/A | N/A | N/A |
| Namdar et al., 2024 [35] | Flaxseed oil | N/A | N/A | N/A | N/A | N/A | N/A |
| Khodadadi et al., 2024 [36] | Flaxseed powder | 4.70 ± 2.07 | 3.47 ± 1.46 | p = 0.012 | N/A | N/A | N/A |
| Tian et al., 2025 [37] | Golden flaxseed powder | N/A | N/A | N/A | N/A | N/A | N/A |
| Maleki Sedgi et al., 2024 [38] | Rapeseed oil | N/A | N/A | N/A | N/A | N/A | N/A |
| Mazloomi et al., 2022 [39] | Spirulina sauce | N/A | N/A | N/A | N/A | N/A | N/A |
| Rafie et al., 2020 [40] | Ginger powder | 2.40 (1.14, 3.58) | 1.82 (0.88, 3.18) | p = 0.001 | N/A | N/A | N/A |
| Sangouni et al., 2020 [41] | Garlic powder | N/A | N/A | N/A | N/A | N/A | N/A |
| Sangouni et al., 2020 [42] | Garlic powder | N/A | N/A | N/A | N/A | N/A | N/A |
| Soleimani et al. [43] | Garlic powder | N/A | N/A | N/A | N/A | N/A | N/A |
| Change in Glucose and Lipid Metabolism Outcomes Along with Inflammatory Status After Interventions with Plant-Based Foods in MASLD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compared Plant-Based Foods | Glucose | Insulin | HOMA-IR | HbA1c | Triglycerides | Cholesterol | LDL-C | HDL-C | hs-CRP | LPS |
| FRD [27] | p < 0.001 * | p < 0.001 * | p < 0.001 * | N/A | p < 0.001 * | p < 0.001 * | p < 0.001 * | p < 0.001 | N/A | N/A |
| Whole oranges [28] | p = 0.09 | p = 0.58 | p = 0.94 | N/A | p = 0.66 | p = 0.07 | p = 0.69 | p = 0.92 | p = 0.79 | N/A |
| WGPs [29] | p = 0.020 | p = 0.015 | p = 0.016 | N/A | p = 0.11 | p = 0.004 | p = 0.014 | p = 0.54 | N/A | N/A |
| HFBs [30] | p = 0.63 | p = 0.52 | N/A | N/A | p = 0.14 | p = 0.04 | p = 0.06 | p = 0.36 | N/A | p = 1 |
| BJ [31] | N/A | N/A | N/A | N/A | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | N/A | N/A |
| GCE [32,44] | p = 0.006 | p = 0.113 | p = 0.028 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | p = 0.32 | p = 0.36 | p = 0.33 | p = 0.04 | N/A | N/A | |
| Sour tea [34] | N/A | N/A | N/A | N/A | p = 0.03 | p = 0.61 | p = 0.55 | p = 0.55 | N/A | N/A |
| Flaxseed oil [35] | p = 0.016 | N/A | N/A | N/A | p = 0.947 | p = 0.420 | p = 0.520 | p = 0.80 | N/A | N/A |
| Flaxseed powder [36] | p = 0.379 | p < 0.001 | p = 0.002 | N/A | p < 0.001 | p = 0.028 | p = 0.552 | p = 0.638 | p = 0.598 | N/A |
| Golden flaxseed powder [37] | NS | NS | N/A | N/A | p < 0.05 | NS | p < 0.05 | p < 0.05 | N/A | N/A |
| Rapeseed oil [38] | p < 0.001 | p < 0.001 | p < 0.001 | N/A | N/A | p = 0.006 | p = 0.07 | N/A | N/A | N/A |
| Spirulina sauce [39] | p = 0.55 | p = 0.08 | p = 0.047 | N/A | p = 0.02 | p = 0.15 | p = 0.17 | p = 0.07 | N/A | N/A |
| Ginger powder [40] | p = 0.029 | p = 0.559 | p = 0.047 | N/A | p = 0.823 | p = 0.026 | p = 0.032 | p = 0.948 | p = 0.006 | N/A |
