Immunomodulatory Effects of Lactobacillus brevis NES-428 in a Hyperthyroidism Mouse Model: Potential Applications for Graves’ Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Lactic Acid Bacteria from Korean Kimchi
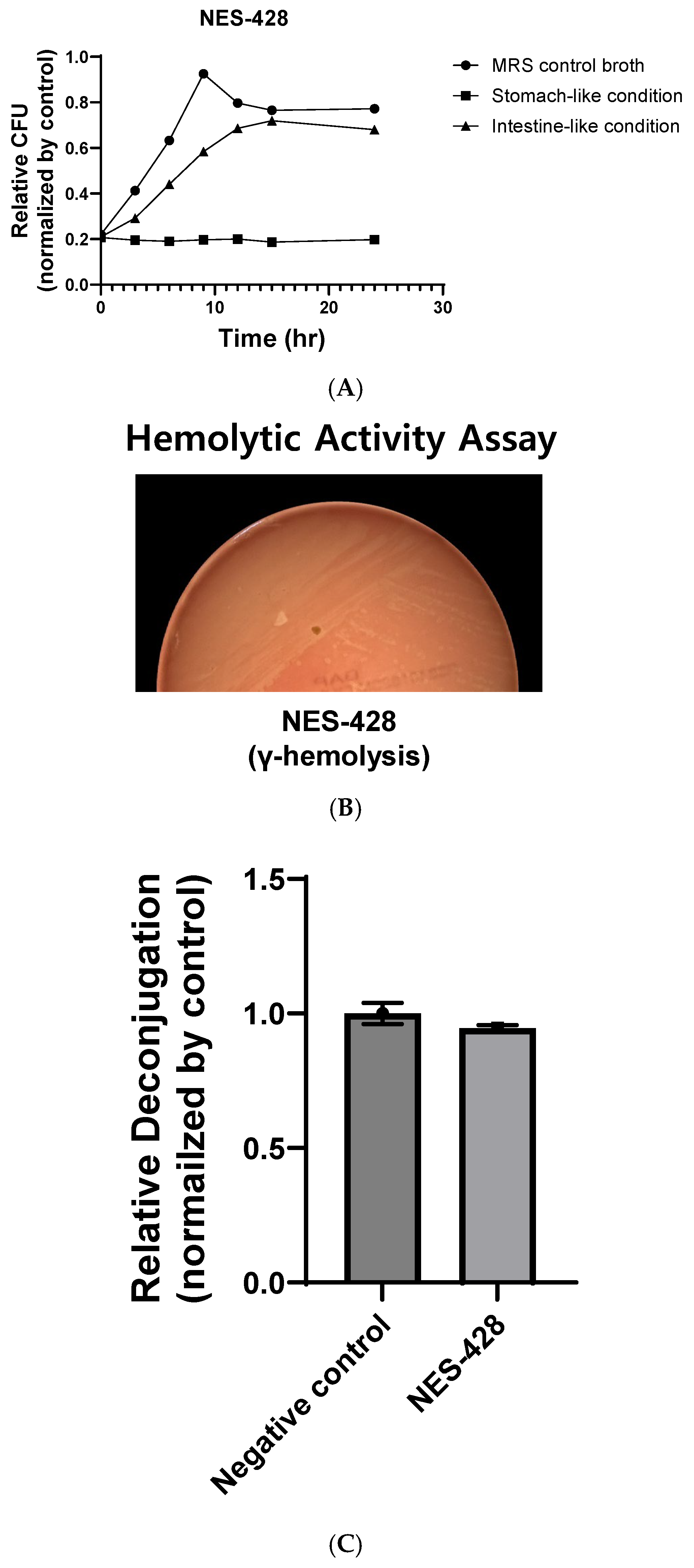
2.2. Acid Tolerance Assay
2.3. Hemolytic Activity Assay
2.4. Bile Salt Hydrolase (BSH) Activity Assay and Protein Quantification
2.5. Plasmid Construction and Adenoviral Vector Packaging
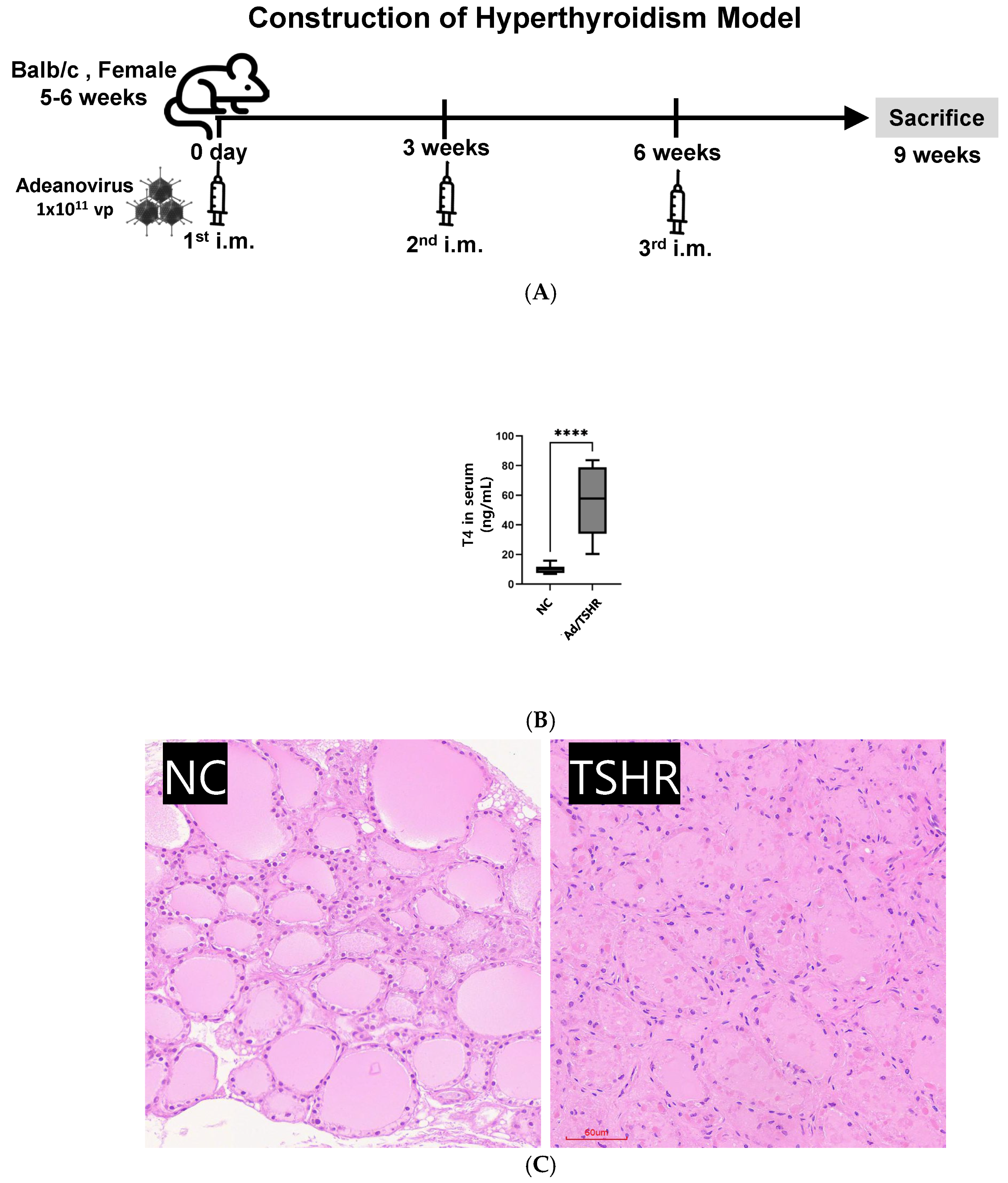
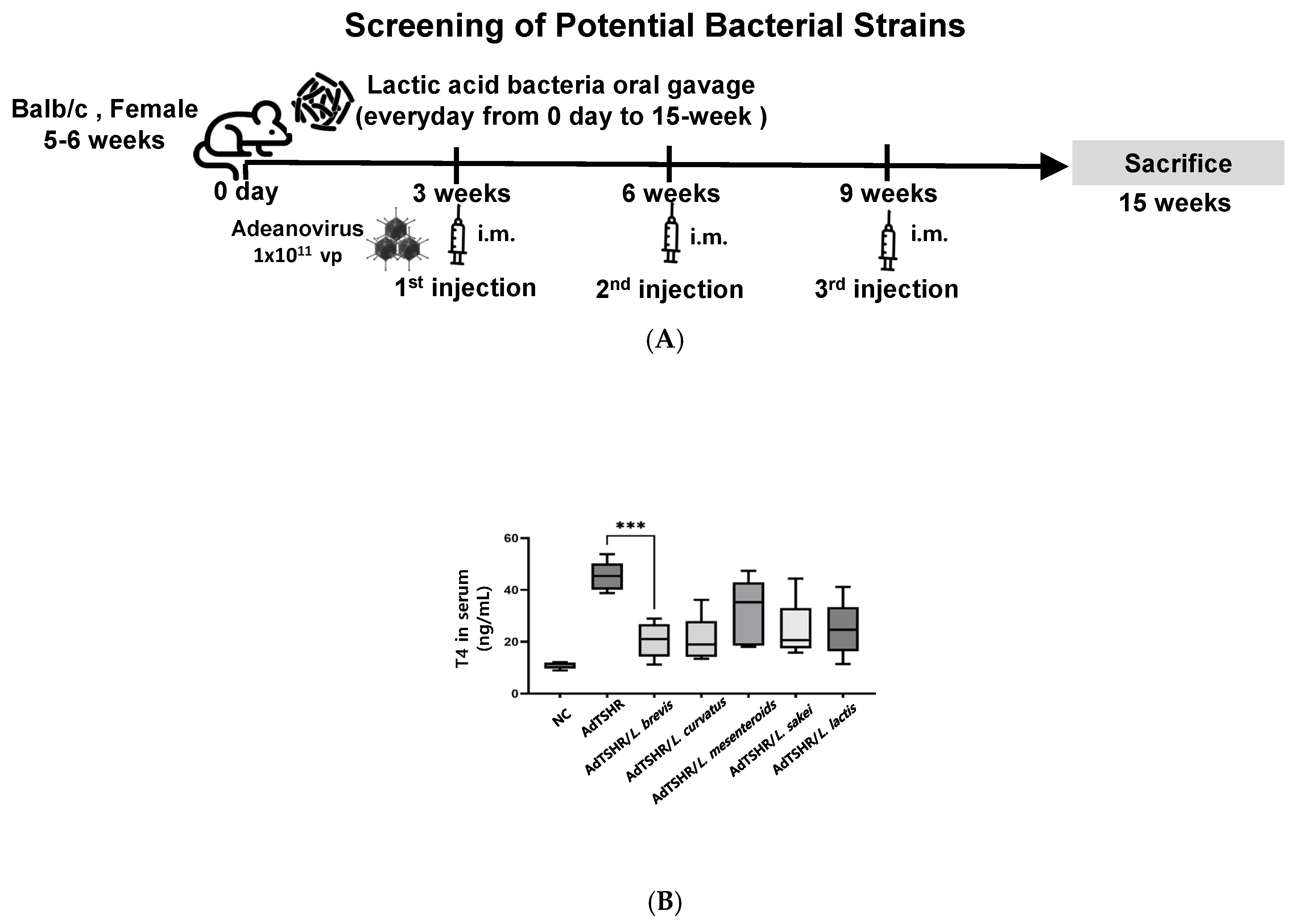
2.6. Constructions of Immunized Mice of Adenovirus Containing TSHR Amino Acid Residues (Ad-TSHR289)
2.7. Measurement of Serum Thyroxine Levels
2.8. Jurkat Cell Culture and Heat-Killed Bacteria Treatment
2.9. RNA Extraction from Jurkat Cell and Spleen Tissue
2.10. mRNA Expression Analysis by qRT-PCR
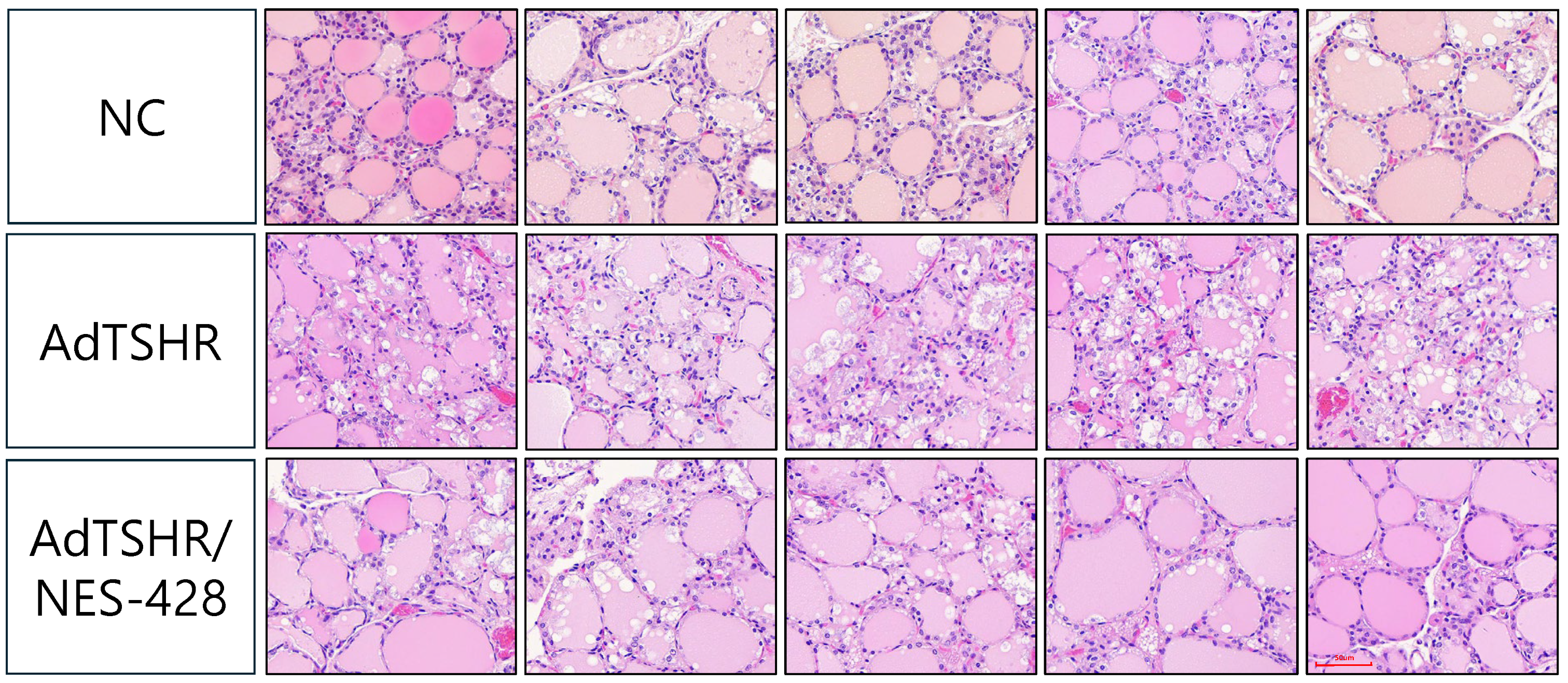
2.11. Histopathological Analysis of Thyroid Tissue
2.12. Ethics Approval
3. Results
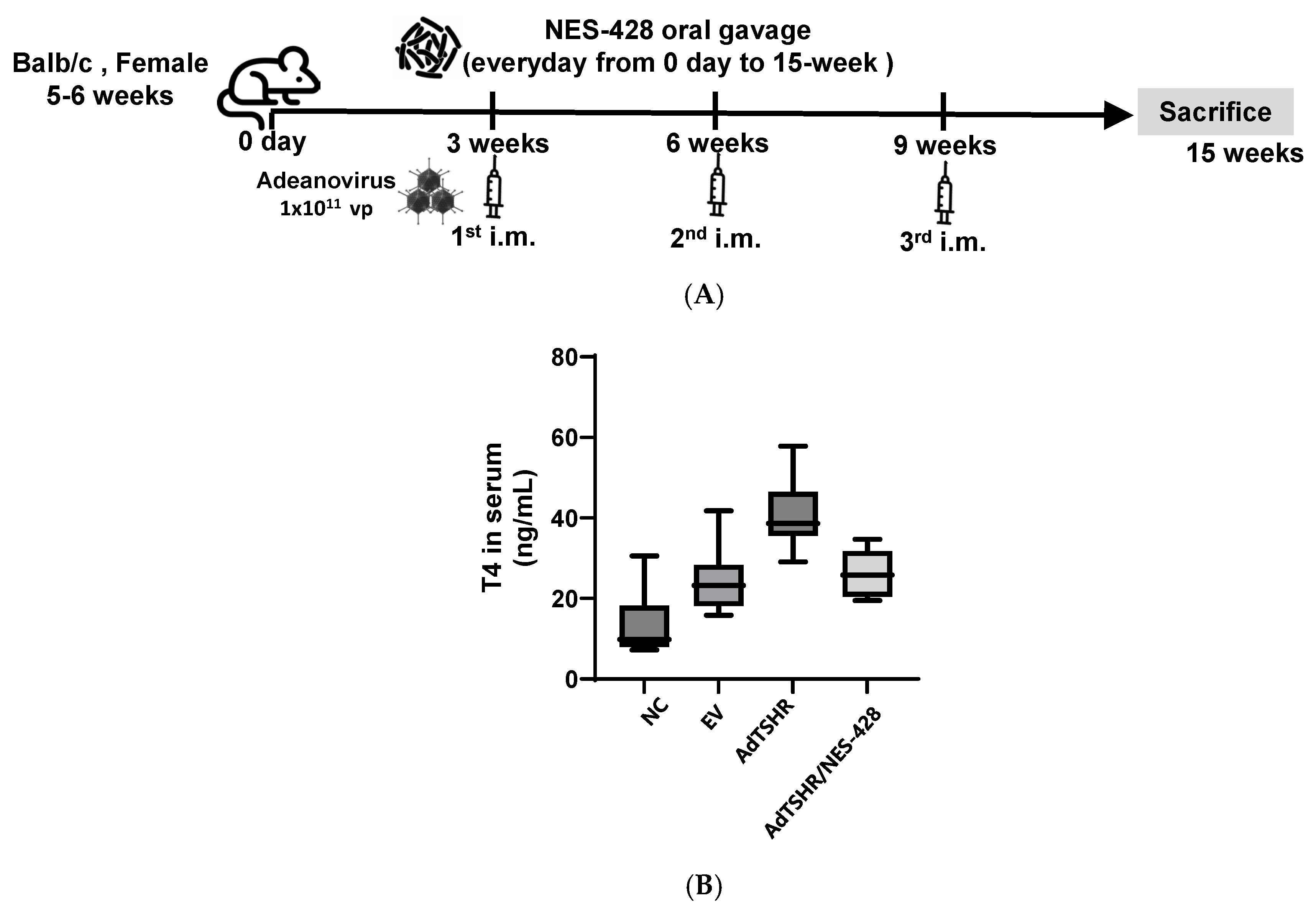
3.1. Construction of Hyperthyroidsm Mouse Model
3.2. Isolation and Identification of Lactic Acid Bacteria from Kimchi
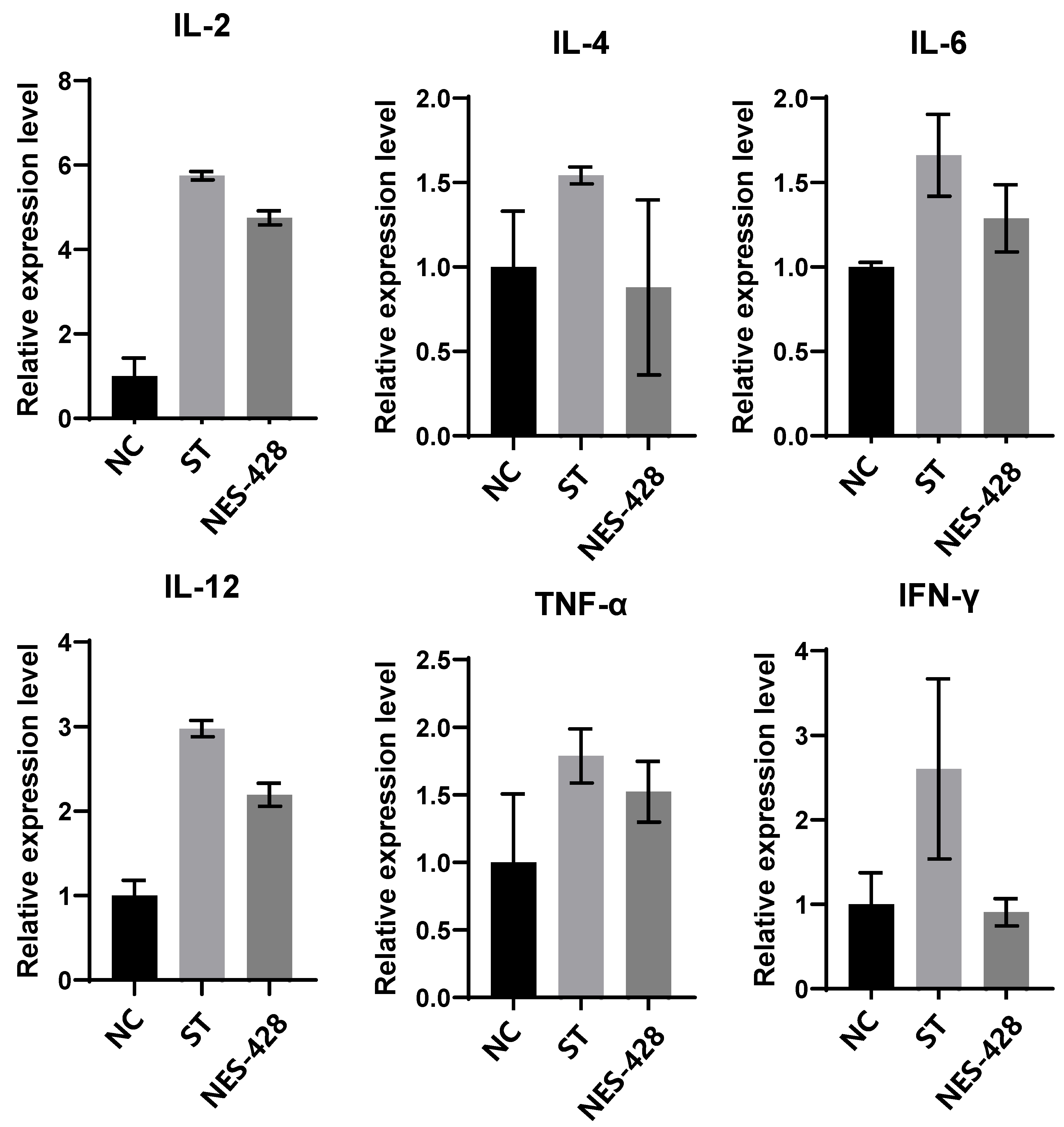
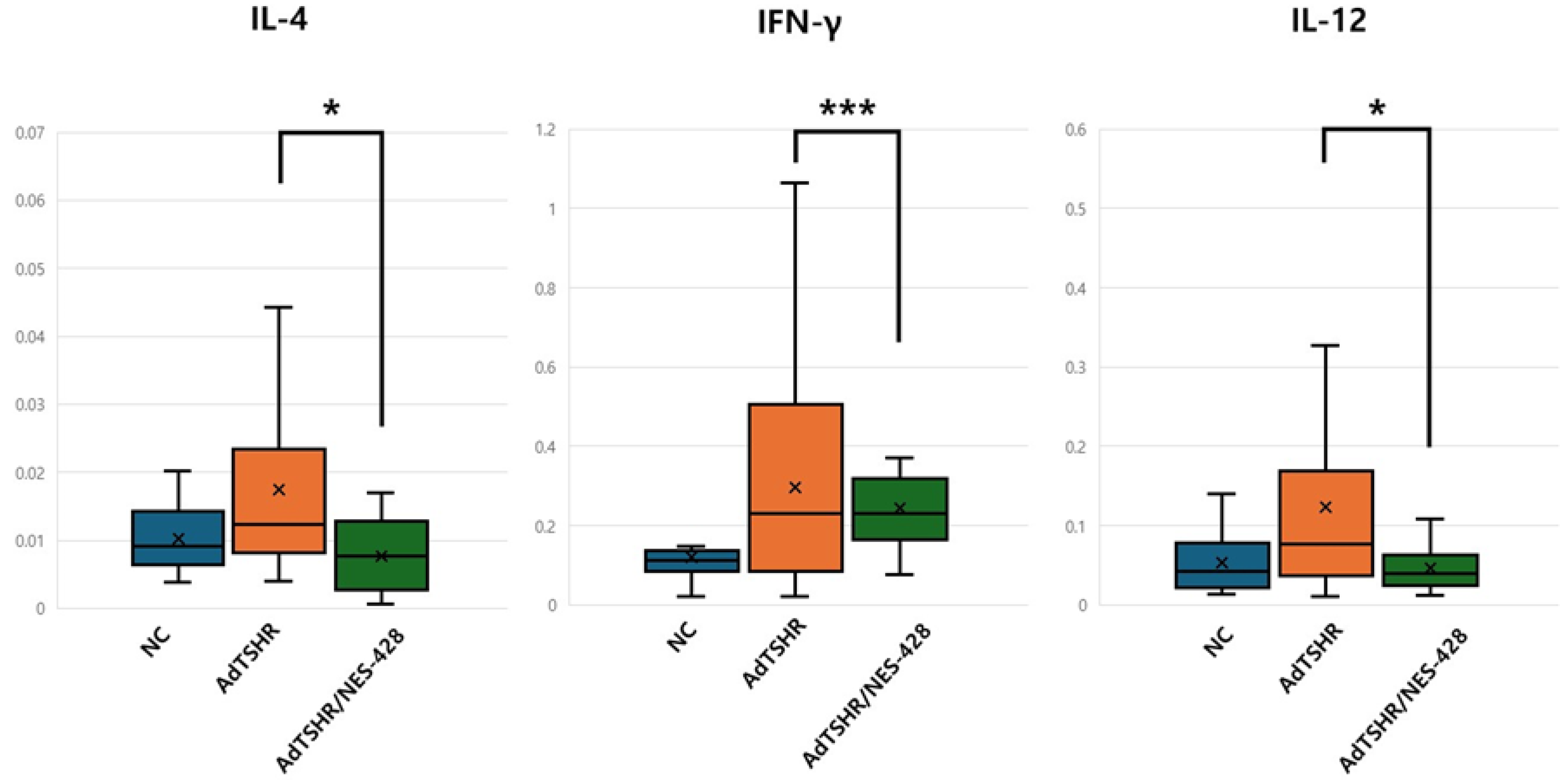
3.3. NES-428 Has an Immunomodulatory Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Correction Statement
References
- Bahn, R.S. Autoimmunity and Graves’ disease. Clin. Pharmacol. Ther. 2012, 91, 577–579. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedüs, L.; Lesser, I.; Perros, P.; Dorris, K.; Kinrade, M.; Troy-Ott, P.; Wuerth, L.; Nori, M. How patients experience thyroid eye disease. Front. Endocrinol. 2023, 14, 1283374. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Ragusa, F.; Elia, G.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Giusti, C.; Gonnella, D.; Cristaudo, A.; et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101387. [Google Scholar] [CrossRef] [PubMed]
