Effect of Apple Cider Vinegar Intake on Body Composition in Humans with Type 2 Diabetes and/or Overweight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
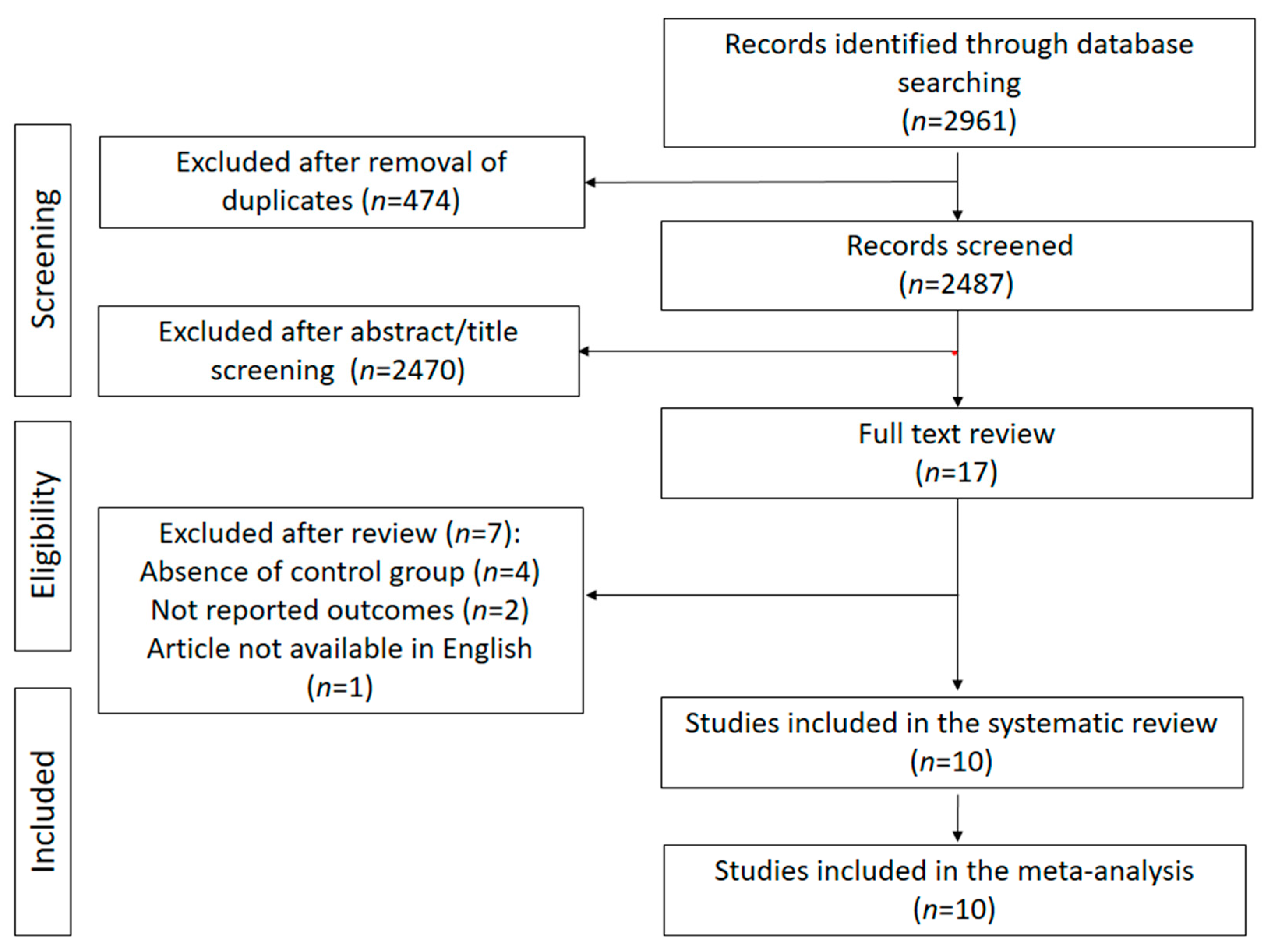
2. Material and Methods
2.1. Selection and Search Strategy
2.2. Inclusion/Exclusion Criteria
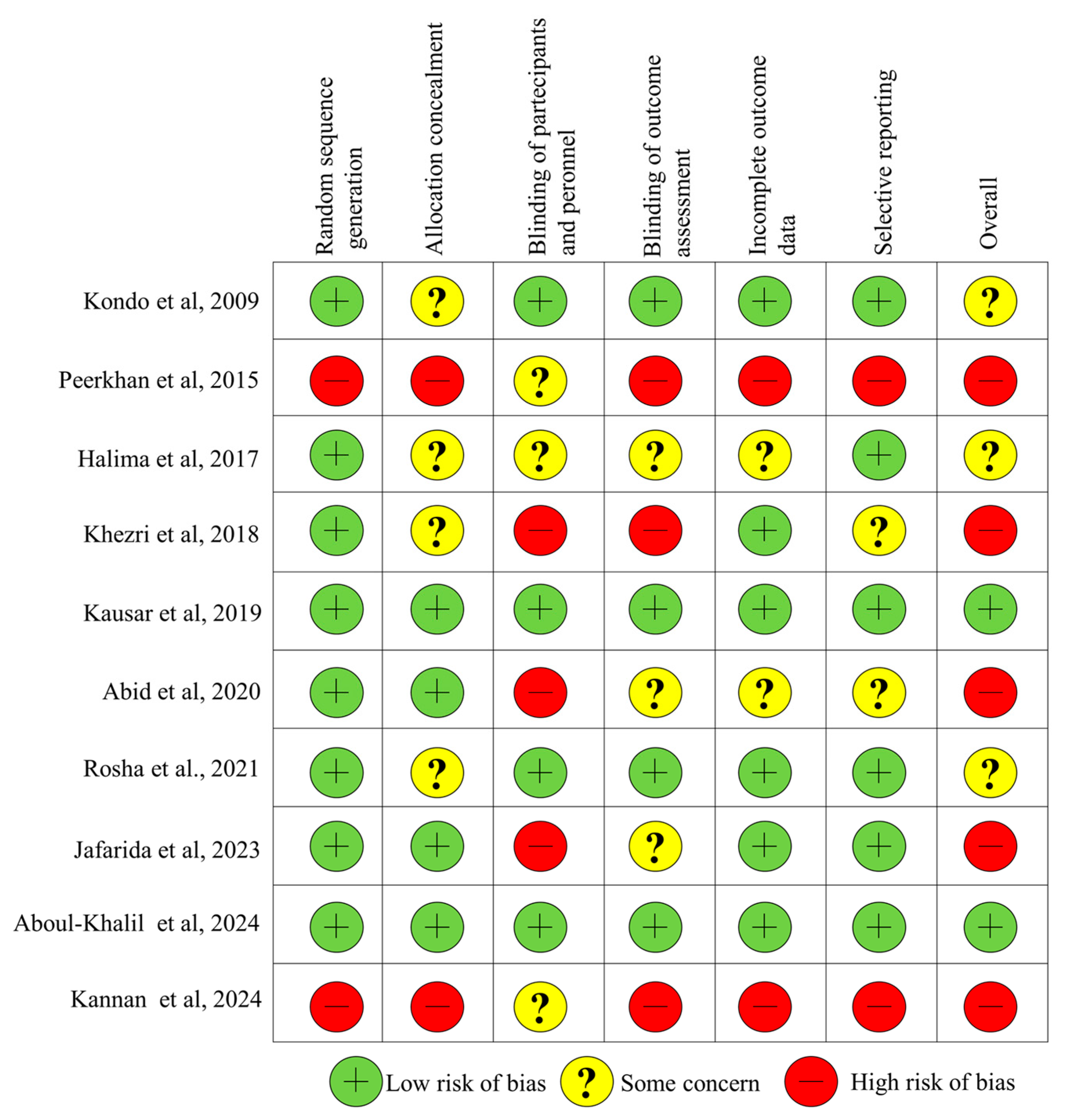
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
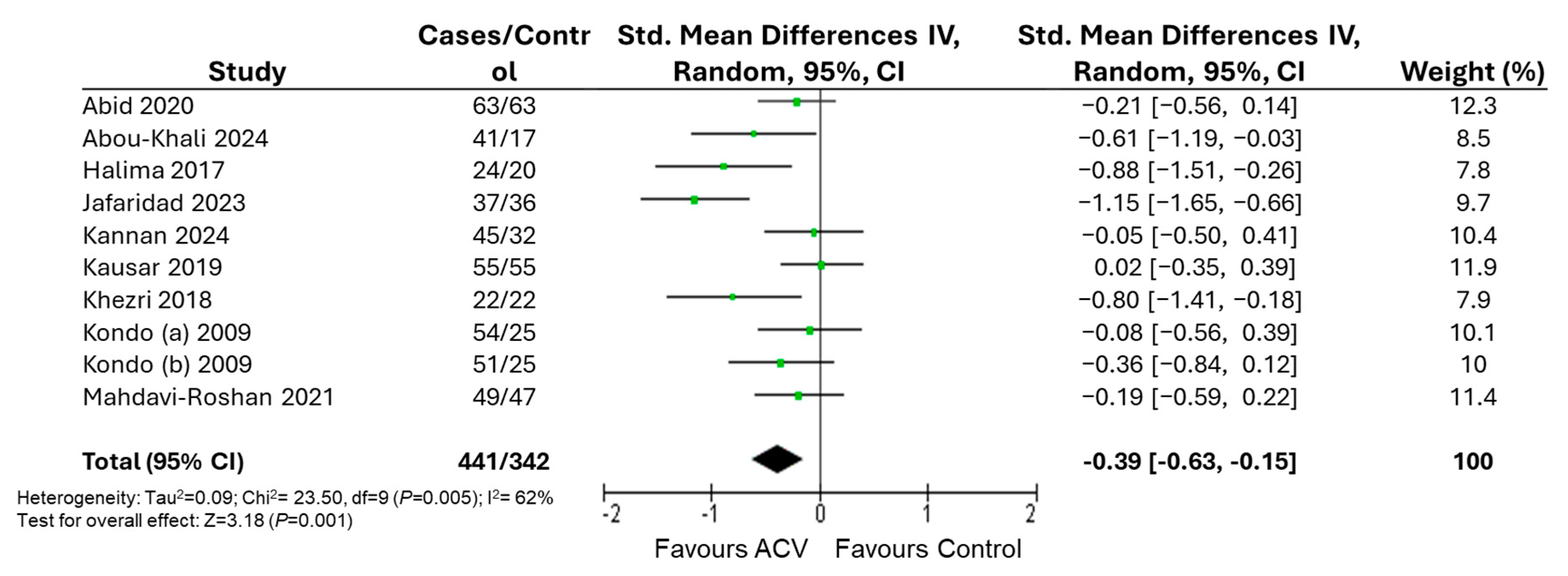
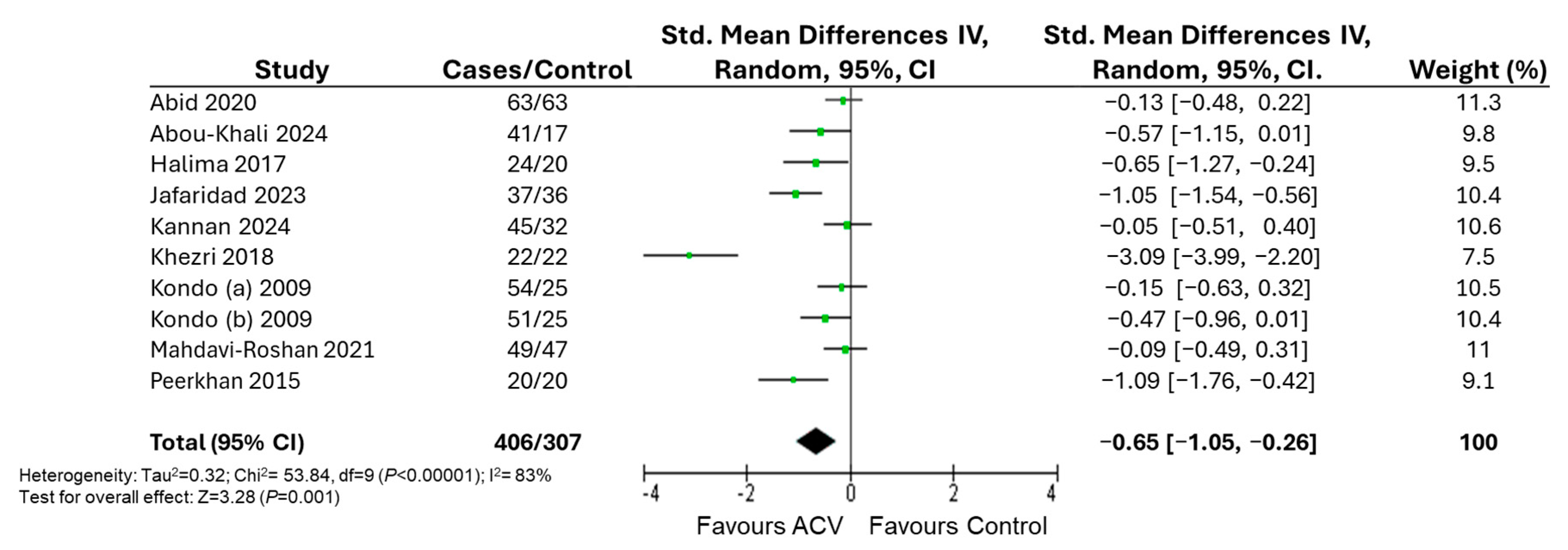
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kanem, N.; Murray, C.J.L.; Horton, R. The Lancet Commission on 21st-Century Global Health Threats. Lancet 2023, 401, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Gujral, U.P.; Quarells, R.C.; Rhodes, E.C.; Shah, M.K.; Obi, J.; Lee, W.; Shamambo, L.; Weber, M.B.; Narayan, K.M.V. Disparities in Diabetes Prevalence and Management by Race and Ethnicity in the USA: Defining a Path Forward. Lancet Diabetes Endocrinol. 2023, 11, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H. World_Obesity_Atlas_2023_Report. 2023. Available online: https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf (accessed on 16 May 2025).
- d’Errico, M.; Pavlova, M.; Spandonaro, F. The Economic Burden of Obesity in Italy: A Cost-of-Illness Study. Eur. J. Health. Econ. 2022, 23, 177–192. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Underweight and Obesity from 1990 to 2022: A Pooled Analysis of 3663 Population-Representative Studies with 222 Million Children, Adolescents, and Adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Adolescent BMI Collaborators. Global, Regional, and National Prevalence of Child and Adolescent Overweight and Obesity, 1990-2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 785–812. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Cesare, M.D.; Powis, J.; Shrikhande, S.; Codato, E.; Zhou, B.; Bixby, H.; Adeoye, M.; Evans, N.; Lara-Breitinger, K.; et al. World_Heart_Report_2025_Online Version. Available online: https://world-heart-federation.org/wp-content/uploads/World_Heart_Report_2025_Online-Version.pdf (accessed on 16 May 2025).
- Soerjomataram, I.; Bray, F. Planning for Tomorrow: Global Cancer Incidence and the Role of Prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M. A Review of Current Guidelines for the Treatment of Obesity. Am. J. Manag. Care 2022, 28, S288–S296. [Google Scholar]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of Obesity: An Update on the Available Medications and Drugs Under Investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-Term Weight-Loss Maintenance: A Meta-Analysis of US Studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araujo-Soares, V.; Sniehotta, F.F. Long Term Maintenance of Weight Loss with Non-Surgical Interventions in Obese Adults: Systematic Review and Meta-Analyses of Randomised Controlled Trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef] [PubMed]
