Identification of Amino Acids That Regulate Angiogenesis and Alter Pathogenesis of a Mouse Model of Choroidal Neovascularization
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Lines and Culture Conditions
2.3. Gene Expression Analysis by Quantitative PCR (qPCR)
2.4. Immunoblotting
2.5. VEGF ELISAs
2.6. Cell Viability Assay
2.7. Tube Formation
2.8. shRNA Knockdown
2.9. RNA-Seq
2.10. GEO Database
2.11. Cell Metabolism Assays
2.12. Choroidal-Retinal Flat Mount and Immunostaining
2.13. Fundus Fluorescein Angiography (FFA)
2.14. Optical Coherence Tomography (OCT)
2.15. Intracellular ROS Production Assays
2.16. Detection of Mitochondrial Membrane Potential
2.17. LC-MS/MS Analysis
2.18. Statistical Analysis
3. Results
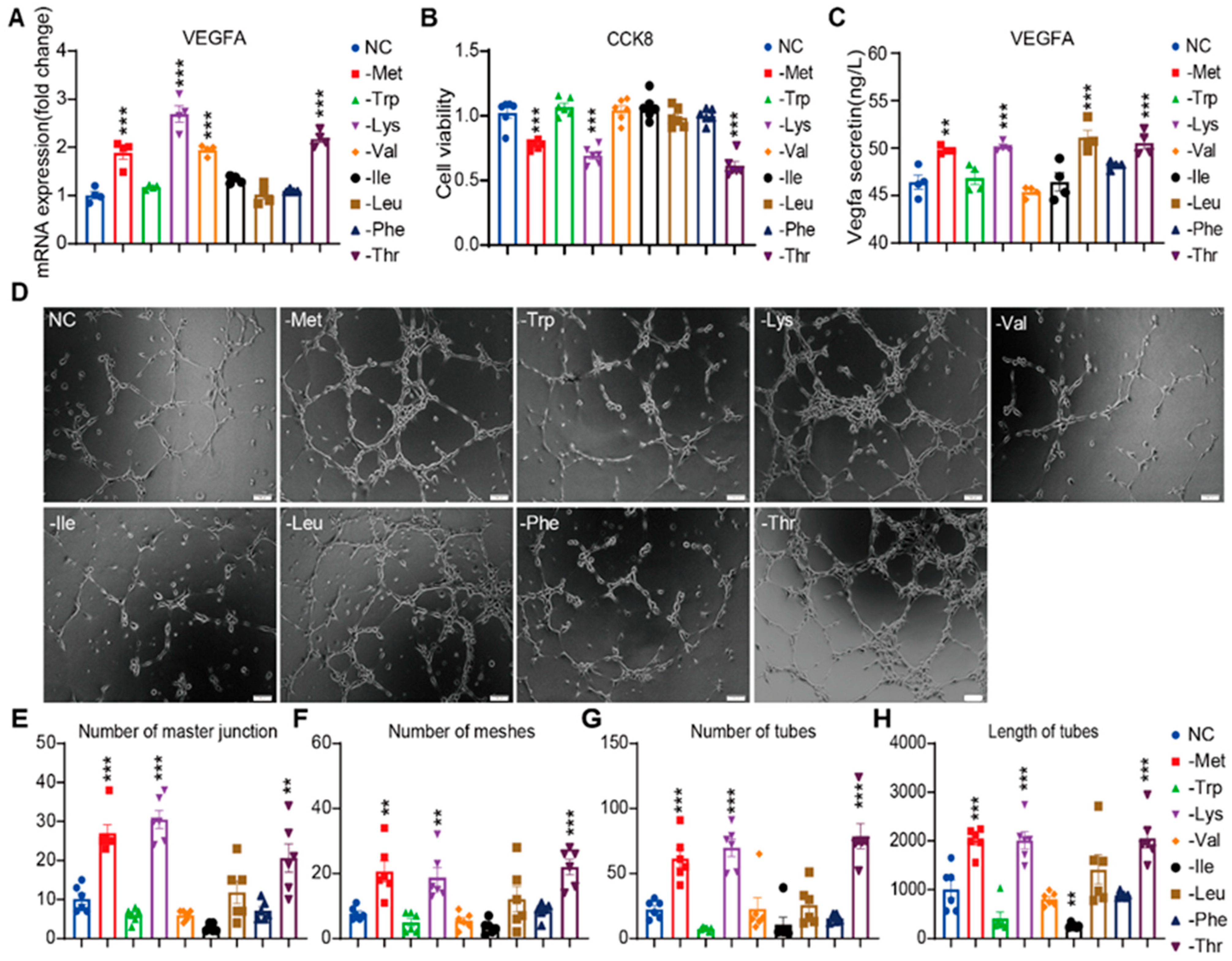
3.1. Essential Amino Acid Restriction Activates Angiogenic Programming in HRMVECs
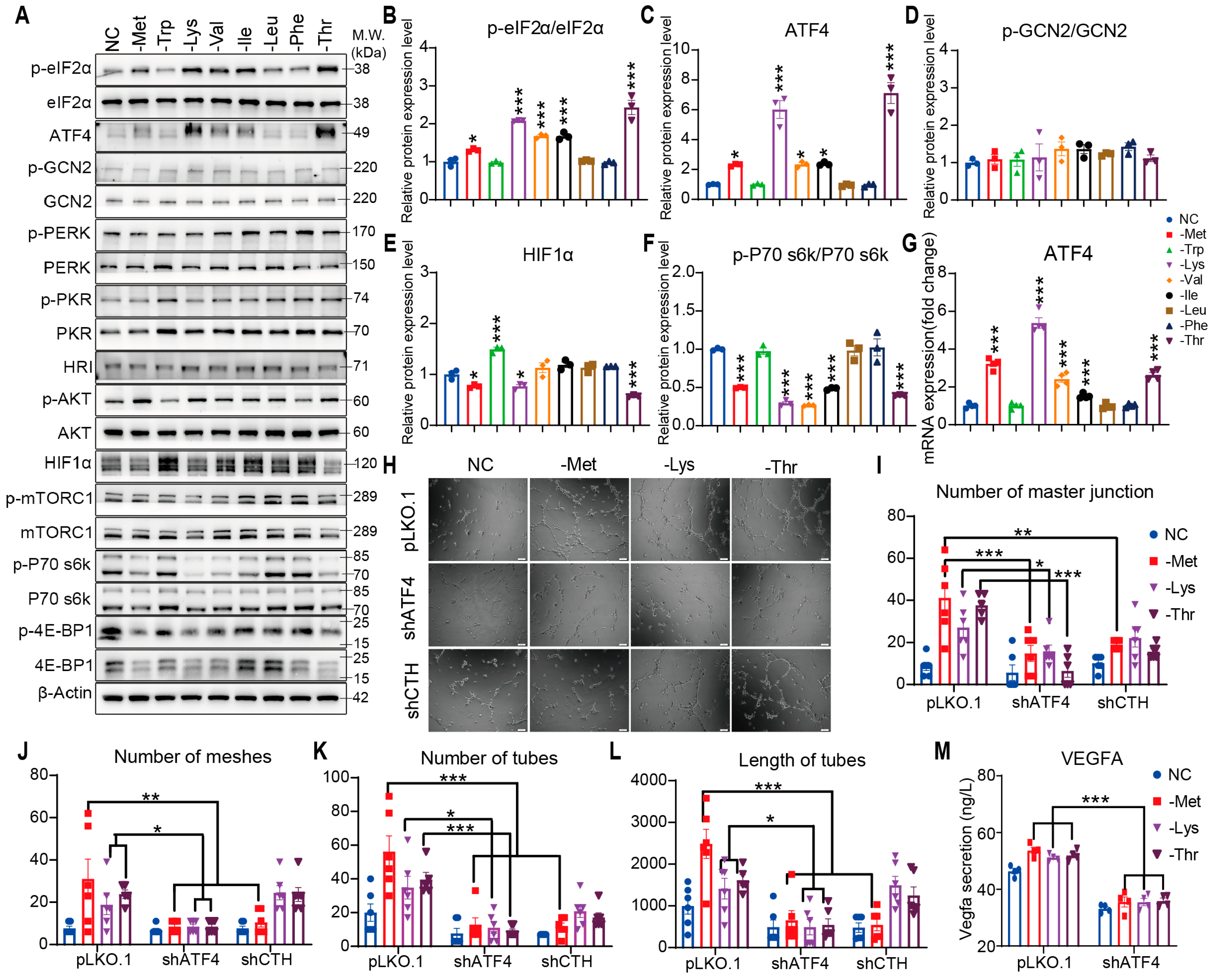
3.2. Promotion of Angiogenesis by Restriction of EAAs Is Mediated by the eIF2α/ATF4 Axis
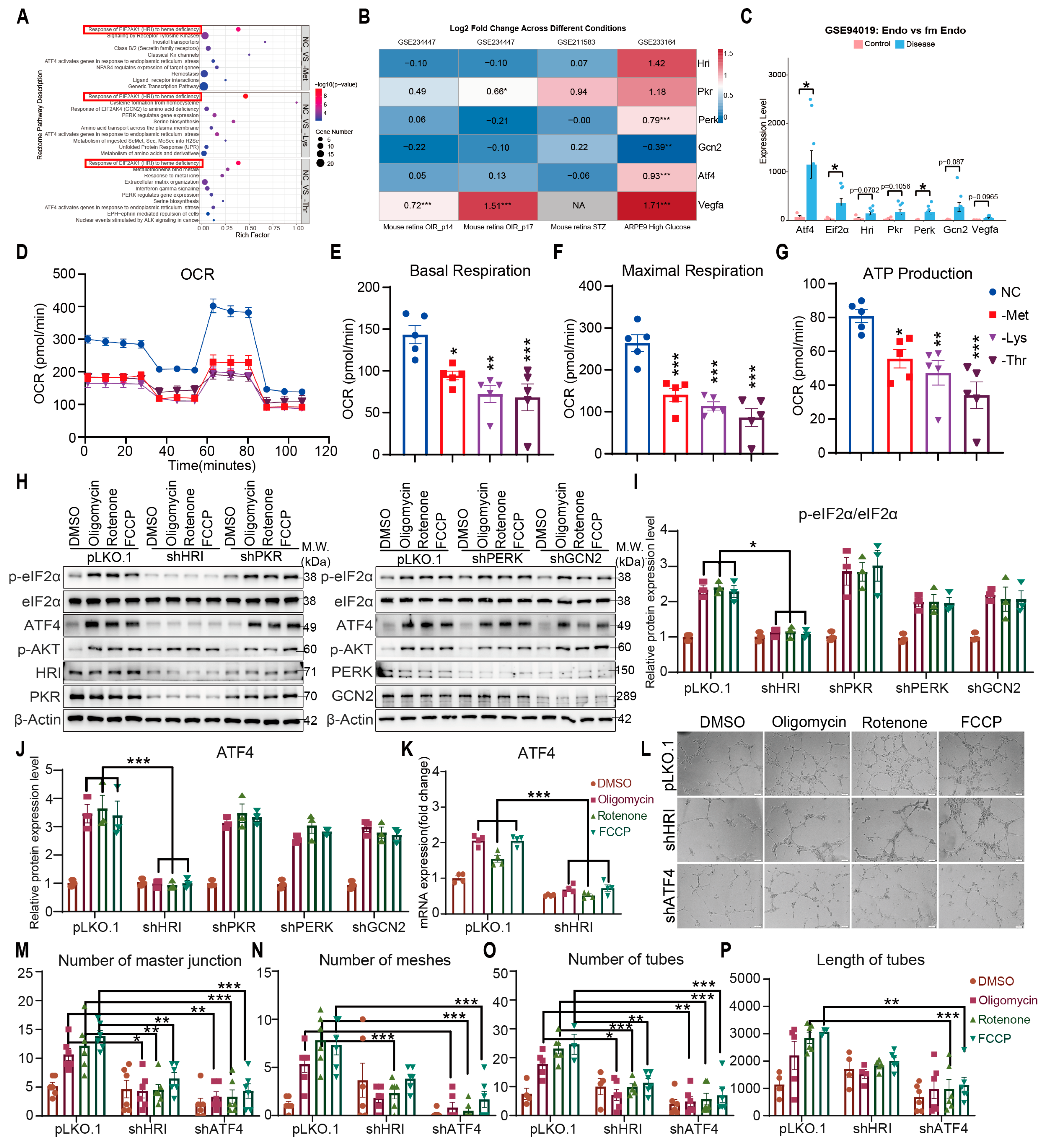
3.3. HRI Couples Mitochondrial Stress to ATF4-Driven Angiogenesis
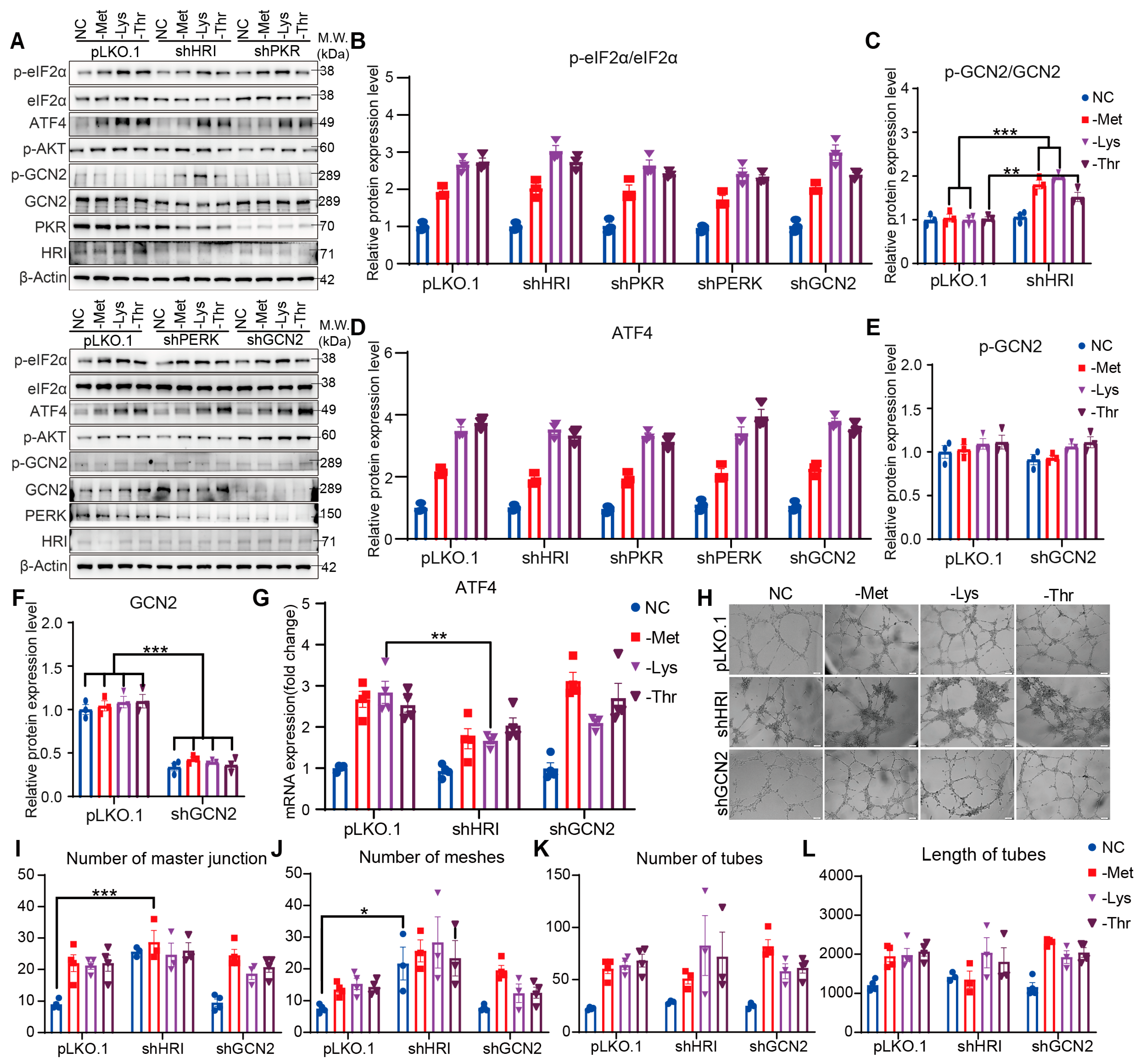
3.4. Knockdown of HRI or GCN2 Alone Fails to Attenuate EAA Restriction-Driven Angiogenesis
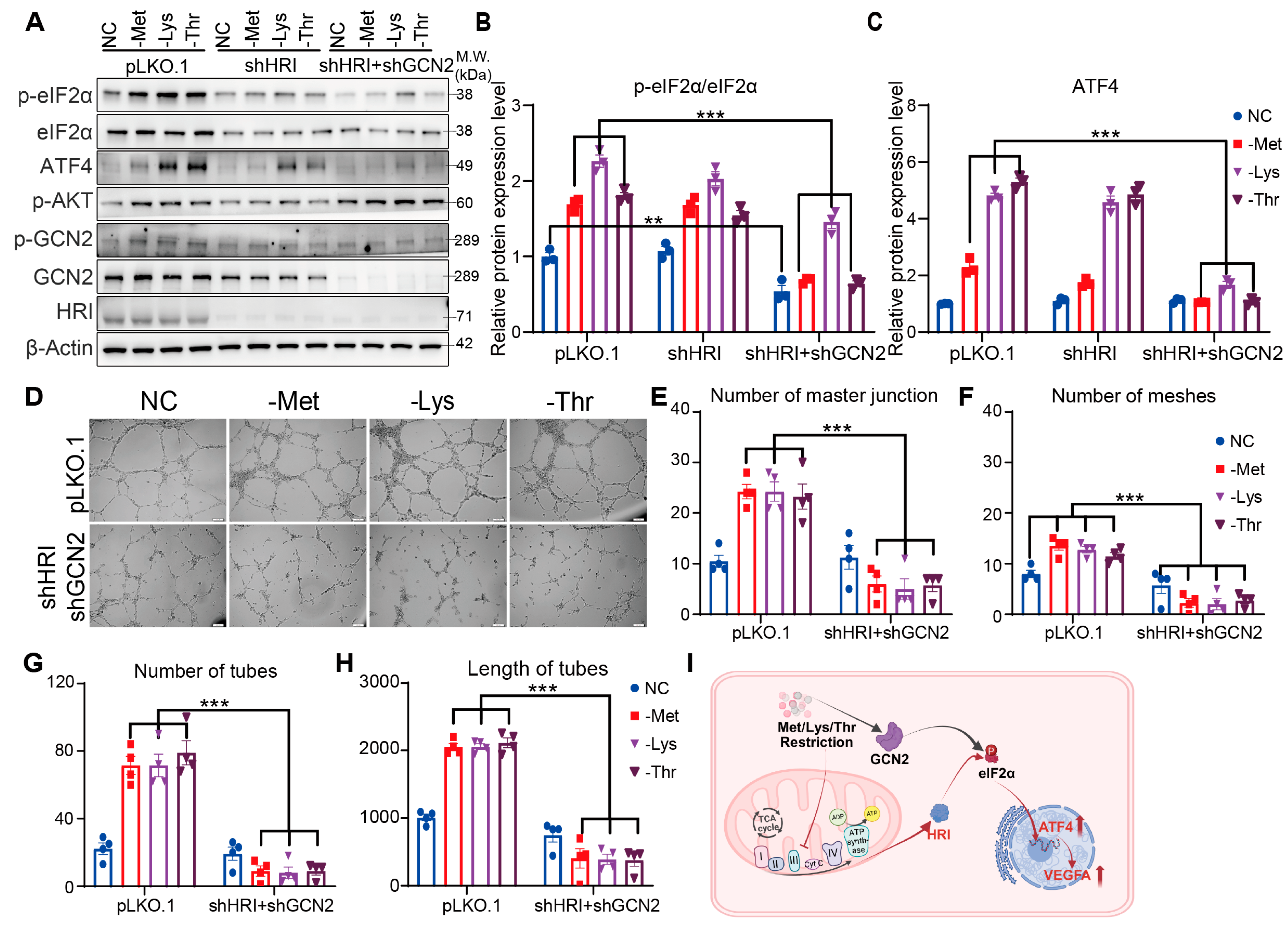
3.5. HRI and GCN2 Cooperatively Drive ATF4-Dependent Angiogenesis Through eIF2α Phosphorylation Under EAA Restriction
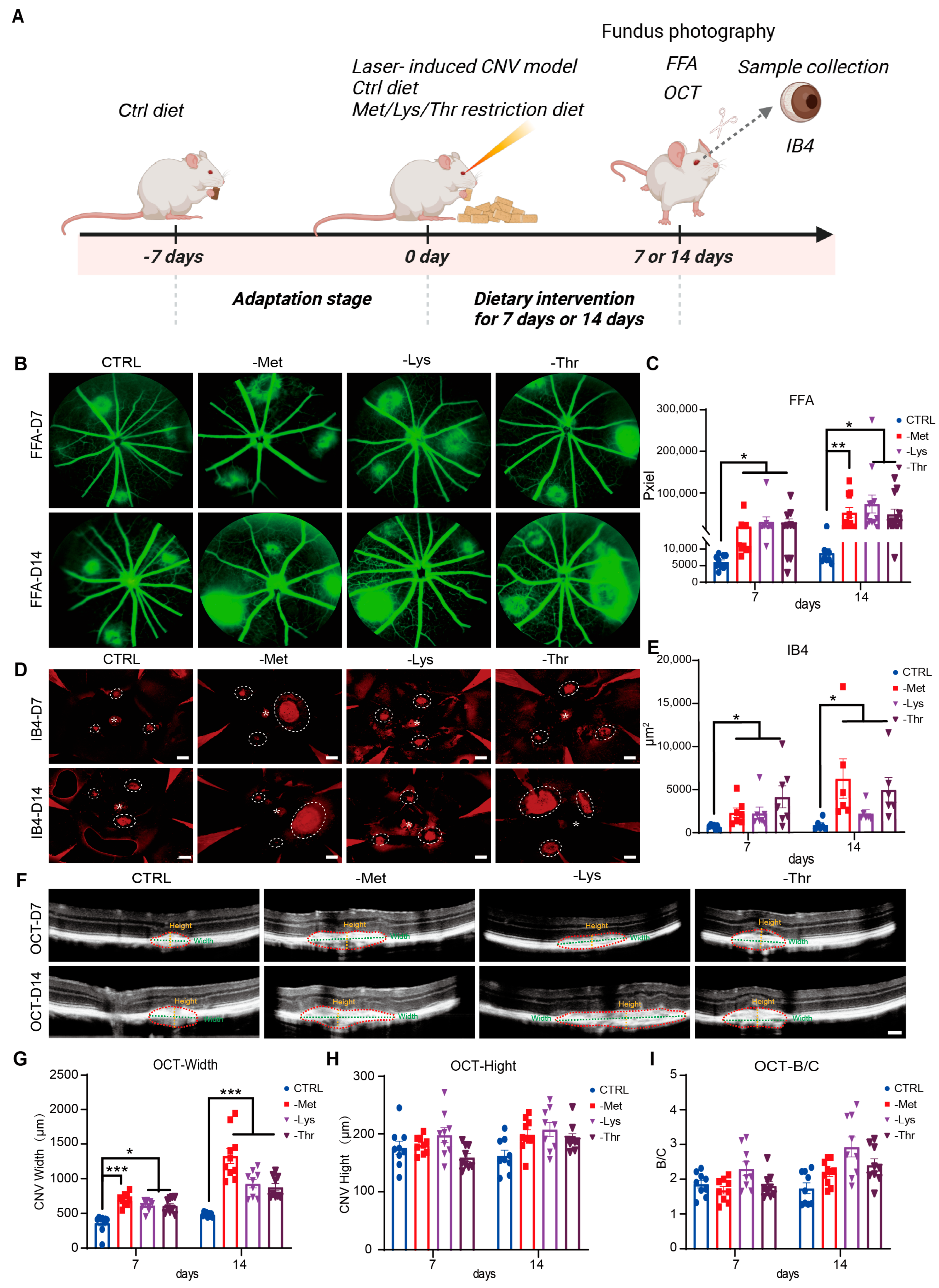
3.6. Dietary Restriction of Methionine, Lysine, and Threonine Exacerbates Choroidal Neovascularization in a Laser-Induced AMD Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | amino acid |
| EAA | essential amino acid |
| AMD | age-related macular degeneration |
| VEGF-A | vascular endothelial growth factor A |
| HRMVECs | human retinal microvascular endothelial cells |
| HRI | heme-regulated inhibitor |
| PKR | protein kinase RNA-activated |