- Kanokwongnuwat, W.; Penpong, N.; Sangsri, C. Incidence and treatment outcomes of Graves’ disease in Thailand: A single-center retrospective observational study. Thyroid Res. 2022, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Hen, K.; Czarnywojtek, A.; Florek, E.; Warmuz-Stangierska, I.; Ruchała, M. The etiology of Graves’ disease--current state of knowledge. Przegl. Lek. 2012, 69, 1132–1134. [Google Scholar]
- Yao, Z.; Guo, F.; Tan, Y.; Zhang, Y.; Geng, Y.; Yang, G.; Wang, S. Causal relationship between inflammatory cytokines and autoimmune thyroid disease: A bidirectional two-sample Mendelian randomization analysis. Front. Immunol. 2024, 15, 1334772. [Google Scholar] [CrossRef]
- Kocjan, T.; Wraber, B.; Repnik, U.; Hojker, S. Changes in Th1/Th2 cytokine balance in Graves’ disease. Pflugers. Arch. 2000, 440 (Suppl. 5), R94–R95. [Google Scholar] [CrossRef]
- Zhou, J.; Bi, M.; Fan, C.; Song, X.; Yang, R.; Zhao, S.; Li, L.; Li, Y.; Teng, W.; Shan, Z. Regulatory T cells but not T helper 17 cells are modulated in an animal model of Graves’ hyperthyroidism. Clin. Exp. Med. 2012, 12, 39–46. [Google Scholar] [CrossRef]
- Subekti, I.; Pramono, L.A. Current diagnosis and management of Graves’ disease. Acta. Med. Indones. 2018, 50, 177–182. [Google Scholar]
- Suzuki, N.; Noh, J.Y.; Hiruma, M.; Kawaguchi, A.; Morisaki, M.; Ohye, H.; Suzuki, M.; Matsumoto, M.; Kunii, Y.; Iwaku, K.; et al. Analysis of antithyroid drug-induced severe liver injury in 18,558 newly diagnosed patients with Graves’ disease in Japan. Thyroid 2019, 29, 1390–1398. [Google Scholar] [CrossRef]
- Bahn, R.S.; Burch, H.S.; Cooper, D.S.; Garber, J.R.; Greenlee, C.M.; Klein, I.L.; Laurberg, P.; McDougall, I.R.; Rivkees, S.A.; Ross, D.; et al. The Role of Propylthiouracil in the Management of Graves’ Disease in Adults: Report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 2009, 19, 673–674. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef]