- Peirson, L.; Fitzpatrick-Lewis, D.; Ciliska, D.; Usman Ali, M.; Raina, P.; Sherifali, D. Strategies for Weight Maintenance in Adult Populations Treated for Overweight and Obesity: A Systematic Review and Meta-Analysis. CMAJ Open 2015, 3, 47. [Google Scholar] [CrossRef]
- Kosmalski, M.; Deska, K.; Bak, B.; Rozycka-Kosmalska, M.; Pietras, T. Pharmacological Support for the Treatment of Obesity-Present and Future. Healthcare 2023, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Alobaida, M.; Alrumayh, A.; Oguntade, A.S.; Al-Amodi, F.; Bwalya, M. Cardiovascular Safety and Superiority of Anti-Obesity Medications. Diabetes Metab. Syndr. Obes. 2021, 14, 3199–3208. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Raj, R.; Elshimy, G.; Zapata, I.; Kannan, L.; Majety, P.; Edem, D.; Correa, R. Adverse Events Related to Tirzepatide. J. Endocr. Soc. 2023, 7, bvad016. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, S.; Montrief, T.; Koyfman, A.; Long, B. High Risk and Low Incidence Diseases: Bariatric Surgery Complications. Am. J. Emerg. Med. 2025, 87, 113–122. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Narain, K.; Scannell, C. Exploring Racial and Ethnic Differences in Utilization of Medications for Obesity Management in a Nationally Representative Survey. J. Racial Ethn. Health Disparities 2024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Washington, T.B.; Johnson, V.R.; Kendrick, K.; Ibrahim, A.A.; Tu, L.; Sun, K.; Stanford, F.C. Disparities in Access and Quality of Obesity Care. Gastroenterol. Clin. N. Am. 2023, 52, 429–441. [Google Scholar] [CrossRef]
- Hidalgo-Lozada, G.M.; Villarruel-Lopez, A.; Nuno, K.; Garcia-Garcia, A.; Sanchez-Nuno, Y.A.; Ramos-Garcia, C.O. Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment. Int. J. Mol. Sci. 2024, 25, 2671. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nurkolis, F.; Won, H.; Yang, J.; Oh, D.; Jo, H.; Choi, J.; Chung, S.; Kurniawan, R.; Kim, B. Could Natural Products Help in the Control of Obesity? Current Insights and Future Perspectives. Molecules 2023, 28, 6604. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Millat, M.S.; Akter, T.; Hossain, M.S.; Islam, M.M.; Mohsin, S.; Ansari, F.; Kabir, A.; Amin, M.N.; Islam, M.S. A Comprehensive Review on Clinically Proven Medicinal Plants in the Treatment of Overweight and Obesity, with Mechanistic Insights. Heliyon 2023, 9, e13493. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoz, K.; Echave, J.; Garcia-Oliveira, P.; Seyyedi-Mansour, S.; Donn, P.; Xiao, J.; Trzaskowska, M.; Prieto, M.A. Polyphenolic Profile, Processing Impact, and Bioaccessibility of Apple Fermented Products. Crit. Rev. Food Sci. Nutr. 2025, 65, 507–526. [Google Scholar] [CrossRef] [PubMed]
- DuPont, M.S.; Bennett, R.N.; Mellon, F.A.; Williamson, G. Polyphenols from Alcoholic Apple Cider are Absorbed, Metabolized and Excreted by Humans. J. Nutr. 2002, 132, 172–175. [Google Scholar] [CrossRef]
