Vitamins, Vascular Health and Disease
Abstract
1. Vitamins in Vascular Health
1.1. Vitamins Essential for Endothelial Health: Folate
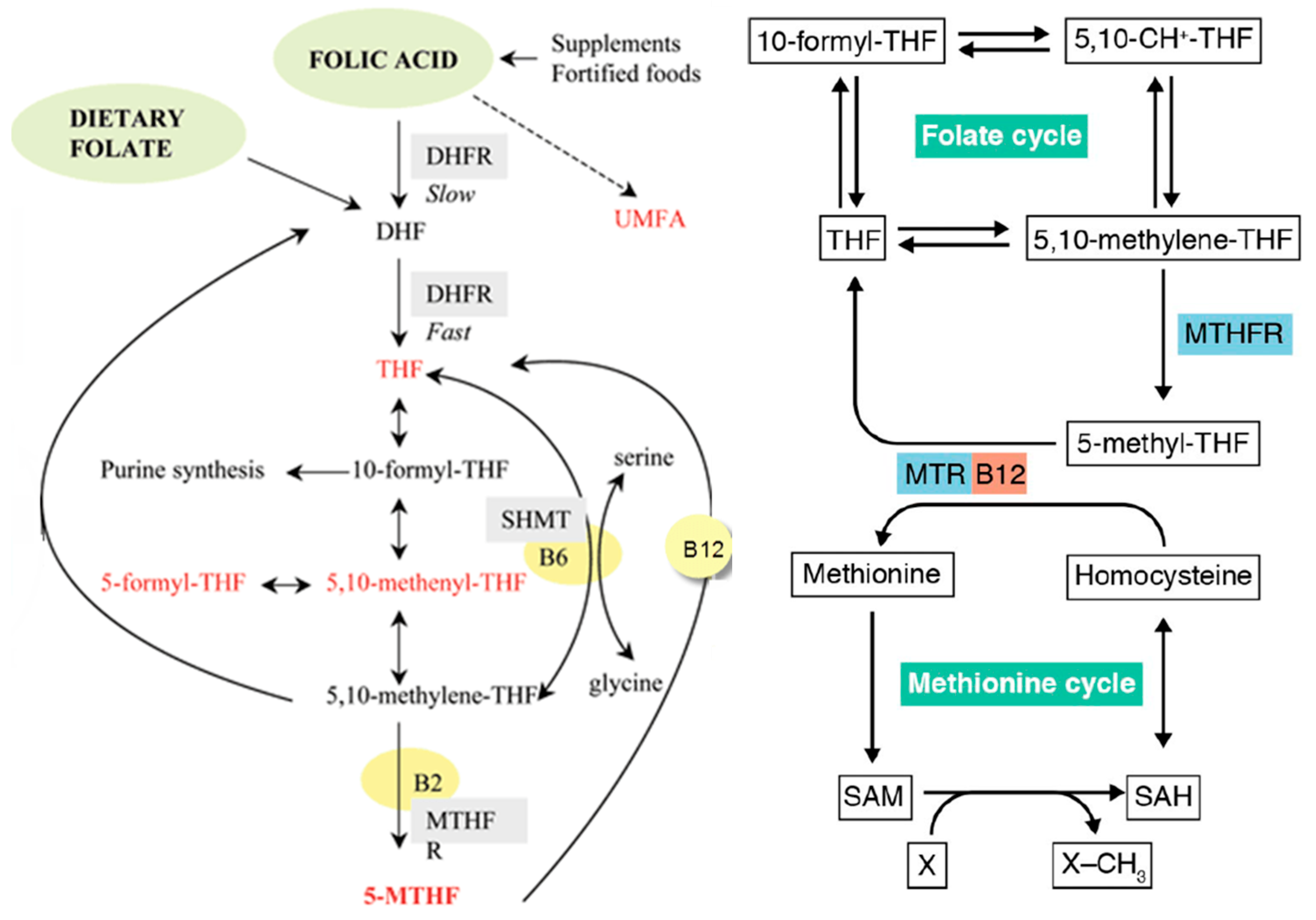
1.1.1. Steps in the Folate Pathway
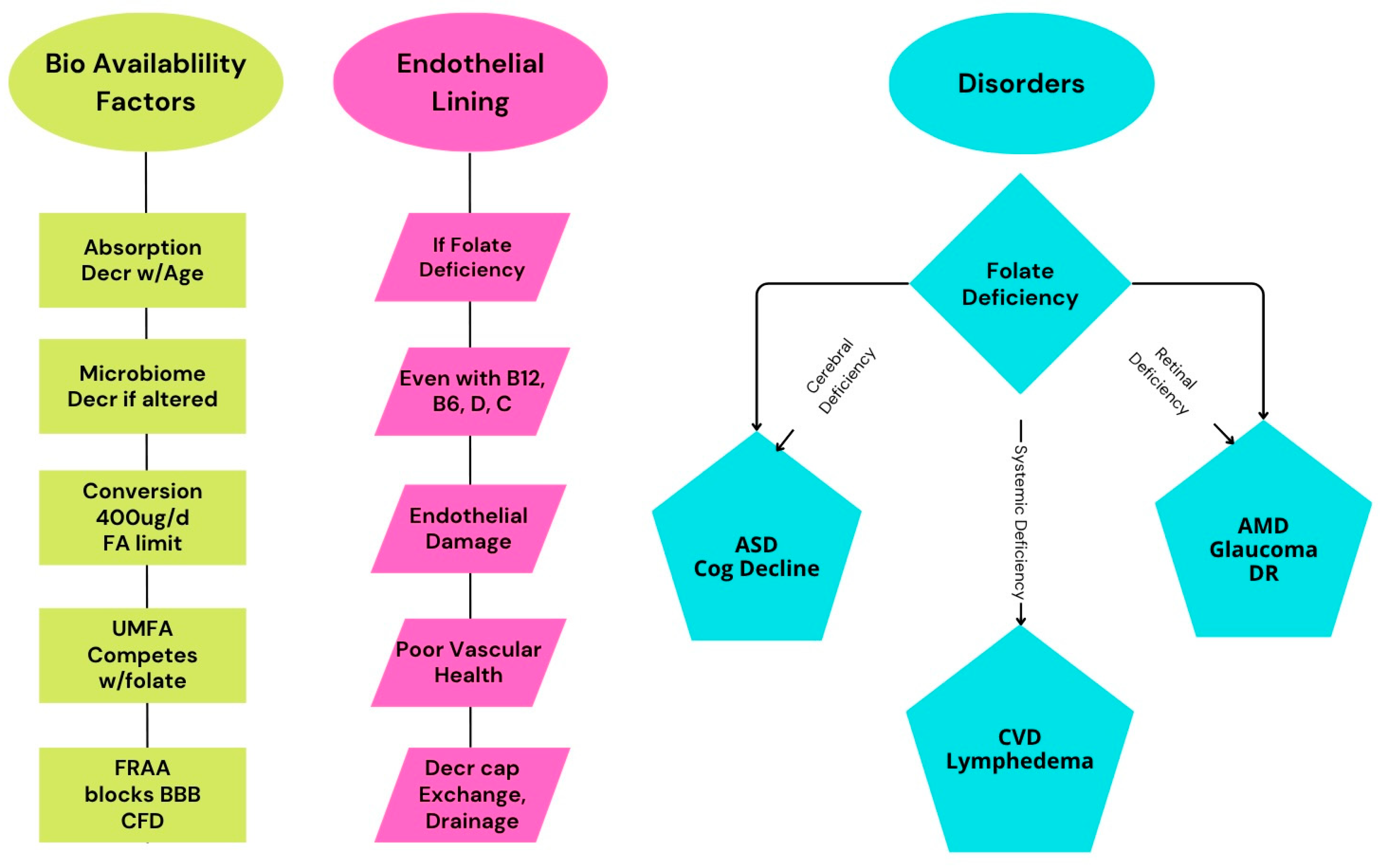
1.1.2. Folate Deficiency/Insufficiency and Vascular Dysfunction
1.2. Vitamins Essential for Endothelial Health: Pyridoxine and Cobalamin
1.3. Vitamins Essential for Endothelial Health: Ascorbic Acid
1.4. Vitamins Essential for Endothelial Health: Vitamin D
1.5. Vitamins Essential for Endothelial Health: Tocopherol and Vitamin K2
1.6. Public Health Aspects
1.6.1. Nutritional Sufficiency
1.6.2. Genetic Polymorphisms
1.6.3. Supplementation and Disease Prevention
2. Folate Sources, Absorption, and Bioavailability
2.1. Folate from Foods
2.2. Bioavailability and Absorption
2.3. Genetic Influences on Folate Metabolism
2.4. Folate Transport into the Brain
3. Cardiovascular Health
3.1. Folate and Endothelial Function
3.2. Evidence for Improved Blood Flow
3.3. Clinical Evidence
4. Peripheral Circulation, Lymphedema, and Glymphatic Function
4.1. Folate and Peripheral Circulation
4.2. Folate and Lymphedema
4.3. Glymphatic System
5. Retinal Vascularization and Retinal Disease
5.1. Retinal Hyperhomocysteine and Vascular Dysfunction
5.2. Retinal Oxidative Stress and Antioxidants
5.3. Clinical Cases
6. Neurodegenerative Disorders: Dementia and Cognitive Decline
