Effect of 6-Shogaol Derived from Ginger (Zingiber officinale) on Dual-Species Biofilm Formation by Streptococcus mutans and Candida albicans
Abstract
1. Introduction
2. Materials and Methods
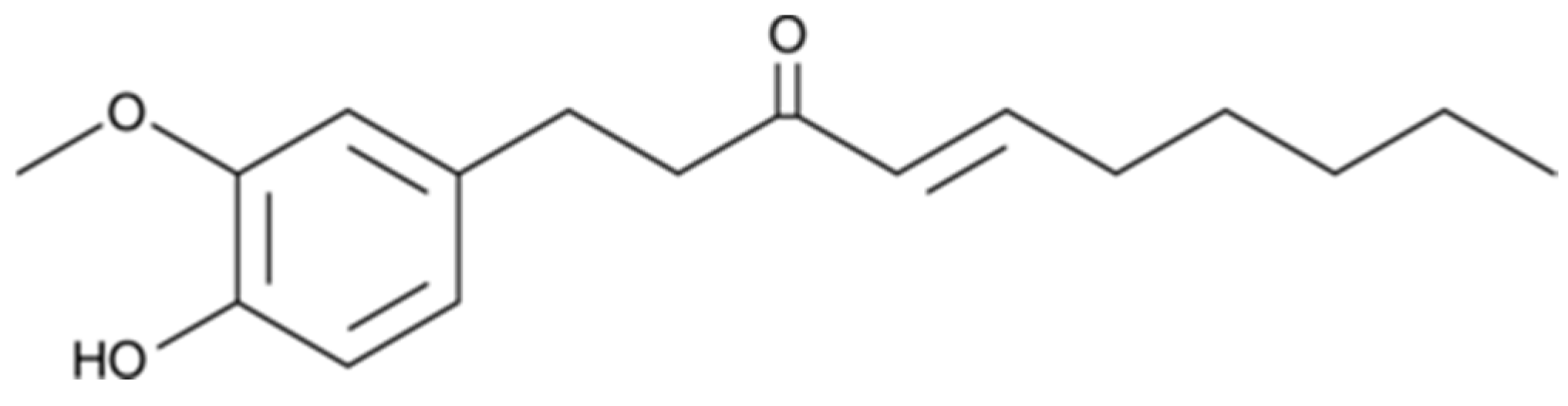
2.1. Chemicals, and Microbial Species and Culture
2.2. Saliva Sample Preparation
2.3. Biofilm Formation Assay
2.4. Biochemical Biofilm Analysis
2.5. Colony-Forming Unit (CFU) Assay
2.6. Confocal Laser Scanning Microscopy (CLSM)
2.7. qPCR Analysis
2.8. Statistical Analysis
3. Results
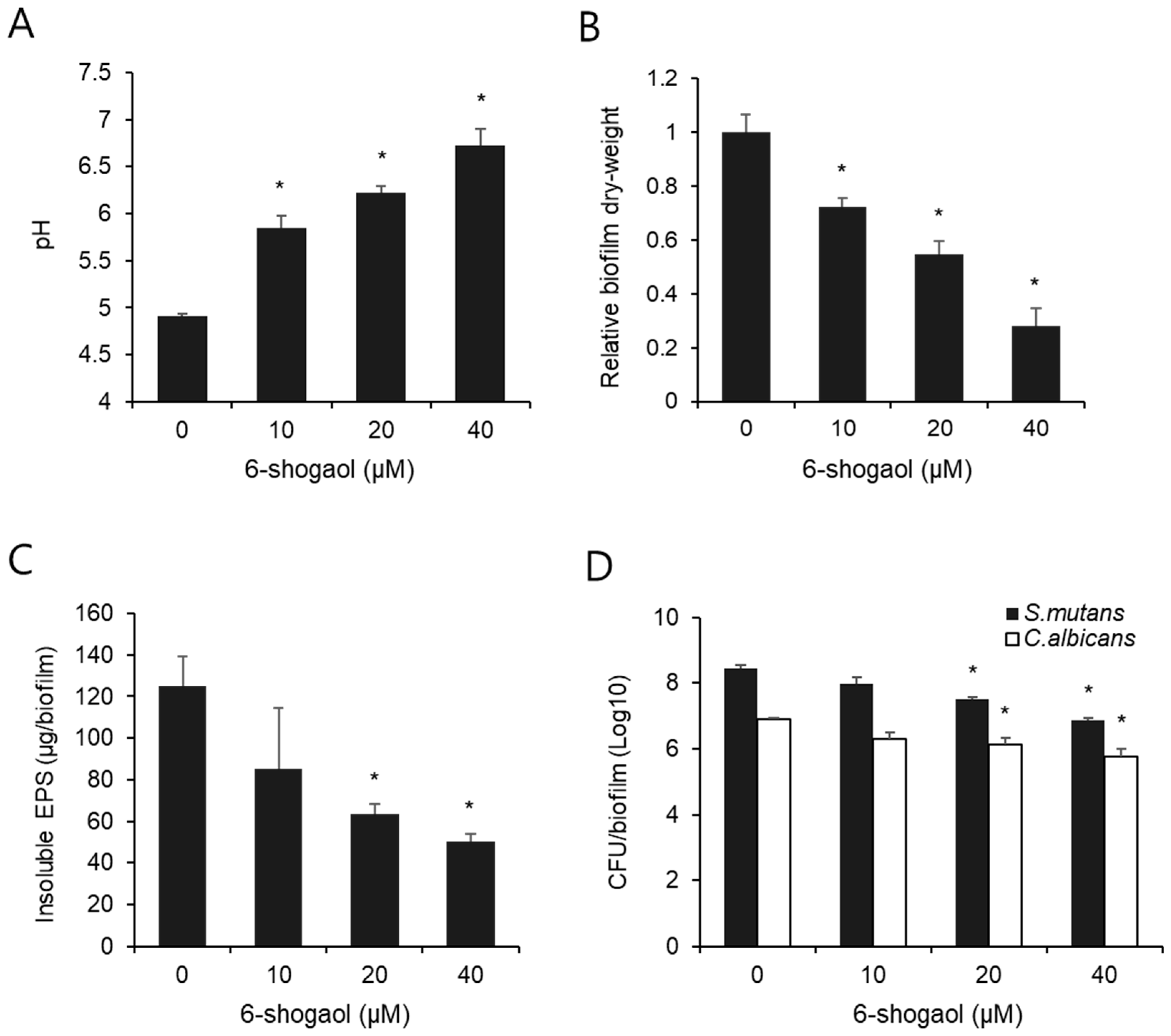
3.1. Effect of 6-Shogaol on Biochemical Properties and CFU Counts of Dual-Species Biofilm by S. mutans and C. albicans
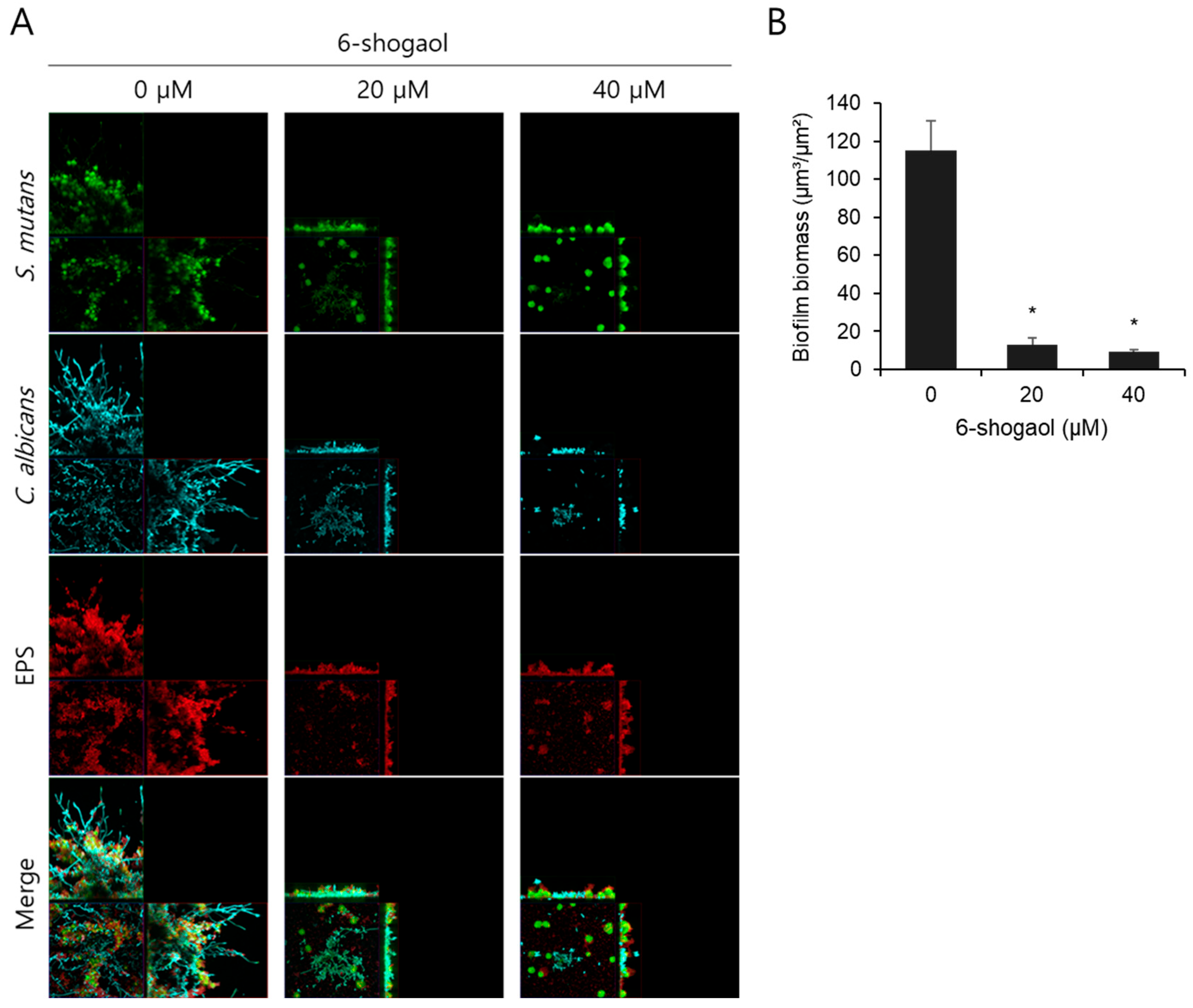
3.2. Effect of 6-Shogaol on the Morphology of Dual-Species Biofilm by S. mutans and C. albicans
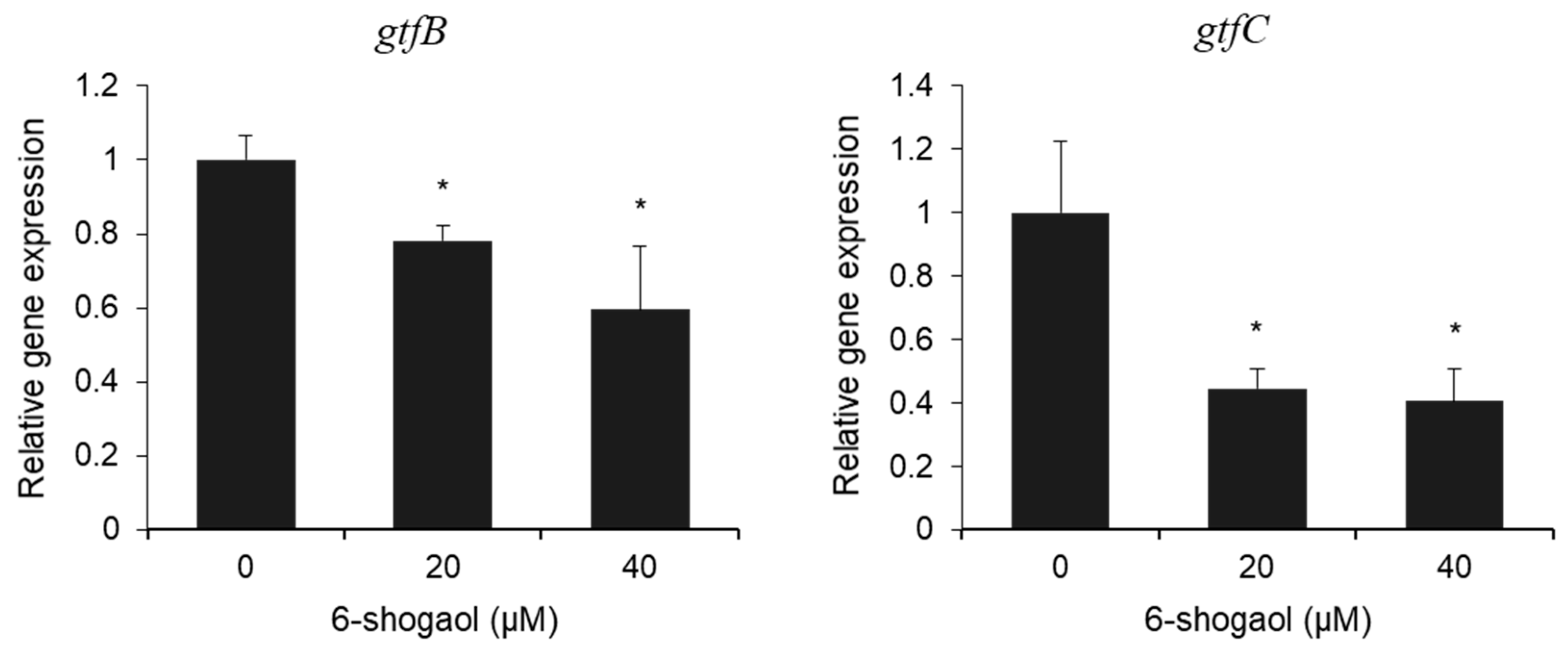
3.3. Effect of 6-Shogaol on the Expression of gtf Genes in Dual-Species Biofilm by S. mutans and C. albicans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S. mutans | Streptococcus mutans |
| EPS | Extracellular polysaccharides |
| C. albicans | Candida albicans |
| HA | Hydroxyapatite |
| CLSM | Confocal laser scanning microscopy |
| CFU | Colony-forming unit |
| qPCR | quantitative polymerase chain reaction |
| SD | Standard deviation |
References
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, A.F.; Koo, H.; Bellato, C.M.; Bedi, G.; Cury, J.A. The role of sucrose in cariogenic dental biofilm formation--new insight. J. Dent. Res. 2006, 85, 878–887. [Google Scholar] [CrossRef]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef]