- Arjmandfard, D.; Behzadi, M.; Sohrabi, Z.; Mohammadi Sartang, M. Effects of Apple Cider Vinegar on Glycemic Control and Insulin Sensitivity in Patients with Type 2 Diabetes: A GRADE-Assessed Systematic Review and Dose-Response Meta-Analysis of Controlled Clinical Trials. Front. Nutr. 2025, 12, 1528383. [Google Scholar] [CrossRef] [PubMed]
- Sohouli, M.H.; Kutbi, E.; Al Masri, M.K.; Dadhkhah, H.; Fatahi, S.; Santos, H.O.; Hekmatdoost, A.; Abu-Zaid, A. Effects of Vinegar Consumption on Cardiometabolic Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Integr. Med. 2022, 55, 102176. [Google Scholar] [CrossRef]
- Hadi, A.; Pourmasoumi, M.; Najafgholizadeh, A.; Clark, C.C.T.; Esmaillzadeh, A. The Effect of Apple Cider Vinegar on Lipid Profiles and Glycemic Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. BMC Complement. Med. Ther. 2021, 21, 179. [Google Scholar] [CrossRef]
- Halima, B.H.; Sonia, G.; Sarra, K.; Houda, B.J.; Fethi, B.S.; Abdallah, A. Apple Cider Vinegar Attenuates Oxidative Stress and Reduces the Risk of Obesity in High-Fat-Fed Male Wistar Rats. J. Med. Food 2018, 21, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Bouderbala, H.; Dib, W.; Kheroua, O.; Saidi, D.; Kaddouri, H. Modulation of the Intestinal Microbiota by Apple Cider Vinegar in Rats Subjected to Cafeteria Diet. Arch. Cardiovasc. Dis. Suppl. 2019, 11, e373–e374. [Google Scholar] [CrossRef]
- Launholt, T.L.; Kristiansen, C.B.; Hjorth, P. Safety and Side Effects of Apple Vinegar Intake and its Effect on Metabolic Parameters and Body Weight: A Systematic Review. Eur. J. Nutr. 2020, 59, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.D.; Keshani, M.; Rouhani, M.H.; Moallem, S.A.; Bagherniya, M.; Sahebkar, A. The Effects of Apple Cider Vinegar on Cardiometabolic Risk Factors: A Systematic Review and Meta-Analysis of Clinical Trials. Curr. Med. Chem. 2025, 32, 2257–2274. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Pujia, C.; Ferro, Y.; Mazza, E.; Maurotti, S.; Montalcini, T.; Pujia, A. The Role of Mobile Apps in Obesity Management: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e66887. [Google Scholar] [CrossRef]
- Kannan, S.; Anandhasayanam, A.; Niranjana, E.S.; Rajamurugan, R.; Akash, B.; Abitha, S.; Nandhamurugan, R. Apple Cider Vinegar Effervescent Tablets on Gut Health, Obesity and User Experience: An Observation. AFSJ 2024, 23, 38. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Ugajin, S.; Kaga, T. Vinegar Intake Reduces Body Weight, Body Fat Mass, and Serum Triglyceride Levels in Obese Japanese Subjects. Biosci. Biotechnol. Biochem. 2009, 73, 1837–1843. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Emaminejad, S.; Parvinroo, S.; Ashouri, A.; Alizadeh, I.; Medicine, I. The Effect of Oxymel Syrup on some Cardiovascular Risk Factors in Overweight and Obese People: A Randomized Controlled Trial Study. Tradit. Integr. Med. 2021, 6. [Google Scholar] [CrossRef]
- Abid, M.; Memon, Z.; Shaheen, S.; Ahmed, F.; Zaman, M.; Agha, F. Comparison of Apple Cider Vinegar and Metformin Combination with Metformin Alone in Newly Diagnosed Type 2 Diabetic Patients: A Randomized Controlled Trial. Int. J. Med. Res. Health Sci. 2020, 9, 1–7. [Google Scholar]
