Is There a Future Without Gluten Restrictions for Celiac Patients? Update on Current Treatments
Abstract
1. Celiac Disease Pathophysiology and Limitations of a Gluten-Free Diet
2. Gluten-Degrading Enzymes
2.1. Fungi-Derived Enzymes
| Origin | Species | Name | Type | Phase, Clinical Trial, Sponsor | Ref. |
|---|---|---|---|---|---|
| Fungi | A. niger | AN-PEP | Serine prolyl endopeptidase | Interventional (NCT02060864, NCT01335503), Phase 1 and Phase 2 (NCT00810654), Phase 4 (NCT04788797)/DSM Food Specialties | [11,12,13,14,15,16] |
| A. niger and A. oryzae | STAN1 | Aspartate aspergillopepsin (ASP) + serine dipeptidyl-peptidase IV (DPPIV) | Phase 1 and phase 2 (NCT00962182)/Heim Pal Children’s Hospital | [23] | |
| A. oryzae | Flavourzyme | Mix of endo- and exo-peptidases | In vitro | [24] | |
| T. rubrum | AMYNOPEP | Leucine aminopeptidase 2 and DPPIV | German Register of Clinical Studies, DRKS00033099 | [21,22] | |
| Bacteria | R. aeria and R. mucilaginosa | R. aeria and R. mucilaginosa | Subtilisin-type serine endopeptidases | In vitro | [25] |
| A. sendaiensis | Zamaglutenase/TAK-062 (from KumaMax and Kuma062) | Serine endopeptidase | Phase 1 (NCT03701555), Phase 2 (NCT05353985)/Takeda | [26,27,28] | |
| B. gladioli | Bga1903 | Serine endopeptidase | In vitro | [29] | |
| A. A8 | Endopeptidase 40 (E40) | Endopeptidase | In vitro | [30] | |
| M. xanthus | MX PEP | Prolyl endopeptidase | In vitro | [31] | |
| F. meningosepticum | FM PEP | Prolyl endopeptidase | In vitro | [32,33] | |
| S. capsulate | SC-PEP | Prolyl endoprotease | In vitro | [34] | |
| B. licheniformis | Alcalase | Serine endopeptidase | In vitro and in vivo | [24,35] | |
| Plant- and food-derived enzymes | N. ventrata | Celiacase (neprosin) | Prolyl endopeptidase | In vitro and in vivo | [36,37] |
| Pineapple (A. comosus) | Bromelain | Cysteine protease | In vitro | [38] | |
| Fig latex (F. carica) | Ficin | Cysteine protease | In vitro | [38] | |
| Hayward Kiwi (A. deliciosa cv. Hayward) | Actinidin | Cysteine protease | In vitro and in vivo | [39,40,41] | |
| Papaya latex (C. papaya) | Papain/Caricain (crude or purified) | Cysteine protease | In vitro and in vivo | [24,35,42] | |
| T. aestivum | Triticain-α | Cysteine protease | In vitro | [43] | |
| Hordeym vulgare (Barley) | EP-B2 | Cysteine endoprotease | In vitro and in vivo | [32] | |
| Combined (Bacteria + Plants) | S. Capsulate + H. vulgare (Barley) | Latiglutenase (ALV003 or IMX003) | Glutamine-specific cysteine peptidase + modified serine prolyl-specific oligopeptidase | Phase 1 (NCT00626184, NCT00669825), Phase 2 (NCT00959114, NCT01255696, NCT01917630, NCT03585478)/Immunogenics, LLC | [44] |
2.2. Bacteria-Derived Enzymes
2.3. Plant- and Food-Derived Enzymes
2.4. Combined Bacterial- and Plant-Derived Enzymes
2.5. Limitations and Strengths of Current Gluten-Degrading Enzymes
- Strengths:
- Precise target: Gluten-degrading enzymes directly target the root problem in CeD, i.e., the GIPs that are resistant to digestion and then trigger immune responses. Their mechanism is rational and well defined, making them appealing therapeutic candidates.
- High safety and tolerability: Due to enzyme specificity, these are usually easily tolerated and do not interfere with other biological pathways.
- Oral delivery: Most researched enzymes are proposed to be delivered orally in a pill format before meals, which is easy and practical for patients.
- Role as a GFD-adjuvant: Some of the enzymes show potential in supporting digestion of inadvertent gluten exposure, which is a practical issue for patients strictly adhering to a GFD.
- Limitations:
- Physiological barriers: Maintaining enzyme activity through the acidic, protease-rich environment of the stomach and achieving effective gluten degradation in real-meal contexts remains challenging, especially in mixed meals with complex matrices. From all the reviewed gluten-degrading enzymes, only a few have managed to overcome this frequent hindrance.
- High gliadin/enzyme ratio: Most of the enzymes developed required a high amount of the active proteins in order to properly degrade gluten in an effective manner.
- Site-specific enzyme activation: Effective gluten-degrading enzymes must be activated at the stomach’s acidic pH, where complete gliadin degradation should occur, and revert to an inactive state as pH rises along the gastrointestinal tract (GIT), as other ways GIPs may persist and trigger downstream immune responses.
- Inconsistent clinical efficacy: Despite promising in vitro and preclinical results, many enzymes have failed to show consistent benefit in human trials. Hence, since no enzyme therapy to date can serve as a standalone alternative to a GFD, even the most promising candidates are currently seen as adjunctive, not curative.
3. Gluten Direct Neutralization
Limitations and Strengths of Current Gluten Neutralization Treatments
- Strengths:
- Early incidence: Since these strategies aim to block gluten passing through the epithelial layer, they act on the very first step of CeD pathophysiology, preventing the derived immune reaction before its initiation.
- Safety and tolerability: The anti-gliadin IgY antibodies from egg yolk are not absorbed systemically, significantly reducing the risk of immune reactions. This makes them particularly safe as oral therapies.
- Ease of production: Anti-gliadin IgY antibodies can be produced in large quantities at relatively low cost, offering a practical advantage over more complex biologics.
- Oral delivery: Both proposed treatments would be delivered orally, which generally show better acceptance scores by patients than other methods (e.g., vaccines).
- Limitations:
- Uncertain site of action: It remains unclear where gluten neutralization primarily occurs, whether in the stomach or further along the GIT, which would allow for GIPs to be generated and absorbed into the lamina propria, thus triggering an immune reaction. Additionally, the stability and resistance of these agents to gastric pH remain insufficiently characterized.
- Limited clinical data: While preclinical studies show promise, robust efficacy data in humans are still lacking. The results of critical phase 2 trials are not yet published, creating uncertainty about real-world effectiveness.
4. Intestinal Permeability Modulators
Limitations and Strengths of Current Intestinal Permeability Modulators
- Strengths:
- Disease target: The focus on intestinal permeability directly addresses a core feature of celiac pathophysiology, i.e., the disruption of TJs, which will then prevent access of GIPs into the lamina propria. Moreover, since these treatments typically act locally within the gut, systemic immune suppression and the risk of broader side effects common with immunomodulators are minimized.
- Safety and tolerability: Both existent strategies have demonstrated good tolerability in phase 1 and 2 trials, with mild or manageable adverse events, making them promising from a safety standpoint.
- Oral delivery: Both proposed treatments would be delivered orally, which generally show better acceptance scores by patients than other methods (e.g., vaccines).
- Symptom relief in some trials: Larazotide acetate has shown symptomatic benefit in several trials, even when histological or biomarker outcomes are inconsistent. This suggests potential value as an adjunct therapy for patients with persistent symptoms despite adherence to a GFD.
- Limitations:
- Indirect activity: IP modulators do not alter gliadin itself; therefore, gliadin levels and GIP production remain unchanged. Their action is limited to preventing peptide translocation into the lamina propria, meaning that even minimal peptide crossing may still be sufficient to initiate CeD pathophysiology.
- Limited clinical effectiveness and inconsistent outcomes: No current IP modulator has demonstrated the ability to fully prevent villous atrophy or histological damage in response to gluten exposure, which is a key therapeutic goal in celiac disease. Moreover, while some studies reported reduced antibody levels or symptoms, others failed to demonstrate significant improvements in intestinal permeability or histological recovery. Results are often dose-dependent and inconsistent across studies.
- Uncertain causality in pathogenesis: It remains unclear whether increased IP is a cause or a consequence of CeD. This ambiguity complicates the rationale for prioritizing it as a standalone therapeutic target.
5. Immune Modulators
5.1. TG2 Inhibitors
5.2. Anti-IL-15 Treatments
| Strategy | Active Principle | Name | Phase, Clinical Trial | Sponsor | Ref. |
|---|---|---|---|---|---|
| TG2 inhibitors | SIRT6 modulator | IMU-856 | Phase 1 (ACTRN12620000901909 or CT-2020-CTN-01997-1) | Immunic Australia Pty Ltd. | [66] |
| TG2 inhibitor | ZED1227 | Phase 1 (2014-003044-13) Phase 2 (2015-005283-42 and 2017-00224-30) | Dr. Falk Pharma GmbH | [68,69] | |
| TG2 inhibitor | GSK3915393 | Phase 1–Discontinued (NCT04604795) | GlaxoSmithKline | [70] | |
| IL-15 inhibitors | IL-15 inhibitor (moAb) | AMG 714 (PRV-015) | Phase 2 (NCT02633020, NCT02637141 and NCT04424927) | Amgen and Provention Bio (Sanofi) | [74,75,76,91] |
| IL-15 inhibitor (moAb) | CALY-002 | Phase 1 (NCT04593251) | Calypso Biotech BV | [77,80] | |
| IL-15 inhibitor (moAb) | TEV-53408 | Phase 1 (NCT06807463) | Teva Branded Pharmaceutical Products R&D LLC | [82,83] | |
| IL-15 and IL-21 inhibitor (gamma chain receptor antagonist) | EQ102 | Phase 1 and 2 (ACTRN12622001449729) | Equillium | [84,85,86,87,88] | |
| IL-15 inhibitor (moAb) | aIL-15 | Ex vivo | ISA-CNR, ELFID * | [90] | |
| Lymphocyte migration inhibitors | CCR9 antagonist | GSK1605786A/vercirnon (formerly CCX282-B) | Phase 2 (NCT00540657) | Amgen (formerly ChemoCentryx) | [92,93] |
| α4β7 integrin inhibitor | PTG-100 | Phase 1 (NCT04524221) | Nielsen Fernandez-Becker | [94,95] | |
| HLA-DQ2 inhibitor | HLA-DQ2 inhibitor | Azidoproline | In vitro | [96,97] | |
| JAK inhibition | Pan-JAK inhibitor | Tofacitinib (Xeljanz) | Phase 2 (EudraCT 2018-001678-10) | VU Medical Center | [98] |
| JAK3 and TEC kinase family inhibitor | Ritlecitinib (LITFULO) | Phase 2 (NCT05636293) | Massachusetts General Hospital/Pfizer | [99,100,101] | |
| CFTR potentiatior | CFTR potentiator | Ivacaftor (Kalydeco) | Case studies | [102,103,104] |
5.3. Lymphocyte Migration Inhibitors
5.4. HLA-DQ2 Inhibitors
5.5. Janus Kinase (JAK) Inhibition
5.6. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Promoter
5.7. Limitations and Strengths of Current Immune Modulators
- Strengths:
- Disease target: Immune modulators aim to intervene directly in the autoimmune cascade triggered by gluten exposure, including T-cell activation, cytokine release, and tissue damage. By targeting elements like IL-15, TG2, JAK-STAT signaling, or HLA-DQ2 binding, these therapies address the core immunopathology of CeD rather than just symptoms.
- Promising early results in specific areas: ZED1227 (TG2 inhibitor) has shown histological improvement in phase 2 trials, marking one of the most promising outcomes in disease-modifying therapy. Tofacitinib and Ritlecitinib (JAK inhibitors) showed a clinical improvement in RCeD-II, suggesting utility in hard-to-treat CeD. AMG 714 provided symptom relief in some cohorts, particularly for patients with non-responsive CeD.
- Mode of delivery: Most treatments are designed to be delivered orally.
- Limitations:
- Safety and tolerability: Tofacitinib was associated with increased visceral adiposity and TEAEs in all patients studied. Long-term safety data for JAK inhibitors and systemic moAbs are still limited, particularly regarding immune suppression and infection risk.
- Mode of delivery: While some treatments are designed to be delivered orally, others, mainly the IL-15 inhibitors, should be administered through an injection, which may decrease patient acceptability.
- Site of action: Most immune modulators act downstream, after gliadin peptides have crossed the epithelial barrier and entered the lamina propria, where they can already trigger the autoimmune cascade. This timing allows for disease activation before therapeutic intervention can occur.
- Symptom relief without mucosal healing: Several immune modulators improved subjective symptoms (e.g., diarrhea, abdominal pain) but failed to correlate with objective markers like IEL counts or villous architecture.
6. Gluten Tolerization
Limitations and Strengths of Current Gluten Tolerization Strategies
- Strengths:
- Disease target: These therapies aim to reprogram the immune system to tolerate gluten by directly targeting antigen-specific T-cell responses, rather than broadly suppressing immunity. This makes them highly specific and theoretically curative, addressing the disease’s underlying cause.
- Promising early results in specific areas: Agents like TAK-101 and KAN-101 have shown clear immunologic effects in clinical trials, including reductions in gliadin-specific IFN-γ-producing T cells, decreased IL-2 levels following gluten challenge and prevention of or reduction in villous atrophy.
- Safety and tolerability: Most candidates, including TAK-101, KAN-101, TPM502, and Nexvax2, demonstrated acceptable safety profiles, with only mild to moderate TEAEs and no serious safety concerns reported.
- Limitations:
- Mode of delivery: Most agents are currently delivered intravenously (e.g., TAK-101, KAN-101, TPM502), which may limit patient acceptability and clinical practicality for long-term treatment or maintenance use.
