Exercise-Induced Changes in Enterohepatic Communication Are Linked to Liver Steatosis Resolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Diets
2.2. Glucose Tolerance Test
2.3. Liver Tissue Sectioning and Staining
2.4. Hepatic TG Quantification
2.5. Serum Biochemical Analysis
2.6. 16S rRNA Gene Sequencing and Bioinformatics Analysis
2.7. Bile Acid Profiling by UHPLC-MS/MS
2.8. Quantitative Real-Time PCR (qPCR) Analysis
2.9. Microbiome and Statistical Analysis
3. Results
3.1. Aerobic Exercise Alleviates High-Fat Diet-Induced Hepatic Steatosis and Liver Injury
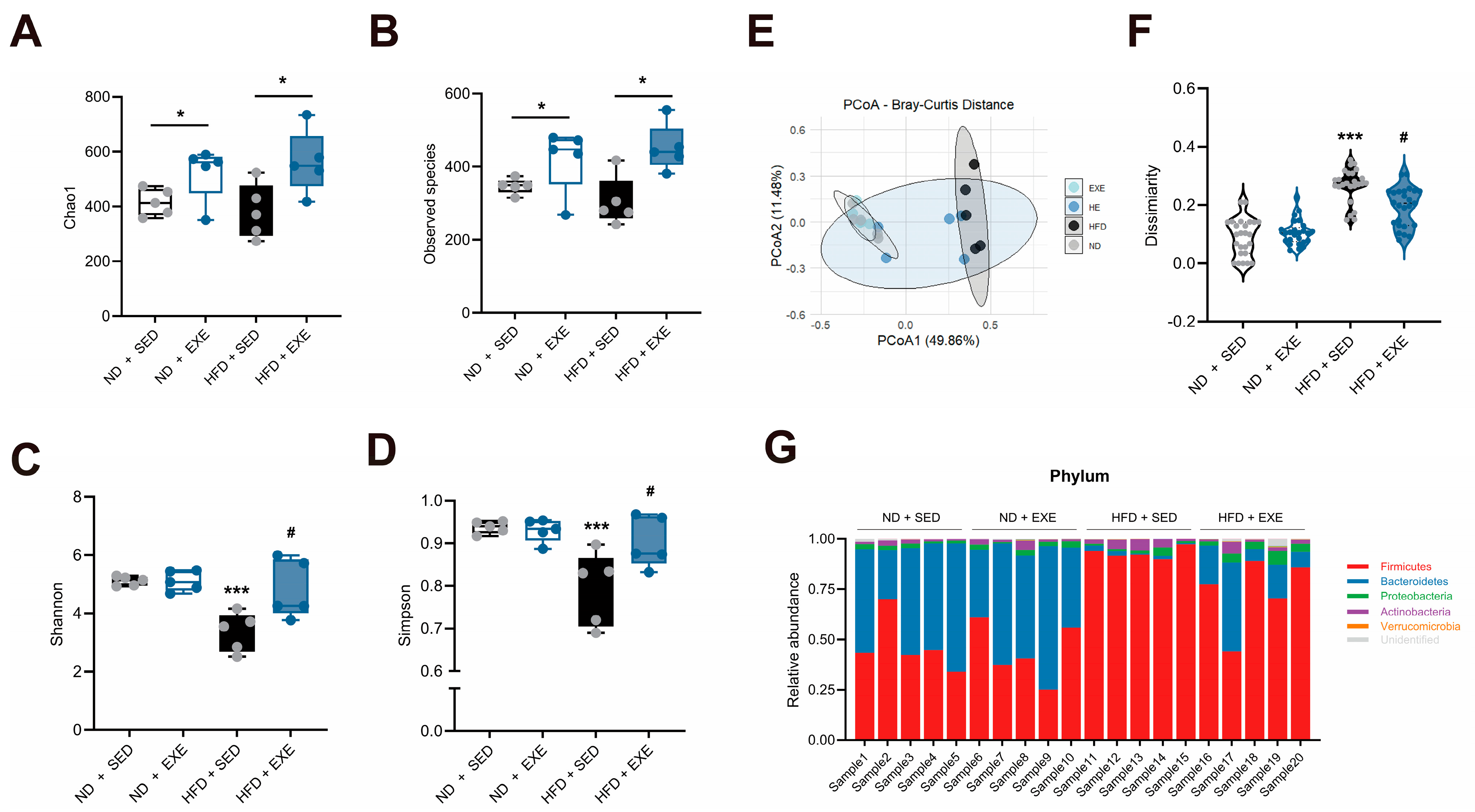
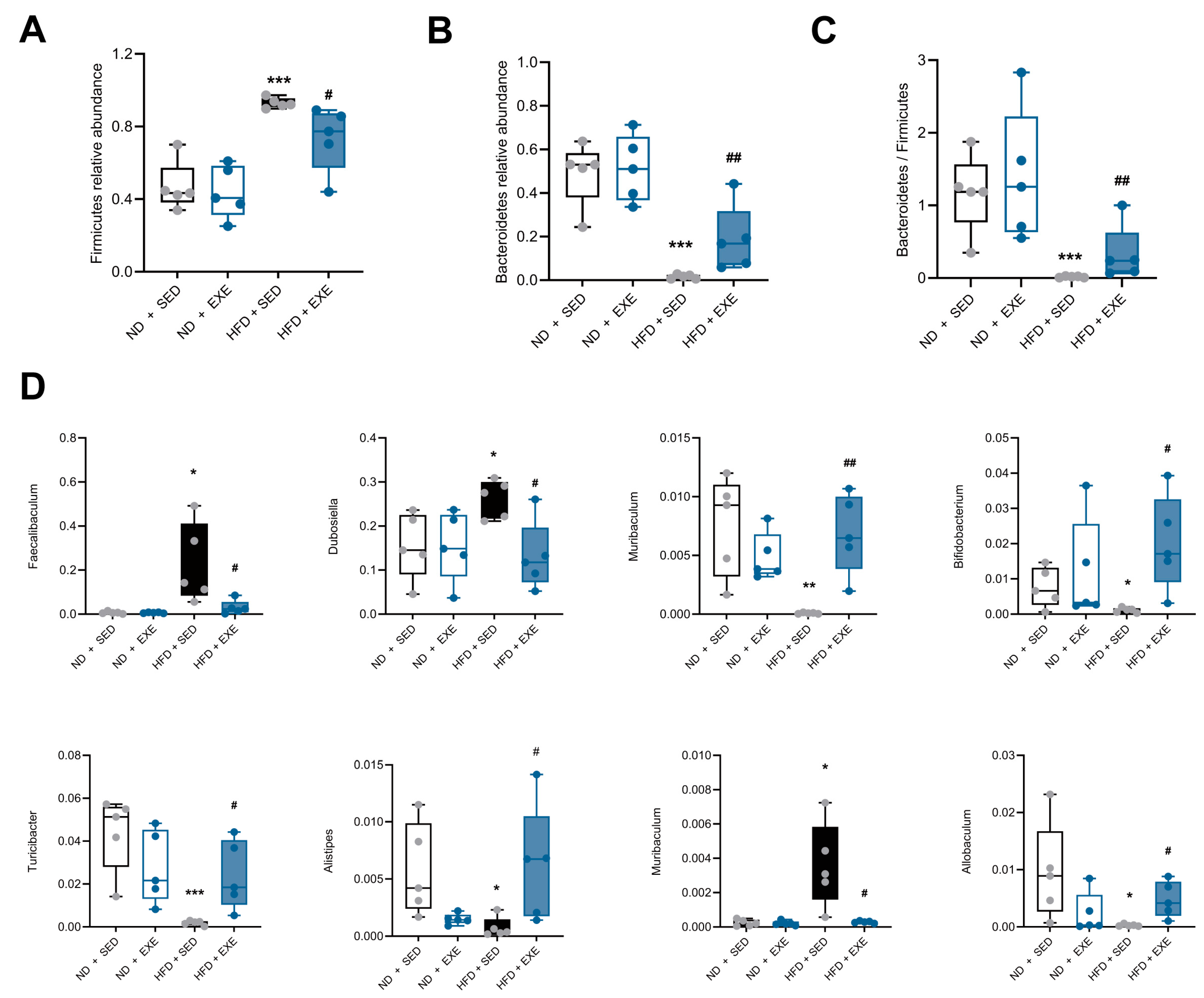
3.2. Aerobic Exercise Restructures Gut Microbial Composition in High-Fat Diet Mouse Models
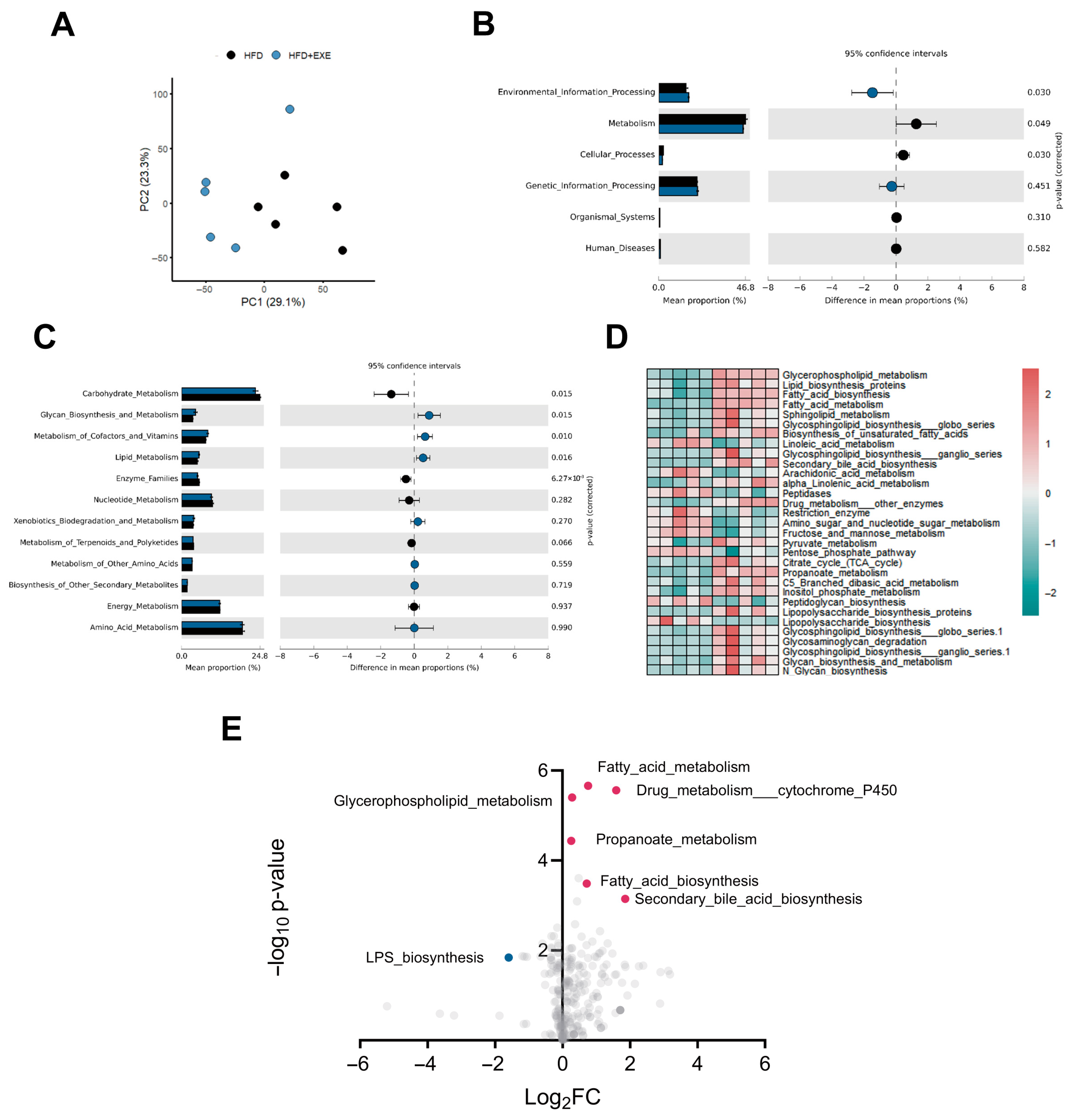
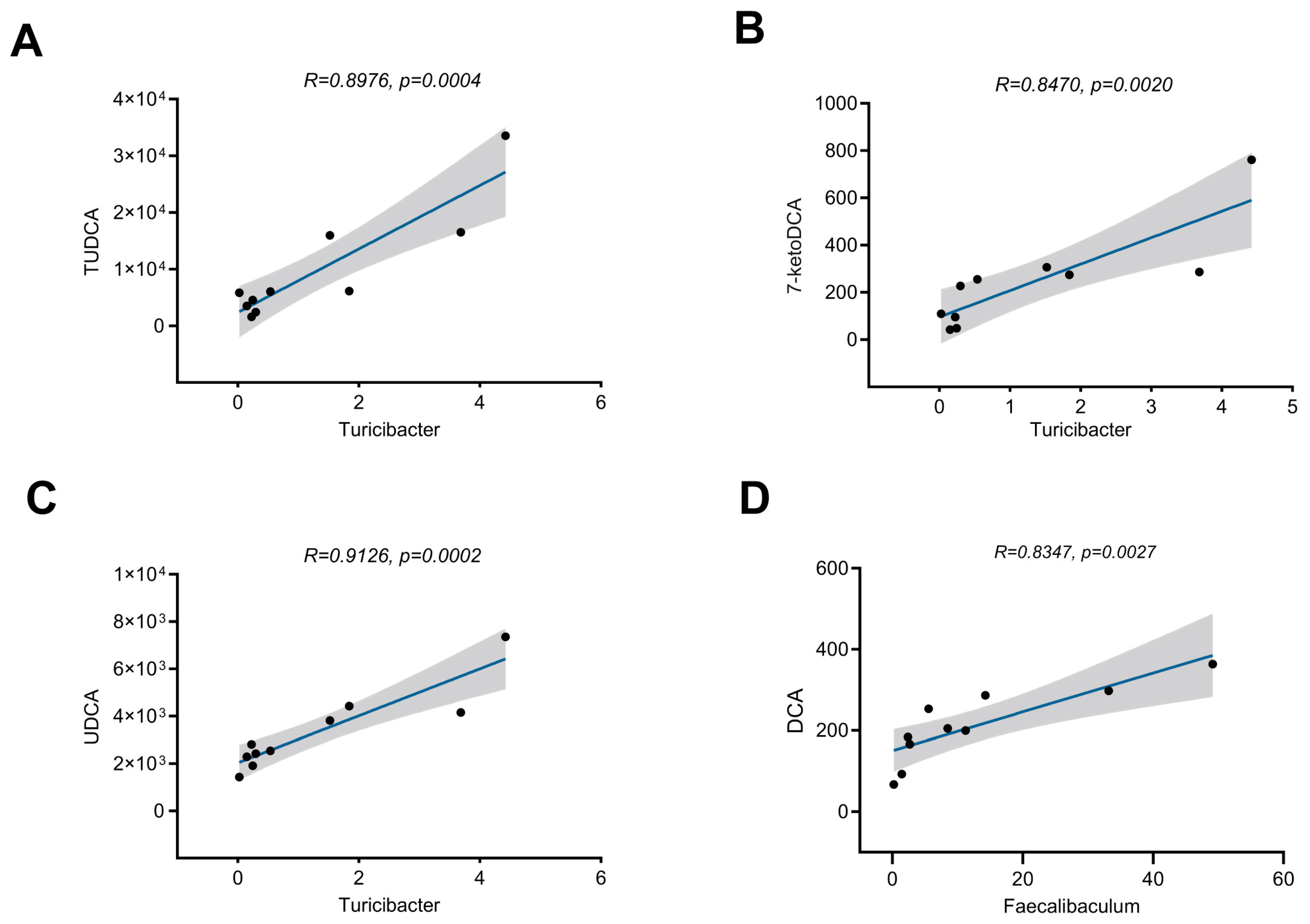
3.3. Aerobic Exercise-Induced Alterations in Gut Microbial Composition Are Associated with Bile Acid Metabolism
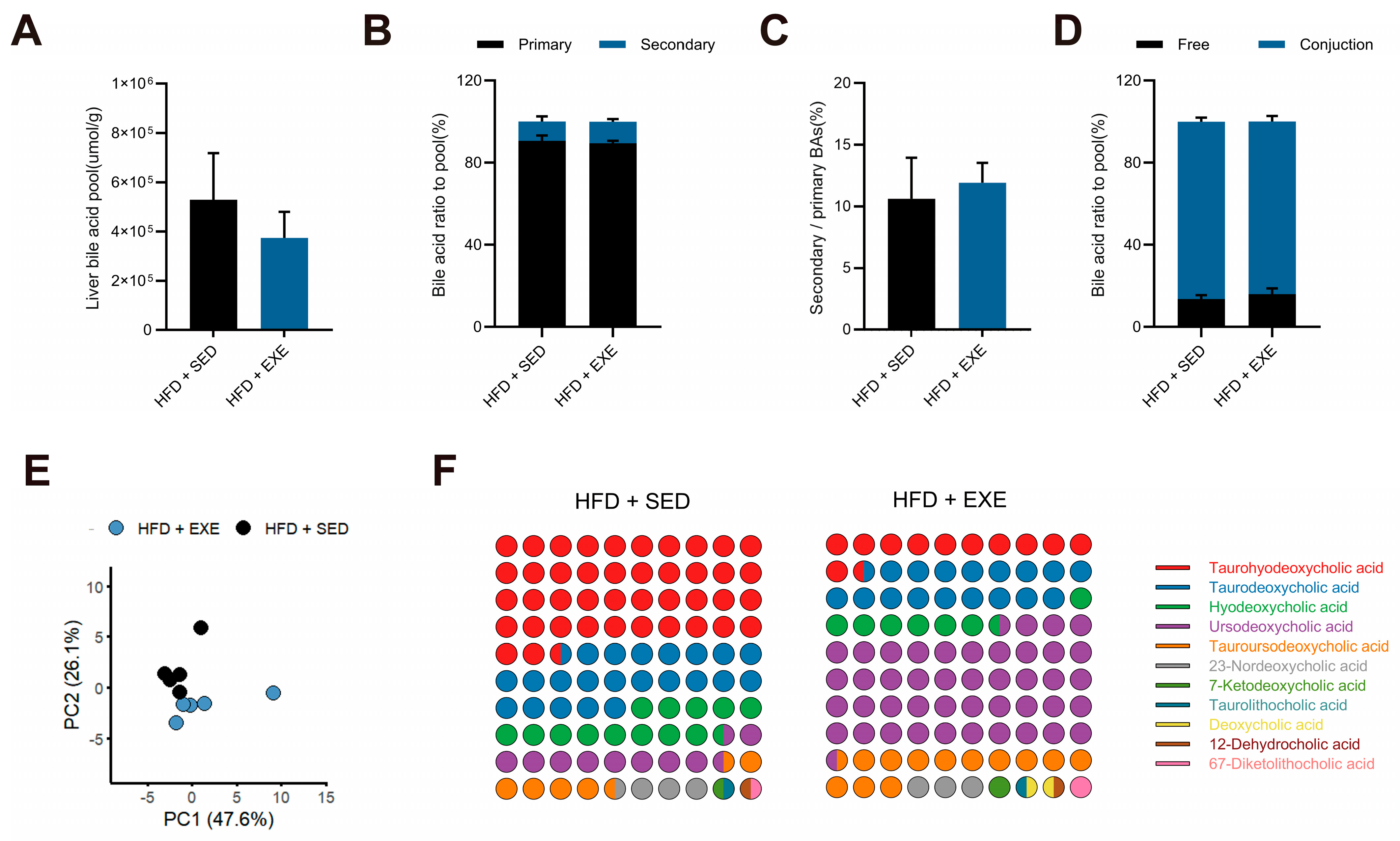
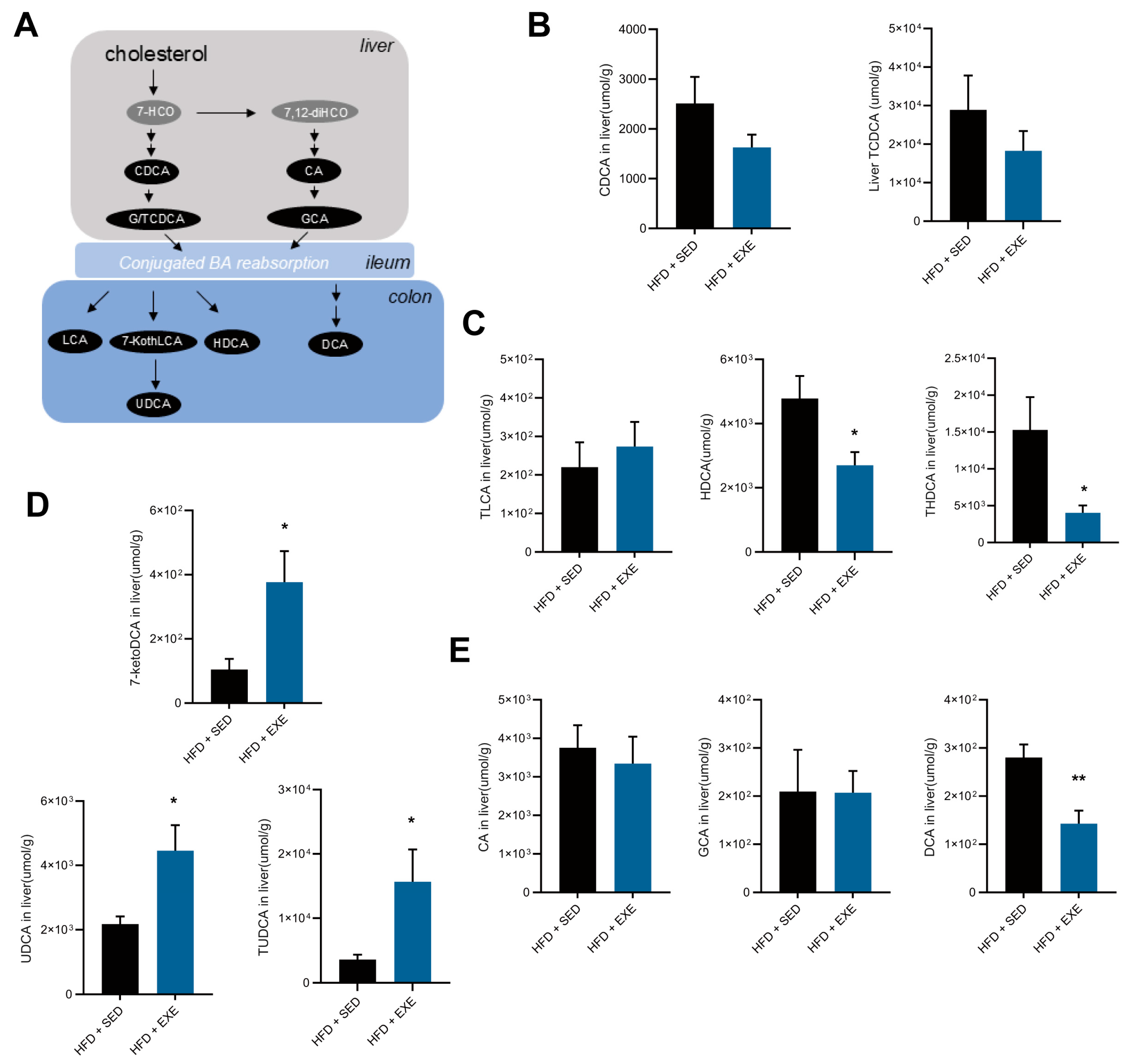
3.4. Aerobic Exercise Restructures the Hepatic Bile Acid Pool in High-Fat Diet-Fed Mice
3.5. Aerobic Exercise Modulates Gut Microbiota to Enhance CDCA-Derived and Reduce CA-Derived Secondary Bile Acid Metabolism
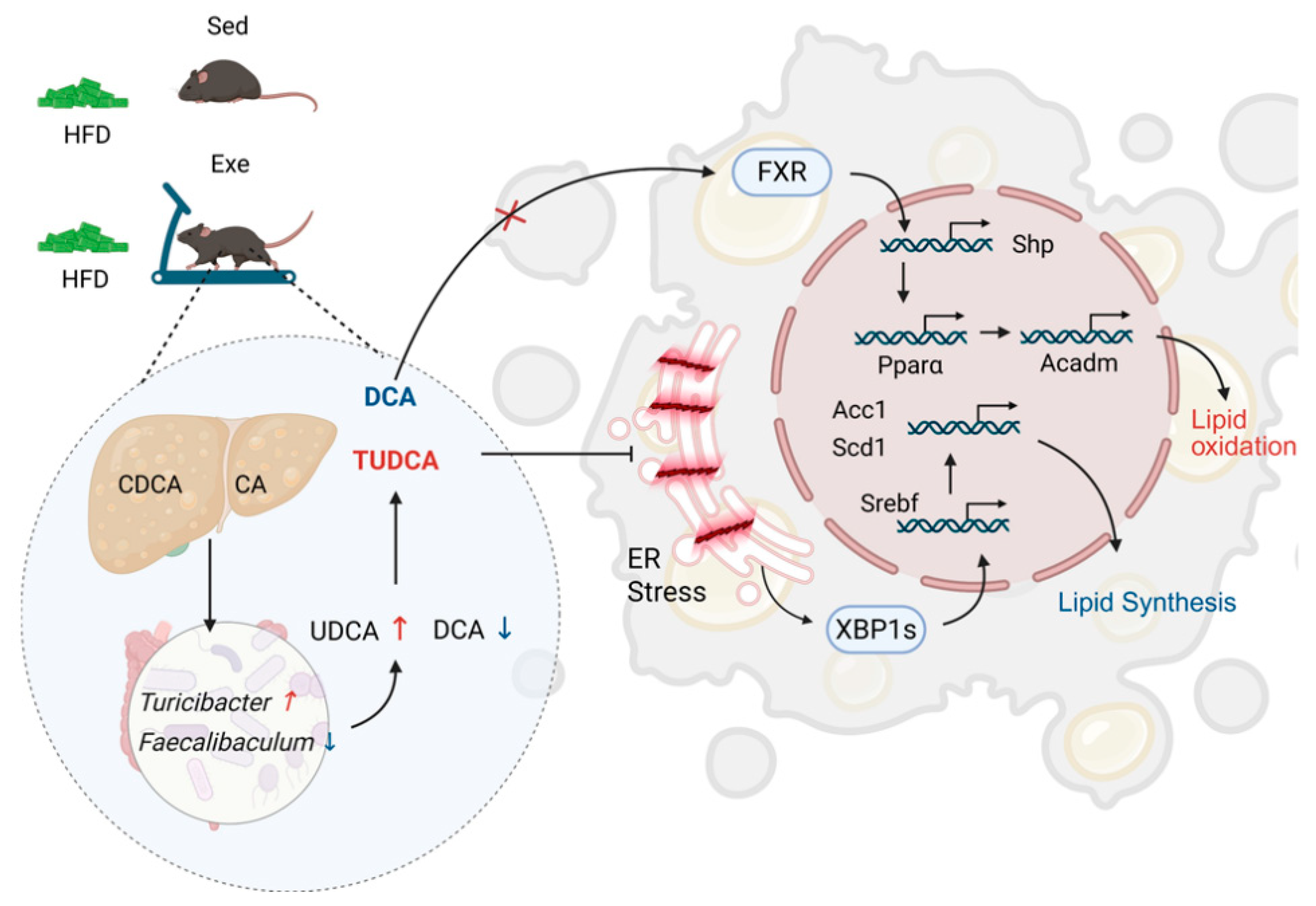
3.6. Aerobic Exercise Attenuates Hepatic Lipid Metabolism via Secondary Bile Acid-Mediated ERS Alleviation and FXR Activation
4. Discussion
4.1. Effects of Long-Term Exercise Training on Gut Microbiota Diversity in HFD-Fed Mice
4.2. Effects of Long-Term Exercise Training on Liver Bile Acid Pool in HFD-Fed Mice
4.3. Potential Molecular Mechanisms of Exercise-Induced Hepatic Steatosis Amelioration