| PERK | protein kinase R-like endoplasmic reticulum kinase |
| GCN2 | general control nonderepressible 2 |
| ATF4 | activating transcription factor 4 |
| CNV | choroidal neovascularization |
| GSH | glutathione |
| OXPHOS | oxidative phosphorylation |
| ROS | reactive oxygen species |
| eIF2α | eukaryotic translation initiation factor 2α |
| SIBS | Shanghai Institute for Biological Sciences |
| CAS | Chinese Academy of Sciences |
| AL | ad libitum |
| M | methionine |
| K | lysine |
| T | threonine |
| ECM | endothelial cell medium |
| ECGS | endothelial cell growth supplement |
| FBS | fetal bovine serum |
| qPCR | quantitative PCR |
| DEG | differential expression genes |
| TPM | transcripts per million reads |
| FFA | fundus fluorescein angiography |
| OCT | optical coherence tomography |
| RPE | retinal pigment epithelium |
| SEM | standard error of the mean |
| ANOVA | analysis of variance |
| HUVECs | human umbilical vein endothelial cells |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| HIF1α | hypoxia-inducible factor 1α |
| CTH | cystathionine gamma-lyase |
| ISR | integrated stress response |
| OCR | oxygen consumption rate |
References
- Jiang, P.; Tan, H.; Peng, Q. Ranibizumab and conbercept for treating wet age-related macular degeneration in China: A systematic review and meta-analysis. Medicine 2021, 100, e27774. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef]
- Zych, M.; Wojnar, W.; Dudek, S.; Kaczmarczyk-Sedlak, I. Rosmarinic and Sinapic Acids May Increase the Content of Reduced Glutathione in the Lenses of Estrogen-Deficient Rats. Nutrients 2019, 11, 803. [Google Scholar] [CrossRef]
- Ascunce, K.; Dhodapkar, R.M.; Huang, D.; Hafler, B.P. Innate immune biology in age-related macular degeneration. Front. Cell Dev. Biol. 2023, 11, 1118524. [Google Scholar] [CrossRef]