- Limited clinical effectiveness and inconsistent outcomes: Nexvax2, despite strong immunological rationale and early safety, failed to reduce symptoms during gluten challenge in a phase 2 trial, so it was discontinued. Results of multiple phase 2 trials have not been made available.
| Strategy | Active Principle | Name | Phase, Clinical Trial | Sponsor | Ref. |
|---|---|---|---|---|---|
| Gluten tolerization | Vaccine containing 5 immunodominant gluten epitopes | Nexvax2 | Phase 1 (NCT00879749, NCT02528799, NCT03543540) Phase 2–terminated (NCT03644069) | Nexpep Pty Ltd. and ImmusanT, Inc. | [114,115,116,117] |
| Gliadin-encapsulated nanoparticles | TAK-101 or TIMP-GLIA | Phase 1 (NCT03486990), Phase 2 (NCT03738475 and NCT04530123) | Takeda | [119,120,126] | |
| Liver-targeting glycosylation signature conjugated to an α-gliadin deamidated peptide | KAN-101 | Phase 1 (NCT04248855, NCT05574010), Phase 2 (NCT05574010 and NCT06001177) | Kanyos Bio, Inc. (Anokion SA) | [121,122,123] | |
| Nanoparticles coupled with CeD-derived relevant antigens | TPM502 | Phase 2 (NCT05660109) | Topas Therapeutics GmbH | [124,125] |
7. Probiotics, Prebiotics, Synbiotics and Postbiotics
7.1. Bifidobacterium
7.2. Lactobacillus
7.3. Others
| Active Principle | Name | Phase, Clinical Trial | Sponsor | Ref. |
|---|---|---|---|---|
| Bifidobacteria | B. infantis NLS-SS | Interventional (NCT01257620) Phase 2 (NCT03271138) | Bai, Julio M.D. and Global Institute of Probiotics | [137,138] |
| B. breve BR03 and B632 | Phase 1 and 2 (NCT02244047) | University Medical Centre Maribor | [139,140,141] | |
| B. longum CECT 7347 (ES1) | Interventional (NCT05339243 and NCT05367427) Phase 2–unknow status (NCT02810301), Maggiore Polyclinic Hospital clinical trial 1370 | Vedic Lifesciences Pvt. Ltd., Instituto de Investigación Hospital Universitario La Paz, Maggiore Polyclinic Hospital clinical, Exzell Pharma Inc. | [142,143,144,145,146,147,148,166] | |
| B. longum NCC2705 | Interventional (NCT03775499) | Société des Produits Nestlé (SPN) | [149,150] | |
| B. lactis | In vitro and in vivo | [152,161] | ||
| Lactobacillus | L. plantarum HEAL9 and L. paracasei 8700:2 | Interventional (NCT03176095 and NCT04014660) | Lund University | [153,154,155] |
| L. brevis KT16-2 | In vitro | [158] | ||
| L. casei ATCC 9595 | In vitro and in vivo | [159,160,161] | ||
| L. plantarum ITM21B, L. paracasei IMPC2.1, L. fermentum, | In vitro and in vivo | [161] | ||
| Bacillus | Bacillus sp. GS 33, 143, 181 and 188 | In vitro | [162,163] | |
| B. vulgatus 20220303-A2 | Ex vivo | [165] | ||
| Multispecies probiotics | VSL#3 | Phase 2 (ACTRN12610000630011) | Metametrix Clinical Laboratory, Diagnostic Insight and Sigma Pharmaceuticals Pty | [167,168] |
| Multispecies probiotic | Interventional (NCT01699191) | University of Bari | [169] | |
| Pentabiocel | Interventional (NCT03857360) | Università Politecnica delle Marche | [170] | |
| P1: B. breve B632 and BR03, P2: L. plantarum LP14, L. casei subsp. paracasei LPC09, L. rhamnosus LR04 | In vitro and in vivo | Pobiotical SpA | [171] | |
| Bacteria & yeast | E. mundtii QAUSD01 and W. anomalus QAUWA03 | In vitro | [164] | |
| Yeast | S. boulardii KK1 | In vivo | [172] | |
| Helminth | N. americanus | Phase 1 (NCT02754609), Phase 1 and 2 (NCT01661933) Phase 2 (NCT00671138 | Princess Alexandra Hospital, The Prince Charles Hospital and James Cook University | [173,174,175] |
| Prebiotics and Postbiotics | Synergy 1 (Oligofructose-enriched inulin) | Interventional (NCT03064997) | Polish Academy of Sciences | [176,177,178,179] |
| Heat-treated B. longum CECT 7347 (HI-ES1) | Interventional (NCT05339243 and NCT05367427) Phase 2–unknow status (NCT02810301), Maggiore Polyclinic Hospital clinical trial 1370 | Vedic Lifesciences Pvt. Ltd., Instituto de Investigación Hospital Universitario La Paz, Maggiore Polyclinic Hospital clinical, Exzell Pharma Inc. | [142,143,144,145,146,147,148,166] |
7.4. Multispecies Probiotics
7.5. Yeast
7.6. Helminth
7.7. Prebiotics, Synbiotics and Postbiotics
7.8. Fecal Microbiota Transplantation (FMT)
7.9. Limitations and Strengths of Current Probiotic, Prebiotic, Synbiotic and Postbiotic Strategies
- Strengths:
- Disease target: CeD is increasingly recognized as a condition involving gut dysbiosis, even in patients adhering to a GFD. The microbial imbalance is associated with inflammation, increased IP and persistent symptoms, making microbiota-targeted therapies highly relevant.
- Uncertain mechanisms of action: A key limitation of probiotic use in CeD is the lack of clear evidence regarding the exact mechanism of action. While some studies suggest potential benefits, the specific pathways through which probiotics exert these effects in the context of gluten-induced autoimmunity remain largely speculative. This uncertainty hampers the development of targeted formulations and limits confidence in their therapeutic efficacy.
- Broad immunomodulatory potential: Certain strains (e.g., B. breve, B. longum, L. casei) have shown abilities to decrease pro-inflammatory cytokines, improve intestinal barrier function, modulate immune cell populations or increase beneficial metabolites like SCFA.
- Symptom relief in some studies: Several probiotic combinations (e.g., B. infantis NLS-SS, ES1 or some multispecies formulations) showed improvements in GI symptoms, including bloating, stool consistency, and GSRS scores, especially in patients on a GFD with persistent symptomatology.
- Safety and tolerability: Across most trials, no serious TEAEs were reported. Even novel combinations or postbiotics (e.g., heat-treated ES1) were well tolerated, including in children.
- Complementary to existing therapies: Generally, these strategies do not aim to replace a GFD but rather enhance its effectiveness by improving symptom control, supporting mucosal healing, and addressing non-responsive or partially responsive CeD.
- Mode of delivery: Most treatments are designed to be delivered orally.
- Limitations:
- Limited clinical efficacy and inconsistent outcomes: Many interventions showed no significant changes in serological markers, histological damage or IP. Some promising in vitro or in vivo studies failed to translate into measurable clinical benefits in humans (e.g., VSL#3, Pentabiocel, S. boulardii).
- Uncertain causality in pathogenesis: Similar to intestinal permeability modulators, it remains unclear whether increased gut dysbiosis is a cause or a consequence of CeD. Hence, the extent of the effect of improvement in the gut microbiota profile in CeD symptomatology and disease biomarkers is unclear.
- Few rigorous or large-scale trials: Most trials were small, exploratory or short in duration, with few phase 2 studies. Critical endpoints like villous atrophy, IEL infiltration and anti-TG2 levels are rarely assessed.
8. Nutraceuticals
8.1. Polyphenols
8.2. Combination of Polyphenols and Other Bioactives
| Strategy | Active Principle | Source | Mechanism of Action | Phase * | Ref. |
|---|---|---|---|---|---|
| Polyphenols | Polyphenols | Green tea extract | Gliadin sequestration | In vitro | [194] |
| (-)-epigallocatechin and (-)-epigallocatechin-3-gallate | Green tea | Gliadin sequestration | In vitro | [195] | |
| Catechin, Procyanidin B3, Procyanidin C2, Epigallocatechin and Epigallocatechin Gallate | Gliadin sequestration | In vitro | [196] | ||
| Polyphenols | Artichoke leaves, cranberries, apples, green tea leaves | Gliadin sequestration | In vitro | [197] | |
| Kuromanin, Callistephin, Oenin, Cyanin, Pelargonin, Malvin (Anthocyanins) | Gliadin sequestration | In vitro | [198] | ||
| Procyanidin B3, B6 and T2 (Tannins) | Grape seed | Gliadin sequestration | In vitro | [202] | |
| Quercetin (Flavonoid) | Gliadin sequestration | In vitro | [200] | ||
| Cyanidin (Anthocyanidin) | Coumarin | Gliadin sequestration | In vitro | [199] | |
| Proanthocyanidins | Peanut skin | Gliadin sequestration | In vitro | [203] | |
| Anthocyanins | Sour cherry extract | Immune and intestinal permeability modulation | In vitro | [204] | |
| Flavonoids | Propolis dry extract ESIT 12® | Immune and gut microbiota modulation | Ex vivo | [205,206] | |
| Curcumin (Diarylheptanoid) | Turmeric (Curcuma longa) | Immune modulation | In vitro | [207] | |
| Resveratrol (Stilbene) | Grapes, red wine | Immune, intestinal permeability and gut microbial modulation | In vitro and in vivo | [208] | |
| Combination of polyphenols & other bioactives | Lycopene (carotenoid), quercetin (flavonoid) and tyrosol (phenolic alcohol) | Tomatoes/Onions, extra virgin olive oil (EVOO), broccoli/white wine, EVOO | Immune modulation | In vitro | [209] |
| Procyanidin B2, theobromine, caffeine (alkaloids) | Cocoa extract | Immune modulation | In vitro | [210] | |
| Epicatechin (flavanol), theobromine (alkaloid) | Chocolate | Immune modulation | Pilot study, ECCEL2 nº 43.18: 4, 2018 | [211] |
8.3. Vitamins
8.4. Fatty Acids
8.5. Terpenes
8.6. Glucosinolates/Isothiocyanates
8.7. Algae
8.8. Limitations and Strengths of Current Nutraceutical Strategies
- Strengths:
- Wide range of bioactive effects: Nutraceuticals offer multiple mechanisms of action relevant to CeD pathogenesis, including gliadin degradation or sequestration, inhibition of TG2 enzyme activity, modulation of IP, anti-inflammatory and antioxidant effects and gut microbiota modulation.
- Safety and tolerability: Since nutraceuticals are naturally occurring, they are generally recognized as safe and are well tolerated, even at higher doses.
- Complementary to existing therapies: Generally, these strategies do not aim to replace a GFD but rather enhance its effectiveness by improving symptom control, supporting mucosal healing and decreasing gluten-derived inflammation.
- Mode of delivery: Most treatments are designed to be delivered orally.
- Limitations:
- Predominance of in vitro and preclinical evidence: Most data originated from in vitro and animal models, with only a few human trials.
- Low bioavailability of certain compounds: Some nutraceuticals suffer from poor stability or absorption, limiting their in vivo efficacy unless specially formulated. This remains a challenge for translation into real-world therapies.
- Confounding from GFD adherence: Several studies are conducted in patients already on a GFD, making it difficult to isolate the effect of nutraceutical intervention from the baseline benefits of dietary gluten exclusion.
- Heterogeneity of compounds and protocols: The category “nutraceuticals” includes vastly diverse compounds, delivery forms, and dosages, making standardization, reproducibility, and comparison between studies difficult.
| Strategy | Active Principle | Source | Mechanism of Action | Phase * | Ref. |
|---|---|---|---|---|---|
| Vitamins | Vitamin D, 1,25-dihydroxy vitamin D3 | Immune and intestinal permeability modulation | In vitro, in vivo, clinical trial, CIEC 53,043,469/050.04–52 | [226,227] | |
| Fatty acids | DHA | Immune and intestinal permeability modulation | In vitro | [230] | |
| DHA and EPA | Fish Oil | Clinical trial, CEP-FAG 2,315,783 | [231] | ||
| Terpenes | Thymoquinone and monoterpenes | Black cumin (N. sativa) oil | Immune modulation | Clinical trial, ECCM-UB | [232] |
| Glucosinolates/ isothiocyanates | Glucoraphanin/Sulforaphane | Broccoli sprouts (B. oleracea var. italica Planck) | Immune and intestinal permeability modulation | In vitro | [233] |
| Algae | C. pyrenoidosa | Chlorella sp. (algae) | Gut microbial modulation | Ex vivo | [234] |
9. Food Modifications
9.1. Bacterial and Enzymatic Degradation of Gluten-Contaning Foodstuffs
9.2. Gluten Genetic Modifications
9.3. Gluten Transamidation
9.4. The Gluten Friendly™ Technology
9.5. Limitations and Strengths of Current Gluten-Containing Food Modifications
- Strengths:
- Source targeting: These strategies aim to prevent the immune response from initiating by modifying gluten before ingestion, with clear health and economic benefits.
- Potential to improve quality of life: By detoxifying gluten in common foods like bread and pasta, these methods could enhance dietary flexibility, reduce cross-contamination risks, and improve patient adherence and satisfaction.
- Cost-effective and scalable: Compared to pharmaceutical development, food-processing interventions are relatively inexpensive, can be integrated into existing manufacturing systems, and scale efficiently, making them a viable solution in broader populations and low-resource settings.
- Safety and tolerability: Most studies report that modified gluten products are well tolerated in CeD patients, with no major adverse effects or symptom relapses when consumed under controlled conditions. This supports their potential for safe dietary integration, although more safety studies should be performed.
- Mode of delivery: By incorporating modified gluten directly into commonly consumed foods (like bread, pasta, or flour), these strategies offer a non-invasive, patient-friendly alternative to pills or injections. This enhances patient acceptability and compliance, especially for long-term management.
- Limitations:
- Predominance of preclinical evidence: Most data originate from in vitro and animal models, with only a few human trials. Many promising findings lack validation in clinical settings, especially with well-defined CeD endpoints like villous atrophy or IEL counts.
- Incomplete or inconsistent detoxification: Not all methods fully remove or neutralize gluten peptides, which may still trigger an immune response in sensitive individuals. Moreover, gluten modification methods may differ in effectiveness depending on the food matrix, processing conditions, and enzyme source, making it challenging to standardize protocols for broad use.
- Mode of delivery: Although modified gluten foods offer a convenient route of delivery, they still require CeD patients to consume specialized or alternative food products. This contrasts with pharmaceutical approaches (like enzymes or immune modulators), which may one day allow patients to eat unmodified gluten-containing foods, potentially offering greater freedom and social normalization.
- Regulatory and consumer acceptance barriers: Strategies involving genetic modification (e.g., CRISPR/Cas9, RNA interference) face regulatory hurdles in many regions (notably the EU), as well as potential consumer resistance.
- Limited clinical validation: Most strategies have not progressed beyond small trials or preclinical models, leaving efficacy in real-world patients uncertain.
