A 12-Week Randomized Controlled Trial of Nutrition and Exercise Education with Dietary Supplementation for Sarcopenia Prevention in Korean Baby Boomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size, Randomization, and Allocation
2.4. Interventions
2.5. Outcome Measures and Data Collection
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Baseline Data of Participants
3.2. Changes in Dietary Intake
3.3. Changes in Body Composition and Physical Function
3.4. Changes in Blood Biomakers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AWGS | Asian Working Group for Sarcopenia |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| COVID-19 | Coronavirus Disease 2019 |
| DiEx | Nutrition and exercise education only |
| DiExSp | Nutrition and exercise education plus dietary supplementation |
| ESCEO | European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein |
| ICSFR | International Conference on Frailty and Sarcopenia Research |
| IRB | Institutional Review Board |
| LDL | Low-density lipoprotein |
| METs | Metabolic Equivalent of Task |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| RCTs | Randomized controlled trials |
| SarQoL-K | Korean version of the Sarcopenia Quality of Life |
| SD | Standard deviation |
| SFA | Saturated fatty acids |
| SPPB | Short Physical Performance Battery |
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2020: Highlights; United Nations: New York, NY, USA, 2020. [Google Scholar]
- Norman, K.; Otten, L. Financial impact of sarcopenia or low muscle mass—A short review. Clin. Nutr. 2019, 38, 1489–1495. [Google Scholar] [CrossRef]
- Goates, S.; Du, K.; Arensberg, M.B.; Gaillard, T.; Guralnik, J.; Pereira, S.L. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J. Frailty Aging 2019, 8, 93–99. [Google Scholar] [CrossRef]
- Sowers, M.R.; Crutchfield, M.; Richards, K.; Wilkin, M.K.; Furniss, A.; Jannausch, M.; Zhang, D.; Gross, M. Sarcopenia is related to physical functioning and leg strength in middle-aged women. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 486–490. [Google Scholar] [CrossRef]
- Robinson, S.; Cooper, C.; Sayer, A.A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. J. Aging Res. 2012, 2012, 510801. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.J.; Jung, S.H. The Ageing Society of Korea and the Population Estimate. Korea J. Popul. Stud. 2011, 34, 113–133. [Google Scholar]
- Han, C.; Hong, Y.-C. Fetal and childhood malnutrition during the Korean War and metabolic syndrome in adulthood. Nutrition 2019, 62, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Lee, G.; Hong, B.; Lee, S.; Kim, S.; Kwon, J. Policy Response to the Retirement and Ageing of Baby Boomers; Ministry of Health and Welfare, Korean Institute of Health and Social Affairs: Seoul, Republic of Korea, 2011.