- Fine, R.L.; Mubiru, D.L.; Kriegel, M.A. Friend or foe? Lactobacillus in the context of autoimmune disease. Adv. Immunol. 2020, 146, 29–56. [Google Scholar] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.I.; Jeong, C.H.; Hong, S.W.; Eun, J.B.; Kim, T.W. Optimizing conditions in the acid tolerance test for potential probiotics using response surface methodology. Microbiol. Spectr. 2022, 10, e0162522. [Google Scholar] [CrossRef] [PubMed]
- Zommara, M.; El-Ghaish, S.; Haertle, T.; Chobert, J.M.; Ghanimah, M. Probiotic and technological characterization of selected Lactobacillus strains isolated from different egyptian cheeses. BMC Microbiol. 2023, 23, 160. [Google Scholar] [CrossRef]
- Tanaka, H.; Hashiba, H.; Kok, J.; Mierau, I. Bile salt hydrolase of Bifidobacterium longum-biochemical and genetic characterization. Appl. Environ. Microbiol. 2000, 66, 2502–2512. [Google Scholar] [CrossRef]
- Chen, C.R.; Pichurin, P.; Nagayama, Y.; Latrofa, F.; Rapoport, B.; McLachlan, S.M. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J. Clin. Investig. 2003, 111, 1897–1904. [Google Scholar] [CrossRef]
- Chen, C.R.; Pichurin, P.; Chazenbalk, G.D.; Aliesky, H.; Nagayama, Y.; McLachlan, S.M.; Rapoport, B. Low-dose immunization with adenovirus expressing the thyroid-stimulating hormone receptor A-subunit deviates the antibody response toward that of autoantibodies in human Graves’ disease. Endocrinology 2004, 145, 228–233. [Google Scholar] [CrossRef]
- Dong, H.; Rowland, I.; Tuohy, K.M.; Thomas, L.V.; Yaqoob, P. Selective effects of Lactobacillus casei Shirota on T cell activation, natural killer cell activity and cytokine production: Probiotics and immune function. Clin. Exp. Immunol. 2010, 161, 378–388. [Google Scholar] [CrossRef]
- Moshkelgosha, S.; Verhasselt, H.L.; Masetti, G.; Covelli, D.; Biscarini, F.; Horstmann, M.; Daser, A.; Westendorf, A.M.; Jesenek, C.; Philipp, S.; et al. Modulating gut microbiota in a mouse model of Graves’ orbitopathy and its impact on induced disease. Microbiome 2021, 9, 45. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef]