4.4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFD | high-fat diet |
| ND | Normal diet |
| ND-SED | Normal diet with sedentary |
| ND-EXE | Normal diet with exercise |
| HFD-SED | High fatty diet with sedentary |
| HFD-EXE | High fatty diet with exercise |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| TG | Triglyceride |
| ER | Endoplasmic reticulum |
| GTT | Glucose tolerance test |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| PCA | Principal component analysis |
| PCoA | Principal coordinates analysis |
| OTUs | Operational Taxonomic Units |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GRP78 | Glucose-Regulated Protein 78 |
| FXR | Farnesoid X receptor |
| Srebp | Sterol Regulatory Element-Binding Protein |
| Acc1 | Acetyl-CoA carboxylase 1 |
| Scd1 | Stearoyl-CoA desaturase 1 |
| Xbp1s/u | X-box binding protein 1 spliced/ unspliced |
| SHP | Short heterodimer partner |
| Pparα | Peroxisome proliferator-activated receptor alpha |
| Acadm | Medium-chain acyl-CoA dehydrogenase |
| IRE1α | Inositol requiring enzyme 1 alpha |
| DGAT2 | Diacylglycerol O-Acyltransferase 2 |
| FASN | Fatty Acid Synthase |
| TUDCA | Tauroursodeoxycholic acid |
| UDCA | Ursodeoxycholic acid |
| THDCA | Taurohyodeoxycholic |
| CDCA | Chenodeoxycholic acid |
| DCA | Deoxycholic acid |
| TDCA | Taurodeoxycholic acid |
| CA | Cholic Acid |
| GCA | Glycocholic acid |
| 7-ketoDCA | 7-ketodeoxycholic acid (7-ketoDCA) |
| SCFA | Short-chain fatty acid |
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef]