- Sunwoo, D. Health status and long-term care needs of the baby boom generation. In Health Welf Policy Forum; Korea Institute for Health and Social Affairs: Seoul, Republic of Korea, 2011; pp. 19–27. [Google Scholar]
- Jang, E.-H.; Han, Y.-J.; Jang, S.-E.; Lee, S. Association between diet quality and sarcopenia in older adults: Systematic review of prospective cohort studies. Life 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Van Elswyk, M.E.; Teo, L.; Lau, C.S.; Shanahan, C.J. Dietary Patterns and the Risk of Sarcopenia: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2022, 6, nzac001. [Google Scholar] [CrossRef]
- Bao, W.; Sun, Y.; Zhang, T.; Zou, L.; Wu, X.; Wang, D.; Chen, Z. Exercise Programs for Muscle Mass, Muscle Strength and Physical Performance in Older Adults with Sarcopenia: A Systematic Review and Meta-Analysis. Aging Dis. 2020, 11, 863–873. [Google Scholar] [CrossRef]
- Beckwee, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Hernandez-Lepe, M.A.; Miranda-Gil, M.I.; Valbuena-Gregorio, E.; Olivas-Aguirre, F.J. Exercise Programs Combined with Diet Supplementation Improve Body Composition and Physical Function in Older Adults with Sarcopenia: A Systematic Review. Nutrients 2023, 15, 1998. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; McCloskey, E.; Bruyere, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertiere, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.-E.; Hwang, H.-S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-I.; Ha, Y.-C.; Kim, M.; Seo, S.-H.; Kim, M.-J.; Lee, G.-Y.; Seo, Y.-M.; Sung, C.; Park, K.-S. Translation and validation of the Korean version of the Sarcopenia Quality of Life (SarQoL-K®) questionnaire and applicability with the SARC-F screening tool. Qual. Life Res. 2021, 30, 603–611. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. General. 2012, 141, 2. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 21, 19–25. [Google Scholar]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated guideline for reporting randomised trials. BMJ 2025, 388, e081123. [Google Scholar] [CrossRef]
- Nowson, C.; O’Connell, S. Protein requirements and recommendations for older people: A review. Nutrients 2015, 7, 6874–6899. [Google Scholar] [CrossRef] [PubMed]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and sarcopenia: Potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.; Tieland, M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: A systematic review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Duan, L.; Zhao, Y.; He, Y.; Li, W.; Cui, J. Associations of micronutrient dietary patterns with sarcopenia among US adults: A population-based study. Front. Nutr. 2024, 11, 1301831. [Google Scholar] [CrossRef]