- Mimee, M.; Tucker, A.C.; Voigt, C.A.; Lu, T.K. Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the Murine gut Microbiota. Cell Syst. 2015, 1, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, H.; Liu, C.; Shang, M.; Wei, T.; Yin, P. Gut microbiome and the role of metabolites in the study of graves’ disease. Front. Mol. Biosci. 2022, 9, 841223. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Shin, Y.H.; Gopalakrishnan, G.; Hennessey, J.; De Groot, L.J. Regulatory T cells in Graves’ disease: Regulatory T cells in Graves’ disease. Clin. Endocrinol. 2009, 71, 587–593. [Google Scholar] [CrossRef]
- Eckstein, A.; Philipp, S.; Goertz, G.; Banga, J.P.; Berchner-Pfannschmidt, U. Lessons from mouse models of Graves’ disease. Endocrine 2020, 68, 265–270. [Google Scholar] [CrossRef]
- Nagayama, Y. Progress in the pathogenesis of Graves’ disease: Approaches from animal models. Nihon. Rinsho. 2006, 64, 2215–2218. [Google Scholar]
- Carneiro, J.R.; Fuzii, H.T.; Kayser, C.; Alberto, F.L.; Soares, F.A.; Sato, E.I.; Andrade, L.E. IL-2, IL-5, TNF-α and IFN-γ mRNA expression in epidermal keratinocytes of systemic lupus erythematosus skin lesions. Clinics 2011, 66, 77–82. [Google Scholar] [CrossRef]
- Miao, X.; Jiang, Y.; Wu, Z.; Liu, H.; Gong, W. BEZ235 prolongs Murine cardiac allograft survival through the autophagy pathway. Transplant. Proc. 2022, 54, 2008–2015. [Google Scholar] [CrossRef]
- Djoba Siawaya, J.F.; Bapela, N.B.; Ronacher, K.; Beyers, N.; van Helden, P.; Walzl, G. Differential expression of interleukin-4 (IL-4) and IL-4 delta 2 mRNA, but not transforming growth factor beta (TGF-beta), TGF-beta RII, Foxp3, gamma interferon, T-bet, or GATA-3 mRNA, in patients with fast and slow responses to antituberculosis treatment. Clin. Vaccine Immunol. 2008, 15, 1165–1170. [Google Scholar] [CrossRef]