- Nazni, P.; Singh, R.; Shobana, R.; Singh, H.; Singh, S.; Singh, K.; Deep Singh, H.; Kumar, S. Assessment of hypoglycemic effects of apple cider vinegar in type 2 diabets. Int. J. Food Nutr. Sci. 2015, 4, 206–209. [Google Scholar]
- Abou-Khalil, R.; Andary, J.; El-Hayek, E. Apple Cider Vinegar for Weight Management in Lebanese Adolescents and Young Adults with Overweight and Obesity: A Randomised, Double-Blind, Placebo-Controlled Study. BMJ Nutr. Prev. Health. 2024, 7, 61–67. [Google Scholar] [CrossRef]
- Jafarirad, S.; Elahi, M.; Mansoori, A.; Khanzadeh, A.; Haghighizadeh, M. The Improvement Effect of Apple Cider Vinegar as a Functional Food on Anthropometric Indices, Blood Glucose and Lipid Profile in Diabetic Patients: A Randomized Controlled Clinical Trial. Front. Clin. Diabetes Healthc. 2023, 4, 1288786. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Abbas, M.A.; Ahmad, H.; Yousef, N.; Ahmed, Z.; Humayun, N.; Ashfaq, H.; Humayun, A. Effect of Apple Cider Vinegar in Type 2 Diabetic Patients with Poor Glycemic Control: A Randomized Placebo Controlled Design ®. Int. J. Med. Res. Health Sci. 2019, 8, 149–159. [Google Scholar]
- Khezri, S.S.; Saidpour, A.; Hosseinzadeh, N.; Amiri, Z. Beneficial Effects of Apple Cider Vinegar on Weight Management, Visceral Adiposity Index and Lipid Profile in Overweight or Obese Subjects Receiving Restricted Calorie Diet: A Randomized Clinical Trial. J. Funct. Foods 2018, 43, 95. [Google Scholar] [CrossRef]
- Hmad Halima, B.; Sarra, K.; Mohamed, S.; Louay, T.; Slama Fethi, B.; Houda, B.J.; Henda, J.; Abdallah, A. Apple Cider Vinegar Ameliorates Hyperglycemia and Hyperlipidemia in Tunisian Type 2 Diabetic Patients. Int. J. Multidiscip. Curr. Res. 2017, 5, 1453–1459. [Google Scholar]
- Khalid, A.S.; Khalid, A.S.; Ashiq, E. Effect of the Apple Cider Vinegar on Weight Management, Blood Glucose Levels and Lipid Profile among Obese/Overweight Adults: A Randomised Control Trial. Pak. J. Med. Health Sci. 2022, 16, 286. [Google Scholar] [CrossRef]
- Abid, M.; Ahmed, F.; Shaheen, S.; Memon, Z.; Shaikh, M.Z.; Agha, F. Evaluating the Synergistic Activity of Metformin and Apple Cider Vinegar in Type 2 Diabetics. J. Pharm. Res. Int. 2020, 31, 1–7. [Google Scholar] [CrossRef]
- Junejo, S.; Karim, N.; Sajid, M.; Jaffri, A. Apple Cider Vinegar Effect on Lipid Profile in Prediabetics. Rawal Med. J. 2024, 49, 723. [Google Scholar]
- Pusparatha, S.B.; Devi, R.G.; Jyothipriya, A. Effects of Apple Cider Vinegar on Diabetic and Obese Patients. Drug Invent. Today 2019, 12, 968. [Google Scholar]
- Shahzad, M.K.; Shahzad, M.A.; Rasool, R.; Laila, U.; Amir, M.; Sheryar, M.; Salam, M.A.; Gul, M. Investigation of Medicinal Properties and Chemical and Biochemical Characterization of Apple Cider Vinegar for Anti-Hyperuricemia in Female Human Subjects in Controlled Randomized Trial. Pak. J. Pharm. Sci. 2023, 36, 277–280. [Google Scholar]