- Sung, J.H.; Son, S.R.; Baek, S.-H.; Kim, B.-J. The association of aerobic, resistance, and combined exercises with the handgrip strength of middle-aged and elderly Korean adults: A nationwide cross-sectional study. BMC Geriatr. 2022, 22, 676. [Google Scholar] [CrossRef]
- Hua-Rui, L.; Shouliang, H.; Zhengze, Y.; Ning, J.; Peihua, L.; Yifei, Z.; Fenglin, P. Optimal dose of resistance training to improve handgrip strength in older adults with sarcopenia: A systematic review and Bayesian model-based network meta-analysis. Front. Physiol. 2025, 16, 1564988. [Google Scholar] [CrossRef]
- Romare, M.; Elcadi, G.H.; Johansson, E.; Tsaklis, P. Relative neuroadaptive effect of resistance training along the descending neuroaxis in older adults. Brain Sci. 2023, 13, 679. [Google Scholar] [CrossRef]
- Tettamanzi, F.; Bagnardi, V.; Louca, P.; Nogal, A.; Monti, G.S.; Mambrini, S.P.; Lucchetti, E.; Maestrini, S.; Mazza, S.; Rodriguez-Mateos, A. A high protein diet is more effective in improving insulin resistance and glycemic variability compared to a mediterranean diet—A cross-over controlled inpatient dietary study. Nutrients 2021, 13, 4380. [Google Scholar] [CrossRef]
- Cheah, K.J.; Cheah, L.J. Benefits and side effects of protein supplementation and exercise in sarcopenic obesity: A scoping review. Nutr. J. 2023, 22, 52. [Google Scholar] [CrossRef]
- Lei, X.; Zhou, Q.; Wang, Y.; Fu, S.; Li, Z.; Chen, Q. Serum and supplemental vitamin D levels and insulin resistance in T2DM populations: A meta-analysis and systematic review. Sci. Rep. 2023, 13, 12343. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Rouhani, P.; Saneei, P. Dietary calcium intake in relation to type-2 diabetes and hyperglycemia in adults: A systematic review and dose–response meta-analysis of epidemiologic studies. Sci. Rep. 2022, 12, 1050. [Google Scholar] [CrossRef]
- Zhang, F.; Huai, R.; Jia, F.; Cui, Y.; Wang, H.; Shen, X. Association between mixed dietary B vitamin intake and insulin resistance in US middle-aged and older adults without diabetes: The Bayesian kernel machine regression approach. Asia Pac. J. Clin. Nutr. 2022, 31, 768–779. [Google Scholar]
- Liu, Z.-j.; Zhu, C.-f. Causal relationship between insulin resistance and sarcopenia. Diabetol. Metab. Syndr. 2023, 15, 46. [Google Scholar] [CrossRef]
- Chapman, I.; Oberoi, A.; Giezenaar, C.; Soenen, S. Rational use of protein supplements in the elderly—Relevance of gastrointestinal mechanisms. Nutrients 2021, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, X.; Feng, Y.; Ainsworth, B.E.; Liu, Y. Effects of resistance training in healthy older people with sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Eur. Rev. Aging Phys. Act. 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Mansson, L.; Pettersson, B.; Rosendahl, E.; Skelton, D.A.; Lundin-Olsson, L.; Sandlund, M. Feasibility of performance-based and self-reported outcomes in self-managed falls prevention exercise interventions for independent older adults living in the community. BMC Geriatr. 2022, 22, 147. [Google Scholar] [CrossRef] [PubMed]