- Lu, S.Y. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Macias-Paz, I.U.; Pérez-Hernández, S.; Tavera-Tapia, A.; Luna-Arias, J.P.; Guerra-Cárdenas, J.E.; Reyna-Beltrán, E. Candida albicans the main opportunistic pathogenic fungus in humans. Rev. Argent. Microbiol. 2023, 55, 189–198. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef]
- Kim, D.; Liu, Y.; Benhamou, R.I.; Sanchez, H.; Simón-Soro, Á.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.R.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.I.; Duarte, S.; Xiao, J.; Mitra, S.; Foster, T.H.; Koo, H. Structural and molecular basis of the role of starch and sucrose in Streptococcus mutans biofilm development. Appl. Environ. Microbiol. 2009, 75, 837–841. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Du, J.; Wu, M.; Huang, X. Current and prospective therapeutic strategies: Tackling Candida albicans and Streptococcus mutans cross-kingdom biofilm. Front. Cell. Infect. Microbiol. 2023, 13, 1106231. [Google Scholar] [CrossRef]
- Fan, F.; Liu, Y.; Liu, Y.; Lv, R.; Sun, W.; Ding, W.; Cai, Y.; Li, W.; Liu, X.; Qu, W. Candida albicans biofilms: Antifungal resistance, immune evasion, and emerging therapeutic strategies. Int. J. Antimicrob. Agents 2022, 60, 106673. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Wang, Y.; Ji, M.; Li, Y.; Zhu, H.; Yan, Y.; Fu, D.; Zou, L.; Ren, B. Natural products from traditional medicine as promising agents targeting at different stages of oral biofilm development. Front. Microbiol. 2022, 13, 955459. [Google Scholar] [CrossRef]