- Guo, X.; Aviles, G.; Liu, Y.; Tian, R.; Unger, B.A.; Lin, Y.T.; Wiita, A.P.; Xu, K.; Correia, M.A.; Kampmann, M. Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature 2020, 579, 427–432. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, Q.Y.; Xu, H.; Xu, A.N.; Liu, D.J.; Li, N.Z.; Chen, Y.; Jin, Y.; Xu, C.H.; Feng, C.Z.; et al. Selective and competitive functions of the AAR and UPR pathways in stress-induced angiogenesis. Cell Discov. 2021, 7, 98. [Google Scholar] [CrossRef]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Trevino-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H(2)S Production. Cell 2018, 173, 117–129.e14. [Google Scholar] [CrossRef] [PubMed]
- Upcin, B.; Szi-Marton, D.; Henke, E.; Ergün, S.; Aktas, B.H. Abstract 688: Targeting tumor associated host cells by modifiers of integrated endoplasmic reticulum stress response. Cancer Res. 2020, 80, 688. [Google Scholar] [CrossRef]
- White, M.C.; Schroeder, R.D.; Zhu, K.; Xiong, K.; McConkey, D.J. HRI-mediated translational repression reduces proteotoxicity and sensitivity to bortezomib in human pancreatic cancer cells. Oncogene 2018, 37, 4413–4427. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hu, Z.; Pan, T.; Chen, L.; Xie, P.; Liu, Q. Complement factor B gene polymorphisms and risk of age-related macular degeneration: A meta-analysis. Eur. J. Ophthalmol. 2020, 30, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Laeger, T.; Albarado, D.C.; Burke, S.J.; Trosclair, L.; Hedgepeth, J.W.; Berthoud, H.R.; Gettys, T.W.; Collier, J.J.; Munzberg, H.; Morrison, C.D. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 that Is Delayed by the Absence of GCN2. Cell Rep. 2016, 16, 707–716. [Google Scholar] [CrossRef]