| Variables | Control Group (n = 9) | DiEx Group (n = 10) | DiExSp Group (n = 12) | p a |
|---|---|---|---|---|
| Age (yrs) | 63.89 ± 2.62 | 63.20 ± 1.99 | 63.58 ± 2.43 | 0.744 |
| Women (%) | 7 (77.8) | 10 (100.0) | 11 (91.7) | 0.361 |
| Smoking status | 1.000 | |||
| Non-smoker | 8 (88.9) | 9 (90.0) | 10 (83.3) | |
| Former smoker | 1 (11.1) | 1 (10.0) | 2 (16.7) | |
| Current smoker | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Alcohol consumption status | 0.819 | |||
| Non-drinker | 5 (55.6) | 4 (40.0) | 6 (50.0) | |
| Drinker | 4 (44.4) | 6 (60.0) | 6 (50.0) | |
| Physical activity (METs-min/week) | 0.838 | |||
| METs < 600 | 1 (11.1) | 0 (0.0) | 1 (8.3) | |
| 600 ≤ METs < 3000 | 6 (66.7) | 8 (80.0) | 7 (58.3) | |
| 3000 ≤ METs | 2 (22.2) | 2 (20.0) | 4 (33.3) | |
| Monthly household income | 0.722 | |||
| Low (<1,000,000 won) | 0 (0.0) | 1 (10.0) | 1 (8.3) | |
| Lower-middle (1,000,000–<2,000,000 won) | 1 (11.1) | 0 (0.0) | 2 (16.7) | |
| Upper-middle (2,000,000–<4,000,000 won) | 6 (66.7) | 5 (50.0) | 4 (33.3) | |
| High (≥4,000,000 won) | 2 (22.2) | 4 (40.0) | 5 (41.7) | |
| Education Levels | 0.273 | |||
| Elementary school or below | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Middle school graduate | 2 (22.2) | 1 (10.0) | 2 (16.7) | |
| High school graduate | 3 (33.3) | 3 (30.0) | 8 (66.7) | |
| College graduate or above | 4 (44.5) | 6 (60.0) | 2 (16.7) | |
| Subjective health status | 2.44 ± 0.73 | 2.70 ± 0.67 | 2.83 ± 0.72 | 0.386 |
| Sarcopenia-specific Quality of Life score b | 75.61 ± 10.91 | 70.84 ± 13.74 | 73.16 ± 8.83 | 0.799 |
| Variables | Control Group (n = 9) | p a | r b | DiEx Group (n = 10) | p a | r b | DiExSp Group (n = 12) | p a | r b | p c | ε2 d | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Week | Baseline | 12-Week | Baseline | 12-Week | |||||||||
| Energy (kcal) | 1275.3 ± 256.4 | 1411.5 ± 470.5 | 0.570 | 0.22 | 1483.7 ± 335.3 | 1473.8 ± 361.7 | 0.922 | 0.05 | 1416.9 ± 275.9 | 1691.8 ± 317.6 | 0.077 | 0.52 | 0.309 | 0.00 |
| Carbohydrate (g) | 181.0 ± 37.4 | 196.2 ± 57.8 | 0.652 | 0.18 | 191.2 ± 54.7 | 200.5 ± 43.0 | 0.695 | 0.15 | 202.9 ± 51.6 | 203.5 ± 35.5 | 0.970 | 0.02 | 0.861 | 0.00 |
| Fat (g) | 37.8 ± 12.4 | 41.6 ± 19.4 | 0.426 | 0.30 | 48.7 ± 15.3 | 42.9 ± 15.6 | 0.432 | 0.27 | 37.1 ± 11.9 | 52.5 ± 15.5 | 0.021 | 0.66 | 0.110 | 0.00 |
| Protein (g) | 55.3 ± 15.8 | 62.0 ± 27.6 | 0.734 | 0.14 | 68.4 ± 17.9 | 71.3 ± 26.6 | 0.922 | 0.05 | 56.5 ± 11.5 | 101.6 ± 24.4 | <0.001 | 0.88 | 0.004 | 0.01 |