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3-EcC | 3-ethoxycarbonylcoumarin |
| AA | Alopecia areata |
| AAc | Arachidonic acid |
| Abs | Antibodies |
| AFU | Active fluorescent units |
| AGY | Immunoglobulin Y antibody from the egg yolk |
| ALT | Alanine aminotransferase |
| AOPP | Advanced oxidation protein product |
| APCs | Antigen presenting cells |
| ASP | Aspergillus niger |
| BF | Bromelain and ficin |
| CCL | CC motif chemokine ligand |
| CCR | CC motif chemokine receptor |
| CeD | Celiac disease |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CFU | Colony-forming units |
| COX-2 | Cyclooxygenase-2 |
| cPLA2 | Cytosolic phospholipase A2 |
| CXCL | CXC motif chemokine ligand |
| DGP | Deamidated gluten peptides |
| DH | Dermatitis herpetiformis |
| DHA | Docosahexanoic acid |
| DPPIV | Dipeptidyl peptidase IV |
| ECGC | (-)-Epigallocatechin-3-gallate |
| EEP | Ethanolic extract of propolis |
| EGC | (-)-Epigallocatechin |
| ELISA | Enzyme-linked immunosorbent assay |
| EoE | Eosinophilic esophagitis |
| FA | Fatty acid |
| FD-4 | FITC-dextran 4000 |
| FM PEP | Flavobacterium meningosepticum |
| GCD | Gluten-containing diet |
| GFD | Gluten-free diet |
| GIP | Gluten immunogenic peptide |
| GIT | Gastrointestinal tract |
| GPx3 | Glutathione peroxidase-3 |
| GSH | Glutathione |
| GSRS | Gastrointestinal symptoms rating scale |
| HDL | High-density lipoprotein |
| HLA | Human leukocyte antigen |
| HPLC | High-performance liquid chromatography |
| IBS | Inflammatory bowel disease |
| IBS-D | Diarrhea-predominant inflammatory bowel disease |
| IEC | Intestinal epithelial cell |
| IEL | Intestinal epithelial lymphocyte |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IP | Intestinal permeability |
| IRF | Interferon regulatory factor |
| JAK | Janus kinase |
| LAMA | Lactulose to mannitol |
| LMR | Lymphocyte-to-monocyte ratio |
| MasCAM | Mucosal addressin cell adhesion molecule |
| MCP | Monocyte chemoattractant protein |
| MDA | Malondialdehyde |
| MHC | Major histocompatibility complex |
| MoAb | Monoclonal antibody |
| MPO | Myeloperoxidase |
| MX PEP | Myxococcus xanthus |
| n-3 | Omega-3 |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NCGS | Non-celiac gluten sensitivity |
| NF-κB | Nuclear factor kappa-light-chain enhancer of activated B cells |
| NK | Natural killer |
| NKT | Natural killer T cells |
| NO | Nitric oxide |
| NOD/DQ8 | HLA-DQ8 non-obese diabetic |
| NOX | NADPH oxidase |
| P(HEMA-co-SS) | Poly(hydroxyethyl methacrylate-co-styrene sulfonate) |
| PBMC | Peripheral blood mononuclear cells |
| PCE | Procyanidin B2-enriched cocoa extract |
| PGE2 | Prostaglandin E2 |
| PLR | Platelet-to-lymphocyte ratio |
| PSPc | Proanthocyanidins |
| pSTAT | Phosphorylated STAT |
| PT-G | Pepsin-trypsin-digested gliadin |
| PUFA | Polyunsaturated fatty acid |
| RCeD-II | Type II refractory CeD |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acids |
| SIRT6 | Sirtuin 6 |
| SOP | Superoxide dismutase |
| Srp | Serine protease inhibitor |
| STAT | Signal transducer and activator of transcription |
| TEAE | Treatment-emergent adverse event |
| TEC | Tyrosine kinase expressed in hepatocellular carcinoma |
| TEER | Transepithelial electrical resistance |
| tg | Transgenic |
| Th | T-helper |
| TLR | Toll-like receptor |
| tTG2 | Tissue transglutaminase 2 |
| VD3 | Vitamin D3 |
| Vh/Cd | Villous height/crypt depth ratio |
References
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo Definitions for Coeliac Disease and Related Terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-Related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Biesiekierski, J.R. What Is Gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef]
- Schalk, K.; Lang, C.; Wieser, H.; Koehler, P.; Scherf, K.A. Quantitation of the Immunodominant 33-Mer Peptide from α-Gliadin in Wheat Flours by Liquid Chromatography Tandem Mass Spectrometry. Sci. Rep. 2017, 7, srep45092. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Verdu, E.F.; Bai, J.C.; Lionetti, E. Coeliac Disease. Lancet 2022, 399, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Makharia, G.K.; Singh, P.; Catassi, C.; Sanders, D.S.; Leffler, D.; Ali, R.A.R.; Bai, J.C. The Global Burden of Coeliac Disease: Opportunities and Challenges. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 313–327. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; Hujoel, I.A.; West, C.P.; Taneja, V.; Prokop, L.J.; Rubio-Tapia, A.; Murray, J.A. Sex Difference in Celiac Disease in Undiagnosed Populations: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1954–1968.e13. [Google Scholar] [CrossRef]
- Heijdra Suasnabar, J.; Meijer, C.R.; Smit, L.; van Overveld, F.; Thom, H.; Keeney, E.; Mearin, M.L.; van den Akker-van Marle, M.E. Long-Term Cost-Effectiveness of Case Finding and Mass Screening for Celiac Disease in Children. Gastroenterology 2024, 167, 1129–1140. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac Disease: Understanding the Gluten-Free Diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Wei, G.; Helmerhorst, E.J.; Darwish, G.; Blumenkranz, G.; Schuppan, D. Gluten Degrading Enzymes for Treatment of Celiac Disease. Nutrients 2020, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Mitea, C.; Havenaar, R.; Wouter Drijfhout, J.; Edens, L.; Dekking, L.; Koning, F.; Dekking, E.H.A. Efficient Degradation of Gluten by a Prolyl Endoprotease in a Gastrointestinal Model: Implications for Coeliac Disease. Gut 2008, 57, 25–32. [Google Scholar] [CrossRef]
- Montserrat, V.; Bruins, M.J.; Edens, L.; Koning, F. Influence of Dietary Components on Aspergillus Niger Prolyl Endoprotease Mediated Gluten Degradation. Food Chem. 2015, 174, 440–445. [Google Scholar] [CrossRef]
- König, J.; Holster, S.; Bruins, M.J.; Brummer, R.J. Randomized Clinical Trial: Effective Gluten Degradation by Aspergillus Niger-Derived Enzyme in a Complex Meal Setting. Sci. Rep. 2017, 7, 13100. [Google Scholar] [CrossRef]
- Salden, B.N.; Monserrat, V.; Troost, F.J.; Bruins, M.J.; Edens, L.; Bartholomé, R.; Haenen, G.R.; Winkens, B.; Koning, F.; Masclee, A.A. Randomised Clinical Study: Aspergillus Niger-Derived Enzyme Digests Gluten in the Stomach of Healthy Volunteers. Aliment. Pharmacol. Ther. 2015, 42, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Tack, G.J.; van de Water, J.M.W.; Bruins, M.J.; Kooy-Winkelaar, E.M.C.; van Bergen, J.; Bonnet, P.; Vreugdenhil, A.C.E.; Korponay-Szabo, I.; Edens, L.; von Blomberg, B.M.E.; et al. Consumption of Gluten with Gluten-Degrading Enzyme by Celiac Patients: A Pilot-Study. World J. Gastroenterol. 2013, 19, 5837–5847. [Google Scholar] [CrossRef]
- Stefanolo, J.P.; Segura, V.; Grizzuti, M.; Heredia, A.; Comino, I.; Costa, A.F.; Puebla, R.; Temprano, M.P.; Niveloni, S.I.; de Diego, G.; et al. Effect of Aspergillus Niger Prolyl Endopeptidase in Patients with Celiac Disease on a Long-Term Gluten-Free Diet. World J. Gastroenterol. 2024, 30, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Mohan Kumar, B.V.; Vijaykrishnaraj, M.; Kurrey, N.K.; Shinde, V.S.; Prabhasankar, P. Prolyl Endopeptidase-Degraded Low Immunoreactive Wheat Flour Attenuates Immune Responses in Caco-2 Intestinal Cells and Gluten-Sensitized BALB/c Mice. Food Chem. Toxicol. 2019, 129, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Wieser, H.; Koehler, P. Production of Gluten-Free Wheat Starch by Peptidase Treatment. J. Cereal. Sci. 2014, 60, 202–209. [Google Scholar] [CrossRef]
- Knorr, V.; Wieser, H.; Koehler, P. Production of Gluten-Free Beer by Peptidase Treatment. Eur. Food Res. Technol. 2016, 242, 1129–1140. [Google Scholar] [CrossRef]
- Camarca, A.; D’Auria, G.; Rotondi Aufiero, V.; Nitride, C.; Giardullo, N.; Sapone, A.; Leffler, D.; Ferranti, P.; Mazzarella, G. Digestion Supplemented with Commercial Proteases: Evaluation of the Fate of Gluten Immunogenic Peptides in Pizza. Food Res. Int. 2025, 220, 117027. [Google Scholar] [CrossRef]
- Tschollar, W.; Mourabit, S. Pharmaceutical Compositions Comprising Aminopeptidase and Dipeptidylpeptidase Polypeptides and Uses Thereof 2023. Available online: https://patents.google.com/patent/WO2025067633A1/en?assignee=AMYRA&oq=AMYRA (accessed on 30 July 2025).
- Mourabit, S.; Römer, S.; Bonner, E.R.; Winter, F.; Tschollar, J.; Tzvetkov, M.V.; Weitschies, W.; Engeli, S.; Tschollar, W. Exopeptidase Combination Enhances the Degradation of Isotopically Labelled Gluten Immunogenic Peptides in Humans. Front. Immunol. 2024, 15, 1425982. [Google Scholar] [CrossRef]
- Ehren, J.; Móron, B.; Martin, E.; Bethune, M.T.; Gray, G.M.; Khosla, C. A Food-Grade Enzyme Preparation with Modest Gluten Detoxification Properties. PLoS ONE 2009, 4, e6313. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Goktepe, I.; Ahmedna, M. The Potential of Papain and Alcalase Enzymes and Process Optimizations to Reduce Allergenic Gliadins in Wheat Flour. Food Chem. 2016, 196, 1338–1345. [Google Scholar] [CrossRef]
- Zamakhchari, M.; Wei, G.; Dewhirst, F.; Lee, J.; Schuppan, D.; Oppenheim, F.G.; Helmerhorst, E.J. Identification of Rothia Bacteria as Gluten-Degrading Natural Colonizers of the Upper Gastro-Intestinal Tract. PLoS ONE 2011, 6, e24455. [Google Scholar] [CrossRef]
- Pultz, I.S.; Hill, M.; Vitanza, J.M.; Wolf, C.; Saaby, L.; Liu, T.; Winkle, P.; Leffler, D.A. Gluten Degradation, Pharmacokinetics, Safety, and Tolerability of TAK-062, an Engineered Enzyme to Treat Celiac Disease. Gastroenterology 2021, 161, 81–93.e3. [Google Scholar] [CrossRef]
- Wolf, C.; Siegel, J.B.; Tinberg, C.; Camarca, A.; Gianfrani, C.; Paski, S.; Guan, R.; Montelione, G.; Baker, D.; Pultz, I.S. Engineering of Kuma030: A Gliadin Peptidase That Rapidly Degrades Immunogenic Gliadin Peptides in Gastric Conditions. J. Am. Chem. Soc. 2015, 137, 13106–13113. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). A Study of TAK-062 in Treatment of Active Celiac Disease in Participants Attempting a Gluten-Free Diet. Available online: https://www.clinicaltrials.gov/study/NCT05353985 (accessed on 7 July 2025).
- Liu, Y.Y.; Lee, C.C.; Hsu, J.H.; Leu, W.M.; Meng, M. Efficient Hydrolysis of Gluten-Derived Celiac Disease-Triggering Immunogenic Peptides by a Bacterial Serine Protease from Burkholderia Gladioli. Biomolecules 2021, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, L.; Taravella, A.; Carrano, L.; Carenzi, G.; Sigurtà, A.; Solinas, N.; De Caro, S.; Di Stasio, L.; Picascia, S.; Laezza, M.; et al. E40, a Novel Microbial Protease Efficiently Detoxifying Gluten Proteins, for the Dietary Management of Gluten Intolerance. Sci. Rep. 2019, 9, 13147. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.; Ehren, J.; Strohmeier, G.; Isaacs, I.; Khosla, C. Fermentation, Purification, Formulation, and Pharmacological Evaluation of a Prolyl Endopeptidase from Myxococcus Xanthus: Implications for Celiac Sprue Therapy. Biotechnol. Bioeng. 2005, 92, 674–684. [Google Scholar] [CrossRef]
- Gass, J.; Vora, H.; Bethune, M.T.; Gray, G.M.; Khosla, C. Effect of Barley Endoprotease EP-B2 on Gluten Digestion in the Intact Rat. J. Pharmacol. Exp. Ther. 2006, 318, 1178–1186. [Google Scholar] [CrossRef]
- Bordusa, F.; Jakubke, H.-D. The Specificity of Prolyl Endopeptidase from Flavobacterium Meningoseptum: Mapping the S H Subsites by Positional Scanning via Acyl Transfer. Bioorganic Med. Chem. 1998, 6, 1775–1780. [Google Scholar] [CrossRef]
- Siegel, M.; Bethune, M.T.; Gass, J.; Ehren, J.; Xia, J.; Johannsen, A.; Stuge, T.B.; Gray, G.M.; Lee, P.P.; Khosla, C. Rational Design of Combination Enzyme Therapy for Celiac Sprue. Chem. Biol. 2006, 13, 649–658. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Li, T.; Pan, H.; Liu, J.; Fan, M.; Qian, H.; Zhang, H.; Ying, H.; Wang, L. Phosphorylation and Enzymatic Hydrolysis with Alcalase and Papain Effectively Reduce Allergic Reactions to Gliadins in Normal Mice. J. Agric. Food Chem. 2019, 67, 6313–6323. [Google Scholar] [CrossRef]
- del Amo-Maestro, L.; Mendes, S.R.; Rodríguez-Banqueri, A.; Garzon-Flores, L.; Girbal, M.; Rodríguez-Lagunas, M.J.; Guevara, T.; Franch, À.; Pérez-Cano, F.J.; Eckhard, U.; et al. Molecular and in Vivo Studies of a Glutamate-Class Prolyl-Endopeptidase for Coeliac Disease Therapy. Nat. Commun. 2022, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Yang, M.; Lee, L.; Zhang, Y.; Sheff, J.G.; Sensen, C.W.; Mrazek, H.; Halada, P.; Man, P.; McCarville, J.L.; et al. Addressing Proteolytic Efficiency in Enzymatic Degradation Therapy for Celiac Disease. Sci. Rep. 2016, 6, 30980. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Maleki, M.S.; Aghamirza Moghim Ali Abadi, H.; Vaziri, B.; Shabani, A.A.; Ghavami, G.; Madanchi, H.; Sardari, S. Bromelain and Ficin Proteolytic Effects on Gliadin Cytotoxicity and Expression of Genes Involved in Cell-Tight Junctions in Caco-2 Cells. Amino Acids 2023, 55, 1601–1619. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Montoya, C.A.; Zou, M.L.; Moughan, P.J.; Drummond, L.N.; Boland, M.J. Effect of Actinidin from Kiwifruit (Actinidia Deliciosa Cv. Hayward) on the Digestion of Food Proteins Determined in the Growing Rat. Food Chem. 2011, 129, 1681–1689. [Google Scholar] [CrossRef]
- Jayawardana, I.A.; Boland, M.J.; Higgs, K.; Zou, M.; Loo, T.; Mcnabb, W.C.; Montoya, C.A. The Kiwifruit Enzyme Actinidin Enhances the Hydrolysis of Gluten Proteins during Simulated Gastrointestinal Digestion. Food Chem. 2021, 341, 128239. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, I.A.; Boland, M.J.; Loo, T.S.; McNabb, W.C.; Montoya, C.A. Actinidin Reduces Gluten-Derived Immunogenic Peptides Reaching the Small Intestine in an in Vitro Semi-Dynamic Gastrointestinal Tract Digestion Model. Food Res. Int. 2022, 159, 111560. [Google Scholar] [CrossRef]
- Buddrick, O.; Cornell, H.J.; Small, D.M. Reduction of Toxic Gliadin Content of Wholegrain Bread by the Enzyme Caricain. Food Chem. 2015, 170, 343–347. [Google Scholar] [CrossRef]
- Savvateeva, L.V.; Gorokhovets, N.V.; Makarov, V.A.; Serebryakova, M.V.; Solovyev, A.G.; Morozov, S.Y.; Reddy, V.P.; Zernii, E.Y.; Zamyatnin, A.A.; Aliev, G. Glutenase and Collagenase Activities of Wheat Cysteine Protease Triticain-α: Feasibility for Enzymatic Therapy Assays. Int. J. Biochem. Cell Biol. 2015, 62, 115–124. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Anderson, R.P.; Ffrench, R.A.; Brown, G.J.; Hodsman, P.; Siegel, M.; Botwick, W.; Shreeniwas, R. The Effects of ALV003 Pre-Digestion of Gluten on Immune Response and Symptoms in Celiac Disease in Vivo. Clin. Immunol. 2010, 134, 289–295. [Google Scholar] [CrossRef]
- Wei, G.; Darwish, G.; Oppenheim, F.G.; Schuppan, D.; Helmerhorst, E.J. Commensal Bacterium Rothia Aeria Degrades and Detoxifies Gluten via a Highly Effective Subtilisin Enzyme. Nutrients 2020, 12, 3724. [Google Scholar] [CrossRef]
- Gass, J.; Khosla, C. Prolyl Endopeptidases. Cell. Mol. Life Sci. 2007, 64, 345–355. [Google Scholar] [CrossRef]
- Gomis-Rüth, F.X.; Del Amo Maestro, L.; Dos Mendes Rei, S.I.; Eckhard, U.; Rodríguez Banqueri, A.; Guevara Puig, T.; Pérez Cano, F.; Rodríguez Lagunas, M.J.; Franch Masferrer, À.; Girbal González, M. Proteína Degradadora de Gluten y Usos de La Misma 2023. Available online: https://patents.google.com/patent/ES3008845A1/es (accessed on 8 July 2025).