- Estes, C.; Chan, H.L.Y.; Chien, R.N.; Chuang, W.; Fung, J.; Goh, G.B.; Hu, T.H.; Huang, J.; Jang, B.K.; Jun, D.W.; et al. Modelling NAFLD disease burden in four Asian regions-2019–2030. Aliment. Pharm. Ther. 2020, 51, 801–811. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Behary, J.; Amorim, N.; Jiang, X.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Nychas, E.; Marfil-Sánchez, A.; Chen, X.; Mirhakkak, M.; Li, H.; Jia, W.; Xu, A.; Nielsen, H.B.; Nieuwdorp, M.; Loomba, R.; et al. Discovery of robust and highly specific microbiome signatures of non-alcoholic fatty liver disease. Microbiome 2025, 13, 10. [Google Scholar] [CrossRef]
- Yang, M.; Qi, X.; Li, N.; Kaifi, J.T.; Chen, S.; Wheeler, A.A.; Kimchi, E.T.; Ericsson, A.C.; Rector, R.S.; Staveley-O’Carroll, K.F.; et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 2023, 14, 228. [Google Scholar] [CrossRef]
- Ni, Y.; Qian, L.; Siliceo, S.L.; Long, X.; Nychas, E.; Liu, Y.; Ismaiah, M.J.; Leung, H.; Zhang, L.; Gao, Q.; et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023, 35, 1530–1547.e8. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Schneider, E.; O’Riordan, K.J.; Clarke, G.; Cryan, J.F. Feeding gut microbes to nourish the brain: Unravelling the diet-microbiota-gut-brain axis. Nat. Metab. 2024, 6, 1454–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Cheng, W.; Dai, Q.; Wei, Z.; Guo, M.; Chen, F.; Qiao, S.; Hu, J.; Wang, J.; et al. Heart-gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion. Cardiovasc. Res. 2023, 119, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef]