| Plant protein (g) | 25.8 ± 6.1 | 28.1 ± 11.5 | 1.000 | 0.02 | 27.7 ± 11.1 | 30.9 ± 8.7 | 0.557 | 0.21 | 25.1 ± 5.9 | 31.6 ± 11.5 | 0.176 | 0.41 | 0.607 | 0.00 |
| Animal protein (g) | 26.1 ± 9.5 | 33.0 ± 20.6 | 0.301 | 0.38 | 37.4 ± 14.3 | 39.9 ± 23.0 | 0.770 | 0.11 | 29.9 ± 9.5 | 42.6 ± 15.6 | 0.034 | 0.61 | 0.199 | 0.00 |
| Total dietary fiber (g) | 24.7 ± 6.5 | 21.0 ± 7.1 | 0.250 | 0.41 | 25.0 ± 9.3 | 22.7 ± 6.0 | 0.625 | 0.18 | 20.9 ± 7.2 | 28.2 ± 8.7 | 0.110 | 0.48 | 0.079 | 0.01 |
| Total sugars (g) | 38.6 ± 16.8 | 29.8 ± 13.5 | 0.301 | 0.38 | 50.9 ± 20.2 | 36.2 ± 17.5 | 0.064 | 0.60 | 40.7 ± 21.6 | 32.9 ± 12.9 | 0.301 | 0.32 | 0.816 | 0.00 |
| Variables | Control Group (n = 9) | p a | r b | DiEx Group (n = 10) | p a | r b | DiExSp Group (n = 12) | p a | r b | p c | ε2 d | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Week | Baseline | 12-Week | Baseline | 12-Week | |||||||||
| Height (cm) | 158.3 ± 8.7 | 158.3 ± 8.7 | - | - | 155.7 ± 4.5 | 155.9 ± 4.3 | - | - | 156.0 ± 6.5 | 156.0 ± 6.5 | - | - | 0.350 | 0.00 |
| Weight (kg) | 58.3 ± 10.3 | 58.1 ± 9.7 | 0.844 | 0.10 | 56.8 ± 6.8 | 57.0 ± 6.6 | 0.449 | 0.24 | 59.5 ± 8.5 | 59.5 ± 8.0 | 0.952 | 0.02 | 0.893 | 0.00 |
| BMI (kg/m2) | 23.2 ± 3.0 | 22.8 ± 2.5 | 0.477 | 0.27 | 23.4 ± 2.3 | 23.4 ± 2.1 | 0.820 | 0.12 | 24.4 ± 3.1 | 24.7 ± 2.8 | 0.339 | 0.26 | 0.705 | 0.00 |
| Skeletal muscle mass (g) | 21.1 ± 4.2 | 21.6 ± 3.9 | 0.207 | 0.45 | 20.3 ± 2.4 | 20.4 ± 2.4 | 0.750 | 0.10 | 21.3 ± 2.6 | 21.1 ± 2.7 | 0.505 | 0.20 | 0.286 | 0.00 |
| Lean body mass (g) | 18.9 ± 4.2 | 18.1 ± 3.8 | 0.516 | 0.26 | 18.9 ± 4.9 | 19.0 ± 4.0 | 0.846 | 0.08 | 19.9 ± 6.1 | 20.3 ± 5.5 | 0.505 | 0.20 | 0.700 | 0.00 |
| Body fat percentage (%) | 32.3 ± 4.5 | 31.1 ± 3.7 | 0.426 | 0.30 | 33.0 ± 5.9 | 33.2 ± 4.5 | 0.770 | 0.11 | 33.0 ± 5.9 | 33.7 ± 5.5 | 0.392 | 0.24 | 0.487 | 0.00 |
| Handgrip strength | 39.8 ± 8.9 | 41.9 ± 10.4 | 0.129 | 0.53 | 32.9 ± 5.9 | 35.7 ± 5.5 | 0.020 | 0.73 | 39.5 ± 9.5 | 42.0 ± 8.2 | 0.027 | 0.63 | 0.724 | 0.00 |
| Variables | Control Group (n = 9) | p a | r b | DiEx Group (n = 10) | p a | r b | DiExSp Group (n = 12) | p a | r b | p c | ε2 d | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Week | Baseline | 12-Week | Baseline | 12-Week | |||||||||
| Total protein (g/dL) | 7.4 ± 0.4 | 7.5 ± 0.3 | 0.281 | 0.45 | 7.3 ± 0.4 | 7.4 ± 0.3 | 1.000 | 0.12 | 7.2 ± 0.5 | 7.2 ± 0.4 | 1.000 | 0.00 | 0.654 | 0.00 |
| Albumin (g/dL) | 4.6 ± 0.2 | 4.7 ± 0.2 | 0.328 | 0.38 | 4.6 ± 0.2 | 4.6 ± 0.2 | 1.000 | 0.00 | 4.6 ± 0.3 | 4.6 ± 0.3 | 0.656 | 0.20 | 0.637 | 0.00 |
| Total bilirubin (mg/dL) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.688 | 0.24 | 0.8 ± 0.4 | 0.7 ± 0.3 | 0.266 | 0.32 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.457 | 0.28 | 0.354 | 0.00 |