| Garlic powder [41,42,43] | p = 0.001 | N/A | N/A | p = 0.001 | p = 0.022 | p = 0.005 | p = 0.005 | p = 0.556 | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| N/A | N/A | N/A | N/A | p < 0.001 | p = 0.02 | p = 0.01 | p < 0.001 | N/A | N/A | |
| p = 0.02 | p = 0.001 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Dietary Intervention with Plant-Based Food | Liver Function Outcomes After Intervention with Plant-Based Foods | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatic Enzymes | Hepatic Steatosis | Liver Fibrosis | ||||||||||||||
| AST | ALT | ALP | CAP | Hepatic Inflammation (FLI) | Grade of Fatty Liver | |||||||||||
| B | PI | p | B | PI | p | B | PI | p | B | PI | p | |||||
| Alami et al., 2022 [27] | FRD | 26.8 ± 11.0 | 74.5 ± 107.8 | p < 0.001 | 38.1 ± 25.3 | 89.1 ± 92.9 | p < 0.001 | 189.4 ± 73.2 | 273.4 ± 128.5 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A |
| Notarnicola et al., 2024 [28] | Whole oranges | 23.42 ± 9.93 | 24.29 ± 7.29 | p = 0.05 | 36.68 ± 23.74 | 34.93 ± 18.50 | p = 0.28 | 67.64 ± 19.99 | 68.74 ± 19.88 | p = 0.58 | N/A | N/A | N/A | N/A | N/A | N/A |
| Dorosti et al., 2020 [29] | WGPs | 27.7 ± 13.6 | 21.9 ± 6.8 | p < 0.001 | 34.6 ± 12.5 | 24.1 ± 12.2 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | B—Normal Liver: 0; Grade 1: 24 (51.1%); Grade 2: 20 (42.6%); Grade 3: 3 (6.4%); PI—Normal Liver: 17 (36.2%); Grade 1: 23 (48.9%); Grade 2: 7 (14.9%); Grade 3: 0 (0%) | N/A |
| Stachowska et al., 2022 [30] | HFBs | 24 (13–40) | N/A | 35 (11–86) | N/A | N/A | N/A | N/A | 277 (224–371)/95% CI: 274.94–310.68 | N/A | N/A | N/A | FibroScan: 5.3 (3.6–9.7)/95% CI: 5.02–6.13 | |||
| Fateh et al., 2023 [31] | BJ | 61.43 ± 8.85 | 58.18 ± 6.33 | p < 0.001 | 37.63 ± 3.45 | 36.61 ± 5.87 | p = 0.320 | 119.0 ± 8.5 | 113.6 ± 7.6 | p < 0.001 | N/A | N/A | N/A | FLI: B—79; PI—42; NS | Change in the liver fat content after intervention—PI—reduction 1 Grade: 23; reduction 2 Grades: 10; no change: 12 | N/A |
| Hosseinabadi et al., 2020 [32], Hosseinabadi et al., 2020 [33] | GCE | 35.71 ± 22.63 | 32.66 ± 16.74 | p = 0.48 | 43.85 ± 25.82 | 44.52 ± 30.08 | p = 0.90 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | B—Normal Liver: 0; Grade 1: 12; Grade 2: 8; Grade 3: 1; PI—Normal Liver: 1; Grade 1: 12; Grade 2: 8; Grade 3: 0 | N/A |
| Izadi et al., 2021 [34] | Sour tea | 45.5 ± 13.4 | 39.8 ± 12.7 | p = 0.04 | 35.16 ± 18.5 | 30.53 ± 13.4 | p = 0.01 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Namdar et al., 2024 [35] | Flaxseed oil | 54.5 ± 19.87 | 33.37 ± 8.07 | p < 0.001 | 89.13 ± 39.30 | 48.17 ± 14.11 | p < 0.001 | 209 ± 50.26 | 167.43 ± 38.15 | p < 0.001 | N/A | N/A | N/A | N/A | B—Normal Liver: 0 (0%); Grade 1: 0 (0%); Grade 2: 48 (87.3%); Grade 3: 7 (12.7%); PI—Normal Liver: 8 (14.54%); Grade 1: 31 (56.36%); Grade 2: 16 (9.1%); Grade 3: 0 (0%) | N/A |