- Lu, C.C.; Kuo, H.C.; Wang, F.S.; Jou, M.H.; Lee, K.C.; Chuang, J.H. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int. J. Mol. Sci. 2014, 16, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Antonios, D.; Rousseau, P.; Larangé, A.; Kerdine-Römer, S.; Pallardy, M. Mechanisms of IL-12 synthesis by human dendritic cells treated with the chemical sensitizer NiSO4. J. Immunol. 2010, 185, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Elkon, K.B.; Ma, X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 2004, 21, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Lee, H.S.; Kim, S.M.; Kim, S.; Lim, J.; Woo, M.; Kim, E.J. Immunostimulatory activity of hydrolyzed and fermented Platycodon grandiflorum extract occurs via the MAPK and NF-κB signaling pathway in RAW 264.7 cells. Nutr. Res. Pract. 2022, 16, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, T.; Yamada, A.; Takami, M.; Suzuki, D.; Saito, Y.; Hiranuma, K.; Enomoto, T.; Morimura, N.; Yamamoto, M.; Iijima, T.; et al. Expression of nephronectin is inhibited by oncostatin M via both JAK/STAT and MAPK pathways. FEBS Open Bio 2015, 5, 303–307. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed. Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Jang, S.E.; Hyam, S.R.; Han, M.J.; Kim, S.Y.; Lee, B.G.; Kim, D.H. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J. Appl. Microbiol. 2013, 115, 888–896. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.; Qiao, N.; Xiao, Y.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Latilactobacillus curvatus: A candidate probiotic with excellent fermentation properties and health benefits. Foods 2020, 9, 1366. [Google Scholar] [CrossRef]
- Lim, S.; Moon, J.H.; Shin, C.M.; Jeong, D.; Kim, B. Effect of Lactobacillus sakei, a probiotic derived from Kimchi, on body fat in Koreans with obesity: A randomized controlled study. Endocrinol. Metab. 2020, 35, 425–434. [Google Scholar] [CrossRef]