- Bhakuni, T.; Norden, P.R.; Ujiie, N.; Tan, C.; Lee, S.K.; Tedeschi, T.; Hsieh, Y.W.; Wang, Y.; Liu, T.; Fawzi, A.A.; et al. FOXC1 regulates endothelial CD98 (LAT1/4F2hc) expression in retinal angiogenesis and blood-retina barrier formation. Nat. Commun. 2024, 15, 4097. [Google Scholar] [CrossRef]
- Fessler, E.; Eckl, E.M.; Schmitt, S.; Mancilla, I.A.; Meyer-Bender, M.F.; Hanf, M.; Philippou-Massier, J.; Krebs, S.; Zischka, H.; Jae, L.T. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature 2020, 579, 433–437. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Guymer, R.; Mones, J.M.; Tufail, A.; Jaffe, G.J. Anti-Vascular Endothelial Growth Factor Use and Atrophy in Neovascular Age-Related Macular Degeneration: Systematic Literature Review and Expert Opinion. Ophthalmology 2020, 127, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, L.; Lv, X.; Liu, N.; Miao, Y.; Zhang, Q.; Xiao, Z.; Li, M.; Yang, Y.; Liu, Z.; et al. Emerging Co-Assembled and Sustained Released Natural Medicinal Nanoparticles for Multitarget Therapy of Choroidal Neovascularization. Adv. Mater. 2024, 36, e2314095. [Google Scholar] [CrossRef]
- Yanai, R.; Yasunaga, G.; Tsuji, S.; Honda, T.; Iwata, A.; Miyagawa, E.; Yoshida, K.; Kishimoto, M.; Sakai, H.; Fujise, Y.; et al. Dietary intake of whale oil-containing omega-3 long-chain polyunsaturated fatty acids attenuates choroidal neovascularization in mice. FASEB J. 2025, 39, e70378. [Google Scholar] [CrossRef]
- Sahye-Pudaruth, S.; Ma, D.W.L. Assessing the Highest Level of Evidence from Randomized Controlled Trials in Omega-3 Research. Nutrients 2023, 15, 1001. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Yang, W.; Kim, H.; Chang, S.; Kim, D.; Chang, M.; Lee, K.; Chung, J.; Kim, J.H. Anti-complement component 5 antibody targeting MG4 domain inhibits choroidal neovascularization. Oncotarget 2017, 8, 45506–45516. [Google Scholar] [CrossRef]