- Phelps, C.M.; Willis, N.B.; Duan, T.; Lee, A.H.; Zhang, Y.; Rodriguez, J.D.M.; Pandey, S.P.; Laughlin, C.R.; Rosen, A.B.I.; McPherson, A.C.; et al. Exercise-induced microbiota metabolite enhances CD8 T cell antitumor immunity promoting immunotherapy efficacy. Cell 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Y.; Yuan, H.; Huang, J.; Fu, L. AMPK binds to Sestrins and mediates the effect of exercise to increase insulin-sensitivity through autophagy. Metabolism 2015, 64, 658–665. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liu, Z.; Ruan, X.; Wang, H.; Zhang, Q.; Cao, L.; Song, L.; Chen, Y.; Sun, Y. Moderate treadmill exercise alleviates nafld by regulating the biogenesis and autophagy of lipid droplet. Nutrients 2022, 14, 4910. [Google Scholar] [CrossRef]
- Chen, B.; Bai, Y.; Tong, F.; Yan, J.; Zhang, R.; Zhong, Y.; Tan, H.; Ma, X. Glycoursodeoxycholic acid regulates bile acids level and alters gut microbiota and glycolipid metabolism to attenuate diabetes. Gut Microbes 2023, 15, 2192155. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. The gut microbiota in obesity and weight management: Microbes as friends or foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Forster, S.C.; Giles, E.M. Exercise, the Gut Microbiome and Gastrointestinal Diseases: Therapeutic Impact and Molecular Mechanisms. Gastroenterology 2025, 169, 48–62. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Z.; Zhang, Y.; Zhang, S.; Cui, Z.; Liu, W.; Li, L.; Xia, J.; Zou, Y.; Qi, Z. ASMT determines gut microbiota and increases neurobehavioral adaptability to exercise in female mice. Commun. Biol. 2023, 6, 1126. [Google Scholar] [CrossRef]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis. Model Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Ablitip, A.; Wang, R.; Luciana, T.; Wei, M.; Ma, X. Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1070. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, X.; Liu, W.; Wei, H.; Liang, W.; Zhou, Y.; Ding, Y.; Ji, F.; Ho-Kwan Cheung, A.; Wong, N.; et al. Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J. Hepatol. 2023, 79, 1352–1365. [Google Scholar] [CrossRef]
- Lai, C.; Chen, L.; Zhong, X.; Tian, X.; Zhang, B.; Li, H.; Zhang, G.; Wang, L.; Sun, Y.; Guo, L. Long-term arsenic exposure decreases mice body weight and liver lipid droplets. Environ. Int. 2024, 192, 109025. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Chen, C.; Zheng, J.; Jiang, B.; Dong, X.; Lou, S.; Luo, J.; Zhang, X.; Zhou, Z.; Luo, Q.; et al. Ampelopsis grossedentata tea alleviating liver fibrosis in BDL-induced mice via gut microbiota and metabolite modulation. npj Sci. Food 2024, 8, 93. [Google Scholar] [CrossRef]
- Li, S.; Zhang, N.; Yang, X.; Huang, T.; Lin, Y.; Jiang, Z.; Yi, Y.; Liu, E. Nobiletin Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Gut Microbiota and Myristoleic Acid Metabolism. J. Agric. Food Chem. 2023, 71, 7312–7323. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, L.; Zhang, L.; Xu, F.; Zhang, C.; Ren, G.; Chang, K.; He, G.; Du, Z.; Le, Y.; et al. Xie Zhuo Tiao Zhi formula modulates intestinal microbiota and liver purine metabolism to suppress hepatic steatosis and pyroptosis in NAFLD therapy. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 121, 155111. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Kaicen, W.; Bian, X.; Yang, L.; Ding, S.; Li, Y.; Li, S.; Zhuge, A.; Li, L. Akkermansia muciniphila alleviates high-fat-diet-related metabolic-associated fatty liver disease by modulating gut microbiota and bile acids. Microb. Biotechnol. 2023, 16, 1924–1939. [Google Scholar] [CrossRef]
