Effect of Behavioral Change Communication and Livestock Feed Intervention on Dietary Practices in a Kenyan Pastoral Community: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
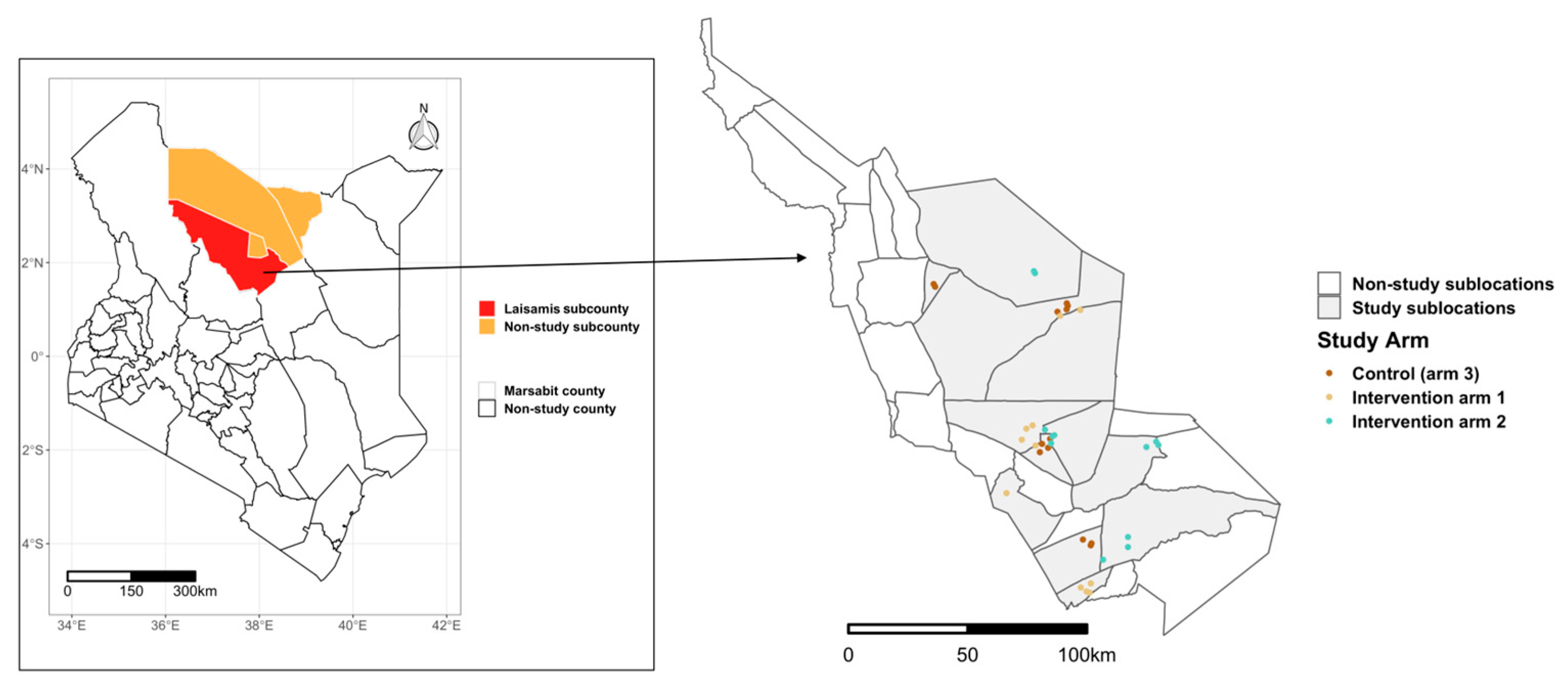
2.1. Study Area
2.2. Data Sources
2.3. Data Collection and Categorization
2.4. Analytical Methods
3. Results
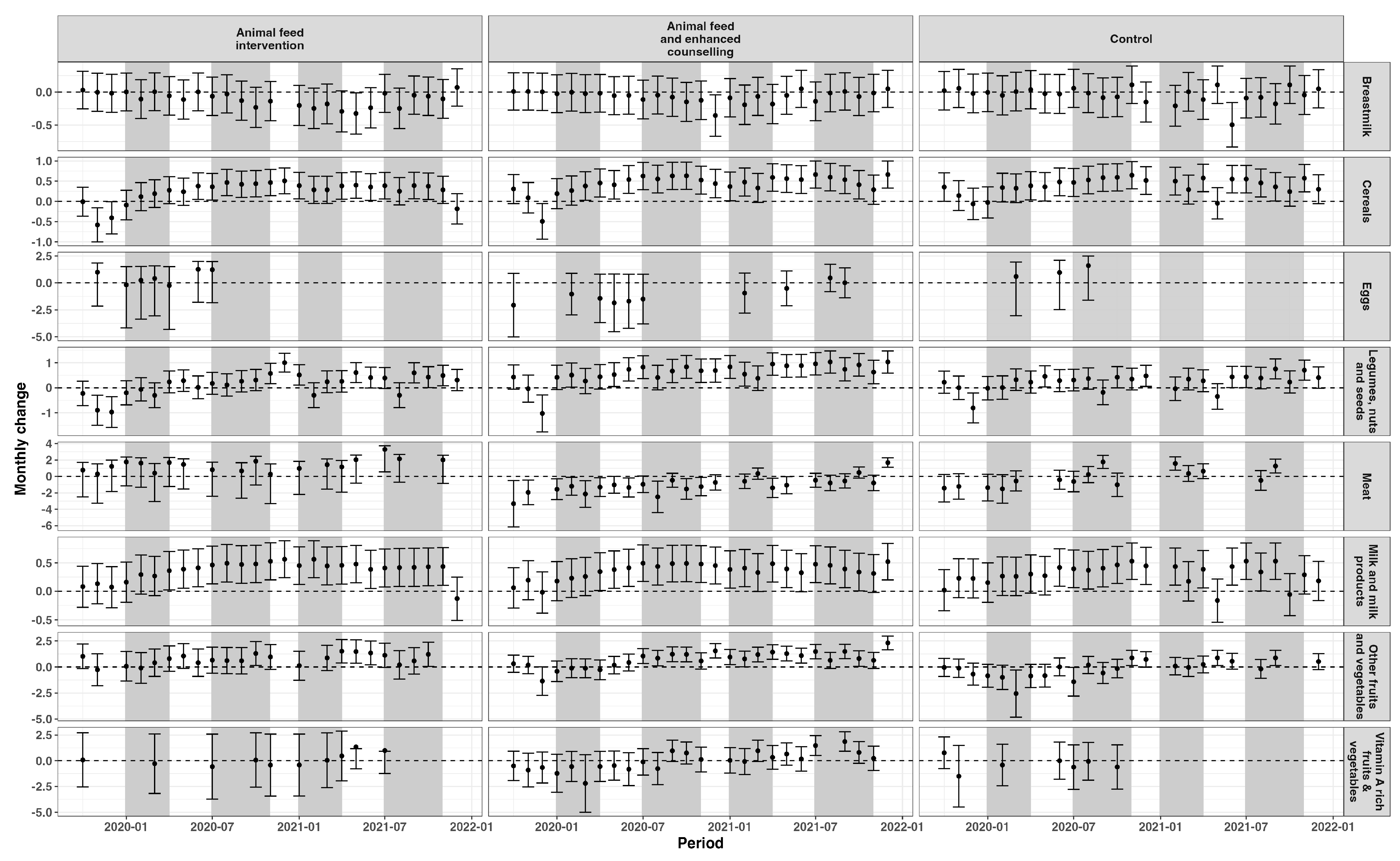
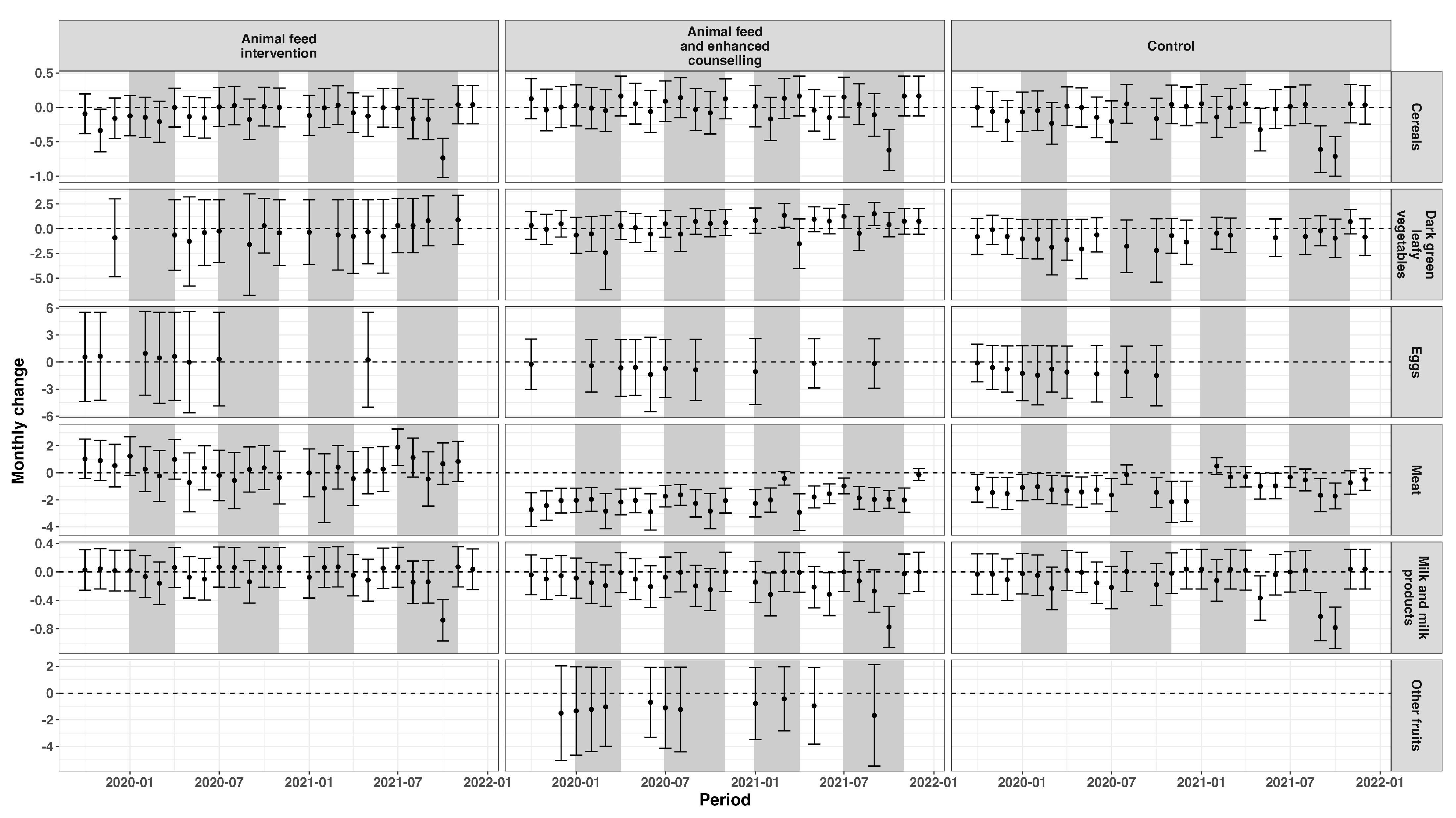
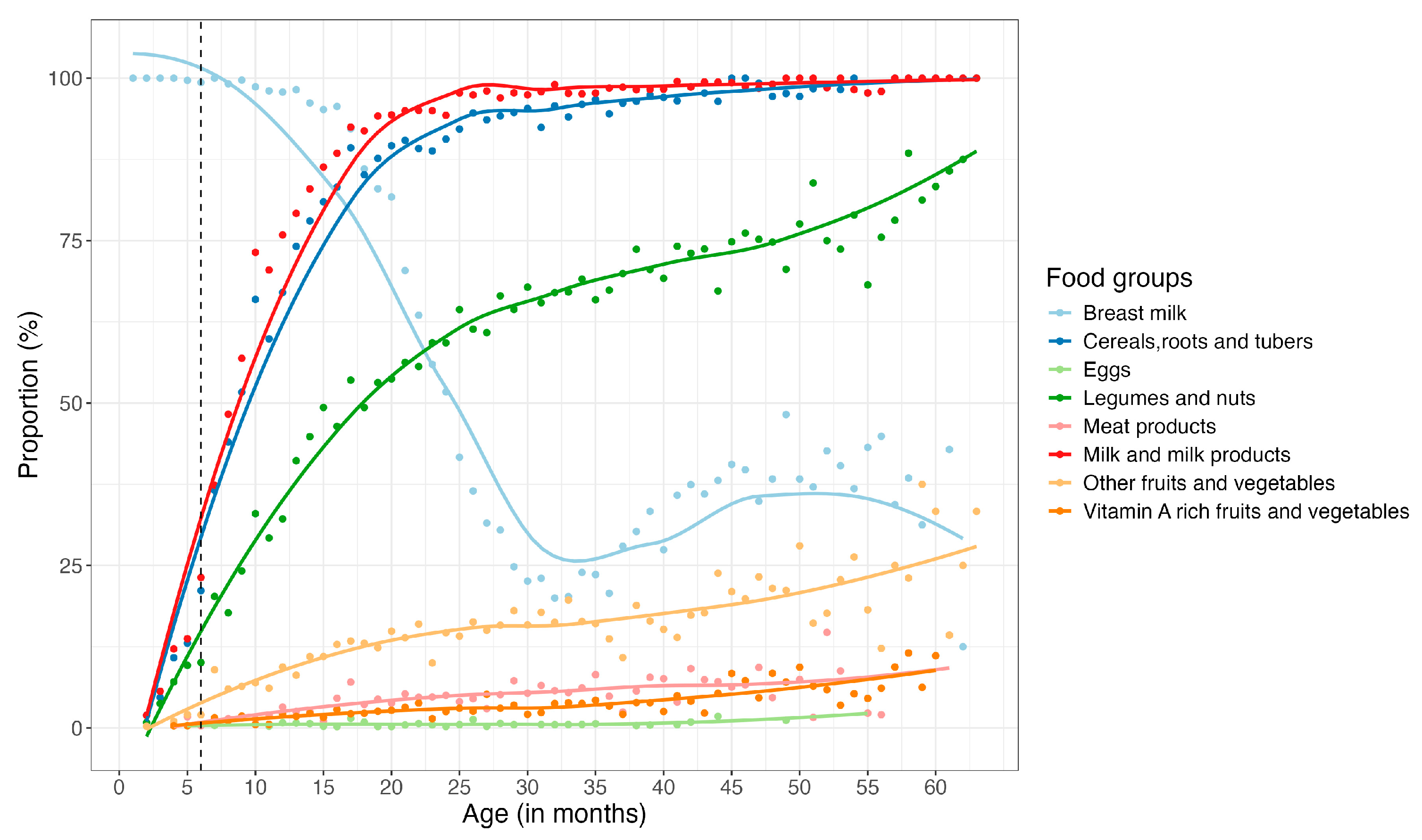
3.1. Dietary Patterns
3.1.1. Children
3.1.2. Women of Reproductive Age
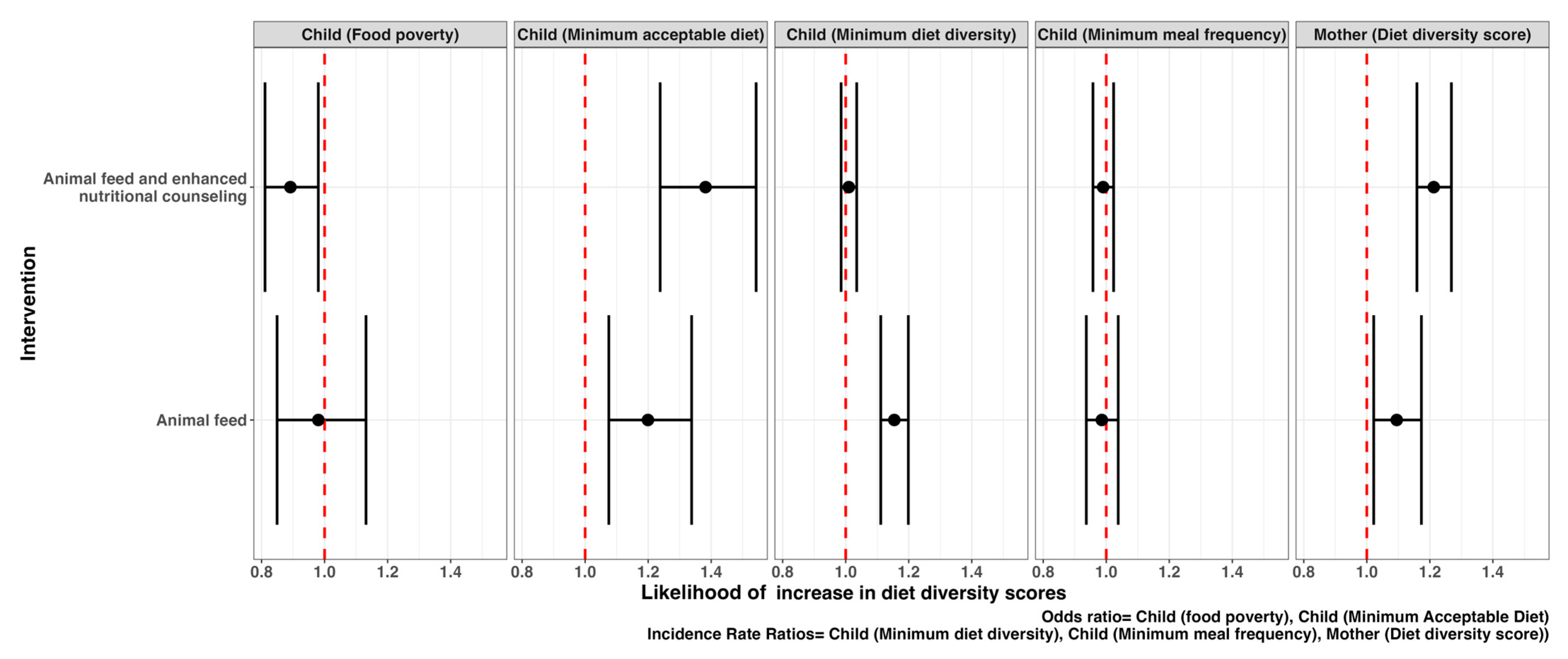
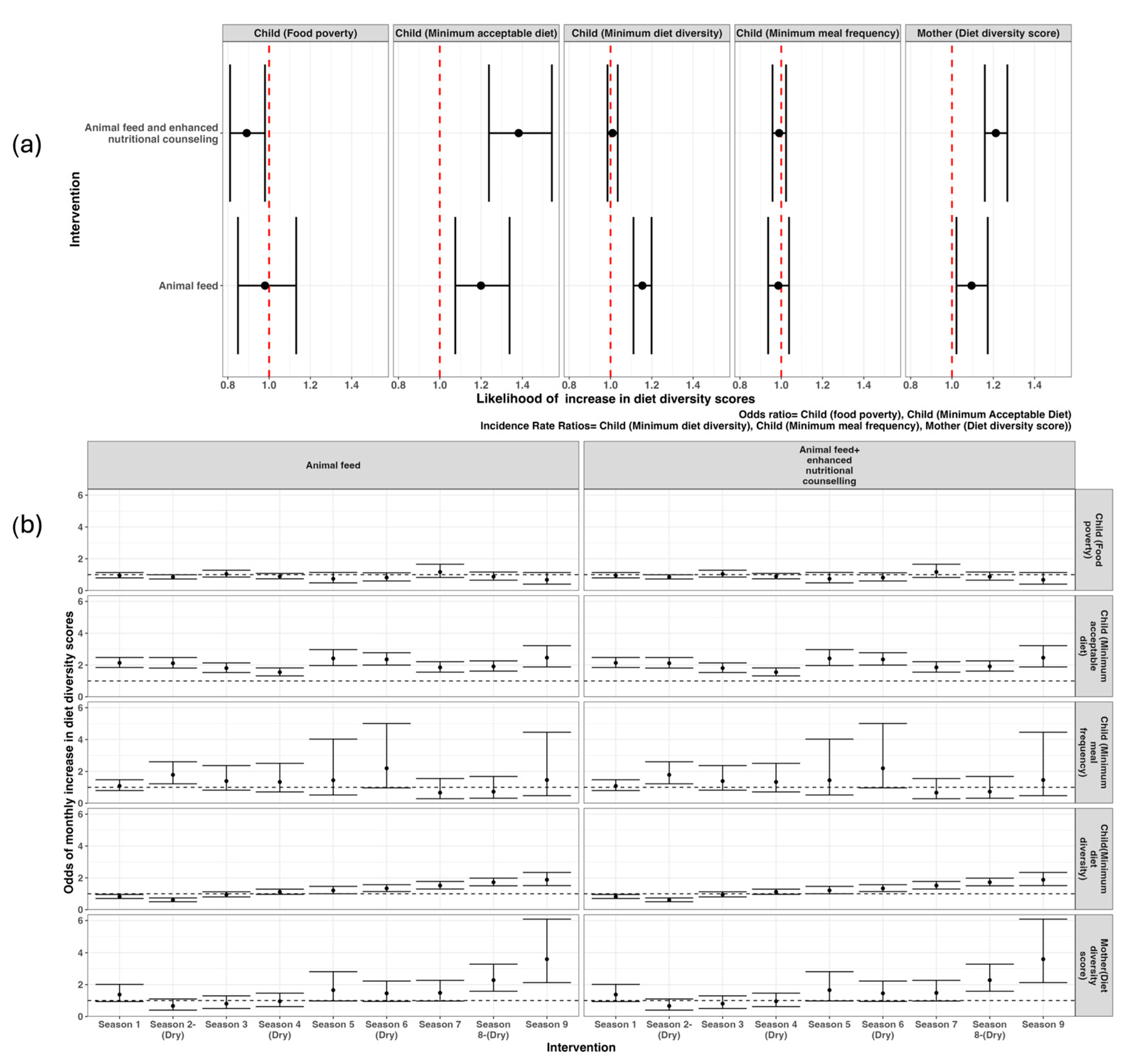
3.2. Dietary Intake Indicators
3.3. Factors Associated with Feeding Patterns Among Households, Infants, Young Children, and Women of Reproductive Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Food Group | Infants < 6 Months (n = 269) | Children 6–23 Months (n = 1009) | Children 24–59 Months (n = 367) |
|---|---|---|---|
| Breast milk (exclusive) | 244 (90%) | 74 (7%) | - |
| Breast milk (non-exclusive) | 28 (10%) | 1025 (93%) | 122 (32%) |
| Breastfed and non-milk liquids | 15 (6%) | - | - |
| Breastfed and animal milk | 10 (4%) | - | - |
