Effects of Pomegranate Extract on IGF-1 Levels and Telomere Length in Older Adults (55–70 Years): Findings from a Randomised Double-Blinded Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Intervention
2.1.1. Participants
2.1.2. Laboratory Analysis
2.1.3. Compliance
2.1.4. Power Calculation and Statistical Analysis
3. Results
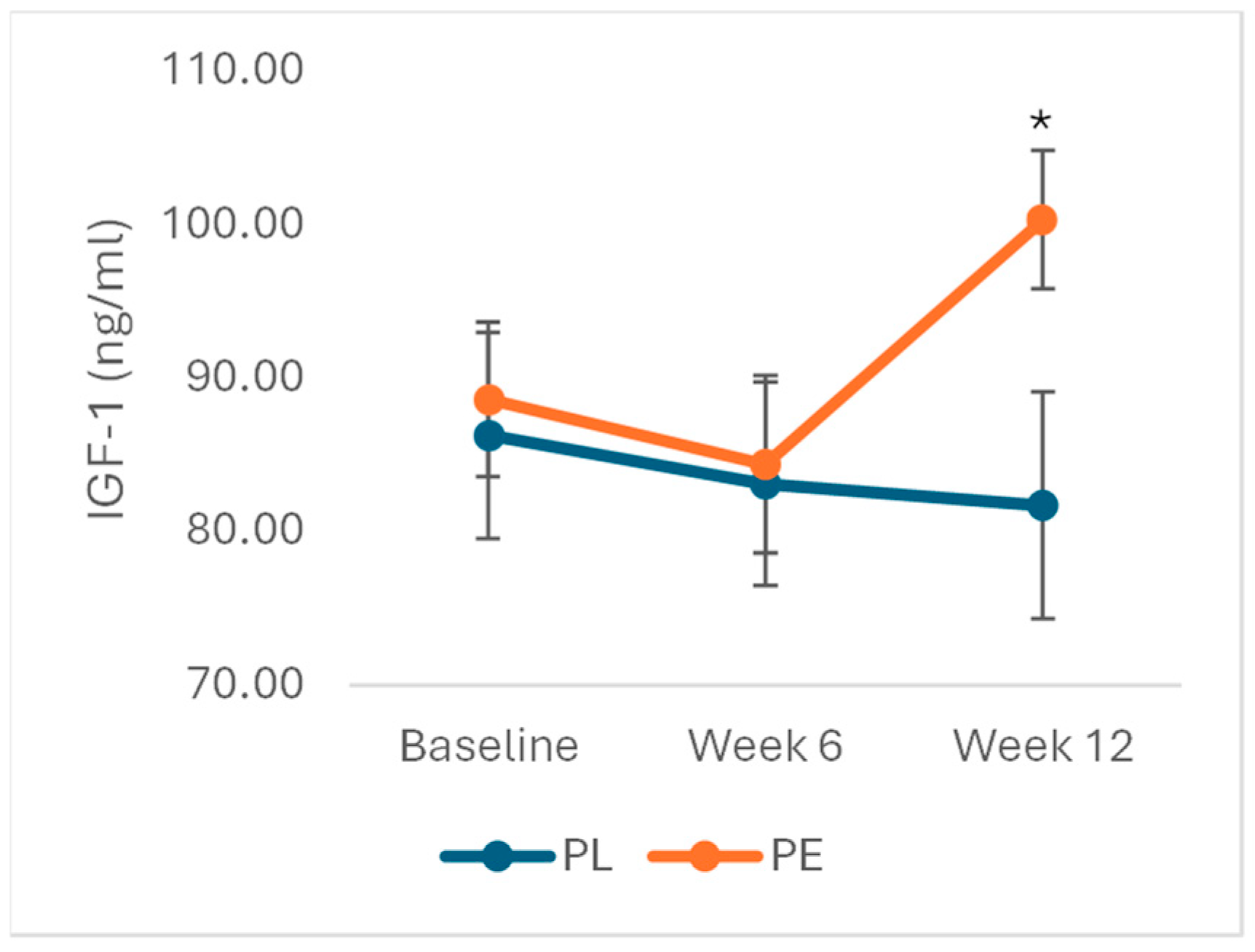
3.1. Effects of Pomegranate Extract on IGF-1 Levels
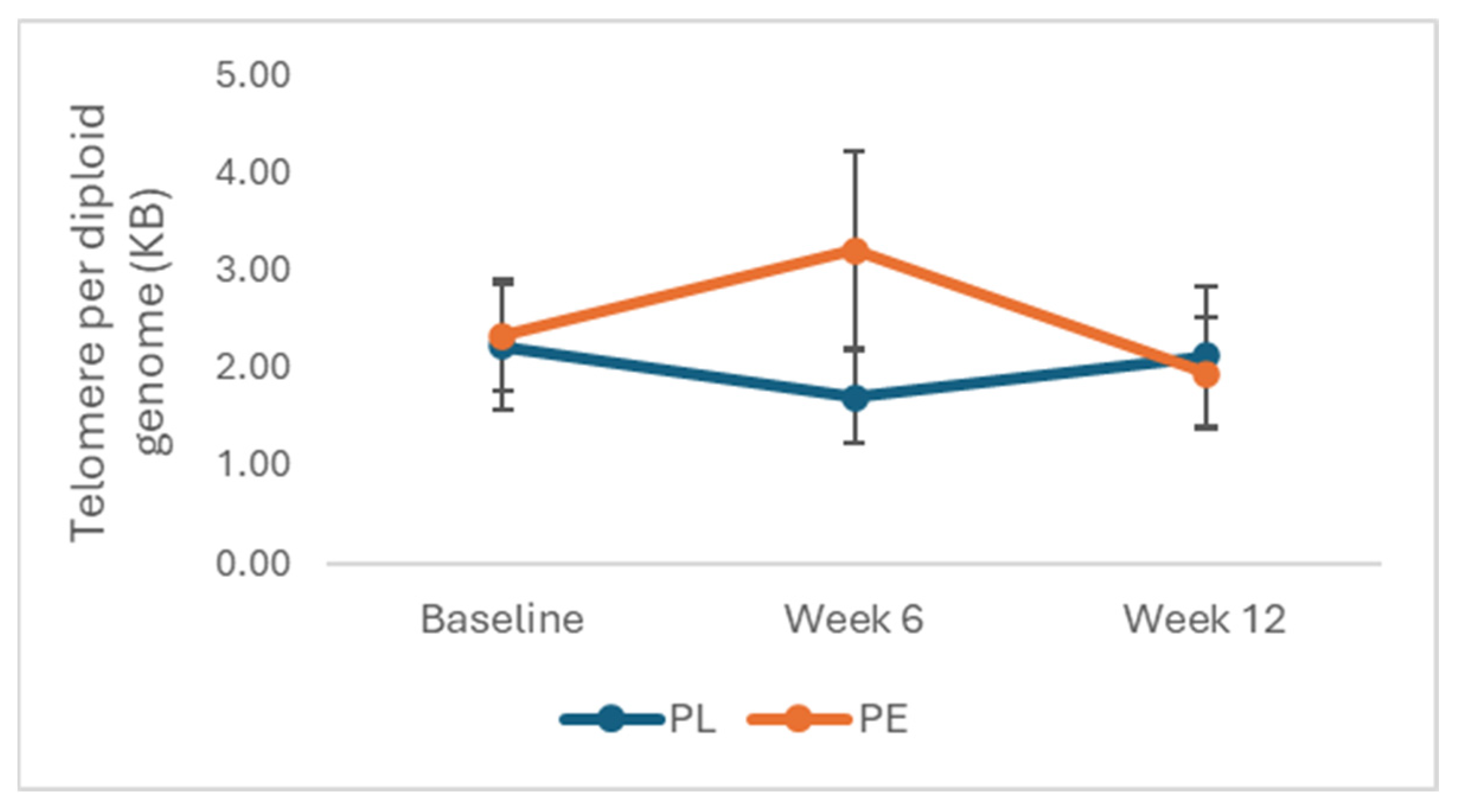
3.2. Effects of PE on Telomere Length
3.3. Changes in Energy Intake and MET Levels
3.4. Impact of Confounding Factors on the Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statemen
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Pomegranate extract |
| PL | Placebo |
| IGF-1 | Insulin growth Factor-1 |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| TNF-α | Tumour necrosis factor-alpha |
References
- WHO. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 22 April 2025).