- Gheflati, A.; Bashiri, R.; Ghadiri-Anari, A.; Reza, J.Z.; Kord, M.T.; Nadjarzadeh, A. The Effect of Apple Vinegar Consumption on Glycemic Indices, Blood Pressure, Oxidative Stress, and Homocysteine in Patients with Type 2 Diabetes and Dyslipidemia: A Randomized Controlled Clinical Trial. Clin. Nutr. ESPEN 2019, 33, 132–138. [Google Scholar] [CrossRef]
- Bashiri, R.; Ghadiri-Anari, A.; Hekmatimoghadam, H.; Dehghani, A.; Najarzadeh, A. The Effect of Apple Vinegar on Lipid Profiles and Anthropometric Indices in Type 2 Diabetes Patients with Dyslipidemia: A Randomized Clinical Trial. J. Shahid Sadoughi Univ. Med. Sci. 2014, 22, 1543–1553. [Google Scholar]
- Williamson, D.A.; Bray, G.A.; Ryan, D.H. Is 5% Weight Loss a Satisfactory Criterion to Define Clinically Significant Weight Loss? Obesity 2015, 23, 2319–2320. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.A.; Soltan; Shehata, M.M.E.M. Antidiabetic and Hypocholesrolemic Effect of Different Types of Vinegar in Rats. Life Sci. J. 2012, 9, 2141–2151. [Google Scholar]
- Hlebowicz, J.; Darwiche, G.; Bjorgell, O.; Almer, L. Effect of Apple Cider Vinegar on Delayed Gastric Emptying in Patients with Type 1 Diabetes Mellitus: A Pilot Study. BMC Gastroenterol. 2007, 7, 46. [Google Scholar] [CrossRef]
- Lin, H.C.; Doty, J.E.; Reedy, T.J.; Meyer, J.H. Inhibition of Gastric Emptying by Acids Depends on pH, Titratable Acidity, and Length of Intestine Exposed to Acid. Am. J. Physiol. 1990, 259, 1025. [Google Scholar] [CrossRef]
- Seok, H.; Lee, J.Y.; Park, E.M.; Park, S.E.; Lee, J.H.; Lim, S.; Lee, B.; Kang, E.S.; Lee, H.C.; Cha, B.S. Balsamic Vinegar Improves High Fat-Induced Beta Cell Dysfunction Via Beta Cell ABCA1. Diabetes Metab. J. 2012, 36, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhao, H.; Sui, Y.; Guan, J.; Chan, J.C.N.; Tong, P.C.Y. White Rice Vinegar Improves Pancreatic Beta-Cell Function and Fatty Liver in Streptozotocin-Induced Diabetic Rats. Acta Diabetol. 2010, 49, 185. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Grahame Hardie, D. AMP-Activated Protein Kinase: A Master Switch in Glucose and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2004, 5, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kang, C.; Qiang, X.; Zhang, X.; Li, S.; Liang, K.; Wang, Y.; Wang, J.; Cao, H.; Wang, M. Beneficial Effect of Vinegar Consumption Associated with Regulating Gut Microbiome and Metabolome. Curr. Res. Food Sci. 2023, 8, 100566. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.S.J.; Soong, R.Y.; Teo, Y.Q.J.; Flolo, T.N.; Chong, B.; Yong, C.L.; Ang, S.H.; Ho, Y.; Chew, N.W.S.; So, J.B.Y.; et al. Anthropometric and Cardiometabolic Effects of Polyphenols in People with Overweight and Obesity: An Umbrella Review. Nutr. Rev. 2024, 82, 1556–1593. [Google Scholar] [CrossRef] [PubMed]