| Tubers, roots, and cereals | 3 (1%) | 613 (58%) | 348 (92%) |
| Pulses/legumes and nuts | 2 (1%) | 371 (34%) | 254 (67%) |
| Dairy products | 3 (1%) | 898 (82%) | 357 (95%) |
| Flesh foods | - | 29 (3%) | 23 (6%) |
| Eggs | - | 7 (1%) | 3 (1%) |
| Vitamin A-rich fruits and vegetables | - | 19 (2%) | 10 (3%) |
| Other fruits and vegetables | 1 (<1%) | 93 (8%) | 44 (12%) |
| Children 6–23 Months | Children 24–59 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Food Group | Overall | Intervention Arm 1 | Intervention Arm 2 | Control | p Value | Overall | Intervention Arm 1 | Intervention Arm 2 | Control | p Value |
| Breastmilk | 87.0% | 84.6% | 89.8% | 86.6% | 0.093 | 31.6% | 29.9% | 34.1% | 30.9% | 0.131 |
| Dairy products | 82.4% | 82.3% | 84.9% | 79.9% | 0.356 | 97.6% | 97.6% | 98.4% | 96.8% | 0.06 |
| Tubers, roots, and cereals | 78.4% | 77.8% | 79.9% | 77.4% | 0.821 | 94.7% | 93.3% | 94.8% | 96.0% | 0.423 |
| Pulses/legumes and nuts | 47.7% | 45.9% | 50.9% | 46.2% | 0.424 | 66.4% | 61.8% | 70.6% | 66.6% | 0.072 |
| Other fruits and vegetables | 15.7% | 8.5% | 25.4% | 11.2% | <0.001 | 16.9% | 9.2% | 26.5% | 14.4% | <0.001 |