6.1. Neurodegenerative Disease and Folate
6.2. Folate Deficiency Linked to Cognitive Decline
6.3. Folate Supplementation Slows Cognitive Decline
6.4. Efficacy of Folate in Slowing Cognitive Decline
6.5. Risks of Excessive Folate in Disease
6.5.1. Excess Folic Acid and Increased Mortality Risk
6.5.2. Excess Folic Acid and Attenuated Cognitive Benefits
6.5.3. Pathways of Harm from Excess Folic Acid
- Folate oversaturation in fortified populations: Over 50% of US CVD patients already meet the recommended folate intake, yet 25% still use supplements [96].
- Interaction with CVD pathophysiology: High folate may accelerate atherosclerosis progression in established CVD through poorly understood mechanisms [106].
- Homocysteine paradox: While folate lowers homocysteine, excessive supplementation in CVD patients shows no mortality benefit despite homocysteine reduction [108].
- Masking vitamin B12 deficiency: High folate intake can mask hematological signs of vitamin B12 deficiency, which is common in older adults. This can allow neurological damage from B12 deficiency to progress undetected, potentially leading to irreversible cognitive and neurological impairment [59].
- Potential for unmetabolized folic acid (UMFA): A high intake of synthetic folic acid (from supplements/fortified foods) can lead to unmetabolized folic acid in the bloodstream, which has been linked to impaired immune function and possibly an increased cancer risk [58,109]. This risk from unmetabolized folic acid, when found during pregnancy and lactation, may also be associated with an increased risk of neurodevelopmental disorders such as autism [110,111], as well as with a tripling in the rate of gestational diabetes [112]. Given that excessive folic acid supplementation is the cause of UMFA, there is reason to reconsider the amount supplemented. In the US, supplementation provides about 200 µg on average, though some individuals have much higher levels, greatly exceeding the 400 µg daily conversion in the body. Switching to L-methylfolate would reduce this risk.
6.5.4. Guidelines for Folate Supplementation in CVD Patients
- Avoid high-dose folic acid supplements (>400 μg/day) in older adults with CVD unless prescribed for a specific deficiency.
- Monitor vitamin B12 status in older adults taking folic acid, especially those at risk of deficiency.
- Prioritize dietary sources of folate (leafy greens and legumes) over high-dose supplements, unless medically indicated.
- When supplementation is warranted, prioritize the use of methylfolate.
- Personalize supplementation based on individual risk factors, comorbidities, and baseline folate/B12 status.
- Consider high-dose treatment with a drug product containing leucovorin or levoleucovring based on specific diagnostic tests (such as genetic tests and the FRAA test).