- Tosi, G.M.; Neri, G.; Barbera, S.; Mundo, L.; Parolini, B.; Lazzi, S.; Lugano, R.; Poletto, E.; Leoncini, L.; Pertile, G.; et al. The Binding of CD93 to Multimerin-2 Promotes Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Bruning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Sarf, E.A.; Dyachenko, E.I.; Bel’skaya, L.V. The Role of Salivary Vascular Endothelial Growth Factor A, Cytokines, and Amino Acids in Immunomodulation and Angiogenesis in Breast Cancer. Biomedicines 2024, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Murdoch, C.E.; Xu, H.; Shi, H.; Duan, D.D.; Ahmed, A.; Gu, Y. Vascular endothelial growth factor signaling requires glycine to promote angiogenesis. Sci. Rep. 2017, 7, 14749. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.T.; Andrade, J.; Armbruster, M.; Shi, C.; Castro, M.; Costa, A.S.H.; Sugino, T.; Eelen, G.; Zimmermann, B.; Wilhelm, K.; et al. A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat. Metab. 2022, 4, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Y.; Ye, L. The Role of Amino Acids in Endothelial Biology and Function. Cells 2022, 11, 1372. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Zhou, H.; Li, G.; Huang, F.; Zhang, J.; Xia, T.; Lei, W.; Zhao, J.; Li, C.; et al. Activating transcription factor 4 regulates angiogenesis under lipid overload via methionine adenosyltransferase 2A-mediated endothelial epigenetic alteration. FASEB J. 2021, 35, e21612. [Google Scholar] [CrossRef]
- Wunderle, C.; Haller, L.; Laager, R.; Bernasconi, L.; Neyer, P.; Stumpf, F.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. The Association of the Essential Amino Acids Lysine, Methionine, and Threonine with Clinical Outcomes in Patients at Nutritional Risk: Secondary Analysis of a Randomized Clinical Trial. Nutrients 2024, 16, 2608. [Google Scholar] [CrossRef]