- Xu, H.; Fang, F.; Wu, K.; Song, J.; Li, Y.; Lu, X.; Liu, J.; Zhou, L.; Yu, W.; Yu, F.; et al. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome 2023, 11, 262. [Google Scholar] [CrossRef]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Hagio, M.; Matsumoto, M.; Yajima, T.; Hara, H.; Ishizuka, S. Voluntary wheel running exercise and dietary lactose concomitantly reduce proportion of secondary bile acids in rat feces. J. Appl. Physiol. 2010, 109, 663–668. [Google Scholar] [CrossRef]
- Kugler, B.A.; Maurer, A.; Fu, X.; Franczak, E.; Ernst, N.; Schwartze, K.; Allen, J.; Li, T.; Crawford, P.A.; Koch, L.G.; et al. Aerobic Capacity and Exercise Mediate Protection Against Hepatic Steatosis via Enhanced Bile Acid Metabolism. Function 2025, 6, zqaf019. [Google Scholar] [CrossRef]
- Meissner, M.; Lombardo, E.; Havinga, R.; Tietge, U.J.F.; Kuipers, F.; Groen, A.K. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 2011, 218, 323–329. [Google Scholar] [CrossRef]
- Gillard, J.; Clerbaux, L.; Nachit, M.; Sempoux, C.; Staels, B.; Bindels, L.B.; Tailleux, A.; Leclercq, I.A. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP Rep. Innov. Hepatol. 2022, 4, 100387. [Google Scholar] [CrossRef]