- Park, M.Y.; Kim, J.; Kim, S.; Whang, K.Y. Lactobacillus curvatus KFP419 and Leuconostoc mesenteroides subsp. mesenteroides KDK411 Isolated from Kimchi Ameliorate Hypercholesterolemia in Rats. J. Med. Food. 2018, 21, 647–653. [Google Scholar] [CrossRef]
- Khalaf, H.; Jass, J.; Olsson, P.-E. Differential cytokine regulation by NF-kappaB and AP-1 in Jurkat T-cells. BMC Immunol. 2010, 11, 26. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Saitoh, O.; McLachlan, S.M.; Rapoport, B.; Kano, H.; Kumazawa, Y. TSH receptor-adenovirus-induced Graves’ hyperthyroidism is attenuated in both interferon-gamma and interleukin-4 knockout mice; implications for the Th1/Th2 paradigm. Clin. Exp. Immunol. 2004, 138, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Ye, X.; Hu, X.; Song, S.; Xu, H.; Niu, M.; Wang, H.; Wang, J. Simultaneous induction of Graves’ hyperthyroidism and Graves’ ophthalmopathy by TSHR genetic immunization in BALB/c mice. PLoS ONE 2017, 12, e0174260. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.; Mazziotti, G.; von Ruhland, C.; Ludgate, M. Reevaluating thyrotropin receptor-induced mouse models of graves’ disease and ophthalmopathy. Endocrinology 2005, 146, 835–844. [Google Scholar] [CrossRef][Green Version]
- McLachlan, S.M.; Nagayama, Y.; Rapoport, B. Insight into Graves’ hyperthyroidism from animal models. Endocr. Rev. 2005, 26, 800–832. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, W.; Lu, G.; Wang, R.; Lv, Z.; Li, D. Insight into mouse models of hyperthyroidism. Front. Endocrinol. 2022, 13, 929750. [Google Scholar] [CrossRef]
- Nagayama, Y. Animal models of Graves’ hyperthyroidism. Endocr. J. 2005, 52, 385–394. [Google Scholar] [CrossRef]
- Köhling, H.L.; Plummer, S.F.; Marchesi, J.R.; Davidge, K.S.; Ludgate, M. The microbiota and autoimmunity: Their role in thyroid autoimmune diseases. Clin. Immunol. 2017, 183, 63–74. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedüs, L. Graves’ disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef]