- Pahlavan, A.; Sharma, G.M.; Pereira, M.; Williams, K.M. Effects of Grain Species and Cultivar, Thermal Processing, and Enzymatic Hydrolysis on Gluten Quantitation. Food Chem. 2016, 208, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Garber, M.E.; Spencer, A.G.; Botwick, W.; Kumar, P.; Williams, R.N.; Kozuka, K.; Shreeniwas, R.; Pratha, V.; Adelman, D.C. Safety, Tolerability, and Activity of ALV003: Results from Two Phase 1 Single, Escalating-Dose Clinical Trials. Dig. Dis. Sci. 2012, 57, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Lähdeaho, M.L.; Kaukinen, K.; Laurila, K.; Vuotikka, P.; Koivurova, O.P.; Kärjä-Lahdensuu, T.; Marcantonio, A.; Adelman, D.C.; Mäki, M. Glutenase ALV003 Attenuates Gluten-Induced Mucosal Injury in Patients with Celiac Disease. Gastroenterology 2014, 146, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Kelly, C.P.; Green, P.H.R.; Marcantonio, A.; Wu, T.T.; Mäki, M.; Adelman, D.C.; Ansari, S.; Ayub, K.; Basile, A.; et al. No Difference Between Latiglutenase and Placebo in Reducing Villous Atrophy or Improving Symptoms in Patients with Symptomatic Celiac Disease. Gastroenterology 2017, 152, 787–798.e2. [Google Scholar] [CrossRef]
- Murray, J.A.; Syage, J.A.; Wu, T.T.; Dickason, M.A.; Ramos, A.G.; Van Dyke, C.; Horwath, I.; Lavin, P.T.; Mäki, M.; Hujoel, I.; et al. Latiglutenase Protects the Mucosa and Attenuates Symptom Severity in Patients with Celiac Disease Exposed to a Gluten Challenge. Gastroenterology 2022, 163, 1510–1521.e6. [Google Scholar] [CrossRef]
- Gujral, N.; Löbenberg, R.; Suresh, M.; Sunwoo, H. In-Vitro and in-Vivo Binding Activity of Chicken Egg Yolk Immunoglobulin y (IgY) against Gliadin in Food Matrix. J. Agric. Food Chem. 2012, 60, 3166–3172. [Google Scholar] [CrossRef]
- Sample, D.A.; Sunwoo, H.H.; Huynh, H.Q.; Rylance, H.L.; Robert, C.L.; Xu, B.W.; Kang, S.H.; Gujral, N.; Dieleman, L.A. AGY, a Novel Egg Yolk-Derived Anti-Gliadin Antibody, Is Safe for Patients with Celiac Disease. Dig. Dis. Sci. 2017, 62, 1277–1285. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Randomized, Double-Blind, Placebo Controlled, Crossover Trial to Evaluate Safety and Efficacy of AGY in Celiac Disease (AGY-010). Available online: https://clinicaltrials.gov/study/NCT03707730?term=agy-010&rank=1 (accessed on 8 July 2025).
- Pinier, M.; Verdu, E.F.; Nasser–Eddine, M.; David, C.S.; Vézina, A.; Rivard, N.; Leroux, J. Polymeric Binders Suppress Gliadin-Induced Toxicity in the Intestinal Epithelium. Gastroenterology 2009, 136, 288–298. [Google Scholar] [CrossRef]
- McCarville, J.L.; Nisemblat, Y.; Galipeau, H.J.; Jury, J.; Tabakman, R.; Cohen, A.; Naftali, E.; Neiman, B.; Halbfinger, E.; Murray, J.A.; et al. BL-7010 Demonstrates Specific Binding to Gliadin and Reduces Gluten-Associated Pathology in a Chronic Mouse Model of Gliadin Sensitivity. PLoS ONE 2014, 9, e109972. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). A Two-Part, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Systemic Exposure of Single Escalating Administrations and Repeated Administration of BL-7010 in Well-Controlled Celiac Patients. Available online: https://clinicaltrials.gov/study/NCT01990885?intr=BL-7010&rank=1 (accessed on 18 June 2025).
- Leffler, D.A.; Kelly, C.P.; Green, P.H.R.; Fedorak, R.N.; Dimarino, A.; Perrow, W.; Rasmussen, H.; Wang, C.; Bercik, P.; Bachir, N.M.; et al. Larazotide Acetate for Persistent Symptoms of Celiac Disease despite a Gluten-Free Diet: A Randomized Controlled Trial. Gastroenterology 2015, 148, 1311–1319.e6. [Google Scholar] [CrossRef]
- Paterson, B.M.; Lammers, K.M.; Arrieta, M.C.; Fasano, A.; Meddings, J.B. The Safety, Tolerance, Pharmacokinetic and Pharmacodynamic Effects of Single Doses of AT-1001 in Coeliac Disease Subjects: A Proof of Concept Study. Aliment. Pharmacol. Ther. 2007, 26, 757–766. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kelly, C.P.; Abdallah, H.Z.; Colatrella, A.M.; Harris, L.A.; Leon, F.; Arterburn, L.A.; Paterson, B.M.; Lan, Z.H.; Murray, J.A. A Randomized, Double-Blind Study of Larazotide Acetate to Prevent the Activation of Celiac Disease during Gluten Challenge. Am. J. Gastroenterol. 2012, 107, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; Green, P.H.R.; Murray, J.A.; Dimarino, A.; Colatrella, A.; Leffler, D.A.; Alexander, T.; Arsenescu, R.; Leon, F.; Jiang, J.G.; et al. Larazotide Acetate in Patients with Coeliac Disease Undergoing a Gluten Challenge: A Randomised Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2013, 37, 252–262. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Study to Evaluate the Efficacy and Safety of Larazotide Acetate for the Relief of CeD Symptoms. Available online: https://clinicaltrials.gov/study/NCT03569007?cond=ced%20in%20syndrome&viewType=Table&checkSpell=&rank=2 (accessed on 8 July 2025).
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac Disease: Role of the Epithelial Barrier. CMGH 2017, 3, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Hoilat, G.J.; Altowairqi, A.K.; Ayas, M.F.; Alhaddab, N.T.; Alnujaidi, R.A.; Alharbi, H.A.; Alyahyawi, N.; Kamal, A.; Alhabeeb, H.; Albazee, E.; et al. Larazotide Acetate for Treatment of Celiac Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101782. [Google Scholar] [CrossRef] [PubMed]
- Daveson, A.J.M.; Stubbs, R.; Polasek, T.M.; Isola, J.; Anderson, R.; Tye-Din, J.A.; Schoeman, M.; Lionnet, C.; Mei, S.L.C.Y.; Mihajlović, J.; et al. Safety, Clinical Activity, Pharmacodynamics, and Pharmacokinetics of IMU-856, a SIRT6 Modulator, in Coeliac Disease: A First-in-Human, Randomised, Double-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Gastroenterol. Hepatol. 2024, 10, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Guo, Y.; Ping, L.; Qiu, Y.; Liu, Q.; Li, Z.; Wang, Z. Protective Effects of SIRT6 Overexpression against DSS-Induced Colitis in Mice. Cells 2020, 9, 1513. [Google Scholar] [CrossRef]
- Schuppan, D.; Mäki, M.; Lundin, K.E.A.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, Ø.; et al. A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef]
- Büchold, C.; Hils, M.; Gerlach, U.; Weber, J.; Pelzer, C.; Heil, A.; Aeschlimann, D.; Pasternack, R. Features of ZED1227: The First-in-Class Tissue Transglutaminase Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac Disease. Cells 2022, 11, 1667. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of GSK3915393 in Healthy Participants and to Evaluate the Interaction Between GSK3915393 and Grapefruit Juice and Itraconazole. Available online: https://clinicaltrials.gov/study/NCT04604795?cond=%22Celiac%20disease%22&rank=9 (accessed on 9 July 2025).
- National Library of Medicine (U.S.). A Study to Evaluate the Efficacy and Safety of GSK3915393 in Participants with Idiopathic Pulmonary Fibrosis (IPF). Available online: https://clinicaltrials.gov/study/NCT06317285?term=Labrador%20Lung&viewType=Table&rank=10 (accessed on 9 July 2025).
- Abadie, V.; Kim, S.M.; Lejeune, T.; Palanski, B.A.; Ernest, J.D.; Tastet, O.; Voisine, J.; Discepolo, V.; Marietta, E.V.; Hawash, M.B.F.; et al. IL-15, Gluten and HLA-DQ8 Drive Tissue Destruction in Coeliac Disease. Nature 2020, 578, 600–604. [Google Scholar] [CrossRef]
- Dunne, M.R.; Byrne, G.; Chirdo, F.G.; Feighery, C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front. Immunol. 2020, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Lähdeaho, M.L.; Scheinin, M.; Vuotikka, P.; Taavela, J.; Popp, A.; Laukkarinen, J.; Koffert, J.; Koivurova, O.P.; Pesu, M.; Kivelä, L.; et al. Safety and Efficacy of AMG 714 in Adults with Coeliac Disease Exposed to Gluten Challenge: A Phase 2a, Randomised, Double-Blind, Placebo-Controlled Study. Lancet Gastroenterol. Hepatol. 2019, 4, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Cellier, C.; Bouma, G.; van Gils, T.; Khater, S.; Malamut, G.; Crespo, L.; Collin, P.; Green, P.H.; Crowe, S.E.; Tsuji, W.; et al. Safety and Efficacy of AMG 714 in Patients with Type 2 Refractory Coeliac Disease: A Phase 2a, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Lancet Gastroenterol. Hepatol. 2019, 4, 960–970. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). PRV-015 in Gluten-Free Diet Non-Responsive Celiac Disease (PROACTIVE). Available online: https://clinicaltrials.gov/study/NCT04424927 (accessed on 9 July 2025).
- Vicari, A.P.; Schoepfer, A.M.; Meresse, B.; Goffin, L.; Léger, O.; Josserand, S.; Guégan, N.; Yousefi, S.; Straumann, A.; Cerf-Bensussan, N.; et al. Discovery and Characterization of a Novel Humanized Anti-IL-15 Antibody and Its Relevance for the Treatment of Refractory Celiac Disease and Eosinophilic Esophagitis. mAbs 2017, 9, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Jabri, B. IL-15: A Central Regulator of Celiac Disease Immunopathology. Immunol. Rev. 2014, 260, 221–234. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Arias, Á.; Tenias, J.M. Systematic Review: The Association between Eosinophilic Oesophagitis and Coeliac Disease. Aliment. Pharmacol. Ther. 2014, 40, 422–434. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Dose Escalation Study to Evaluate an Experimental New Treatment (CALY-002) in Healthy Subjects and Subjects with Celiac Disease and Eosinophilic Esophagitis. Available online: https://clinicaltrials.gov/study/NCT04593251?intr=CALY-002&rank=1 (accessed on 10 July 2025).
- Koenig, L.; Ben-Eliezer, I.; Tao, T.P.; Winter, A.; Grossman, M. Modeling Human Natural Killer Cell Development and Drug Response in a Microfluidic Bone Marrow Model. Front. Immunol. 2025, 16, 1499397. [Google Scholar] [CrossRef] [PubMed]
- Schnir, V.; Rigby, M.; Calota, M.R.; Gross, N.; Echenique, I.; Lammerich, A.; Liu, Y.; Robinson, R.; Wangsa, J.; Morrow, V. Su1326 Developing TEV-53408 for the Treatment of Celiac Disease: Summary of Preliminary Results from the First in Human Phase 1 Study in Healthy Volunteers. Gastroenterology 2024, 166, S-730. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Trial to Assess the Efficacy and Safety of TEV-53408 in Adults with Celiac Disease ClinicalTrials.Gov ID NCT06807463. Available online: https://clinicaltrials.gov/study/NCT06807463 (accessed on 10 July 2025).
- Tiet, P.; Giovannone, A.J.; Al-Mawsawi, L.; Ampudia, J.; Marrocco, V.; Connelly, S.; Ng, C. Γc Receptor Antagonist, EQ102, Prevents the NK and T Cell-Mediated Responses Driven by IL-15 and IL-21. J. Immunol. 2023, 210, 219–243. [Google Scholar] [CrossRef]
- Equillium Equillium Announces Initiation of Phase 1 Study of EQ102, A First-in-Class Multi-Cytokine Inhibitor of IL-15 and IL-21 Targeting Celiac Disease. Available online: https://www.equilliumbio.com/investors/press-releases/news-details/2022/Equillium-Announces-Initiation-of-Phase-1-study-of-EQ102-A-First-in-Class-Multi-Cytokine-Inhibitor-of-IL-15-and-IL-21-Targeting-Celiac-Disease/default.aspx (accessed on 10 July 2022).
- Australian New Zealand Clinical Trials Registry a Randomized, Double-Blind, Placebo-Controlled Phase I Study to Assess the Safety and Tolerability of Subcutaneous EQ102 Administered in a Single and Multiple Ascending Dose Schedule. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=384358 (accessed on 10 July 2022).
- National Library of Medicine (U.S.). Study of EQ101 in Adult Subjects with Moderate to Severe Alopecia Areata. Available online: https://clinicaltrials.gov/study/NCT05589610?term=EQ101&rank=1 (accessed on 10 July 2022).
- Equillium Equillium Announces Update on Multi-Cytokine Inhibitors EQ101 & EQ102 in Development for Alopecia Areata and Celiac Disease. Available online: https://www.equilliumbio.com/investors/press-releases/news-details/2023/Equillium-Announces-Update-on-Multi-Cytokine-Inhibitors-EQ101--EQ102-in-Development-for-Alopecia-Areata-and-Celiac-Disease/default.aspx (accessed on 10 July 2025).
- Equillium Equillium Announces Preclinical Data from New Orally Deliverable Multi-Cytokine Inhibitor in Presentation at the 18th Annual Peptide Therapeutics Symposium. Available online: https://www.equilliumbio.com/investors/press-releases/news-details/2023/Equillium-Announces-Preclinical-Data-from-New-Orally-Deliverable-Multi-Cytokine-Inhibitor-in-Presentation-at-the-18th-Annual-Peptide-Therapeutics-Symposium/default.aspx (accessed on 11 July 2025).
- Rotondi Aufiero, V.; Iacomino, G.; De Chiara, G.; Picariello, E.; Iaquinto, G.; Troncone, R.; Mazzarella, G. Neutralizing IL-15 Inhibits Tissue-Damaging Immune Response in Ex Vivo Cultured Untreated Celiac Intestinal Mucosa. Cells 2025, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). A Study to Evaluate the Efficacy and Safety of AMG 714 in Adult Patients with Celiac Disease. Available online: https://clinicaltrials.gov/study/NCT02637141?cond=celiac&intr=prv-015&rank=2&tab=results (accessed on 9 July 2025).
- National Library of Medicine (U.S.). A Phase II Study of CCX282-B in Patients with Celiac Disease. Available online: https://clinicaltrials.gov/study/NCT00540657?term=NCT00540657&rank=1 (accessed on 10 July 2022).
- Walters, M.J.; Wang, Y.; Lai, N.; Baumgart, T.; Zhao, B.N.; Dairaghi, D.J.; Bekker, P.; Ertl, L.S.; Penfold, M.E.T.; Jaen, J.C.; et al. Characterization of CCX282-B, an Orally Bioavailable Antagonist of the CCR9 Chemokine Receptor, for Treatment of Inflammatory Bowel Disease. J. Pharmacol. Exp. Ther. 2010, 335, 61–69. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). PTG-100 for Patients with Celiac Disease. Available online: https://www.clinicaltrials.gov/study/NCT04524221 (accessed on 11 July 2022).