- Chang, J.; Pan, Y.; Jiang, F.; Xu, W.; Wang, Y.; Wang, L.; Hu, B. Mechanism of CXCL8 regulation of methionine metabolism to promote angiogenesis in gliomas. Discov. Oncol. 2024, 15, 614. [Google Scholar] [CrossRef]
- Jonsson, W.O.; Margolies, N.S.; Mirek, E.T.; Zhang, Q.; Linden, M.A.; Hill, C.M.; Link, C.; Bithi, N.; Zalma, B.; Levy, J.L.; et al. Physiologic Responses to Dietary Sulfur Amino Acid Restriction in Mice Are Influenced by Atf4 Status and Biological Sex. J. Nutr. 2021, 151, 785–799. [Google Scholar] [CrossRef]
- Statzer, C.; Meng, J.; Venz, R.; Bland, M.; Robida-Stubbs, S.; Patel, K.; Petrovic, D.; Emsley, R.; Liu, P.; Morantte, I.; et al. ATF-4 and hydrogen sulfide signalling mediate longevity in response to inhibition of translation or mTORC1. Nat. Commun. 2022, 13, 967. [Google Scholar] [CrossRef]
- Wang, R.H.; Chu, Y.H.; Lin, K.T. The Hidden Role of Hydrogen Sulfide Metabolism in Cancer. Int. J. Mol. Sci. 2021, 22, 6562. [Google Scholar] [CrossRef]
- Obacz, J.; Avril, T.; Rubio-Patino, C.; Bossowski, J.P.; Igbaria, A.; Ricci, J.E.; Chevet, E. Regulation of tumor-stroma interactions by the unfolded protein response. FEBS J. 2019, 286, 279–296. [Google Scholar] [CrossRef]
- Cherubini, A.; Zito, E. ER stress as a trigger of UPR and ER-phagy in cancer growth and spread. Front. Oncol. 2022, 12, 997235. [Google Scholar] [CrossRef]
- Varone, E.; Decio, A.; Chernorudskiy, A.; Minoli, L.; Brunelli, L.; Ioli, F.; Piotti, A.; Pastorelli, R.; Fratelli, M.; Gobbi, M.; et al. The ER stress response mediator ERO1 triggers cancer metastasis by favoring the angiogenic switch in hypoxic conditions. Oncogene 2021, 40, 1721–1736. [Google Scholar] [CrossRef]
- Raines, L.N.; Huang, S.C. How the Unfolded Protein Response Is a Boon for Tumors and a Bane for the Immune System. Immunohorizons 2023, 7, 256–264. [Google Scholar] [CrossRef]