| Flesh foods | 7.4% | 2.7% | 9.1% | 10.4% | 0.092 | 6.4% | 4.3% | 6.8% | 8.2% | 0.057 |
| Vitamin A-rich fruits and vegetables | 4.8% | 1.4% | 7.1% | 2.1% | 0.009 | 5.1% | 1.2% | 7.9% | 3.3% | <0.001 |
| Eggs | 1.6% | 1.0% | 2.1% | 1.2% | 0.214 | 1.4% | 1.1% | 1.1% | 2.1% | 0.015 |
| Eggs/flesh foods | 7.7% | 2.9% | 9.9% | 10.5% | 0.066 | 6.7% | 4.4% | 7.2% | 8.7% | 0.018 |
| Fruits/vegetables | 16.5% | 8.6% | 27.1% | 11.6% | <0.001 | 17.6% | 9.5% | 27.8% | 14.9% | <0.001 |
| Baseline | Study Period | ||||
|---|---|---|---|---|---|
| Food Group | Women (n = 1734) | Intervention Arm 1 | Intervention Arm 2 | Control | p Value |
| Dairy products | 92.7% | 92.8% | 91.4% | 91.3% | 0.573 |
| Grains, roots, and tubers | 92.2% | 89.8% | 90.0% | 90.6% | 0.892 |
| Pulses | 72.8% | 66.5% | 70.8% | 67.3% | 0.166 |
| Other vegetables | 15.1% | 10.7% | 27.2% | 13.2% | <0.001 |
| Flesh foods | 8.2% | 5.5% | 9.0% | 8.7% | 0.058 |
| Dark green leafy vegetables | 1.7% | 6.0% | 8.8% | 3.2% | 0.037 |
| Other vitamin A-rich fruits and vegetables | 1.0% | 1.4% | 3.7% | 2.0% | 0.002 |
| Eggs | 0.6% | 1.0% | 2.0% | 7.1% | 0.035 |