- Sandborn, W.J.; Mattheakis, L.C.; Modi, N.B.; Pugatch, D.; Bressler, B.; Lee, S.; Bhandari, R.; Kanwar, B.; Shames, R.; D’Haens, G.; et al. PTG-100, an Oral A4β7 Antagonist Peptide: Preclinical Development and Phase 1 and 2a Studies in Ulcerative Colitis. Gastroenterology 2021, 161, 1853–1864.e10. [Google Scholar] [CrossRef]
- Kapoerchan, V.V.; Wiesner, M.; Hillaert, U.; Drijfhout, J.W.; Overhand, M.; Alard, P.; van der Marel, G.A.; Overkleeft, H.S.; Koning, F. Design, Synthesis and Evaluation of High-Affinity Binders for the Celiac Disease Associated HLA-DQ2 Molecule. Mol. Immunol. 2010, 47, 1091–1097. [Google Scholar] [CrossRef]
- Kapoerchan, V.V.; Wiesner, M.; Overhand, M.; van der Marel, G.A.; Koning, F.; Overkleeft, H.S. Design of Azidoproline Containing Gluten Peptides to Suppress CD4+ T-Cell Responses Associated with Celiac Disease. Bioorg. Med. Chem. 2008, 16, 2053–2062. [Google Scholar] [CrossRef]
- Dieckman, T.; Schumann, M.; Beaumont, H.; Bontkes, H.J.; Koning, F.; Bouma, G.; Byrnes, V.; McCarthy, J.; Neefjes-Borst, A.; Loddenkemper, C.; et al. Enduring Clinical Remission in Refractory Celiac Disease Type II with Tofacitinib: An Open-Label Clinical Study. Clin. Gastroenterol. Hepatol. 2024, 22, 2334–2336. [Google Scholar] [CrossRef]
- Blair, H.A. Ritlecitinib: First Approval. Drugs 2023, 83, 1315–1321. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Double Blind, Placebo-Controlled Trial to Establish Safety and Efficacy of Ritlecitinib in Celiac Disease Patients in Remission. Available online: https://clinicaltrials.gov/study/NCT05636293?cond=celiac%20disease&intr=ritlecitinib&rank=1 (accessed on 11 July 2025).
- Choung, R.S.; Ramakrishna, J.; Pradhan, V.; King, B.; Guttman-Yassky, E.; Peeva, E.; Murray, J.A. Ritlecitinib, a JAK3 /TEC Inhibitor, Modulates the Markers of Celiac Autoimmunity in Alopecia Areata and Vitiligo Patients. Arch. Dermatol. Res. 2025, 317, 280. [Google Scholar] [CrossRef]
- Hjelm, M.; Shaikhkhalil, A.K. Celiac Disease in Patients with Cystic Fibrosis on Ivacaftor: A Case Series. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Safe, M.; Gifford, A.J.; Jaffe, A.; Ooi, C.Y. Resolution of Intestinal Histopathology Changes in Cystic Fibrosis after Treatment with Ivacaftor. Ann. Am. Thorac. Soc. 2016, 13, 297–298. [Google Scholar] [CrossRef]
- Villella, V.R.; Venerando, A.; Cozza, G.; Esposito, S.; Ferrari, E.; Monzani, R.; Spinella, M.C.; Oikonomou, V.; Renga, G.; Tosco, A.; et al. A Pathogenic Role for Cystic Fibrosis Transmembrane Conductance Regulator in Celiac Disease. EMBO J. 2019, 38, e100101. [Google Scholar] [CrossRef]
- Escudero-Hernández, C.; Martín, Á.; de Pedro Andrés, R.; Fernández-Salazar, L.; Garrote, J.A.; Bernardo, D.; Arranz, E. Circulating Dendritic Cells from Celiac Disease Patients Display a Gut-Homing Profile and Are Differentially Modulated by Different Gliadin-Derived Peptides. Mol. Nutr. Food Res. 2020, 64, e1900989. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Rovedatti, L.; Rosado, M.M.; Carsetti, R.; Corazza, G.R.; MacDonald, T.T. Increased Expression of Mucosal Addressin Cell Adhesion Molecule 1 in the Duodenum of Patients with Active Celiac Disease Is Associated with Depletion of Integrin A4β7-Positive T Cells in Blood. Hum. Pathol. 2009, 40, 699–704. [Google Scholar] [CrossRef]
- Brown, N.K.; Guandalini, S.; Semrad, C.; Kupfer, S.S. A Clinician’s Guide to Celiac Disease HLA Genetics. Am. J. Gastroenterol. 2019, 114, 1587–1592. [Google Scholar] [CrossRef]
- Lei, H.; Crawford, M.S.; McCole, D.F. Jak-Stat Pathway Regulation of Intestinal Permeability: Pathogenic Roles and Therapeutic Opportunities in Inflammatory Bowel Disease. Pharmaceuticals 2021, 14, 840. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Perera, P.Y.; Waldmann, T.A.; Hiroi, T.; Perera, L.P. Tofacitinib, a Janus Kinase Inhibitor Demonstrates Efficacy in an IL-15 Transgenic Mouse Model That Recapitulates Pathologic Manifestations of Celiac Disease. J. Clin. Immunol. 2013, 33, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Vanuytsel, T.; Hiele, M. Celiac Disease Remission with Tofacitinib: A Case Report. Ann. Intern. Med. 2020, 173, 585. [Google Scholar] [CrossRef]
- Kahn, J.S.; Moody, K.; Rosmarin, D. Significant Improvement of Dermatitis Herpetiformis with Tofacitinib. Dermatol. Online J. 2021, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lenfant, M.; De Hertogh, G.; Verstockt, B. Tofacitinib for Celiac Disease and Microscopic Colitis: Killing Two Birds with One Stone. Acta Gastroenterol. Belg. 2023, 86, 374–376. [Google Scholar] [CrossRef]
- Maiuri, L.; Villella, V.R.; Raia, V.; Kroemer, G. The Gliadin-CFTR Connection: New Perspectives for the Treatment of Celiac Disease. Ital. J. Pediatr. 2019, 45, 40. [Google Scholar] [CrossRef]
- Daveson, A.J.M.; Ee, H.C.; Andrews, J.M.; King, T.; Goldstein, K.E.; Dzuris, J.L.; MacDougall, J.A.; Williams, L.J.; Treohan, A.; Cooreman, M.P.; et al. Epitope-Specific Immunotherapy Targeting CD4-Positive T Cells in Celiac Disease: Safety, Pharmacokinetics, and Effects on Intestinal Histology and Plasma Cytokines with Escalating Dose Regimens of Nexvax2 in a Randomized, Double-Blind, Placebo-Controlled Phase 1 Study. EBioMedicine 2017, 26, 78–90. [Google Scholar] [CrossRef]
- Goel, G.; King, T.; Daveson, A.J.; Andrews, J.M.; Krishnarajah, J.; Krause, R.; Brown, G.J.E.; Fogel, R.; Barish, C.F.; Epstein, R.; et al. Epitope-Specific Immunotherapy Targeting CD4-Positive T Cells in Coeliac Disease: Two Randomised, Double-Blind, Placebo-Controlled Phase 1 Studies. Lancet Gastroenterol. Hepatol. 2017, 2, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Truitt, K.E.; Daveson, A.J.M.; Ee, H.C.; Goel, G.; MacDougall, J.; Neff, K.; Anderson, R.P. Randomised Clinical Trial: A Placebo-Controlled Study of Subcutaneous or Intradermal NEXVAX2, an Investigational Immunomodulatory Peptide Therapy for Coeliac Disease. Aliment. Pharmacol. Ther. 2019, 50, 547–555. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Daveson, A.J.M.; Goel, G.; Goldstein, K.E.; Hand, H.L.; Neff, K.M.; Popp, A.; Taavela, J.; Maki, M.; Isola, J.; et al. Efficacy and Safety of Gluten Peptide-Based Antigen-Specific Immunotherapy (Nexvax2) in Adults with Coeliac Disease after Bolus Exposure to Gluten (RESET CeD): An Interim Analysis of a Terminated Randomised, Double-Blind, Placebo-Controlled Phase 2 Study. Lancet Gastroenterol. Hepatol. 2023, 8, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Tye-Din, J. Discontinuation of the Nexvax2 Phase 2 Trial. Aust. Coeliac 2019, 21. [Google Scholar]
- Kelly, C.P.; Murray, J.A.; Leffler, D.A.; Getts, D.R.; Bledsoe, A.C.; Smithson, G.; First, M.R.; Morris, A.; Boyne, M.; Elhofy, A.; et al. TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Gastroenterology 2021, 161, 66–80.e8. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Dose-Ranging Study of the Efficacy and Safety of TAK-101 for Prevention of Gluten-Specific T Cell Activation in Participants with Celiac Disease on a Gluten-Free Diet. Available online: https://clinicaltrials.gov/study/NCT04530123?intr=TAK%20101&rank=1 (accessed on 11 July 2025).
- Murray, J.A.; Wassaf, D.; Dunn, K.; Arora, S.; Winkle, P.; Stacey, H.; Cooper, S.; Goldstein, K.E.; Manchanda, R.; Kontos, S.; et al. Safety and Tolerability of KAN-101, a Liver-Targeted Immune Tolerance Therapy, in Patients with Coeliac Disease (ACeD): A Phase 1 Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 735–747. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Study of Safety, Tolerability, Pharmacodynamics, and Pharmacokinetics of KAN-101 in Celiac Disease (AceD-It). Available online: https://www.clinicaltrials.gov/study/NCT05574010 (accessed on 14 July 2025).
- National Library of Medicine (U.S.). A Study of Efficacy, Safety, and Tolerability of KAN-101 in People with Celiac Disease (SynCeD). Available online: https://clinicaltrials.gov/study/NCT06001177 (accessed on 14 July 2025).
- Topas Therapeutics Topas Therapeutics’ TPM502 Achieves Gluten-Specific Tolerance Induction, Positive Safety Profile in Phase 2a Trial in Celiac Disease Patients. Available online: https://topas-therapeutics.com/topas-therapeutics-tpm502-achieves-gluten-specific-tolerance-induction-positive-safety-profile-in-phase-2a-trial-in-celiac-disease-patients/ (accessed on 14 July 2025).
- National Library of Medicine (U.S.). A Study to Assess the Safety of TPM502 in Adults with Celiac Disease. Available online: https://clinicaltrials.gov/study/NCT05660109?term=TPM502&rank=1 (accessed on 14 July 2025).
- Corrêa, J. A Randomized, Double-Blind, Placebo-Controlled, Phase 1/2a Trial Protocol to Assess the Safety and Efficacy of TAK-101 Administered by Microneedles in Patients with Celiac Disease. Princ. Pract. Clin. Res. J. 2022, 8, 37–48. [Google Scholar] [CrossRef]
- Cenit, M.C.; Olivares, M.; Codoñer-Franch, P.; Sanz, Y. Intestinal Microbiota and Celiac Disease: Cause, Consequence or Co-Evolution? Nutrients 2015, 7, 6900–6923. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Reikvam, D.H.; Erofeev, A.; Sandvik, A.; Grcic, V.; Jahnsen, F.L.; Gaustad, P.; McCoy, K.D.; Macpherson, A.J.; Meza-Zepeda, L.A.; Johansen, F.E. Depletion of Murine Intestinal Microbiota: Effects on Gut Mucosa and Epithelial Gene Expression. PLoS ONE 2011, 6, e17996. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific Duodenal and Faecal Bacterial Groups Associated with Paediatric Coeliac Disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalances in Faecal and Duodenal Bifidobacterium Species Composition in Active and Non-Active Coeliac Disease. BMC Microbiol. 2008, 8, 232. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.; et al. Duodenal and Faecal Microbiota of Celiac Children: Molecular, Phenotype and Metabolome Characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Pisarello, M.L.J.; Vintiñi, E.O.; González, S.N.; Pagani, F.; Medina, M.S. Decrease in Lactobacilli in the Intestinal Microbiota of Celiac Children with a Gluten-Free Diet, And Selection of Potentially Probiotic Strains. Can J. Microbiol. 2014, 61, 32–37. [Google Scholar] [CrossRef]
- Wacklin, P.; Laurikka, P.; Lindfors, K.; Collin, P.; Salmi, T.; Lähdeaho, M.L.; Saavalainen, P.; Mäki, M.; Mättö, J.; Kurppa, K.; et al. Altered Duodenal Microbiota Composition in Celiac Disease Patients Suffering from Persistent Symptoms on a Long-Term Gluten-Free Diet. Am. J. Gastroenterol. 2014, 109, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Wacklin, P.; Kaukinen, K.; Tuovinen, E.; Collin, P.; Lindfors, K.; Partanen, J.; Mäki, M.; Mättuö, J. The Duodenal Microbiota Composition of Adult Celiac Disease Patients Is Associated with the Clinical Manifestation of the Disease. Inflamm. Bowel. Dis. 2013, 19, 934–941. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Smecuol, E.; Hwang, H.J.; Sugai, E.; Corso, L.; Cherñavsky, A.C.; Bellavite, F.P.; Gonza’lez, A.; Voda’novich, F.; Moreno, M.L.; Va’zquez, H.; et al. Exploratory, Randomized, Double-Blind, Placebo-Controlled Study on the Effects of Bifidobacterium Infantis Natren Life Start Strain Super Strain in Active Celiac Disease. J. Clin. Gastroenterol. 2013, 47, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Constante, M.; Temprano, M.P.; Costa, A.F.; Moreno, M.L.; Pinto-Sanchez, M.I.; Vázquez, H.; Stefanolo, J.P.; Gonzalez, A.F.; D’Adamo, C.R.; et al. Effect of Bifidobacterium Infantis NLS Super Strain in Symptomatic Coeliac Disease Patients on Long-Term Gluten-Free Diet—An Exploratory Study. Benef. Microbes 2020, 11, 527–534. [Google Scholar] [CrossRef]
- Klemenak, M.; Dolinšek, J.; Langerholc, T.; Di Gioia, D.; Mičetić-Turk, D. Administration of Bifidobacterium Breve Decreases the Production of TNF-α in Children with Celiac Disease. Dig. Dis. Sci. 2015, 60, 3386–3392. [Google Scholar] [CrossRef]
- Quagliariello, A.; Aloisio, I.; Cionci, N.B.; Luiselli, D.; D’Auria, G.; Martinez-Priego, L.; Pérez-Villarroya, D.; Langerholc, T.; Primec, M.; Mičetić-Turk, D.; et al. Effect of Bifidobacterium Breve on the Intestinal Microbiota of Coeliac Children on a Gluten Free Diet: A Pilot Study. Nutrients 2016, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Primec, M.; Klemenak, M.; Di Gioia, D.; Aloisio, I.; Bozzi Cionci, N.; Quagliariello, A.; Gorenjak, M.; Mičetić-Turk, D.; Langerholc, T. Clinical Intervention Using Bifidobacterium Strains in Celiac Disease Children Reveals Novel Microbial Modulators of TNF-α and Short-Chain Fatty Acids. Clin. Nutr. 2019, 38, 1373–1381. [Google Scholar] [CrossRef]
- Laparra, J.M.; Olivares, M.; Gallina, O.; Sanz, Y. Bifidobacterium Longum CECT 7347 Modulates Immune Responses in a Gliadin-Induced Enteropathy Animal Model. PLoS ONE 2012, 7, e30744. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Olivares, M.; Sanz, Y. Oral Administration of Bifidobacterium Longum CECT 7347 Ameliorates Gliadin-Induced Alterations in Liver Iron Mobilisation. Br. J. Nutr. 2013, 110, 1828–1836. [Google Scholar] [CrossRef]
- Olivares, M.; Castillejo, G.; Varea, V.; Sanz, Y. Double-Blind, Randomised, Placebo-Controlled Intervention Trial to Evaluate the Effects of Bifidobacterium Longum CECT 7347 in Children with Newly Diagnosed Coeliac Disease. Br. J. Nutr. 2014, 112, 30–40. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bergomas, F.; Marraccini, P.; Ingenito, M.R.; Ferrari, L.; Vigna, L. Pilot Study on Non-Celiac Gluten Sensitivity: Effects of Bifidobacterium Longum Es1 Co-Administered with a Gluten-Free Diet. Minerva Gastroenterol. Dietol. 2020, 66, 187–193. [Google Scholar] [CrossRef]
- National Library of Medicine. Efficacy of Probiotic ES1 for the Treatment of Non-Celiac Gluten Sensitivity. Available online: https://clinicaltrials.gov/study/NCT02810301 (accessed on 15 July 2025).