| Blood glucose (mg/dL) | 89.6 ± 5.3 | 90.9 ± 5.4 | 0.516 | 0.27 | 99.9 ± 10.6 | 98.9 ± 8.6 | 0.879 | 0.06 | 95.9 ± 8.4 | 94.6 ± 10.1 | 0.713 | 0.11 | 0.723 | 0.00 |
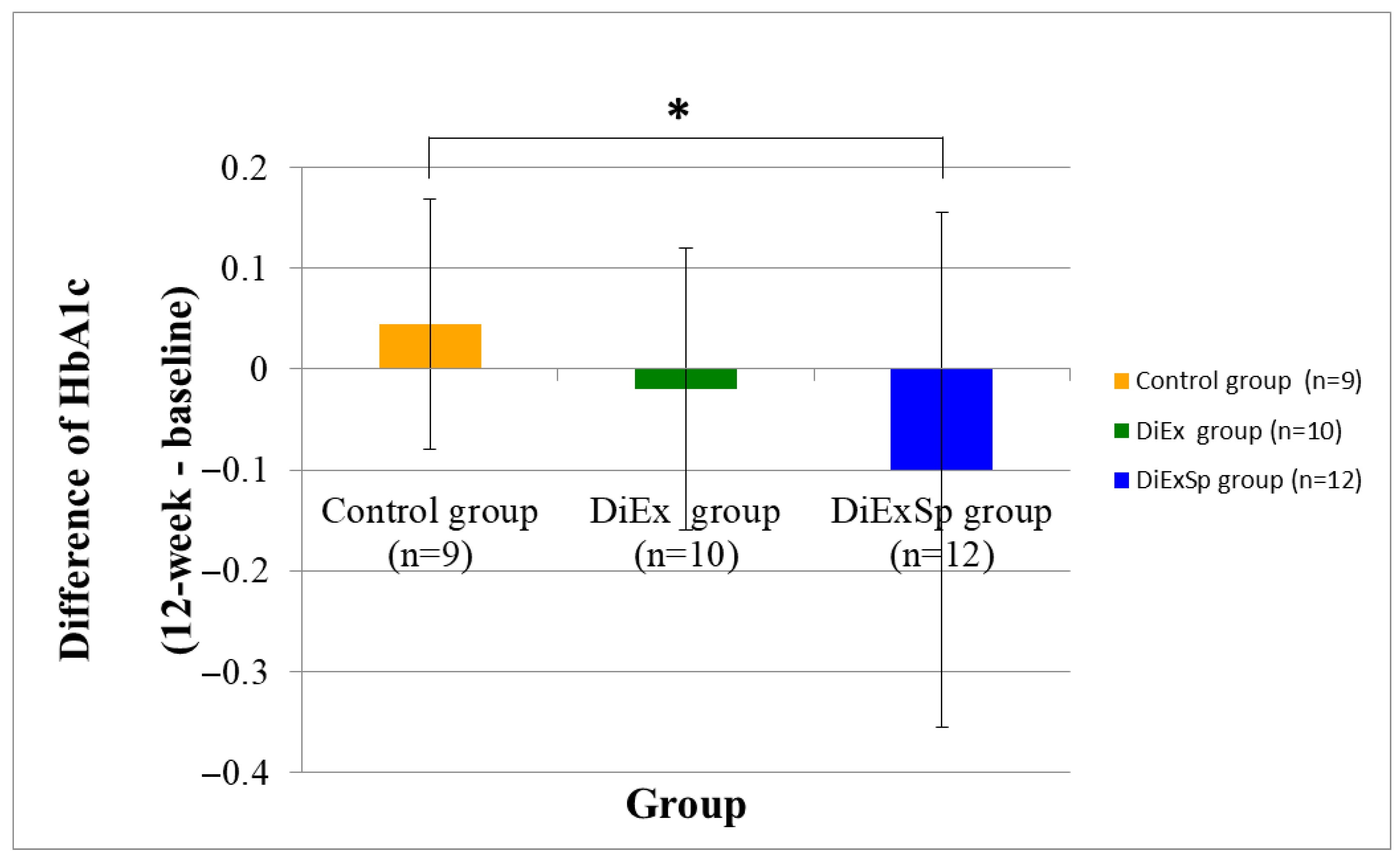
| HbA1c (%) | 5.8 ± 0.3 | 5.8 ± 0.2 | 0.438 | 0.54 | 6.1 ± 0.3 | 6.1 ± 0.3 | 0.275 | 0.32 | 6.1 ± 0.3 | 6.0 ± 0.4 | 0.233 | 0.55 | 0.043 | 0.01 |
| Insulin (μIU/mL) | 6.4 ± 4.6 | 5.7 ± 2.3 | 0.922 | 0.05 | 7.0 ± 4.3 | 7.5 ± 2.8 | 0.406 | 0.37 | 9.1 ± 7.0 | 7.0 ± 4.4 | 0.065 | 0.36 | 0.176 | 0.00 |
| BUN (mg/dL) | 16.6 ± 4.4 | 16.6 ± 3.0 | 1.000 | 0.04 | 15.9 ± 3.0 | 16.4 ± 3.6 | 0.766 | 0.11 | 16.3 ± 2.9 | 16.2 ± 3.4 | 0.957 | 0.02 | 0.964 | 0.00 |
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.586 | 0.20 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.914 | 0.02 | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.241 | 0.38 | 0.725 | 0.00 |
| Total cholesterol (mg/dL) | 193.1 ± 47.3 | 200.3 ± 34.5 | 0.453 | 0.28 | 181.8 ± 35.5 | 190.1 ± 31.9 | 0.369 | 0.31 | 183.0 ± 38.9 | 183.3 ± 38.8 | 0.985 | 0.01 | 0.582 | 0.00 |
| HDL cholesterol (mg/dL) | 62.9 ± 12.2 | 70.7 ± 17.8 | 0.055 | 0.65 | 67.1 ± 11.5 | 67.4 ± 13.7 | 1.000 | 0.00 | 58.3 ± 7.1 | 60.2 ± 9.5 | 0.551 | 0.22 | 0.163 | 0.00 |
| LDL cholesterol (mg/dL) | 112.8 ± 45.5 | 111.6 ± 32.8 | 0.992 | 0.02 | 94.0 ± 24.5 | 102.8 ± 27.0 | 0.264 | 0.37 | 101.5 ± 41.0 | 100.7 ± 39.1 | 0.979 | 0.01 | 0.347 | 0.00 |
| Triglycerides (mg/dL) | 87.1 ± 27.5 | 90.0 ± 34.9 | 0.934 | 0.04 | 104.2 ± 42.5 | 100.8 ± 27.4 | 0.752 | 0.11 | 116.1 ± 39.1 | 112.4 ± 26.9 | 0.953 | 0.02 | 0.970 | 0.00 |
| Vitamin D (ng/mL) | 37.7 ± 19.6 | 38.1 ± 22.6 | 0.590 | 0.18 | 45.7 ± 18.8 | 40.7 ± 17.5 | 0.125 | 0.50 | 28.2 ± 10.4 | 29.1 ± 10.8 | 0.677 | 0.14 | 0.179 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.-H.; Lee, S. A 12-Week Randomized Controlled Trial of Nutrition and Exercise Education with Dietary Supplementation for Sarcopenia Prevention in Korean Baby Boomers. Nutrients 2025, 17, 3008. https://doi.org/10.3390/nu17183008
Jang E-H, Lee S. A 12-Week Randomized Controlled Trial of Nutrition and Exercise Education with Dietary Supplementation for Sarcopenia Prevention in Korean Baby Boomers. Nutrients. 2025; 17(18):3008. https://doi.org/10.3390/nu17183008
Chicago/Turabian StyleJang, Eun-Hee, and Seungmin Lee. 2025. "A 12-Week Randomized Controlled Trial of Nutrition and Exercise Education with Dietary Supplementation for Sarcopenia Prevention in Korean Baby Boomers" Nutrients 17, no. 18: 3008. https://doi.org/10.3390/nu17183008
APA StyleJang, E.-H., & Lee, S. (2025). A 12-Week Randomized Controlled Trial of Nutrition and Exercise Education with Dietary Supplementation for Sarcopenia Prevention in Korean Baby Boomers. Nutrients, 17(18), 3008. https://doi.org/10.3390/nu17183008