| Study, Year (Country) | Study Design and Duration (Weeks) | Type Participants (Number of Subjects) | BMI Mean ± SD (kg/m2) | Intervention | Comparator | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Abid et al., 2020 (Pakistan) [42] | Randomized, open-label, controlled trial (12 weeks) | Adults with newly diagnosed type 2 diabetes (n. 126) | 30.56 ± 5.36 | 750 mg of metformin plus 2 tablespoons of ACV per day | 750 mg of metformin alone per day | Weight and BMI, FBS, and HbA1c | Significant reduction only in FBS and HbA1c in the group that also took ACV compared to metformin alone |
| Abou-Khalil et al., 2024 (Lebanon) [44] | Four-arm, randomized, double-blind, placebo-controlled study (12 weeks) | Overweight and obese young adults (n. 57) | 26.51 ± 3.5 | 5, 10, and 15 mL of ACV diluted in 250 mL of water daily | Water with taste and appearance similar to the treatment | Body composition, lipid and glucose parameters | The treatment group showed significant improvements in anthropometric outcomes compared to placebo |
| Halima et al., 2017 (Tunisia) [48] | Parallel, randomized, controlled clinical trial (4 weeks) | Adults diagnosed with type 2 diabetes (n. 26) | / | 15 mL of ACV daily | Water | Body composition, lipid and glucose parameters | Significant reduction in BMI, FBS, and Tg in the treatment groups compared to placebo |
| Jafaridad et al., 2023 (Iran) [45] | Parallel, randomized, controlled, and open-label clinical trial (8 weeks) | Adults with type 2 diabetes (n. 80) | 29.49 ± 5.09 | 30 mL of ACV diluted in 100 mL of water daily with recommendations for a healthy diet | Recommendations for a healthy diet | Body composition, lipid and glucose parameters | Significant post-treatment differences in body composition, HbA1c, and lipid profile were observed between the two groups |
| Kannan et al., 2024 (India) [39] | Parallel, randomized, controlled clinical trial (60 day) | Overweight/obese adults (n. 77) | / | Daily effervescent ACV tablet (~14 mL of ACV) combined with mild exercise and a low-sugar diet | Only the exercise and diet regimen | Body composition, lipid and glucose parameters. Food intake and various digestive issues | Significant reductions in anthropometric parameters and digestive issues were observed in the treatment groups compared to placebo |
| Kausar et al., 2019 (Pakistan) [46] | Single-blind, randomized, placebo-controlled trial (12 weeks) | Adults with type 2 diabetes (n. 110) | / | 15 mL of ACV diluted in 200 mL of water daily, along with the usual diet and general dietary advice regarding high- and low-glycemic foods | 15 ml of artificial flavor of apple cider vinegar, along with the usual diet and general dietary advice regarding high- and low-glycemic foods | HbA1c, FBS, and lipid and anthropometric parameters | Significant reductions in lipid and glycemic profiles were observed in the intervention group. No differences in anthropometric parameters between groups |
| Khezri et al., 2018 (Iran) [47] | Two-arm, parallel, randomized controlled trial (12 weeks) | Overweight/obese adults (n. 44) | 32.1 ± 4.9 | 30 mL/day of ACV plus restricted-calorie diet | Restricted-calorie diet alone | Body composition and lipid parameters | Significant reduction in weight, BMI, LDL, and Tg and significant increase in HDL in the treatment group compared to the control group |