- Antonelli, A.; Fallahi, P.; Elia, G.; Ragusa, F.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Gonnella, D.; Giusti, C.; Virili, C.; et al. Graves’ disease: Clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101388. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, A.K.; Halder, A.; Jain, M.; Chaturvedi, P.K.; Mathew, M.; Sharma, J.B. Association of raised levels of IL-4 and anti-TPO with hyperprolactinemia. Am. J. Reprod. Immunol. 2019, 81, e13085. [Google Scholar] [CrossRef] [PubMed]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between gut Microbiota and host immunity: Impact on inflammation and immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- D’Amico, F.; Pugliese, N.; Peyrin-Biroulet, L.; Danese, S. Efficacy of anti-TNFα drugs in patients with stricturing Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 347–353. [Google Scholar] [CrossRef]
- Nirschl, C.J.; Suárez-Fariñas, M.; Izar, B.; Prakadan, S.; Dannenfelser, R.; Tirosh, I.; Liu, Y.; Zhu, Q.; Devi, K.S.P.; Carroll, S.L.; et al. IFNγ-dependent tissue-immune homeostasis is co-opted in the tumor microenvironment. Cell 2017, 170, 127–141.e15. [Google Scholar] [CrossRef]
- Kiani, A.; García-Cózar, F.J.; Habermann, I.; Laforsch, S.; Aebischer, T.; Ehninger, G.; Rao, A. Regulation of interferon-gamma gene expression by nuclear factor of activated T cells. Blood 2001, 98, 1480–1488. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′ → 3′) |
|---|---|
| 27F | AGAGTTTGATCMTGGCTCAG |
| 337F | ACTCCTACGGGAGGCAGCAG |
| 518F | CCAGCAGCCGCGGTAATAC |
| 785F | GGATTAGATACCCTGGTA |
| 518R | ATTACCGCGGCTGCTGG |
| 783R | ACAGGGTATCTAATCCTGTT |
| 805R | GACTACCAGGGTATCTAATCC |
| 907R | CCGTCAATTCMTTTRAGTTT |
| 1492R | GGTTACCTTGTTACGACTT |
| Gene | Sequence(5′ → 3′) | Ref. |
|---|---|---|
| human IL-2 | AAGAATCCCAAACTAACCAGGAT | [21] |
| TCTAGACATGAAGATGTTTCAGTTCTC | ||
| mouse IL-2 | TGATGGACCTACAGGAGCTCCTGAG | [22] |
| GAGTCAAATCCAGAACATGCCGCAG | ||