| Khodadadi et al., 2024 [36] | Flaxseed powder | 28.13 ± 16.94 | 17.00 ± 7.05 | p < 0.001 | 24.67 ± 8.39 | 19.29 ± 5.83 | p = 0.003 | N/A | N/A | N/A | 306.62 ± 32.77 | 259.62 ± 38.48 | p < 0.001 | N/A | N/A | Fibrosis score—B: 6.01 ± 1.96; PI: 4.75 ± 1.29; p < 0.001 |
| Tian et al., 2025 [37] | Golden flaxseed powder | 23.00 (21.50, 28.00) | 18.00 (16.00, 23.00) | p < 0.05 | 40.00 (34.00, 57.00) | 37.00 (28.00, 49.00) | NS | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Maleki Sedgi et al., 2024 [38] | Rapeseed oil | 27.5 ± 12.1 | 20.1 ± 6.2 | p < 0.001 | 42.7 ± 31.9 | 28.3 ± 14.3 | p < 0.001 | 167.4 ± 42.5 | 173.6 ± 44.1 | p = 0.105 | N/A | N/A | N/A | N/A | N/A | N/A |
| Mazloomi et al., 2022 [39] | Spirulina sauce | 23.13 ± 2.71 | 18.95 ± 2.72 | p < 0.001 | 38.86 ± 4.09 | 33.25 ± 4.52 | p < 0.001 | 43.17 ± 5.73 | 40.55 ± 4.24 | p = 0.08 | N/A | N/A | N/A | N/A | N/A | N/A |
| Rafie et al., 2020 [40] | Ginger powder | 32.69 ± 5.23 | 31.08 ± 7.85 | p = 0.312 | 42.04 ± 8.92 | 32.21 ± 7.12 | p < 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | FLI: B—89.43 (52.5,97.3); PI—85.21 (39.2,96); p < 0.001 | Change in the liver fat content after intervention—B: Grade 1–12 (12%); Grade 2–8 (34.78%); Grade 3–3 (13.04%); PI: Grade 1- 13 (56.52%); Grade 2–7 (30.43%); Grade 3–3 (13.04%) | N/A |
| Soleimani et al., 2020 [43] | Garlic powder | 48.3 ± 11.6 | 42.2 ± 11.2 | p = 0.001 | 57.8 ± 13.9 | 47.2 ± 16.1 | p = 0.001 | N/A | N/A | N/A | MASLD progression PI change in hepatic steatosis—improved: 51.1%; unchanged: 46.8%; worsened: 2.1%; p < 0.001 | N/A | N/A | N/A | ||
| Sangouni et al., 2020 [42] | Garlic powder | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | MASLD stage at B—Mild: 14 (29.7%); Moderate: 29 (61.7%); Severe: 4 (8.6%) | N/A |
| Sangouni et al., 2020 [41] | Garlic powder | 22.8± 11.1 | 20.6 ± 8.6 | N/A | 30.9± 15.7 | 26.0± 13.2 | N/A | 203.8± 56.9 | 200.3± 49.0 | N/A | N/A | N/A | N/A | N/A | B—Grade 1: 11 (24.4%); Grade 2: 29 (64.4%); Grade 3: 5 (11.2%); PI—reduction 1 Grade: 28 (62.2%); reduction 2 Grades: 2 (4.4%); no change: 15 (33.7%); 1 Grade increase: 0 (0%) | N/A |
| Change in Liver Function Outcomes After Interventions with Plant-Based Foods in MASLD | |||||||
|---|---|---|---|---|---|---|---|
| Dietary Intervention with Plant-Based Food | Hepatic Enzymes | Hepatic Steatosis | Liver Fibrosis | ||||
| AST | ALT | ALP | CAP | Hepatic Inflammation (FLI) | Grade of Fatty liver | ||