- Huang, H.; Kim, M.O.; Kim, K.R. Anticancer effects of 6-shogaol via the AKT signaling pathway in oral squamous cell carcinoma. J. Appl. Oral Sci. 2021, 29, e20210209. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Choi, P.; Ham, J.; Park, J.G.; Lee, J. Antibiofilm and Antivirulence Activities of 6-Gingerol and 6-Shogaol Against Candida albicans Due to Hyphal Inhibition. Front. Cell. Infect. Microbiol. 2018, 8, 299. [Google Scholar] [CrossRef]
- Hasan, S.; Danishuddin, M.; Khan, A.U. Inhibitory effect of zingiber officinale towards Streptococcus mutans virulence and caries development: In vitro and in vivo studies. BMC Microbiol. 2015, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Liu, Y.; Dhall, A.; Bawazir, M.; Koo, H.; Hwang, G. Synergism of Streptococcus mutans and Candida albicans Reinforces Biofilm Maturation and Acidogenicity in Saliva: An In Vitro Study. Front. Cell. Infect. Microbiol. 2020, 10, 623980. [Google Scholar] [CrossRef]
- de Sousa, D.L.; Lima, R.A.; Zanin, I.C.; Klein, M.I.; Janal, M.N.; Duarte, S. Effect of Twice-Daily Blue Light Treatment on Matrix-Rich Biofilm Development. PLoS ONE 2015, 10, e0131941. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146 Pt 10, 2395–2407. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Zhang, L.; Hieawy, A.; Shen, Y. Evaluation of extracellular polymeric substances matrix volume, surface roughness and bacterial adhesion property of oral biofilm. J. Dent. Sci. 2023, 18, 1723–1730. [Google Scholar] [CrossRef]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zeng, Y.; Rustchenko, E.; Huang, X.; Wu, T.T.; Falsetta, M.L. Dual transcriptome of Streptococcus mutans and Candida albicans interplay in biofilms. J. Oral Microbiol. 2023, 15, 2144047. [Google Scholar] [CrossRef]
- Ellepola, K.; Liu, Y.; Cao, T.; Koo, H.; Seneviratne, C.J. Bacterial GtfB Augments Candida albicans Accumulation in Cross-Kingdom Biofilms. J. Dent. Res. 2017, 96, 1129–1135. [Google Scholar] [CrossRef]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Bowen, W.H.; Burne, R.A.; Kuramitsu, H.K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 1993, 61, 3811–3817. [Google Scholar] [CrossRef]
- Bischoff-Kont, I.; Fürst, R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef]
- Koo, H.; Falsetta, M.L.; Klein, M.I. The exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef]
- Lobo, C.I.V.; Rinaldi, T.B.; Christiano, C.M.S.; De Sales Leite, L.; Barbugli, P.A.; Klein, M.I. Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J. Oral Microbiol. 2019, 11, 1581520. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Zero, D.T.; van Houte, J.; Russo, J. Enamel demineralization by acid produced from endogenous substrate in oral streptococci. Arch. Oral Biol. 1986, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Van Loveren, C.; Fielmich, A.M.; Ten Brink, B. Comparison of the effects of fluoride and the ionophore nigericin on acid production by Streptococcus mutans and the resultant in vitro enamel demineralization. J. Dent. Res. 1987, 66, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.J.; Khan, F.; Tabassum, N.; Kim, Y.M. Alteration of oral microbial biofilms by sweeteners. Biofilm 2024, 7, 100171. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, X.; Wang, C.; Song, J.; Xu, J.; Liu, X.; Qian, Y.; Suo, H. New strategies and mechanisms for targeting Streptococcus mutans biofilm formation to prevent dental caries: A review. Microbiol. Res. 2023, 278, 127526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, E.-H.; Hwang, G.; Kim, K.-R. Effect of 6-Shogaol Derived from Ginger (Zingiber officinale) on Dual-Species Biofilm Formation by Streptococcus mutans and Candida albicans. Nutrients 2025, 17, 2999. https://doi.org/10.3390/nu17182999
Jung E-H, Hwang G, Kim K-R. Effect of 6-Shogaol Derived from Ginger (Zingiber officinale) on Dual-Species Biofilm Formation by Streptococcus mutans and Candida albicans. Nutrients. 2025; 17(18):2999. https://doi.org/10.3390/nu17182999
Chicago/Turabian StyleJung, Eun-Ha, Geelsu Hwang, and Ki-Rim Kim. 2025. "Effect of 6-Shogaol Derived from Ginger (Zingiber officinale) on Dual-Species Biofilm Formation by Streptococcus mutans and Candida albicans" Nutrients 17, no. 18: 2999. https://doi.org/10.3390/nu17182999
APA StyleJung, E.-H., Hwang, G., & Kim, K.-R. (2025). Effect of 6-Shogaol Derived from Ginger (Zingiber officinale) on Dual-Species Biofilm Formation by Streptococcus mutans and Candida albicans. Nutrients, 17(18), 2999. https://doi.org/10.3390/nu17182999