- Naghibi, M.; Pont-Beltran, A.; Lamelas, A.; Llobregat, L.; Martinez-Blanch, J.F.; Rojas, A.; Álvarez, B.; López Plaza, B.; Arcos Castellanos, L.; Chenoll, E.; et al. Effect of Postbiotic Bifidobacterium Longum CECT 7347 on Gastrointestinal Symptoms, Serum Biochemistry, and Intestinal Microbiota in Healthy Adults: A Randomised, Parallel, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2024, 16, 3952. [Google Scholar] [CrossRef]
- Srivastava, S.; Basak, U.; Naghibi, M.; Vijayakumar, V.; Parihar, R.; Patel, J.; Jadon, P.S.; Pandit, A.; Dargad, R.R.; Khanna, S.; et al. A Randomized Double-Blind, Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Live Bifidobacterium Longum CECT 7347 (ES1) and Heat-Treated Bifidobacterium Longum CECT 7347 (HT-ES1) in Participants with Diarrhea-Predominant Irritable Bowel Syndrome. Gut Microbes 2024, 16, 2338322. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, H.J.; Wiepjes, M.; Motta, J.P.; Schulz, J.D.; Jury, J.; Natividad, J.M.; Pinto-Sanchez, I.; Sinclair, D.; Rousset, P.; Martin-Rosique, R.; et al. Novel Role of the Serine Protease Inhibitor Elafin in Gluten-Related Disorders. Am. J. Gastroenterol. 2014, 109, 748–756. [Google Scholar] [CrossRef]
- McCarville, J.L.; Dong, J.; Caminero, A.; Bermudez-Brito, M.; Jury, J.; Murray, J.A.; Duboux, S.; Steinmann, M.; Delley, M.; Tangyu, M.; et al. A Commensal Bifidobacterium Longum Strain Prevents Gluten-Related Immunopathology in Mice through Expression of a Serine Protease Inhibitor. Appl. Environ. Microbiol. 2017, 83, e01323-17. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Probiotic BL NCC 2705 and Gluten Sensitivity. Available online: https://clinicaltrials.gov/study/NCT03775499?intr=BL%20NCC&rank=1 (accessed on 15 July 2025).
- Lindfors, K.; Blomqvist, T.; Juuti-Uusitalo, K.; Stenman, S.; Venäläinen, J.; Mäki, M.; Kaukinen, K. Live Probiotic Bifidobacterium Lactis Bacteria Inhibit the Toxic Effects Induced by Wheat Gliadin in Epithelial Cell Culture. Clin. Exp. Immunol. 2008, 152, 552–558. [Google Scholar] [CrossRef]
- Oscarsson, E.; Håkansson, Å.; Andrén Aronsson, C.; Molin, G.; Agardh, D. Effects of Probiotic Bacteria Lactobacillaceae on the Gut Microbiota in Children with Celiac Disease Autoimmunity: A Placebo-Controlled and Randomized Clinical Trial. Front. Nutr. 2021, 8, 680771. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, Å.; Aronsson, C.A.; Brundin, C.; Oscarsson, E.; Molin, G.; Agardh, D. Effects of Lactobacillus Plantarum and Lactobacillus Paracasei on the Peripheral Immune Response in Children with Celiac Disease Autoimmunity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 1925. [Google Scholar] [CrossRef]
- Jenickova, E.; Andrén Aronsson, C.; Mascellani Bergo, A.; Cinek, O.; Havlik, J.; Agardh, D. Effects of Lactiplantibacillus Plantarum and Lacticaseibacillus Paracasei Supplementation on the Faecal Metabolome in Children with Coeliac Disease Autoimmunity: A Randomised, Double-Blinded Placebo-Controlled Clinical Trial. Front. Nutr. 2023, 10, 1183963. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Prevention Av Autoimmunitet Med Laktobaciller (PAL). Available online: https://clinicaltrials.gov/study/NCT04014660?intr=laktobaciller&rank=1 (accessed on 15 July 2025).
- Duar, R.M.; Clark, K.J.; Patil, P.B.; Hernández, C.; Brüning, S.; Burkey, T.E.; Madayiputhiya, N.; Taylor, S.L.; Walter, J. Identification and Characterization of Intestinal Lactobacilli Strains Capable of Degrading Immunotoxic Peptides Present in Gluten. J. Appl. Microbiol. 2015, 118, 515–527. [Google Scholar] [CrossRef]
- Kunduhoglu, B.; Hacioglu, S. Probiotic Potential and Gluten Hydrolysis Activity of Lactobacillus Brevis KT16-2. Probiotics Antimicrob. Proteins 2021, 13, 720–733. [Google Scholar] [CrossRef] [PubMed]
- D’Arienzo, R.; Maurano, F.; Luongo, D.; Mazzarella, G.; Stefanile, R.; Troncone, R.; Auricchio, S.; Ricca, E.; David, C.; Rossi, M. Adjuvant Effect of Lactobacillus Casei in a Mouse Model of Gluten Sensitivity. Immunol. Lett. 2008, 119, 78–83. [Google Scholar] [CrossRef]
- D’Arienzo, R.; Stefanile, R.; Maurano, F.; Mazzarella, G.; Ricca, E.; Troncone, R.; Auricchio, S.; Rossi, M. Immunomodulatory Effects of Lactobacillus Casei Administration in a Mouse Model of Gliadin-Sensitive Enteropathy. Scand. J. Immunol. 2011, 74, 335–341. [Google Scholar] [CrossRef]
- D’Arienzo, R.; Maurano, F.; Lavermicocca, P.; Ricca, E.; Rossi, M. Modulation of the Immune Response by Probiotic Strains in a Mouse Model of Gluten Sensitivity. Cytokine 2009, 48, 254–259. [Google Scholar] [CrossRef]
- Rashmi, B.S.; Gayathri, D. Draft Genome Sequence of the Gluten-Hydrolyzing Bacterium Bacillus Subtilis GS 188, Isolated from Wheat Sourdough. Genome Announc. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Rashmi, B.S.; Gayathri, D. Molecular Characterization of Gluten Hydrolysing Bacillus Sp. and Their Efficacy and Biotherapeutic Potential as Probiotics Using Caco-2 Cell Line. J. Appl. Microbiol. 2017, 123, 759–772. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Usman, K.; Imran, M. Isolation and Characterization of Gluten-Degrading Enterococcus Mundtii and Wickerhamomyces Anomalus, Potential Probiotic Strains from Indigenously Fermented Sourdough (Khamir). LWT 2018, 91, 271–277. [Google Scholar] [CrossRef]
- Tran, T.; Senger, S.; Baldassarre, M.; Brosnan, R.A.; Cristofori, F.; Crocco, M.; De Santis, S.; Elli, L.; Faherty, C.S.; Francavilla, R.; et al. Novel Bacteroides Vulgatus Strain Protects against Gluten-Induced Break of Human Celiac Gut Epithelial Homeostasis: A Pre-Clinical Proof-of-Concept Study. Pediatr. Res. 2024, 95, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramón, D.; et al. Heat-Treated Bifidobacterium Longum CECT-7347: A Whole-Cell Postbiotic with Antioxidant, Anti-Inflammatory, and Gut-Barrier Protection Properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; Myers, S.P.; Rolfe, M. Probiotics and the Microbiome in Celiac Disease: A Randomised Controlled Trial. Evid.-Based Complement. Altern. Med. 2016, 2016, 9048574. [Google Scholar] [CrossRef]
- De Angelis, M.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; De Simone, C.; Silano, M.; De Vincenzi, M.; Losito, I.; Gobbetti, M. VSL#3 Probiotic Preparation Has the Capacity to Hydrolyze Gliadin Polypeptides Responsible for Celiac Sprue. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 80–93. [Google Scholar] [CrossRef]
- Francavilla, R.; Piccolo, M.; Francavilla, A.; Polimeno, L.; Semeraro, F.; Cristofori, F.; Castellaneta, S.; Barone, M.; Indrio, F.; Gobbetti, M.; et al. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients with Persistent IBS-Type Symptoms: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Clin. Gastroenterol. 2019, 53, E117–E125. [Google Scholar] [CrossRef]
- Lionetti, E.; Dominijanni, V.; Iasevoli, M.; Cimadamore, E.; Acquaviva, I.; Gatti, S.; Monachesi, C.; Catassi, G.; Pino, A.; Faragalli, A.; et al. Effects of the Supplementation with a Multispecies Probiotic on Clinical and Laboratory Recovery of Children with Newly Diagnosed Celiac Disease: A Randomized, Placebo-Controlled Trial. Dig. Liver Dis. 2023, 55, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Monzani, R.; Saverio, V.; Gagliardi, M.; Pańczyszyn, E.; Raia, V.; Villella, V.R.; Bona, G.; Pane, M.; Amoruso, A.; et al. Probiotics Supplements Reduce Er Stress and Gut Inflammation Associated with Gliadin Intake in a Mouse Model of Gluten Sensitivity. Nutrients 2021, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Papista, C.; Gerakopoulos, V.; Kourelis, A.; Sounidaki, M.; Kontana, A.; Berthelot, L.; Moura, I.C.; Monteiro, R.C.; Yiangou, M. Gluten Induces Coeliac-like Disease in Sensitised Mice Involving IgA, CD71 and Transglutaminase 2 Interactions That Are Prevented by Probiotics. Lab. Investig. 2012, 92, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; Miller, G.C.; Marquart, L.; Llewellyn, S.; Gupta, R.; Becker, L.; Clouston, A.D.; Welch, C.; Sidorenko, J.; Wallace, L.; et al. Randomized, Placebo Controlled Trial of Experimental Hookworm Infection for Improving Gluten Tolerance in Celiac Disease. Clin. Transl. Gastroenterol. 2020, 11, e00274. [Google Scholar] [CrossRef]
- Daveson, A.J.; Jones, D.M.; Gaze, S.; McSorley, H.; Clouston, A.; Pascoe, A.; Cooke, S.; Speare, R.; Macdonald, G.A.; Anderson, R.; et al. Effect of Hookworm Infection on Wheat Challenge in Celiac Disease—A Randomised Double-Blinded Placebo Controlled Trial. PLoS ONE 2011, 6, e17366. [Google Scholar] [CrossRef]
- Giacomin, P.; Zakrzewski, M.; Jenkins, T.P.; Su, X.; Al-Hallaf, R.; Croese, J.; De Vries, S.; Grant, A.; Mitreva, M.; Loukas, A.; et al. Changes in Duodenal Tissue-Associated Microbiota Following Hookworm Infection and Consecutive Gluten Challenges in Humans with Coeliac Disease. Sci. Rep. 2016, 6, 36797. [Google Scholar] [CrossRef]
- Feruś, K.; Drabińska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. A Randomized, Placebo-Controlled, Pilot Clinical Trial to Evaluate the Effect of Supplementation with Prebiotic Synergy 1 on Iron Homeostasis in Children and Adolescents with Celiac Disease Treated with a Gluten-Free Diet. Nutrients 2018, 10, 1818. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Jarocka-Cyrta, E. The Effect of Oligofructose-Enriched Inulin Supplementation on Gut Microbiota, Nutritional Status and Gastrointestinal Symptoms in Paediatric Coeliac Disease Patients on a Gluten-Free Diet: Study Protocol for a Pilot Randomized Controlled Trial. Nutr. J. 2017, 16, 47. [Google Scholar] [CrossRef]
- Drabińska, N.; Krupa-Kozak, U.; Ciska, E.; Jarocka-Cyrta, E. Plasma Profile and Urine Excretion of Amino Acids in Children with Celiac Disease on Gluten-Free Diet after Oligofructose-Enriched Inulin Intervention: Results of a Randomised Placebo-Controlled Pilot Study. Amino Acids 2018, 50, 1451–1460. [Google Scholar] [CrossRef]
- Drabińska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The Effect of Oligofructose-Enriched Inulin on Faecal Bacterial Counts and Microbiota-Associated Characteristics in Celiac Disease Children Following a Gluten-Free Diet: Results of a Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; De Angelis, M.; Rizzello, C.G.; Cavallo, N.; Dal Bello, F.; Gobbetti, M. Selected Probiotic Lactobacilli Have the Capacity to Hydrolyze Gluten Peptides during Simulated Gastrointestinal Digestion. Appl. Environ. Microbiol. 2017, 83, e00376-17. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Hirata, T.; Sakugawa, H.; Watanabe, T.; Miyagi, S.; Maeshiro, T.; Chinen, T.; Kawane, M.; Zaha, O.; Nakayoshi, T.; et al. An Inverse Relationship Between Autoimmune Liver Diseases and Strongyloides Stercoralis Infection. Am. J. Trop. Med. Hyg. 2007, 76, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Summers, R. Trichuris Suis Seems to Be Safe and Possibly Effective in the Treatment of Inflammatory Bowel Disease. Am. J. Gastroenterol. 2003, 98, 2034–2041. [Google Scholar] [CrossRef]
- Ruyssers, N.E.; De Winter, B.Y.; De Man, J.G.; Ruyssers, N.D.; Van Gils, A.J.; Loukas, A.; Pearson, M.S.; Weinstock, J.V.; Pelckmans, P.A.; Moreels, T.G. Schistosoma Mansoni Proteins Attenuate Gastrointestinal Motility Disturbances during Experimental Colitis in Mice. World J. Gastroenterol. 2010, 16, 703–712. [Google Scholar] [CrossRef]
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.A.; Weinstock, J.V. Trichuris Suis Therapy for Active Ulcerative Colitis: A Randomized Controlled Trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M. Association between Parasite Infection and Immune Responses in Multiple Sclerosis. Ann. Neurol. 2007, 61, 97–108. [Google Scholar] [CrossRef]
- Bager, P.; Arnved, J.; Rønborg, S.; Wohlfahrt, J.; Poulsen, L.K.; Westergaard, T.; Petersen, H.W.; Kristensen, B.; Thamsborg, S.; Roepstorff, A.; et al. Trichuris Suis Ova Therapy for Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Allergy Clin. Immunol. 2010, 125, 123–130.e3. [Google Scholar] [CrossRef]
- Drabińska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. Intestinal Permeability in Children with Celiac Disease after the Administration of Oligofructose-Enriched Inulin into a Gluten-Free Diet—Results of a Randomized, Placebo-Controlled, Pilot Trial. Nutrients 2020, 12, 1736. [Google Scholar] [CrossRef]
- van Beurden, Y.H.; van Gils, T.; van Gils, N.A.; Kassam, Z.; Mulder, C.J.J.; Aparicio-Pagés, N. Serendipity in Refractory Celiac Disease: Full Recovery of Duodenal Villi and Clinical Symptoms after Fecal Microbiota Transfer. J. Gastrointest. Liver Dis. 2016, 25, 385–388. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Safety and Efficacy of Fecal Microbiota Transplantation. Available online: https://clinicaltrials.gov/study/NCT04014413?intr=fecal%20microbiota%20transplantation&page=2&rank=18 (accessed on 16 July 2025).