| FRD [27] | p < 0.001 * | p < 0.001 * | p < 0.001 * | N/A | N/A | N/A | N/A |
| Whole oranges [28] | p = 0.11 | p = 0.45 | p = 0.66 | p < 0.004 | N/A | N/A | N/A |
| WGPs [29] | p < 0.001 | p < 0.001 | N/A | N/A | N/A | p < 0.001 | N/A |
| HFBs [30] | p = 1 | p = 1 | N/A | p = 0.04 | N/A | N/A | N/A |
| BJ [31] | p = 0.014 | p < 0.001 | p < 0.001 | N/A | p < 0.001 | N/A | N/A |
| GCE [32,44] | p = 0.757 | p = 0.268 | N/A | N/A | N/A | N/A | N/A |
| p = 0.086 | p = 0.26 | N/A | N/A | N/A | p = 0.76 | N/A | |
| Sour tea [34] | p = 0.004 | p = 0.01 | N/A | N/A | N/A | N/A | N/A |
| Flaxseed oil [35] | p = 0.010 | p = 0.047 | p < 0.001 | N/A | N/A | N/A | N/A |
| Flaxseed powder [36] | p < 0.001 | p = 0.406 | N/A | p = 0.276 | N/A | N/A | p = 0.032 |
| Golden flaxseed powder [37] | p = 0.05 | NS | N/A | N/A | N/A | N/A | N/A |
| Rapeseed oil [38] | p = 0.119 | p = 0.051 | p = 0.004 | N/A | N/A | p < 0.001 | N/A |
| Spirulina sauce [39] | p = 0.02 | p = 0.03 | p = 0.70 | N/A | N/A | N/A | N/A |
| Ginger powder [40] | N/A | N/A | N/A | N/A | p = 0.116 | N/A | N/A |
| Garlic powder [41,42,43] | p = 0.001 | p = 0.001 | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | p = 0.29 | N/A | |
| p = 0.010 | p < 0.001 | p = 0.65 | N/A | N/A | p = 0.001 | N/A | |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek, J.M.; Zablocka-Slowinska, K.; Pieczynska, J.; Clavero Mestres, H.; Auguet, T. Systematic Review of the Effects of Plant-Based Foods on Metabolic Outcomes in Adults with MASLD and Comorbidities Such as Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2025, 17, 3020. https://doi.org/10.3390/nu17183020
Jurek JM, Zablocka-Slowinska K, Pieczynska J, Clavero Mestres H, Auguet T. Systematic Review of the Effects of Plant-Based Foods on Metabolic Outcomes in Adults with MASLD and Comorbidities Such as Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients. 2025; 17(18):3020. https://doi.org/10.3390/nu17183020
Chicago/Turabian StyleJurek, Joanna Michalina, Katarzyna Zablocka-Slowinska, Joanna Pieczynska, Helena Clavero Mestres, and Teresa Auguet. 2025. "Systematic Review of the Effects of Plant-Based Foods on Metabolic Outcomes in Adults with MASLD and Comorbidities Such as Obesity, Metabolic Syndrome, and Type 2 Diabetes" Nutrients 17, no. 18: 3020. https://doi.org/10.3390/nu17183020
APA StyleJurek, J. M., Zablocka-Slowinska, K., Pieczynska, J., Clavero Mestres, H., & Auguet, T. (2025). Systematic Review of the Effects of Plant-Based Foods on Metabolic Outcomes in Adults with MASLD and Comorbidities Such as Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients, 17(18), 3020. https://doi.org/10.3390/nu17183020