- Wilund, K.R.; Feeney, L.A.; Tomayko, E.J.; Chung, H.R.; Kim, K. Endurance exercise training reduces gallstone development in mice. J. Appl. Physiol. 2008, 104, 761–765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Brit. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Syeda, U.A. Exercise Training Independent of Intensity Lowers Plasma Bile Acids in Prediabetes. Med. Sci. Sports Exerc. 2024, 56, 1009–1017. [Google Scholar] [CrossRef]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef]
- Kemis, J.H.; Linke, V.; Barrett, K.L.; Boehm, F.J.; Traeger, L.L.; Keller, M.P.; Rabaglia, M.E.; Schueler, K.L.; Stapleton, D.S.; Gatti, D.M.; et al. Genetic determinants of gut microbiota composition and bile acid profiles in mice. PLoS Genet. 2019, 15, e1008073. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Li, X.; Zhang, R.; Tang, W.; Li, H.; Wang, S.; Shen, T.; et al. Naringin Attenuates High Fat Diet Induced Non-alcoholic Fatty Liver Disease and Gut Bacterial Dysbiosis in Mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Angelini, G.; Castagneto-Gissey, L.; Salinari, S.; Bertuzzi, A.; Anello, D.; Pradhan, M.; Zschätzsch, M.; Ritter, P.; Le Roux, C.W.; Rubino, F.; et al. Upper gut heat shock proteins HSP70 and GRP78 promote insulin resistance, hyperglycemia, and non-alcoholic steatohepatitis. Nat. Commun. 2022, 13, 7715. [Google Scholar] [CrossRef]
- Li, Q.; Yao, H.; Wang, Y.; Wu, Y.; Thorne, R.F.; Zhu, Y.; Wu, M.; Liu, L. circPRKAA1 activates a Ku80/Ku70/SREBP-1 axis driving de novo fatty acid synthesis in cancer cells. Cell Rep. 2022, 41, 111707. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ren, L.P.; Wang, C.; Zhu, Y.J.; Xing, H.Y.; Zhao, J.; Song, G.Y. Role of X-box binding protein-1 in fructose-induced de novo lipogenesis in HepG2 cells. Chin. Med. J. 2018, 131, 2310–2319. [Google Scholar] [CrossRef]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.P.; Liao, B.; Wang, S.; et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023, 35, 1752–1766.e8. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.C.; Massart, J.; de Boer, J.F.; Porsmyr-Palmertz, M.; Martínez-Redondo, V.; Agudelo, L.Z.; Sinha, I.; Meierhofer, D.; Ribeiro, V.; Björnholm, M.; et al. Bioenergetic cues shift FXR splicing towards FXRα2 to modulate hepatic lipolysis and fatty acid metabolism. Mol. Metab. 2015, 4, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, M.; Mu, W.; Luo, H.; Guo, L. Higd1a facilitates exercise-mediated alleviation of fatty liver in diet-induced obese mice. Metab. Clin. Exp. 2022, 134, 155241. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Xia, J.; Zhang, S.; Guo, Y.; Liu, W.; Qi, Z. Exercise-Induced Changes in Enterohepatic Communication Are Linked to Liver Steatosis Resolution. Nutrients 2025, 17, 2962. https://doi.org/10.3390/nu17182962
Zou Y, Xia J, Zhang S, Guo Y, Liu W, Qi Z. Exercise-Induced Changes in Enterohepatic Communication Are Linked to Liver Steatosis Resolution. Nutrients. 2025; 17(18):2962. https://doi.org/10.3390/nu17182962
Chicago/Turabian StyleZou, Yong, Jie Xia, Sen Zhang, Yingjie Guo, Weina Liu, and Zhengtang Qi. 2025. "Exercise-Induced Changes in Enterohepatic Communication Are Linked to Liver Steatosis Resolution" Nutrients 17, no. 18: 2962. https://doi.org/10.3390/nu17182962
APA StyleZou, Y., Xia, J., Zhang, S., Guo, Y., Liu, W., & Qi, Z. (2025). Exercise-Induced Changes in Enterohepatic Communication Are Linked to Liver Steatosis Resolution. Nutrients, 17(18), 2962. https://doi.org/10.3390/nu17182962