- Singh, S.; Razak, M.A.; Sangam, S.R.; Viswanath, B.; Begum, P.S.; Rajagopal, S. The Impact of Functional Food and Nutraceuticals in Health. In Therapeutic Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 23–47. [Google Scholar]
- Jahdkaran, M.; Asri, N.; Esmaily, H.; Rostami-Nejad, M. Potential of Nutraceuticals in Celiac Disease. Tissue Barriers 2024, 13, 2374628. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Van Buiten, C.B.; Elias, R.J. Gliadin Sequestration as a Novel Therapy for Celiac Disease: A Prospective Application for Polyphenols. Int. J. Mol. Sci. 2021, 22, 595. [Google Scholar] [CrossRef] [PubMed]
- Van Buiten, C.B.; Lambert, J.D.; Elias, R.J. Green Tea Polyphenols Mitigate Gliadin-Mediated Inflammation and Permeability in Vitro. Mol. Nutr. Food Res. 2018, 62, e1700879. [Google Scholar] [CrossRef]
- Xie, F.; Huang, Q.; Fang, F.; Chen, S.; Wang, Z.; Wang, K.; Fu, X.; Zhang, B. Effects of Tea Polyphenols and Gluten Addition on in Vitro Wheat Starch Digestion Properties. Int. J. Biol. Macromol. 2019, 126, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Brás, N.F.; Pérez-Gregorio, M.; Fernandes, I.; Mateus, N.; Freitas, V. A Multi-Spectroscopic Study on the Interaction of Food Polyphenols with a Bioactive Gluten Peptide: From Chemistry to Biological Implications. Food Chem. 2019, 299, 125051. [Google Scholar] [CrossRef]
- Pérot, M.; Lupi, R.; Guyot, S.; Delayre-Orthez, C.; Gadonna-Widehem, P.; Thébaudin, J.Y.; Bodinier, M.; Larré, C. Polyphenol Interactions Mitigate the Immunogenicity and Allergenicity of Gliadins. J. Agric. Food Chem. 2017, 65, 6442–6451. [Google Scholar] [CrossRef]
- Taddei, P.; Zanna, N.; Tozzi, S. Raman Characterization of the Interactions between Gliadins and Anthocyanins. J. Raman Spectrosc. 2013, 44, 1435–1439. [Google Scholar] [CrossRef]
- Tozzi, S.; Zanna, N.; Taddei, P. Study on the Interaction between Gliadins and a Coumarin as Molecular Model System of the Gliadins-Anthocyanidins Complexes. Food Chem. 2013, 141, 3586–3597. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yang, Y.; Zhao, J.; Zhang, Y.; Li, L.; Wang, Q.; Ming, J. Interaction between Wheat Gliadin and Quercetin under Different PH Conditions Analyzed by Multi-Spectroscopy Methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117937. [Google Scholar] [CrossRef]
- Yu, T.; Xie, Y.; Wang, Z.; Li, J.; Shen, Y.; Yuan, J.; Gao, J.; Fakruddin, M.; Wu, Y.; Chen, H. Quercetin Ameliorates Celiac-Related Intestinal Inflammation Caused by Wheat Gluten through Modulating Oxidative Stress, Th1/Th2/Treg Balance, and Intestinal Microflora Structure. Food Funct. 2024, 15, 9343–9356. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Bessa Pereira, C.; Dias, R.; Mateus, N.; De Freitas, V. New-Level Insights into the Effects of Grape Seed Polyphenols on the Intestinal Processing and Transport of a Celiac Disease Immunodominant Peptide. J. Agric. Food Chem. 2021, 69, 13474–13486. [Google Scholar] [CrossRef]
- Wang, N.; Cui, C.; Xu, C.; Ren, H.; Wang, F.; Yu, Q.; Zhang, G. Effect and Mechanism of Peanut Skin Proanthocyanidins on Gliadin-Induced Caco-2 Celiac Disease Model Cells. Clin. Immunol. 2022, 245, 109100. [Google Scholar] [CrossRef]
- Klusóczki, Á.; Oláh, B.; Hosszú, D.; Fenyvesi, F.; Remenyik, J.; Homoki, J.; Gyöngyösi, A.; Bácskay, I.; Váradi, J. Effectiveness of Anthocyanin-Rich Sour Cherry Extract on Gliadin-Induced Caco-2 Barrier Damage. Nutrients 2023, 15, 4022. [Google Scholar] [CrossRef]
- Medjeber, O.; Touri, K.; Rafa, H.; Djeraba, Z.; Belkhelfa, M.; Boutaleb, A.F.; Arroul-Lammali, A.; Belguendouz, H.; Touil-Boukoffa, C. Ex Vivo Immunomodulatory Effect of Ethanolic Extract of Propolis during Celiac Disease: Involvement of Nitric Oxide Pathway. Inflammopharmacology 2018, 26, 1469–1481. [Google Scholar] [CrossRef]
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the Effects of a Standardized Polyphenol Mixture Extracted from Poplar-Type Propolis on Healthy and Diseased Human Gut Microbiota. Biomed. Pharmacother. 2022, 148, 112759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.B.; Mantha, A.K.; Dhiman, M. Mitigation of Gliadin-Induced Inflammation and Cellular Damage by Curcumin in Human Intestinal Cell Lines. Inflammation 2021, 44, 873–889. [Google Scholar] [CrossRef]
- Yu, T.; Xie, Y.; Yuan, J.; Gao, J.; Xiao, Z.; Wu, Y.; Chen, H. The Nutritional Intervention of Resveratrol Can Effectively Alleviate the Intestinal Inflammation Associated with Celiac Disease Induced by Wheat Gluten. Front. Immunol. 2022, 13, 878186. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, Quercetin and Tyrosol Prevent Macrophage Activation Induced by Gliadin and IFN-γ. Eur. J. Pharmacol. 2007, 566, 192–199. [Google Scholar] [CrossRef]
- Périz, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Best, I.; Pastor-Soplin, S.; Castell, M.; Massot-Cladera, M. Influence of Consumption of Two Peruvian Cocoa Populations on Mucosal and Systemic Immune Response in an Allergic Asthma Rat Model. Nutrients 2022, 14, 410. [Google Scholar] [CrossRef] [PubMed]
- Abril-Gil, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castellote, C.; Franch, À.; Castell, M. A Diet Enriched with Cocoa Prevents IgE Synthesis in a Rat Allergy Model. Pharmacol. Res. 2012, 65, 603–608. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Casadesús, G.; Lee, H.G.; Zhu, X.; McShea, A.; Perry, G.; Pérez-Cano, F.J.; Smith, M.A.; Castell, M. Neuroprotective Effect of Cocoa Flavonids on in Vitro Oxidative Stress. Eur. J. Nutr. 2009, 48, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Abril-Gil, M.; Torres, S.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Impact of Cocoa Polyphenol Extracts on the Immune System and Microbiota in Two Strains of Young Rats. Br. J. Nutr. 2014, 112, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Garcia-Aloy, M.; Saldana-Ruiz, S.; Cambras, T.; González-Domínguez, R.; Franch, À.; Pérez-Cano, F.J.; Andres-Lacueva, C.; Castell, M. Role of Theobromine in Cocoa’s Metabolic Properties in Healthy Rats. J. Agric. Food Chem. 2019, 67, 3605–3614. [Google Scholar] [CrossRef]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Theobromine Is Responsible for the Effects of Cocoa on the Antibody Immune Status of Rats. J. Nutr. 2018, 148, 464–471. [Google Scholar] [CrossRef]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health Benefits of Methylxanthines in Cacao and Chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Cocoa and Cocoa Fibre Differentially Modulate IgA and IgM Production at Mucosal Sites. Br. J. Nutr. 2016, 115, 1539–1546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kramer, K.; Yeboah-Awudzi, M.; Magazine, N.; King, J.M.; Xu, Z.; Losso, J.N. Procyanidin B2 Rich Cocoa Extracts Inhibit Inflammation in Caco-2 Cell Model of in Vitro Celiac Disease by down-Regulating Interferon-Gamma- or Gliadin Peptide 31-43-Induced Transglutaminase-2 and Interleukin-15. J. Funct. Foods 2019, 57, 112–120. [Google Scholar] [CrossRef]
- Raguzzini, A.; Poce, G.; Consalvi, S.; Toti, E.; Palmacci, F.; Biava, M.; Peluso, I. Chocolate Consumers and Lymphocyte-to-Monocyte Ratio: A Working Hypothesis from a Preliminary Report of a Pilot Study in Celiac Subjects. Antioxidants 2019, 8, 440. [Google Scholar] [CrossRef]
- Du, J.; Chen, S.; Shi, J.; Zhu, X.; Ying, H.; Zhang, Y.; Chen, S.; Shen, B.; Li, J. The Association between the Lymphocyte-Monocyte Ratio and Disease Activity in Rheumatoid Arthritis. Clin. Rheumatol. 2017, 36, 2689–2695. [Google Scholar] [CrossRef]
- Girbal González, M.; Rodríguez-Banqueri, A.; Eckhard, U.; Gomis-Rüth, F.; Rodríguez-Lagunas, M.J.; Franch, À.; Pérez-Cano, F.J. Caracterización de La Composición y La Función de La Microbiota Intestinal En Un Modelo Preclínico de Celiaquía. XVI Workshop Soc. Española Microbiota Probióticos Y Prebióticos Barc. 2025, 6, 149. [Google Scholar]
- Girbal González, M.; Mendes, S.; Rodríguez-Banqueri, A.; Eckhard, U.; Gomis-Rüth, F.X.; Rodríguez-Lagunas, M.J.; Franch, À.; Pérez-Cano, F.J. Coeliac Disease Progression Is Preclinically Modulated by Cocoa Intake. In Proceedings of the 57th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), Abstract E-books 2025, Helsinki, Finland, 14–17 May 2025; Volume 6, p. 997. [Google Scholar]
- Paterson, D.; Pierce, M.; Peck, E. The Treatment of Coeliac Disease with Vitamin B Complex and Concentrated Liver. Arch. Dis. Child. 1944, 19, 99–110. [Google Scholar] [CrossRef]
- Dickey, W.; Ward, M.; Whittle, C.R.; Kelly, M.T.; Pentieva, K.; Horigan, G.; Patton, S.; Mcnulty, H. Homocysteine and Related B-Vitamin Status in Coeliac Disease: Effects of Gluten Exclusion and Histological Recovery. Scand. J. Gastroenterol. 2008, 43, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Valente, F.X.; Do Nascimento Campos, T.; De Sousa Moraes, L.F.; Miranda Hermsdorff, H.H.; De Morais Cardoso, L.; Pinheiro-Sant’ana, H.M.; Barros Gilberti, F.A.; Do Carmo Gouveia Peluzio, M. B Vitamins Related to Homocysteine Metabolism in Adults Celiac Disease Patients: A Cross-Sectional Study. Nutr. J. 2015, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Tokgöz, Y.; Terlemez, S.; Karul, A. Fat Soluble Vitamin Levels in Children with Newly Diagnosed Celiac Disease, a Case Control Study. BMC Pediatr. 2018, 18, 130. [Google Scholar] [CrossRef]
- Dong, S.; Singh, T.P.; Wei, X.; Yao, H.; Wang, H. Protective Effect of 1,25-Dihydroxy Vitamin D3 on Pepsin–Trypsin-Resistant Gliadin-Induced Tight Junction Injuries. Dig. Dis. Sci. 2018, 63, 92–104. [Google Scholar] [CrossRef]
- Piątek-Guziewicz, A.; Dąbek, A.; Przybylska-Feluś, M.; Zagrodzki, P.; Mach, T.; Zwolińska-Wcisło, M. Oral Vitamin E Supplementation in Reducing Nitrosative Stress in Adults Treated for Celiac Disease. Pol. Arch. Intern. Med. 2020, 130, 711–713. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Araya, M.; Rodríguez, J.M.; Roncoroni, L.; Elli, L.; Alvarez, J.D.P.L.; Valenzuela, R. Interplay of N-3 Polyunsaturated Fatty Acids, Intestinal Inflammation, and Gut Microbiota in Celiac Disease Pathogenesis. Nutrients 2025, 17, 621. [Google Scholar] [CrossRef] [PubMed]