- Emanuelli, G.; Nassehzadeh-Tabriz, N.; Morrell, N.W.; Marciniak, S.J. The integrated stress response in pulmonary disease. Eur. Respir. Rev. 2020, 29, 200184. [Google Scholar] [CrossRef]
- Wang, L.; Wen, J.; Sun, Y.; Yang, X.; Ma, Y.; Tian, X. Knockdown of NUPR1 inhibits angiogenesis in lung cancer through IRE1/XBP1 and PERK/eIF2alpha/ATF4 signaling pathways. Open Med. 2023, 18, 20230796. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Paccard, C.; Chiron, M.; Tabernero, J. Impact of Prior Bevacizumab Treatment on VEGF-A and PlGF Levels and Outcome Following Second-Line Aflibercept Treatment: Biomarker Post Hoc Analysis of the VELOUR Trial. Clin. Cancer Res. 2020, 26, 717–725. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Park, Y.; Reyna-Neyra, A.; Philippe, L.; Thoreen, C.C. mTORC1 Balances Cellular Amino Acid Supply with Demand for Protein Synthesis through Post-transcriptional Control of ATF4. Cell Rep. 2017, 19, 1083–1090. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, Z.; Yang, S.; Lu, G.; Nie, Y.; Ren, Y.; Xue, Y.; Shi, J.S.; Xu, Z.H.; Geng, Y. High methionine intake alters gut microbiota and lipid profile and leads to liver steatosis in mice. Food Funct. 2024, 15, 8053–8069. [Google Scholar] [CrossRef]
- Kosakamoto, H.; Obata, F.; Kuraishi, J.; Aikawa, H.; Okada, R.; Johnstone, J.N.; Onuma, T.; Piper, M.D.W.; Miura, M. Early-adult methionine restriction reduces methionine sulfoxide and extends lifespan in Drosophila. Nat. Commun. 2023, 14, 7832. [Google Scholar] [CrossRef]
- Xin, C.; Cai, M.; Jia, Q.; Huang, R.; Li, R.; Wang, J.; Li, Z.; Zhao, Q.; Liu, T.; Zhuang, W.; et al. Dietary sulfur amino acid restriction improves metabolic health by reducing fat mass. Life Metab. 2025, 4, loaf009. [Google Scholar] [CrossRef]
- Darawshi, O.; Yassin, O.; Shmuel, M.; Wek, R.C.; Mahdizadeh, S.J.; Eriksson, L.A.; Hatzoglou, M.; Tirosh, B. Phosphorylation of GCN2 by mTOR confers adaptation to conditions of hyper-mTOR activation under stress. J. Biol. Chem. 2024, 300, 107575. [Google Scholar] [CrossRef]