- Rodríguez-Rodero, S.; Fernández-Morera, J.L.; Menéndez-Torre, E.; Calvanese, V.; Fernández, A.F.; Fraga, M.F. Aging genetics and aging. Aging Dis. 2011, 2, 186–195. [Google Scholar]
- O’sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef]
- Hsieh, A.Y.; Saberi, S.; Ajaykumar, A.; Hukezalie, K.; Gadawski, I.; Sattha, B.; Côté, H.C. Optimization of a relative telomere length assay by monochromatic multiplex real-time quantitative PCR on the LightCycler 480: Sources of variability and quality control considerations. J. Mol. Diagn. 2016, 18, 425–437. [Google Scholar] [CrossRef]
- An, R.; Yan, H. Body weight status and telomere length in US middle-aged and older adults. Obes. Res. Clin. Pract. 2017, 11, 51–62. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Shen, G.; Lo, K.; Huang, J.Y.; Liu, L.; Yu, Y.L.; Chen, C.L.; Zhang, B.; Feng, Y.Q. The Relationship between Mean Telomere Length and Blood Pressure: Results from the National Health and Education National Surveys. SSRN 2019. [Google Scholar] [CrossRef]
- Von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Barrett-Connor, E.; Criqui, M.H.; Kritz-Silverstein, D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2004, 89, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Sullivan, L.M.; D’Agostino, R.B.; Roubenoff, R.; Harris, T.; Sawyer, D.B.; Levy, D.; Wilson, P.W. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: The Framingham Heart Study. Ann. Intern. Med. 2003, 139, 642–648. [Google Scholar] [CrossRef]
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.Y.; Delafontaine, P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol. Metab. 2010, 21, 245–254. [Google Scholar] [CrossRef]
- Serri, O.; Li, L.; Maingrette, F.; Jaffry, N.; Renier, G. Enhanced lipoprotein lipase secretion and foam cell formation by macrophages of patients with growth hormone deficiency: Possible contribution to increased risk of atherogenesis? J. Clin. Endocrinol. Metab. 2004, 89, 979–985. [Google Scholar] [CrossRef][Green Version]
- Sukhanov, S.; Higashi, Y.; Shai, S.Y.; Vaughn, C.; Mohler, J.; Li, Y.; Song, Y.H.; Titterington, J.; Delafontaine, P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2684–2690. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Kaplan, R.C.; Fitzpatrick, A.L.; Pollak, M.N.; Gardner, J.P.; Jenny, N.S.; McGinn, A.P.; Kuller, L.H.; Strickler, H.D.; Kimura, M.; Psaty, B.M.; et al. Insulin-like growth factors and leukocyte telomere length: The cardiovascular health study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 1103–1106. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; McGinn, A.P.; Strickler, H.D.; Rohan, T.E.; Pollak, M.; Cappola, A.R.; Kuller, L.; Xue, X.; Newman, A.B.; Strotmeyer, E.S.; et al. Insulin-like growth factor-(IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm. IGF Res. 2008, 18, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Q.C.; Dos Santos, T.W.; Fortunato, I.M.; Ribeiro, M.L. The molecular mechanism of polyphenols in the regulation of ageing hallmarks. Int. J. Mol. Sci. 2023, 24, 5508. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.T.; Schlotz, N.; Schreiner, M.; Lamy, E. Short-term dietary intervention with cooked but not raw brassica leafy vegetables increases telomerase activity in CD8+ lymphocytes in a randomized human trial. Nutrients 2019, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Woo, J.; Suen, E.; Leung, J.; Tang, N. Chinese tea consumption is associated with longer telomere length in elderly Chinese men. Br. J. Nutr. 2010, 103, 107–113. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.H.; Kang, N.; Kim, K.N.; Jeon, Y.J. The effects of marine algal polyphenols, phlorotannins, on skeletal muscle growth in C2C12 muscle cells via smad and IGF-1 signaling pathways. Mar. Drugs 2021, 19, 266. [Google Scholar] [CrossRef]
- Chen, X.; Le, Y.; Tang, S.Q.; He, W.Y.; He, J.; Wang, Y.H.; Wang, H.B. Painful Diabetic Neuropathy Is Associated with Compromised Microglial IGF-1 Signaling Which Can Be Rescued by Green Tea Polyphenol EGCG in Mice. Oxidative Med. Cell. Longev. 2022, 2022, 6773662. [Google Scholar] [CrossRef]
- Tatari, M.; Jadidi, E.; Shahmansouri, E. Study of some physiological responses of different pomegranate (Punica granatum L.) cultivars under drought stress to screen for drought tolerance. Int. J. Fruit Sci. 2020, 20 (Suppl. 2), 1798–1813. [Google Scholar] [CrossRef]
- Tinebra, I.; Scuderi, D.; Sortino, G.; Mazzaglia, A.; Farina, V. Pomegranate cultivation in Mediterranean climate: Plant adaptation and fruit quality of ‘Mollar de Elche’ and ‘Wonderful’ cultivars. Agronomy 2021, 11, 156. [Google Scholar] [CrossRef]
- Alshinnawy, A.S.; El-Sayed, W.M.; Sayed, A.A.; Salem, A.M.; Taha, A.M. Telomerase activator-65 and pomegranate peel improved the health status of the liver in aged rats; multi-targets involved. Iran. J. Basic Med. Sci. 2021, 24, 842–850. [Google Scholar]
- Farhat, G.; Malla, J.; Vadher, J.; Al-Dujaili, E.A. Effects of Pomegranate Extract on Inflammatory Markers and Cardiometabolic Risk Factors in Adults Aged 55–70 Years: A Randomised Controlled Parallel Trial. Nutrients 2025, 17, 1235. [Google Scholar] [CrossRef]
- Farhat, G.; Malla, J.; Al-Dujaili, E.A.; Vadher, J.; Nayak, P.; Drinkwater, K. Impact of Pomegranate Extract Supplementation on Physical and Cognitive Function in Community-Dwelling Older Adults Aged 55–70 Years: A Randomised Double-Blind Clinical Trial. Geriatrics 2025, 10, 29. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/publications/i/item/9789240002654 (accessed on 27 November 2024).
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.J.; Fenech, M. A quantitative PCR method for measuring absolute telomere length. Biol. Proced. Online 2011, 13, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. ROLE of IGF-1 system in the modulation of longevity: Controversies and new insights from a centenarians’ perspective. Front. Endocrinol. 2019, 10, 431907. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wu, Y.H.; Liu, L.K.; Lee, W.J.; Hwang, A.C.; Peng, L.N.; Lin, M.-H.; Chen, L.K. Association among serum insulin-like growth factor-1, frailty, muscle mass, bone mineral density, and physical performance among community-dwelling middle-aged and older adults in Taiwan. Rejuvenation Res. 2018, 21, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Handayaningsih, A.E.; Iguchi, G.; Fukuoka, H.; Nishizawa, H.; Takahashi, M.; Yamamoto, M.; Herningtyas, E.-H.; Okimura, Y.; Kaji, H.; Chihara, K.; et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 2011, 152, 912–921. [Google Scholar] [CrossRef]