7. Neurodevelopmental Disorders: Autism Spectrum Disorder
7.1. Folate’s Role in Brain Formation During Pregnancy
7.2. Maternal Folate Deficiency and Autism Risk
7.2.1. Impaired DNA Synthesis and Neuronal Repair
7.2.2. Homocysteine Accumulation and Neurotoxicity
7.2.3. Disrupted Methylation and Epigenetic Regulation
7.2.4. Altered Neurotransmitter Synthesis
7.2.5. Oxidative Stress and Inflammation
7.3. Critical Periods for Folate and Autism
7.3.1. Insights from Folate Supplementation in Reducing Risk of Autism
7.3.2. DNA Synthesis and Cellular Proliferation
- Neural Tube Closure. During the first month of pregnancy, folate is critical for the closure of the neural tube—a process that, if disrupted, results in severe neural tube defects such as spina bifida and anencephaly. This is why periconceptional folic acid supplementation is universally recommended to women planning pregnancy [119,136,137,138].
- Epigenetic Regulation and Gene Expression. Folate acts as a methyl donor in one-carbon metabolism, which is required for DNA methylation. Proper methylation regulates the gene expression patterns necessary for normal brain development. Disrupted methylation due to folate deficiency can lead to long-lasting neurodevelopmental and cognitive changes in offspring [119,136,137].
- Prevention of Cell Death (Apoptosis). Adequate maternal folate reduces apoptosis (programmed cell death) in developing brain regions, ensuring proper formation of neural circuits [137].
7.3.3. Insights from Folate Supplementation in Reducing Risk of Autism
- Very high maternal folate/B12 levels late in pregnancy may be associated with an increased ASD risk, suggesting that moderation is important [132].
- Plasma/whole-blood folate levels alone are less predictive than supplement use, possibly due to timing and individual metabolism [132].
- The use of leucovorin during the periconceptional period and throughout gestation may mitigate the risk associated with gene variants and maternal FRAA [135].
7.4. Leucovorin Clinical Trials
- Bypasses Blocked Folate Transport. Leucovorin is rapidly metabolized to L-methylfolate, which can cross the blood–brain barrier via alternative transporters, even when folate receptor alpha is blocked by autoantibodies or the individual has a genetic polymorphism reducing active transport.
- Supports Mitochondrial Function. Leucovorin may enhance mitochondrial energy production, reduce oxidative stress, and improve neuronal metabolism.
- Restores Methylation. As a methyl donor, it supports DNA methylation and neurotransmitter synthesis, processes often disrupted in ASD.
8. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L-5-MTHF | 5-methyl-(6S)-tetrahydrofolate, L-methylfolate |
| MTHFR | MTHF reductase |
| DHFR | Dihydrofolate reductase |
| CVD | Cardiovascular disease |
| FMD | Flow-mediated diffusion |
| CAD | Coronary artery disease |
| VEGF | Vascular endothelial growth factor |
| UMFA | Unmetabolized folic acid |
| DFE | Dietary folate equivalent |
| CFD | Cerebral folate deficiency |
| AMD | Age-related macular degeneration |
| MCI | Mild cognitive impairment |
| ASD | Autism spectrum disorder |
References
- Josifova, T.; Konieczka, K.; Schötzau, A.; Flammer, J. The Effect of a Specific Vitamin Supplement Containing L-Methylfolate (Ocufolin Forte) in Patients with Neovascular Age-Related Macular Degeneration. Adv. Ophthalmol. Pract. Res. 2025, 5, 135–141. [Google Scholar] [CrossRef]
- Ayoub, G. Treatment of Primary Lymphedema Following Lessons from Endothelin-Driven Retinal Edema, a Case Report. Heal. TIMES Schweiz. Ärztejournal J. Médecins Suisses 2024, 14, 10–13. [Google Scholar]
- Stanhewicz, A.E.; Kenney, W.L. Role of Folic Acid in Nitric Oxide Bioavailability and Vascular Endothelial Function. Nutr. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef]
- Baddam, S.; Khan, K.M.; Jialal, I. Folic Acid Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development—Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Doshi, S.N.; McDowell, I.F.W.; Moat, S.J.; Lang, D.; Newcombe, R.G.; Kredan, M.B.; Lewis, M.J.; Goodfellow, J. Folate Improves Endothelial Function in Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1196–1202. [Google Scholar] [CrossRef]