| hIL-4 | AGATCATCGGCATTTTGAAC | [23] |
| TTTGGCACATCCATCTCCG | ||
| mIL-4 | ATCATCGGCATTTTGAACGAGGTC | [24] |
| ACCTTGGAAGCCCTACAGACGA | ||
| hIL-6 | GTCAACTCCATCTGCCCTTCAG | [25] |
| GGTCTGTTGTGGGTGGTATCCT | ||
| mIL-6 | CCCCAATTTCCAATGCTCTCC | [22] |
| CGCACTAGGTTTGCCGAGTA | ||
| hIL-12 | GACATTCTGCGTTCAGGTCCAG | [26] |
| CATTTTTGCGGCAGATGACCGTG | ||
| mIL-12 | AAATGAAGCTCTGCATCCTGC | [27] |
| TCACCCTGTTGATGGTCACG | ||
| hTNF-α | CCGAGGCAGTCAGATCATCTT | [21] |
| AGCTGCCCCTCAGCTTGA | ||
| mTNF-α | ACCCTCACACTCACAAACCA | [22] |
| ATAGCAAATCGGCTGACGGT | ||
| hIFN-γ | TGTAGCGGATAATGGAACTCTTTT | [21] |
| AATTTGGCTCTGCATTATT | ||
| mIFN-γ | GAACTGGCAAAAGGATGGTGA | [22] |
| TGTGGGTTGTTGACCTCAAAC | ||
| hGAPDH | AGGTTGTCTCCTGCGACT | [28] |
| TGCTGTAGCCGTATTCATTGTCA | ||
| mGAPDH | AAATGGTGAAGGTCGGTGTG | [22] |
| TGAAGGGGTCGTTGATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-G.; Lee, D.-H.; Kang, S.; Koh, J.; Yun, C.-W. Immunomodulatory Effects of Lactobacillus brevis NES-428 in a Hyperthyroidism Mouse Model: Potential Applications for Graves’ Disease. Nutrients 2025, 17, 2967. https://doi.org/10.3390/nu17182967
Lee M-G, Lee D-H, Kang S, Koh J, Yun C-W. Immunomodulatory Effects of Lactobacillus brevis NES-428 in a Hyperthyroidism Mouse Model: Potential Applications for Graves’ Disease. Nutrients. 2025; 17(18):2967. https://doi.org/10.3390/nu17182967
Chicago/Turabian StyleLee, Min-Gyu, Dong-Hyun Lee, Suzie Kang, Jongho Koh, and Cheol-Won Yun. 2025. "Immunomodulatory Effects of Lactobacillus brevis NES-428 in a Hyperthyroidism Mouse Model: Potential Applications for Graves’ Disease" Nutrients 17, no. 18: 2967. https://doi.org/10.3390/nu17182967
APA StyleLee, M.-G., Lee, D.-H., Kang, S., Koh, J., & Yun, C.-W. (2025). Immunomodulatory Effects of Lactobacillus brevis NES-428 in a Hyperthyroidism Mouse Model: Potential Applications for Graves’ Disease. Nutrients, 17(18), 2967. https://doi.org/10.3390/nu17182967