| Other fruits | 0.3% | 1.2% | 1.2% | 1.7% | 0.060 |
| Nuts and seeds | 0% | 0% | 0% | 0% | |

References
- Onyango, A.W.; Jean-Baptiste, J.; Samburu, B.; Mahlangu, T.L.M. Regional Overview on the Double Burden of Malnutrition and Examples of Program and Policy Responses: African Region. Ann. Nutr. Metab. 2019, 75, 127–130. [Google Scholar] [CrossRef]
- Birungi, A.; Koita, Y.; Roopnaraine, T.; Matsiko, E.; Umugwaneza, M. Behavioural drivers of suboptimal maternal and child feeding practices in Rwanda: An anthropological study. Matern. Child Nutr. 2023, 19, e13420. [Google Scholar] [CrossRef]
- Katoch, O.R. Determinants of malnutrition among children: A systematic review. Nutrition 2022, 96, 111565. [Google Scholar] [CrossRef] [PubMed]
- Development Initiatives. Global Nutrition Report Action on Equity to End Malnutrition. Bristol, UK. 2020. Available online: https://globalnutritionreport.org/reports/2020-global-nutrition-report/ (accessed on 10 April 2025).
- Aboagye, R.G.; Seidu, A.A.; Ahinkorah, B.O.; Arthur-Holmes, F.; Cadri, A.; Dadzie, L.K.; Hagan, J.E., Jr.; Eyawo, O.; Yaya, S. Dietary diversity and undernutrition in children aged 6–23 months in Sub-Saharan Africa. Nutrients 2021, 13, 3431. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.; Previdelli, Á.N.; Fisberg, R.M.; Kovalskys, I.; Fisberg, M.; Herrera-Cuenca, M.; Cortes Sanabria, L.Y.; Yepez Garcia, M.C.; Rigotti, A.; Liria-Domínguez, M.R.; et al. Dietary diversity and micronutrients adequacy in women of childbearing age: Results from elans study. Nutrients 2020, 12, 1994. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Aguayo, V.M.; Krishna, V.; Nair, R. Household food insecurity and children’s dietary diversity and nutrition in India. Evidence from the comprehensive nutrition survey in Maharashtra. Matern. Child Nutr. 2017, 13, e12447. [Google Scholar] [CrossRef] [PubMed]
- FAO; USAID. Minimum Dietary Diversity for Women—A Guide to Measurement. 2016. Available online: www.fao.org/publications (accessed on 1 May 2024).
- WHO; UNICEF. Indicators for Assessing Infant and Young Child Feeding Practices: Definitions and Measurement Methods. Geneva. 2021. Available online: https://www.who.int/publications/i/item/9789240018389 (accessed on 1 April 2024).