- Ayoub, G.; Luo, Y. Ischemia from Retinal Vascular Hypertension in Normal Tension Glaucoma: Neuroprotective Role of Folate. Am. J. Biomed. Sci. Res. 2023, 20, 861. [Google Scholar] [CrossRef]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.M. Folate and Folic Acid Metabolism: A Significant Nutrient-Gene-Environment Interaction. Med. Res. Arch. 2023, 11, 3824. [Google Scholar] [CrossRef]
- Zheng, Y.; Cantley, L.C. Toward a Better Understanding of Folate Metabolism in Health and Disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef]
- Obeid, R. The Metabolic Burden of Methyl Donor Deficiency with Focus on the Betaine Homocysteine Methyltransferase Pathway. Nutrients 2013, 5, 3481–3495. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Rezaiian, F.; Saadati, S.; Naseri, K.; Ashtary-Larky, D.; Yousefi, M.; Golalipour, E.; Clark, C.C.T.; Rastgoo, S.; Asbaghi, O. The Effects of Folic Acid Supplementation on Endothelial Function in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2023, 22, 12. [Google Scholar] [CrossRef]
- Sakakeeny, L.; Roubenoff, R.; Obin, M.; Fontes, J.D.; Benjamin, E.J.; Bujanover, Y.; Jacques, P.F.; Selhub, J. Plasma Pyridoxal-5-Phosphate Is Inversely Associated with Systemic Markers of Inflammation in a Population of U.S. Adults. J. Nutr. 2012, 142, 1280–1285. [Google Scholar] [CrossRef]
- Green, R.; Miller, J.W. Vitamin B12 Deficiency. Vitam. Horm. 2022, 119, 405–439. [Google Scholar] [CrossRef] [PubMed]
- Julian, T.; Syeed, R.; Glascow, N.; Angelopoulou, E.; Zis, P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients 2020, 12, 2221. [Google Scholar] [CrossRef]
- McNulty, H.; Pentieva, K.; Hoey, L.; Ward, M. Homocysteine, B-Vitamins and CVD. Proc. Nutr. Soc. 2008, 67, 232–237. [Google Scholar] [CrossRef]
- Deepika; Kumari, A.; Singh, S.; Ahmad, M.F.; Chaki, D.; Poria, V.; Kumar, S.; Saini, N.; Yadav, N.; Sangwan, N.; et al. Vitamin D: Recent Advances, Associated Factors, and Its Role in Combating Non-Communicable Diseases. NPJ Sci. Food 2025, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Ali, S.H.; Naeem, A.; Savul, S.; Afridi, M.S.I.; Kamran, N.; Fazal, F.; Khawer, S.; Savul, I.S.; Najeeb, H.; et al. Current Evidence and Future Perspectives of the Best Supplements for Cardioprotection: Have We Reached the Final Chapter for Vitamins? Rev. Cardiovasc. Med. 2022, 23, 381. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Cheng, R.Z.; Pludowski, P.; Wimalawansa, S.J. Vitamin D and Cardiovascular Health: A Narrative Review of Risk Reduction Evidence. Nutrients 2025, 17, 2102. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Peng, Y.; Wang, M.; Wang, W.; Shi, F.; Wang, Y.; Hua, L. The Effect of Vitamin D Supplementation on Endothelial Function: An Umbrella Review of Interventional Meta-Analyses. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103871. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar] [CrossRef]
- Al Mheid, I.; Patel, R.; Murrow, J.; Morris, A.; Rahman, A.; Fike, L.; Kavtaradze, N.; Uphoff, I.; Hooper, C.; Tangpricha, V.; et al. Vitamin D Status Is Associated with Arterial Stiffness and Vascular Dysfunction in Healthy Humans. J. Am. Coll. Cardiol. 2011, 58, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hariri, E.; Kassis, N.; Iskandar, J.-P.; Schurgers, L.J.; Saad, A.; Abdelfattah, O.; Bansal, A.; Isogai, T.; Harb, S.C.; Kapadia, S. Vitamin K2—A Neglected Player in Cardiovascular Health: A Narrative Review. Open Heart 2021, 8, e001715. [Google Scholar] [CrossRef]
- Ryu, T.; Chae, S.Y.; Lee, J.; Han, J.W.; Yang, H.; Chung, B.S.; Yang, K. Multivitamin Supplementation and Its Impact in Metabolic Dysfunction-Associated Steatotic Liver Disease. Sci. Rep. 2025, 15, 8675. [Google Scholar] [CrossRef]
- Folate (Folic Acid)—Vitamin B9 • The Nutrition Source. Available online: https://nutritionsource.hsph.harvard.edu/folic-acid/ (accessed on 4 August 2025).