- Alberti, C.; Chevenne, D.; Mercat, I.; Josserand, E.; Armoogum-Boizeau, P.; Tichet, J.; Léger, J. Serum concentrations of insulin-like growth factor (IGF)-1 and IGF binding protein-3 (IGFBP-3), IGF-1/IGFBP-3 ratio, and markers of bone turnover: Reference values for French children and adolescents and z-score comparability with other references. Clin. Chem. 2011, 57, 1424–1435. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate supplementation accelerates recovery of muscle damage and soreness and inflammatory markers after a weightlifting training session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: A systematic review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef]
- Bachagol, D.; Joseph, G.S.; Ellur, G.; Patel, K.; Aruna, P.; Mittal, M.; China, S.P.; Singh, R.P.; Sharan, K. Stimulation of liver IGF-1 expression promotes peak bone mass achievement in growing rats: A study with pomegranate seed oil. J. Nutr. Biochem. 2018, 52, 18–26. [Google Scholar] [CrossRef]
- Sheng, R.; Gu, Z.L.; Xie, M.L. Epigallocatechin gallate, the major component of polyphenols in green tea, inhibits telomere attrition mediated cardiomyocyte apoptosis in cardiac hypertrophy. Int. J. Cardiol. 2013, 162, 199–209. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Diet and aging: The role of polyphenol-rich diets in slow down the shortening of telomeres: A review. Antioxidants 2023, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Queen, B.L.; Tollefsbol, T.O. Polyphenols and aging. Curr. Aging Sci. 2010, 3, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Epel, E. How “reversible” is telomeric aging? Cancer Prev. Res. 2012, 5, 1163–1168. [Google Scholar] [CrossRef]

| Target | Sequencer |
|---|---|
| Telomere Forward Primer | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGG TTTGGGTT |
| Telomere Reverse Primer | GGCTTGCCTTACCCTTACCCTTACCC TTACCCTTACCCT |
| Telomere Standard | (TTAGGG)14 |
| 36B4 Forward Primer | CAGCAAGTGGGAAGGTGTAATCC |
| 36B4 Reverse Primer | CCCATTCTATCATCAACGGGTACAA |
| 36B4 Standard | CAGCAAGTGGGAAGGTGTAATCCGTCTCCACAGACAAGGCCAGGACTCGTTTGTACCCGTTGATGATAGAATGGG |
| (a) | ||
| 0.25 ng of template DNA/Standard | ||
| 100 nM forward primer | ||
| 100 nM Reverse primer | ||
| (b) | ||
| Reaction Stage | Temperature | Time |
| Enzyme Activation | 95 °C | 5 min |
| Denaturation | 95 °C | 30 s (40 cycles) |
| Annealing/Extension | 53 °C | 1 min (40 cycles) |
| Signal Stabilization 1 | 4 °C | 5 min |
| Signal Stabilization 2 | 90 °C | 5 min |
| Hold | 4 °C | infinite |
| (c) | ||
| Standard | Quantity, pg−μL | Kb of Telomere |
| A | 1.875 | 7.38 × 106 |
| B | 0.938 | 3.69 × 106 |
| C | 0.469 | 1.84 × 106 |
| D | 0.234 | 9.22 × 105 |
| E | 0.117 | 4.61 × 105 |
| F | 0.059 | 2.30 × 105 |
| G | 0.029 | 1.15 × 105 |
| (a) | ||
| 60 ng of template DNA/Standard | ||
| 100 nM forward primer | ||
| 100 nM Reverse primer | ||
| 2× Quantinova SYBR Green Mastermix | ||
| RNase/DNase free water | ||
| (b) | ||