| Kondo et al., 2009 (Japan) [40] | Three-arm, parallel, randomized controlled trial (12 weeks) | Obese adults (n. 104) | 27.2 ± 1.8 | 15 mL of ACV daily | Water | Body composition and lipid parameters | Significant reduction in BMI and Tg in the treatment group compared to the control group |
| Kondo et al., 2009 (Japan) [40] | Three-arm, parallel, randomized controlled trial (12 weeks) | Obese adults (n. 101) | 27.0 ± 1.7 | 30 mL of ACV daily | Water | Body composition and lipid parameters | Significant reduction in BMI and Tg in the treatment group compared to the control group |
| Peerkhan et al., 2015 (India) [43] | Parallel, randomized, controlled clinical trial (12 weeks) | Adults with type 2 diabetes (n. 40) | 30 mL of ACV daily | No intervention | Body composition, glucose and lipid parameters | Significant reduction in BMI, WHR, FBS, post prandial blood sugar levels, and HbA1c between groups | |
| Roshan M et al., 2021 (Iran) [41] | Two-arm, randomized controlled trial (4 weeks) | Overweight/obese adults (n. 96) | 29.91 ± 4.51 | 30 mL of ACV diluted in 250 cc of water daily | 250 cc of water daily | Anthropometric measurements, lipid parameters, and FBS | Significant reduction in weight and total Chol in the treatment group compared to the control group |
| Variables | Sensitivity Analysis | Number of Participants | Effect Size SMD (95% CI) | I2 (%) | p-Value | |
|---|---|---|---|---|---|---|
| Body weight | Excluding studies at high risk of bias | 463 | −0.29 [−0.54, −0.04] | 39 | 0.02 | |
| BMI | Excluding studies at high risk of bias | 353 | −0.33 [−0.55, −0.11] | 0 | 0.004 | |
| WC | Excluding studies at high risk of bias | 309 | −0.17 [−0.40, 0.07] | 0 | 0.16 | |
| WHR | Excluding studies at high risk of bias | 251 | −0.06 [−0.32, 0.20] | 0 | 0.65 | |
| Variables | Subgroup | Number of participants | Effect Size SMD (95% CI) | I2 (%) | p-value | |
| Body weight | Subject | Obese | 430 | −0.29 [−0.51, −0.08] | 16 | 0.008 |
| T2DM | 449 | −0.44 [−0.85, −0.03] | 78 | 0.03 | ||
| Duration | 4–8 weeks | 547 | −0.32 [−0.61, −0.04] | 58 | 0.03 | |
| 12 weeks | 493 | −0.27 [−0.50, −0.05] | 31 | 0.02 | ||
| Dosage | 5–15 mL/day | 494 | −0.24 [−0.48, 0.01] | 41 | 0.06 | |
| 30 mL/day | 289 | −0.60 [−1.05, −0.15] | 70 | 0.009 | ||
| Control | Water | 353 | −0.37 [−0.63, −0.11] | 26 | 0.006 | |
| Other | 430 | −0.41 [−0.83, 0.01] | 78 | 0.06 | ||
| No drugs | 657 | −0.42 [−0.70, −0.15] | 65 | 0.003 | ||
| Only diet with or without physical activity | 304 | −0.47 [−1.05, 0.10] | 83 | 0.11 | ||
| BMI | Subject | Obese | 430 | −0.65 [−1.24, −0.05] | 88 | 0.03 |
| T2DM | 283 | −0.70 [−1.22, −0.18] | 75 | 0.008 | ||
| Duration | 4–8 weeks | 470 | −0.30 [−0.60, 0.00] | 56 | 0.05 | |
| 12 weeks | 365 | −0.90 [−1.66, −0.15] | 90 | 0.02 | ||
| Dosage | 5–15 mL/day | 384 | −0.24 [−0.45, −0.03] | 5 | 0.03 | |
| 30 mL/day | 329 | −0.90 [−1.53, −0.27] | 90 | 0.005 | ||
| Control | Water | 353 | −0.33 [−0.55, −0.11] | 0 | 0.004 | |