- Vincentini, O.; Quaranta, M.G.; Viora, M.; Agostoni, C.; Silano, M. Docosahexaenoic Acid Modulates in Vitro the Inflammation of Celiac Disease in Intestinal Epithelial Cells via the Inhibition of CPLA 2. Clin. Nutr. 2011, 30, 541–546. [Google Scholar] [CrossRef]
- Costa, A.; Brito, G.A.P.D. Aerobic Exercise Associated with Fish Oil Supplementation Decreases C-Reactive Protein and Interleukin-6 in Celiac Disease Patients. J. Nutr. Metab. 2022, 2022, 3908675. [Google Scholar] [CrossRef]
- Osman, M.T.; Al-Duboni, G.; Taha, B.I.; Muhamed, L.A. Refractory Coeliac Disease; Role of Nigella Sativa as Immunomodulator. J. Adv. Med. Med. Res. 2012, 2, 527–535. [Google Scholar] [CrossRef]
- Sonzogni, E.; Martinelli, G.; Fumagalli, M.; Maranta, N.; Pozzoli, C.; Bani, C.; Marrari, L.A.; Di Lorenzo, C.; Sangiovanni, E.; Dell’Agli, M.; et al. In Vitro Insights into the Dietary Role of Glucoraphanin and Its Metabolite Sulforaphane in Celiac Disease. Nutrients 2024, 16, 2743. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An Emerging Trend in Functional Foods for the Prevention of Cardiovascular Disease and Diabetes: Marine Algal Polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358. [Google Scholar] [CrossRef]
- van der Linde, C.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Swann, J.R.; Costabile, A. An in Vitro Pilot Fermentation Study on the Impact of Chlorella Pyrenoidosa on Gut Microbiome Composition and Metabolites in Healthy and Coeliac Subjects. Molecules 2021, 26, 2330. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E. Hydrolytic Enzymes and Their Directly and Indirectly Effects on Gluten and Dough Properties: An Extensive Review. Food Sci. Nutr. 2021, 9, 3988–4006. [Google Scholar] [CrossRef] [PubMed]
- Akeroyd, M.; Van Zandycke, S.; Den Hartog, J.; Mutsaers, J.; Edens, L.; Van DenBerg, M.; Christis, C. AN-PEP, Proline-Specific Endopeptidase, Degrades All Known Immunostimulatory Gluten Peptides in Beer Made from Barley Malt. J. Am. Soc. Brew. Chem. 2016, 74, 91–99. [Google Scholar] [CrossRef]
- Hooshiyar, M.; Ebrahimipour, G.; Baghaei, K.; Saadi, M.I. Investigating the Effect of Cloned AN-PEP Enzyme in Yeast on Gluten Degradation and Rheological Features of Dough. Infect. Epidemiol Microbiol. 2021, 7, 337–345. [Google Scholar] [CrossRef]
- Hardt, N.A.; Boom, R.M.; van der Goot, A.J. Starch Facilitates Enzymatic Wheat Gluten Hydrolysis. LWT 2015, 61, 557–563. [Google Scholar] [CrossRef]
- Merz, M.; Kettner, L.; Langolf, E.; Appel, D.; Blank, I.; Stressler, T.; Fischer, L. Production of Wheat Gluten Hydrolysates with Reduced Antigenicity Employing Enzymatic Hydrolysis Combined with Downstream Unit Operations. J. Sci. Food Agric. 2016, 96, 3358–3364. [Google Scholar] [CrossRef] [PubMed]
- Mandile, R.; Picascia, S.; Parrella, C.; Camarca, A.; Gobbetti, M.; Greco, L.; Troncone, R.; Gianfrani, C.; Auricchio, R. Lack of Immunogenicity of Hydrolysed Wheat Flour in Patients with Coeliac Disease after a Short-Term Oral Challenge. Aliment. Pharmacol. Ther. 2017, 46, 440–446. [Google Scholar] [CrossRef]
- Gallo, M.; Nigro, F.; Passannanti, F.; Nanayakkara, M.; Lania, G.; Parisi, F.; Salameh, D.; Budelli, A.; Barone, M.V.; Nigro, R. Effect of PH Control during Rice Fermentation in Preventing a Gliadin P31-43 Entrance in Epithelial Cells. Int. J. Food. Sci. Nutr. 2019, 70, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Păcularu-Burada, B.; Ceoromila (Cantaragiu), A.M.; Vasile, M.A.; Bahrim, G.E. Novel Insights into Different Kefir Grains Usefulness as Valuable Multiple Starter Cultures to Achieve Bioactive Gluten-Free Sourdoughs. LWT 2022, 165, 113670. [Google Scholar] [CrossRef]
- Zhou, Y.; Ouyang, B.; Duan, M.; Lv, X.; Zhou, X. Biological Characteristics of the Gluten-Free Sourdough System Fermented by Lactobacillus Plantarum ST-III and Its Effect on Dough Quality and Nutritional Value during Freezing. Food Chem. X 2022, 14, 100350. [Google Scholar] [CrossRef]
- Jouanin, A.; Gilissen, L.J.W.J.; Boyd, L.A.; Cockram, J.; Leigh, F.J.; Wallington, E.J.; van den Broeck, H.C.; van der Meer, I.M.; Schaart, J.G.; Visser, R.G.F.; et al. Food Processing and Breeding Strategies for Coeliac-Safe and Healthy Wheat Products. Food Res. Int. 2018, 110, 11–21. [Google Scholar] [CrossRef]
- Ribeiro, M.; Rodriguez-Quijano, M.; Nunes, F.M.; Carrillo, J.M.; Branlard, G.; Igrejas, G. New Insights into Wheat Toxicity: Breeding Did Not Seem to Contribute to a Prevalence of Potential Celiac Disease’s Immunostimulatory Epitopes. Food Chem. 2016, 213, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Camerlengo, F.; Sestili, F.; Silvestri, M.; Colaprico, G.; Margiotta, B.; Ruggeri, R.; Lupi, R.; Masci, S.; Lafiandra, D. Production and Molecular Characterization of Bread Wheat Lines with Reduced Amount of α-Type Gliadins. BMC Plant Biol. 2017, 17, 11–21. [Google Scholar] [CrossRef]
- Jouanin, A.; Schaart, J.G.; Boyd, L.A.; Cockram, J.; Leigh, F.J.; Bates, R.; Wallington, E.J.; Visser, R.G.F.; Smulders, M.J.M. Outlook for Coeliac Disease Patients: Towards Bread Wheat with Hypoimmunogenic Gluten by Gene Editing of α- And γ-Gliadin Gene Families. BMC Plant Biol. 2019, 19, 33. [Google Scholar] [CrossRef]
- Jouanin, A.; Boyd, L.; Visser, R.G.F.; Smulders, M.J.M. Development of Wheat with Hypoimmunogenic Gluten Obstructed by the Gene Editing Policy in Europe. Front. Plant Sci. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Huilian, C.; Fu, L. Detoxification Mechanism and the Impact of Transamidation-Modified Gliadin on Celiac-Based Gluten Sensitivity: The Potential of Unlocking Gluten Tolerance in Functional Food. J. Agric. Food Chem. 2025, 73, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Gil-Humanes, J.; Pistón, F.; Tollefsen, S.; Sollid, L.M.; Barro, F. Effective Shutdown in the Expression of Celiac Disease-Related Wheat Gliadin T-Cell Epitopes by RNA Interference. Proc. Natl. Acad. Sci. USA 2010, 107, 17023–17028. [Google Scholar] [CrossRef]
- Gianfrani, C.; Siciliano, R.A.; Facchiano, A.M.; Camarca, A.; Mazzeo, M.F.; Costantini, S.; Salvati, V.M.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; et al. Transamidation of Wheat Flour Inhibits the Response to Gliadin of Intestinal T Cells in Celiac Disease. Gastroenterology 2007, 133, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Salvati, V.M.; Iaquinto, G.; Stefanile, R.; Capobianco, F.; Luongo, D.; Bergamo, P.; Maurano, F.; Giardullo, N.; Malamisura, B.; et al. Reintroduction of Gluten Following Flour Transamidation in Adult Celiac Patients: A Randomized, Controlled Clinical Study. Clin. Dev. Immunol. 2012, 2012, 329150. [Google Scholar] [CrossRef]
- Heredia-Sandoval, N.G.; Islas-Rubio, A.R.; Cabrera-Chávez, F.; Calderón De La Barca, A.M. Transamidation of Gluten Proteins during the Bread-Making Process of Wheat Flour to Produce Breads with Less Immunoreactive Gluten. Food Funct. 2014, 5, 1813–1818. [Google Scholar] [CrossRef]
- De Sena, V.; Mazzarella, G.; Rossi, M. Effects of Transamidation vs. Fermentation to Reduce the Gluten Content of the Triticum Monococcum Wheat Cultivar Hammurabi: Analysis of Biochemical, Baking and Sensory Parameters. Int. J. Food Sci. Technol. 2024, 59, 1927–1934. [Google Scholar] [CrossRef]
- Lamacchia, C.; Di Luccia, A.; Gianfrani, C. Method for the Detoxification of Gluten Proteins from Grains of Cereal 2015. U.S. Patent Application No. 14/432,461, 1 October 2015. [Google Scholar]
- Open Access Government Gluten Friendly Technology Fixes Proteins in Wheat Kernels. Available online: https://www.openaccessgovernment.org/gluten-friendly-technology-kernels/36411/ (accessed on 17 July 2025).
- Andriulli, A.; Bevilacqua, A.; Palmieri, O.; Latiano, A.; Fontana, R.; Gioffreda, D.; Castellana, S.; Mazza, T.; Panza, A.; Menzaghi, C.; et al. Healthy and Pro-Inflammatory Gut Ecology Plays a Crucial Role in the Digestion and Tolerance of a Novel Gluten FriendlyTM Bread in Celiac Subjects: A Randomized, Double Blind, Placebo Control in Vivo Study. Food Funct. 2022, 13, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). In Vivo Effects of the Gluten Friendly Bread in Coeliac Disease. Available online: https://clinicaltrials.gov/study/NCT03168490?intr=gluten%20friendly&rank=1disease (accessed on 17 July 2025).
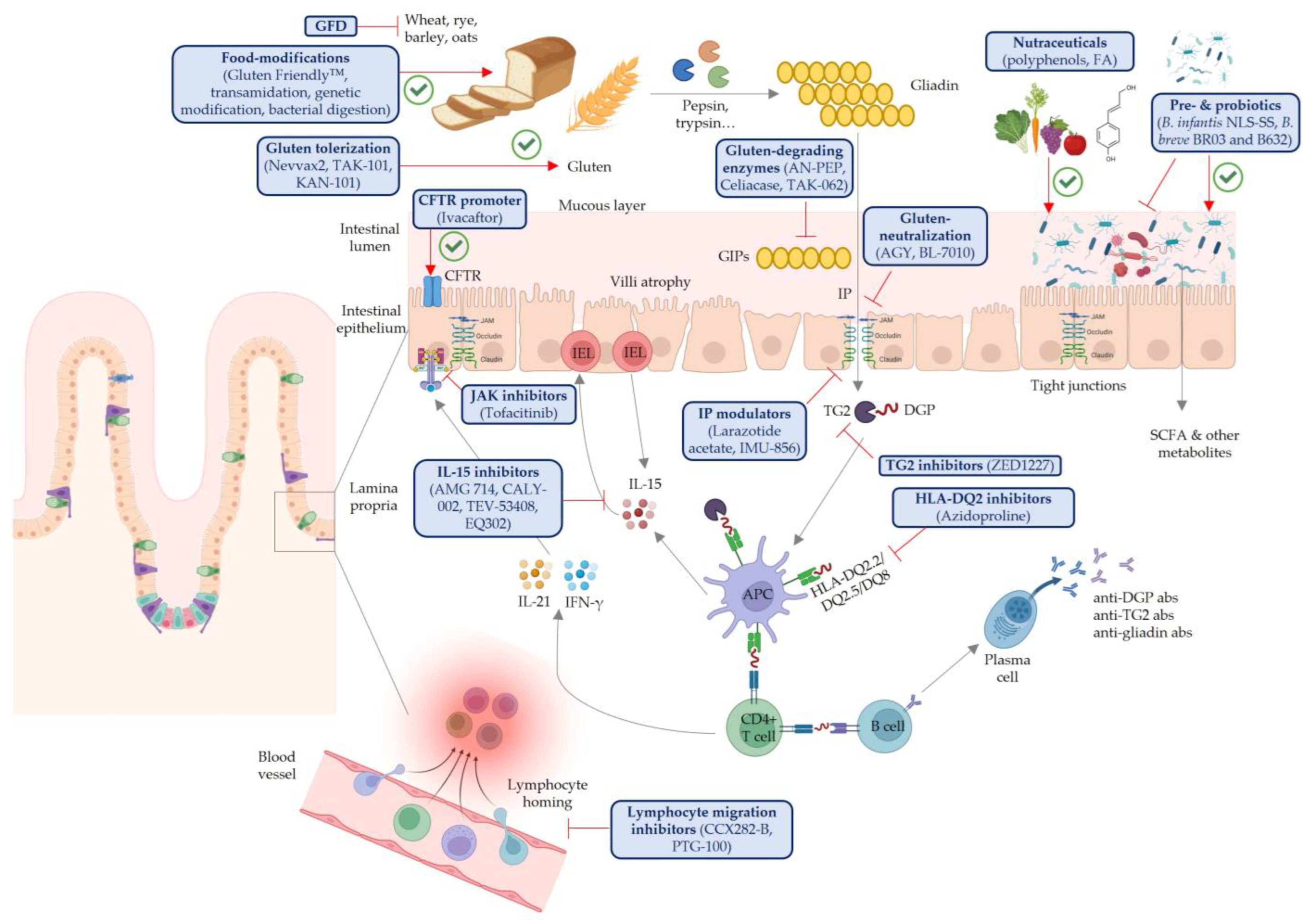
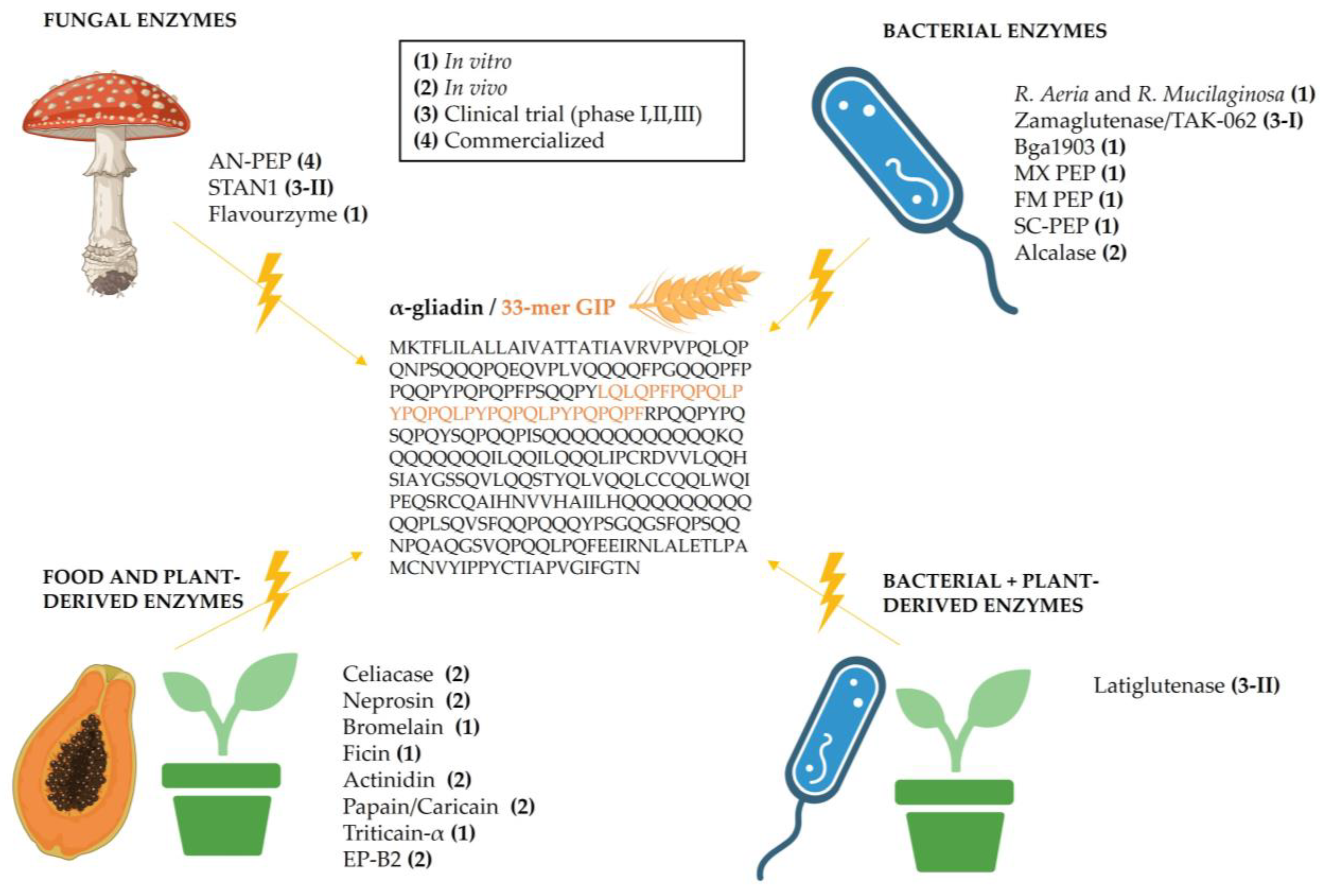
| Strategy | Active Principle | Name | Phase, Clinical Trial | Sponsor | Ref. |
|---|---|---|---|---|---|
| Gluten neutralization | Anti-gliadin IgY from the egg yolk of hypersensitized hens | AGY-010 | Phase 1 (NCT01765647), Phase 2 (NCT03707730) | Igy Inc. | [53,54,55] |
| (P(HEMA-co-SS)) copolymer | BL-7010 | Phase 1 and 2 (NCT01990885) | BioLineRx, Ltd. | [56,57,58] | |
| Intestinal permeability modulators | Octapeptide inhibitor of paracellular permeability (TJs modulator) | Larazotide acetate (AT-1001) | Phase 1 (NCT00386165, NCT00386490), Phase 2 (NCT00362856, NCT00492960, NCT00620451, NCT00889473, NCT01396213), Phase 3–terminated (NCT03569007) | 9 Meters Biopharma, Inc. | [59,60,61,62,63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girbal-González, M.; Pérez-Cano, F.J. Is There a Future Without Gluten Restrictions for Celiac Patients? Update on Current Treatments. Nutrients 2025, 17, 2960. https://doi.org/10.3390/nu17182960
Girbal-González M, Pérez-Cano FJ. Is There a Future Without Gluten Restrictions for Celiac Patients? Update on Current Treatments. Nutrients. 2025; 17(18):2960. https://doi.org/10.3390/nu17182960
Chicago/Turabian StyleGirbal-González, Marina, and Francisco J. Pérez-Cano. 2025. "Is There a Future Without Gluten Restrictions for Celiac Patients? Update on Current Treatments" Nutrients 17, no. 18: 2960. https://doi.org/10.3390/nu17182960
APA StyleGirbal-González, M., & Pérez-Cano, F. J. (2025). Is There a Future Without Gluten Restrictions for Celiac Patients? Update on Current Treatments. Nutrients, 17(18), 2960. https://doi.org/10.3390/nu17182960