- KNBS; ICF. Kenya Demographic and Health Survey 2022 Key Indicators Report. 2023. Available online: www.dhsprogram.com (accessed on 1 May 2024).
- Kenya National Bureau of Statistics. Kenya Demographic and Health Survey 2014. 2014. Available online: www.dhsprogram.com (accessed on 1 May 2024).
- Mulaw, G.F.; Feleke, F.W.; Mare, K.U. Only one in four lactating mothers met the minimum dietary diversity score in the pastoral community, Afar region, Ethiopia: A community-based cross-sectional study. J. Nutr. Sci. 2021, 10, e41. [Google Scholar] [CrossRef]
- Mayanja, M.; Rubaire-Akiiki, C.; Morton, J.; Young, S.; Greiner, T. Diet Diversity in Pastoral and Agro-pastoral Households in Ugandan Rangeland Ecosystems. Ecol. Food Nutr. 2015, 54, 529–545. [Google Scholar] [CrossRef]
- Mengistu, G.; Moges, T.; Samuel, A.; Baye, K. Energy and nutrient intake of infants and young children in pastoralist communities of Ethiopia. Nutrition 2017, 41, 1–6. [Google Scholar] [CrossRef]
- Schelling, E.; Daoud, S.; Daugla, D.M.; Diallo, P.; Tanner, M.; Zinsstag, J. Morbidity and nutrition patterns of three nomadic pastoralist communities of Chad. Acta Trop. 2005, 95, 16–25. [Google Scholar] [CrossRef]
- Geletaw, A.; Egata, G.; Weldegebreal, F.; Kibr, G.; Semaw, M. Nutritional Status and Associated Factors among Primary Schoolchildren from Pastoral Communities, Mieso-Mulu District, Sitti Zone, Somali Regional State, Eastern Ethiopia: Institution-Based Cross-Sectional Study. J. Nutr. Metab. 2021, 2021, 6630620. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.; Kerven, C.; Calo, M.; Manske, M.; Catley, A. Milk Matters: A Literature Review of Pastoralist Nutrition and Programming Responses. 2009. Feinstein International Center, Tufts University and Save the Children, Addis Ababa. Available online: https://fic.tufts.edu/publication-item/milk-matters-a-literature-review-of-pastoralist-nutrition-and-programming-responses/ (accessed on 10 January 2022).
- Getabalew, M.; Alemneh, T. Dairy Production and Milk Consumption in Pastoral Areas of Ethiopia. Arch. Anim. Husb. Dairy Sci. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Loutan, L.; Lamotte, J.-M. Seasonal variations in nutrition among a group of nomadic pastoralists in Niger. Lancet 1984, 323, 945–947. [Google Scholar] [CrossRef]
- FAO; UNICEF; Washington State University. Seasonality of Malnutrition: Community Knowledge on Patterns and Causes of Undernutrition in Children and Women in Laisamis, Marsabit County, Kenya; UNICEF: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Homewood, K.M. Development and the ecology of maasai pastoralist food and nutrition. Ecol. Food Nutr. 1992, 29, 61–80. [Google Scholar] [CrossRef]
- Safari, J.G.; Kirwa, M.K.; Mandara, C.G. Food insecurity in pastoral communities of Ngorongoro conservation area, Tanzania. Agric. Food Secur. 2022, 11, 36. [Google Scholar] [CrossRef]
- Vossenaar, M.; Knight, F.A.; Tumilowicz, A.; Hotz, C.; Chege, P.; Ferguson, E.L. Context-specific complementary feeding recommendations developed using Optifood could improve the diets of breast-fed infants and young children from diverse livelihood groups in northern Kenya. Public Health Nutr. 2017, 20, 971–983. [Google Scholar] [CrossRef]
- Ahoya, B.; Kavle, J.A.; Straubinger, S.; Gathi, C.M. Accelerating progress for complementary feeding in Kenya: Key government actions and the way forward. Matern. Child Nutr. 2019, 15, e12723. [Google Scholar] [CrossRef]
- Jans, C.; Mulwa Kaindi, D.W.; Meile, L. Innovations in food preservation in pastoral zones. OIE Rev. Sci. Tech. 2016, 35, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Government of the Republic of Kenya Ministry of Health. Division of Nutrition National Maternal, Infant and Young Child Nutrition Policy Guidelines. 2013. Available online: https://nutritionhealth.or.ke/resources/nutrition-policies-laws/ (accessed on 10 January 2022).
- Muema, J.; Mutono, N.; Kisaka, S.; Ogoti, B.; Oyugi, J.; Bukania, Z.; Daniel, T.; Njuguna, J.; Kimani, I.; Makori, A.; et al. The impact of livestock interventions on nutritional outcomes of children younger than 5 years old and women in Africa: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1166495. [Google Scholar] [CrossRef]
- County Government of Marsabit. Maternal, Infant and Young Children Nutrition Knowledge Attitude and Practices Baseline Survey for Marsabit County. 2018. Available online: https://nutritionhealth.or.ke/reports-capacity-assessment-reports/miycn-assessments-reports/#toggle-id-3 (accessed on 10 January 2022).
- Thumbi, S.M.; Muema, J.; Mutono, N.; Njuguna, J.; Jost, C.; Boyd, E.; Tewoldeberhan, D.; Mutua, I.; Gacharamu, G.; Wambua, F.; et al. The Livestock for Health Study: A Field Trial on Livestock Interventions to Prevent Acute Malnutrition Among Women and Children in Pastoralist Communities in Northern Kenya. Food Nutr. Bull. 2023, 44, 119–123. [Google Scholar] [CrossRef]
- UNICEF. Child Food Poverty. Nutrition Deprivation in Early Childhood. Child Nutrition Report. New York. 2024. Available online: www.unicef.org (accessed on 1 May 2024).