| Other | 360 | −0.83 [−1.48, −0.19] | 90 | 0.01 | ||
| No drugs | 587 | −0.73 [−1.17, −0.29] | 84 | 0.001 | ||
| Only diet with or without physical activity | 194 | −1.35 [−2.76, 0.07] | 95 | 0.06 | ||
| WC | Subject | Obese | 353 | −0.17 [−0.39, 0.05] | 0 | 0.12 |
| T2DM | 73 | −1.08 [−1.58, −0.59] | - | <0.001 | ||
| Duration | 4–8 weeks | 426 | −0.25 [−0.56, 0.06] | 53 | 0.11 | |
| 12 weeks | 257 | −0.25 [−0.51, 0.01] | 0 | 0.06 | ||
| Dosage | 5–15 mL/day | 213 | −0.26 [−0.63, 0.10] | 0 | 0.16 | |
| 30 mL/day | 213 | −0.38 [−0.87, 0.11] | 75 | 0.13 | ||
| Control | Water | 251 | −0.17 [−0.40, 0.07] | 0 | 0.16 | |
| Other | 175 | −0.66 [−1.52, 0.21] | 80 | 0.14 | ||
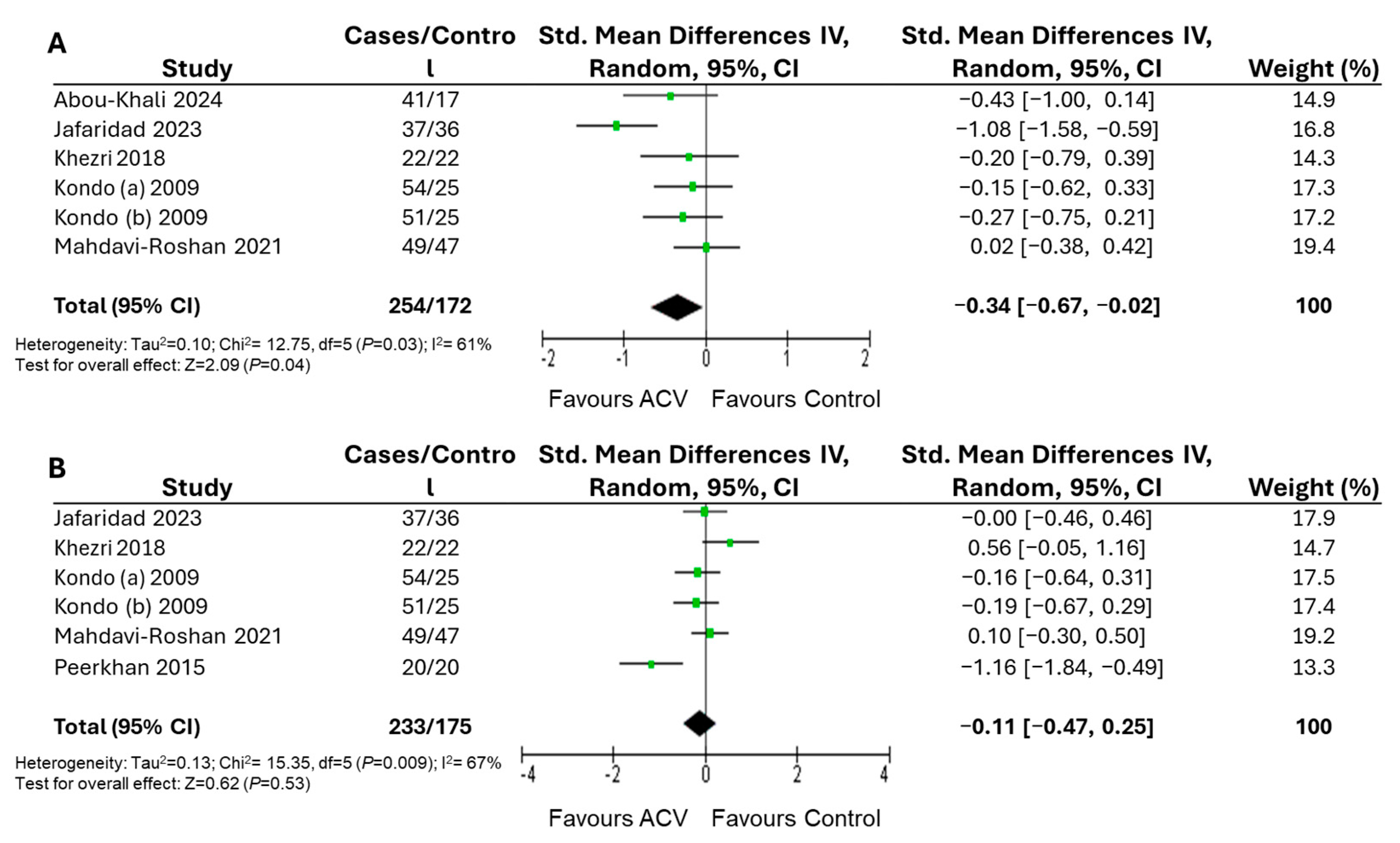
| No drugs | 426 | −0.34 [−0.67, −0.02] | 62 | 0.04 | ||
| Only diet with or without physical activity | 117 | −0.66 [−1.52, −0.21] | 80 | 0.14 | ||
| WHR | Subject | Obese | 295 | 0.04 [−0.25, 0.34] | 32 | 0.77 |
| T2DM | 113 | −0.55 [−1.69, 0.58] | 87 | 0.34 | ||
| Duration | 4–8 weeks | 368 | 0.01 [−0.20, 0.22] | 0 | 0.93 | |
| 12 weeks | 239 | −0.22 [−0.81, 0.37] | 78 | 0.46 | ||
| Dosage | 5–15 mL/day | 79 | −0.33 [−0.55, −0.11] | - | - | |
| 30 mL/day | 329 | −0.11 [−0.55, 0.33] | 74 | 0.63 | ||
| Control | Water | 251 | −0.06 [−0.32, 0.20] | 0 | 0.65 | |
| Other | 157 | −0.19 [−1.07, 0.70] | 86 | 0.68 | ||
| No drugs | 117 | −0.11 [−0.47, 0.25] | 67 | 0.53 | ||
| Only diet with or without physical activity | 117 | 0.24 [−0.30, 0.78] | 52 | 0.38 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagna, A.; Ferro, Y.; Noto, F.R.; Bruno, R.; Aragao Guimaraes, A.; Pujia, C.; Mazza, E.; Maurotti, S.; Montalcini, T.; Pujia, A. Effect of Apple Cider Vinegar Intake on Body Composition in Humans with Type 2 Diabetes and/or Overweight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2025, 17, 3000. https://doi.org/10.3390/nu17183000
Castagna A, Ferro Y, Noto FR, Bruno R, Aragao Guimaraes A, Pujia C, Mazza E, Maurotti S, Montalcini T, Pujia A. Effect of Apple Cider Vinegar Intake on Body Composition in Humans with Type 2 Diabetes and/or Overweight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2025; 17(18):3000. https://doi.org/10.3390/nu17183000
Chicago/Turabian StyleCastagna, Alberto, Yvelise Ferro, Francesca Rita Noto, Rossella Bruno, Analucia Aragao Guimaraes, Carmelo Pujia, Elisa Mazza, Samantha Maurotti, Tiziana Montalcini, and Arturo Pujia. 2025. "Effect of Apple Cider Vinegar Intake on Body Composition in Humans with Type 2 Diabetes and/or Overweight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 17, no. 18: 3000. https://doi.org/10.3390/nu17183000
APA StyleCastagna, A., Ferro, Y., Noto, F. R., Bruno, R., Aragao Guimaraes, A., Pujia, C., Mazza, E., Maurotti, S., Montalcini, T., & Pujia, A. (2025). Effect of Apple Cider Vinegar Intake on Body Composition in Humans with Type 2 Diabetes and/or Overweight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 17(18), 3000. https://doi.org/10.3390/nu17183000