- Shanmugam, R.; Anderson, R.; Schiemann, A.H.; Sattlegger, E. Evidence that Xrn1 is in complex with Gcn1, and is required for full levels of eIF2α phosphorylation. Biochem. J. 2024, 481, 481–498. [Google Scholar] [CrossRef]
- Swanda, R.V.; Ji, Q.; Wu, X.; Yan, J.; Dong, L.; Mao, Y.; Uematsu, S.; Dong, Y.; Qian, S.-B. Lysosomal cystine governs ferroptosis sensitivity in cancer via cysteine stress response. Mol. Cell 2023, 83, 3347–3359.e9. [Google Scholar] [CrossRef]
- Sekine, Y.; Houston, R.; Fessler, E.; Jae, L.T.; Narendra, D.P.; Sekine, S. A mitochondrial iron-sensing pathway regulated by DELE1. bioRxiv 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wu, J.; Zhao, Y.; Zhu, J.; Zhu, X.; Chen, Y.; Wu, J. Identification of Amino Acids That Regulate Angiogenesis and Alter Pathogenesis of a Mouse Model of Choroidal Neovascularization. Nutrients 2025, 17, 3006. https://doi.org/10.3390/nu17183006
Li C, Wu J, Zhao Y, Zhu J, Zhu X, Chen Y, Wu J. Identification of Amino Acids That Regulate Angiogenesis and Alter Pathogenesis of a Mouse Model of Choroidal Neovascularization. Nutrients. 2025; 17(18):3006. https://doi.org/10.3390/nu17183006
Chicago/Turabian StyleLi, Chenchen, Jiawen Wu, Yingke Zhao, Jing Zhu, Xinyu Zhu, Yan Chen, and Jihong Wu. 2025. "Identification of Amino Acids That Regulate Angiogenesis and Alter Pathogenesis of a Mouse Model of Choroidal Neovascularization" Nutrients 17, no. 18: 3006. https://doi.org/10.3390/nu17183006
APA StyleLi, C., Wu, J., Zhao, Y., Zhu, J., Zhu, X., Chen, Y., & Wu, J. (2025). Identification of Amino Acids That Regulate Angiogenesis and Alter Pathogenesis of a Mouse Model of Choroidal Neovascularization. Nutrients, 17(18), 3006. https://doi.org/10.3390/nu17183006