- DeLay, N.D.; Thumbi, S.M.; Vanderford, J.; Otiang, E.; Ochieng, L.; Njenga, M.K.; Palmer, G.H.; Marsh, T.L. Linking calving intervals to milk production and household nutrition in Kenya. Food Secur. 2020, 12, 309–325. [Google Scholar] [CrossRef]
- Mosites, E.M.; Rabinowitz, P.M.; Thumbi, S.M.; Montgomery, J.M.; Palmer, G.H.; May, S.; Rowhani-Rahbar, A.; Neuhouser, M.L.; Walson, J.L. The relationship between livestock ownership and child stunting in three countries in eastern Africa using national survey data. PLoS ONE 2015, 10, e0136686. [Google Scholar] [CrossRef]
- McElwain, T.F.; Thumbi, S.M. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev. Sci. Tech. 2017, 36, 423–433. [Google Scholar] [CrossRef]
- Kuche, D.; Moss, C.; Eshetu, S.; Ayana, G.; Salasibew, M.; Dangour, A.D.; Allen, E. Factors associated with dietary diversity and length-for-age z-score in rural Ethiopian children aged 6–23 months: A novel approach to the analysis of baseline data from the Sustainable Undernutrition Reduction in Ethiopia evaluation. Matern. Child Nutr. 2020, 16, e12852. [Google Scholar] [CrossRef]
- Savy, M.; Martin-Prével, Y.; Sawadogo, P.; Kameli, Y.; Delpeuch, F. Use of variety/diversity scores for diet quality measurement: Relation with nutritional status of women in a rural area in Burkina Faso. Eur. J. Clin. Nutr. 2005, 59, 703–716. [Google Scholar] [CrossRef]
- Megersa, B. Dietary Intake of Infant and Young Children and Assessment of Dietary Adequacy Indicators in a Pastoral Setting, Southern Ethiopia. Nutr. Diet. Suppl. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Musyoka, M.M.; Bukachi, S.A.; Muga, G.O.; Otiang, E.; Kwoba, E.N.; Thumbi, S.M. Addressing child and maternal nutrition: A qualitative study on food prescriptions and proscriptions determining animal source food consumption in rural Kenya. Food Secur. 2023, 15, 901–917. [Google Scholar] [CrossRef]
- Chege, P.M.; Kimiywe, J.O.; Ndungu, Z.W. Influence of culture on dietary practices of children under five years among Maasai pastoralists in Kajiado, Kenya. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Katenga-Kaunda, L.Z.; Iversen, P.O.; Kamudoni, P.R.; Holmboe-Ottesen, G.; Fjeld, H.E. Food-based nutrition counselling and education intervention for improved diets of pregnant women in rural Malawi: A qualitative study of factors influencing dietary behaviour change. Public Health Nutr. 2022, 25, 2436–2447. [Google Scholar] [CrossRef] [PubMed]
- Gebregziabher, H.; Kahsay, A.; Gebrearegay, F.; Berhe, K.; Gebremariam, A.; Gebretsadik, G.G. Food taboos and their perceived reasons among pregnant women in Ethiopia: A systematic review, 2022. BMC Pregnancy Childbirth 2023, 23, 116. [Google Scholar] [CrossRef]
- Mengie, T.; Dessie, Y.; Egata, G.; Muche, T.; Habtegiorgis, S.D.; Getacher, L. Food taboos and associated factors among agro-pastoralist pregnant women: A community-based cross-sectional study in Eastern Ethiopia. Heliyon 2022, 8, e10923. [Google Scholar] [CrossRef] [PubMed]
- Vall, E.; Sib, O.; Vidal, A.; Delma, J.B. Dairy farming systems driven by the market and low-cost intensification in West Africa: The case of Burkina Faso. Trop. Anim. Health Prod. 2021, 53, 288. [Google Scholar] [CrossRef]
- Heckert, J.; Olney, D.K.; Ruel, M.T. Is women’s empowerment a pathway to improving child nutrition outcomes in a nutrition-sensitive agriculture program?: Evidence from a randomized controlled trial in Burkina Faso. Soc. Sci. Med. 2019, 233, 93–102. [Google Scholar] [CrossRef]
- Soofi, S.B.; Khan University, A.; Adu-Bonsaaoh, K.; Biswas, B.; Desalegn, M.; Kebira, J.Y. Short birth interval and its associated factors among multiparous women in Mieso agro-pastoralist district, Eastern Ethiopia: A community-based cross-sectional study. Front. Glob. Women’s Health 2022, 3, 801394. [Google Scholar]
- Manley, J.; Gitter, S.; Slavchevska, V. How Effective are Cash Transfer Programmes at Improving Nutritional Status? A Rapid Evidence Assessment of Programmes’ Effects on Anthropometric Outcomes. 2012. Available online: http://eppi.ioe.ac.uk (accessed on 5 May 2025).
- Hagen-Zanker, J.; Pellerano, L.; Bastagli, F.; Harman, L.; Barca, V.; Sturge, G.; Schmidt, T.; Laing, C. Briefing Shaping policy for development: The impact of cash transfers on women and girls A summary of the evidence. 2017. Available online: https://odi.org/en/publications/the-impact-of-cash-transfers-on-women-and-girls/ (accessed on 5 May 2025).