| Reaction Stage | Temperature | Time |
| Denaturation | 95 °C | 10 min |
| Denaturation | 95 °C | 15 s (40 cycles) |
| Annealing/Extension | 60 °C | 1 min (40 cycles) |
| Dissociation Curve | ||
| Denaturation | 95 °C | 10 s |
| Dissociation | 60–95 °C | 25 min |
| (c) | ||
| Standard | Quantity, pg−μL | Diploid Copies |
| A | 200 | 2.63 × 109 |
| B | 20 | 2.63 × 108 |
| C | 2 | 2.63 × 107 |
| D | 0.2 | 2.63 × 106 |
| E | 0.02 | 2.63 × 105 |
| F | 0.002 | 2.63 × 104 |
| G | 0.0002 | 2.63 × 103 |
| Characteristics | All Participants (n = 72) | PE Group (n = 37) | PL Group (n = 35) |
|---|---|---|---|
| Age at baseline, y | 61.22 (4.31) | 60.46 (3.90) | 62.03 (4.62) |
| Sex (n) | |||
| Female | 44 | 23 | 21 |
| Male | 28 | 14 | 14 |
| Occupation category (n) | |||
| Professional Occupations | 32 | 13 | 19 |
| Managers, Directors & Senior Officials | 6 | 3 | 3 |
| Health-related Professions | 5 | 1 | 4 |
| Associate Professional &Technical Occupations | 3 | 1 | 2 |
| Administrative & Secretarial Occupations | 4 | 4 | 0 |
| Unemployed | 2 | 2 | 0 |
| Sales & Customer Service Occupations | 2 | 2 | 0 |
| Caring, Leisure & Other Service Occupations | 1 | 1 | 0 |
| Retired | 17 | 7 | 10 |
| Ethnicity (n) | |||
| White British | 60 | 32 | 28 |
| White Irish | 3 | 1 | 2 |
| Indian | 1 | 0 | 1 |
| Other white | 2 | 0 | 2 |
| Mixed race | 2 | 2 | 0 |
| Black | 1 | 0 | 1 |
| Other | 3 | 2 | 1 |
| Smoking status (n) | |||
| Smoker | 3 | 1 | 2 |
| Non-smoker | 69 | 36 | 35 |
| MET (MET-minutes/week) | 731 (72) | 742 (33) | 727 (45) |
| BMI (kg/m2) | 23.91 (3.25) | 23.80 (3.20) | 24.01 (3.34) |
| Waist circumference (cm) | 84.75 (10.28) | 83.54 (9.82) | 86.04 (10/73) |
| Waist-to-hip ratio | 0.84 (0.07) | 0.83 (0.07) | 0.85 (0.07) |
| Body fat percentage (%) | 22.03 (10.02) | 22.68 (9.43) | 21.34 (10.69) |
| SBP (mmHg) | 128.97 (12.48) | 128.43 (13.57) | 129.54 (11.38) |
| DBP (mmHg) | 81.38 (8.60) | 80.74 (9.54) | 82.06 (7.55) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhat, G.; Malla, J.; Hanson, L.; Vadher, J.; Al-Dujaili, E.A.S. Effects of Pomegranate Extract on IGF-1 Levels and Telomere Length in Older Adults (55–70 Years): Findings from a Randomised Double-Blinded Controlled Trial. Nutrients 2025, 17, 2974. https://doi.org/10.3390/nu17182974
Farhat G, Malla J, Hanson L, Vadher J, Al-Dujaili EAS. Effects of Pomegranate Extract on IGF-1 Levels and Telomere Length in Older Adults (55–70 Years): Findings from a Randomised Double-Blinded Controlled Trial. Nutrients. 2025; 17(18):2974. https://doi.org/10.3390/nu17182974
Chicago/Turabian StyleFarhat, Grace, Jhama Malla, Liam Hanson, Jay Vadher, and Emad A. S. Al-Dujaili. 2025. "Effects of Pomegranate Extract on IGF-1 Levels and Telomere Length in Older Adults (55–70 Years): Findings from a Randomised Double-Blinded Controlled Trial" Nutrients 17, no. 18: 2974. https://doi.org/10.3390/nu17182974
APA StyleFarhat, G., Malla, J., Hanson, L., Vadher, J., & Al-Dujaili, E. A. S. (2025). Effects of Pomegranate Extract on IGF-1 Levels and Telomere Length in Older Adults (55–70 Years): Findings from a Randomised Double-Blinded Controlled Trial. Nutrients, 17(18), 2974. https://doi.org/10.3390/nu17182974