- Carroll, R.J.; Midthune, D.; Subar, A.F.; Shumakovich, M.; Freedman, L.S.; Thompson, F.E.; Kipnis, V. Taking advantage of the strengths of 2 different dietary assessment instruments to improve intake estimates for nutritional epidemiology. Am. J. Epidemiol. 2012, 175, 340–347. [Google Scholar] [CrossRef] [PubMed]



| Food Group Number | Children (6–23 Months) | Children (24–59 Months) | Women |
|---|---|---|---|
| 1 | Cereals, roots, and tubers | Cereals, roots, and tubers | Cereals, roots, and tubers |
| 2 | Vitamin A-rich fruits and vegetables | Vitamin A-rich fruits and vegetables | Dark green leafy vegetables |
| 3 | Other vitamin A-rich fruits and vegetables | ||
| 4 | Other fruits and vegetables | Other fruits and vegetables | Other fruits |
| 5 | Other vegetables | ||
| 6 | Flesh foods | Flesh foods | Flesh foods |
| 7 | Eggs | Eggs | Eggs |
| 8 | Legumes, nuts, and seeds | Legumes, nuts, and seeds | Legumes |
| 9 | Nuts and seeds | ||
| 10 | Dairy products | Dairy products | Dairy products |
| 11 | Breastmilk | - | - |
| Total Food Groups | 8 | 7 | 10 |
| Diet Scores | |||
| Minimum Dietary Diversity Score (MDD) | Consumption of at least 5/8 food groups | Consumption of at least 4/7 food groups | Consumption of at least 5/10 food groups |
| Minimum Meal Frequency (MMF) | 6–8 months: At least 2 meals a day 9–23 months: At least 3 meals a day Non-breastfed children: At least 4 meals a day | At least 4 meals a day | - |
| Minimum Acceptable Diet (MAD) | Attainment of both minimum dietary diversity and minimum meal frequency | - | |
| Infants and Young Children | Women of Reproductive Age | ||||
|---|---|---|---|---|---|
| Dependent Variables → | Minimum Dietary Diversity (MDD) Odds Ratio (95% CI) | Minimum Meal Frequency (MMF) Odds Ratio (95% CI) | Minimum Acceptable Diet (MAD) Odds Ratio (95% CI) | Minimum Dietary Diversity-Women (MDD-W) Odds Ratio (95% CI) | |
| Factors ↓ | |||||
| Study arm | |||||
| Control arm | Reference | Reference | Reference | Reference | |
| Animal feed only | 1.78 (1.60, 1.98) | 2.19 (1.56, 3.07) | 1.77 (1.53, 2.04) | 1.55 (1.23, 1.89) | |
| Animal feed and enhanced nutritional counselling | 2.54 (2.30, 2.79) | 1.61 (1.08, 2.39) | 3.10 (2.66, 3.62) | 4.22 (3.29, 5.42) | |
| Gender | |||||
| Female | Reference | Reference | Reference | ||
| Male | 0.83 (0.67, 1.02) | 1.19 (0.94, 1.51) | 1.07 (1.00, 1.15) | - | |
| Household head primary occupation | |||||
| Livestock herding | Reference | Reference | Reference | Reference | |
| Non-livestock herding | 0.47 (0.42, 0.52) | 1.18 (0.69, 2.03) | 2.29 (2.02, 2.60) | 2.81 (2.24, 3.54) | |
| Household head education status | |||||
| Received formal education | 2.33 (2.10, 2.60) | 1.53 (0.74, 3.16) | 0.47 (0.40, 0.57) | 0.93 (0.70, 1.24) | |
| No formal education | Reference | Reference | Reference | Reference | |
| Women’s primary occupation | |||||
| Livestock herding | Reference | Reference | Reference | Reference | |
| Non-livestock herding | 1.75 (1.62, 1.89) | 1.43 (1.09, 1.88) | 1.21 (1.13, 1.30) | 2.41 (1.93, 3.01) | |
| Women’s education status | |||||
| Received formal education | 3.02 (2.69, 3.38) | 2.53 (1.19, 5.38) | 0.93 (0.78, 1,11) | 5.29 (3.91, 7.18) | |
| No formal education | Reference | Reference | Reference | Reference | |
| Herd dynamics | |||||
| Births (count) | 1.002 (1.001, 1.004) | 1.01 (0.99, 1.02) | 0.98 (0.95, 1.12) | 1.21 (0.85, 1.66) | |
| Purchases (count) | 1.03 (1.01, 1.06) | 0.84 (0.76, 0.93) | 1.02 (1.01, 1.07) | 4.73 (2.53, 8.85) | |
| Cash transfer | 0.90 (0.87, 0.94) | 1.08 (0.93, 1.26) | 0.95 (0.89, 0.97) | 0.73 (0.54, 0.89) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutono, N.; Muema, J.; Bukania, Z.; Kimani, I.; Boyd, E.; Mutua, I.; Gacharamu, G.; Wambua, F.; Makori, A.; Njuguna, J.; et al. Effect of Behavioral Change Communication and Livestock Feed Intervention on Dietary Practices in a Kenyan Pastoral Community: A Randomized Controlled Trial. Nutrients 2025, 17, 2997. https://doi.org/10.3390/nu17182997
Mutono N, Muema J, Bukania Z, Kimani I, Boyd E, Mutua I, Gacharamu G, Wambua F, Makori A, Njuguna J, et al. Effect of Behavioral Change Communication and Livestock Feed Intervention on Dietary Practices in a Kenyan Pastoral Community: A Randomized Controlled Trial. Nutrients. 2025; 17(18):2997. https://doi.org/10.3390/nu17182997
Chicago/Turabian StyleMutono, Nyamai, Josphat Muema, Zipporah Bukania, Irene Kimani, Erin Boyd, Immaculate Mutua, George Gacharamu, Francis Wambua, Anita Makori, Joseph Njuguna, and et al. 2025. "Effect of Behavioral Change Communication and Livestock Feed Intervention on Dietary Practices in a Kenyan Pastoral Community: A Randomized Controlled Trial" Nutrients 17, no. 18: 2997. https://doi.org/10.3390/nu17182997
APA StyleMutono, N., Muema, J., Bukania, Z., Kimani, I., Boyd, E., Mutua, I., Gacharamu, G., Wambua, F., Makori, A., Njuguna, J., Jost, C., Osman, A. M., Souza, D., Palmer, G. H., Yoder, J., & Thumbi, S. M. (2025). Effect of Behavioral Change Communication and Livestock Feed Intervention on Dietary Practices in a Kenyan Pastoral Community: A Randomized Controlled Trial. Nutrients, 17(18), 2997. https://doi.org/10.3390/nu17182997