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Manolis, A.S. Role of Vitamins in Cardiovascular Health: Know Your Facts-Part 2. Curr. Vasc. Pharmacol. 2023, 21, 399–423. [Google Scholar] [CrossRef]
- Institute for Natural Medicine Staff. Fact Sheet: Folate (Vitamin B9) & Folic Acid; Institute for Natural Medicine: Seattle, WA, USA, 2024. [Google Scholar]
- Hardy, K.K.; Embry, L.; Kairalla, J.A.; Helian, S.; Devidas, M.; Armstrong, D.; Hunger, S.; Carroll, W.L.; Larsen, E.; Raetz, E.A.; et al. Neurocognitive Functioning of Children Treated for High-Risk B-Acute Lymphoblastic Leukemia Randomly Assigned to Different Methotrexate and Corticosteroid Treatment Strategies: A Report from the Children’s Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2700–2707. [Google Scholar] [CrossRef]
- Caudill, M.A. Folate Bioavailability: Implications for Establishing Dietary Recommendations and Optimizing Status. Am. J. Clin. Nutr. 2010, 91, 1455S–1460S. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Folate. Available online: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ (accessed on 4 August 2025).
- Zheng, J.; Wang, X.; Wu, B.; Qiao, L.; Zhao, J.; Pourkheirandish, M.; Wang, J.; Zheng, X. Folate (Vitamin B9) Content Analysis in Bread Wheat (Triticum aestivum L.). Front. Nutr. 2022, 9, 933358. [Google Scholar] [CrossRef]
- Merrell, B.J.; McMurry, J.P. Folic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tate, C.; Shuman, A.; Nice, S.; Salehi, P. The Critical Role of Folate in Prenatal Health and a Proposed Shift from Folic Acid to 5-Methyltetrahydrofolate Supplementation. Georget. Med. Rev. 2024, 8, 124570. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic Acid Improves Verbal Communication in Children with Autism and Language Impairment: A Randomized Double-Blind Placebo-Controlled Trial. Mol. Psychiatry 2018, 23, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Bjørke-Monsen, A.-L.; Ueland, P.M. Folate—A Scoping Review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10258. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.J.; Heid, M.K.; Peerson, J.M.; Bills, N.D. Bioavailability of Food Folates and Evaluation of Food Matrix Effects with a Rat Bioassay. J. Nutr. 1991, 121, 445–453. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.A.; Groom, A.; Potter, C.; Coneyworth, L.J.; Ford, D.; Mathers, J.C.; Relton, C.L. Genetic and Non-Genetic Influences during Pregnancy on Infant Global and Site Specific DNA Methylation: Role for Folate Gene Variants and Vitamin B12. PLoS ONE 2012, 7, e33290. [Google Scholar] [CrossRef]
- Coppedè, F. The Genetics of Folate Metabolism and Maternal Risk of Birth of a Child with Down Syndrome and Associated Congenital Heart Defects. Front. Genet. 2015, 6, 223. [Google Scholar] [CrossRef]
- van der Linden, I.J.M.; Afman, L.A.; Heil, S.G.; Blom, H.J. Genetic Variation in Genes of Folate Metabolism and Neural-Tube Defect Risk. Proc. Nutr. Soc. 2006, 65, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Au, K.; Findley, T.; Northrup, H. Finding the Genetic Mechanisms of Folate Deficiency and Neural Tube Defects—Leaving No Stone Unturned. Am. J. Med. Genet. A. 2017, 173, 3042–3057. [Google Scholar] [CrossRef]
- Devogelaere, T.; Schôtzau, A. The Effects of Vitamin Supplementation Containing L-Methylfolate (Ocufolin® Forte) on Retinal Venous Pressure and Homocysteine Plasma Levels in Patients with Glaucoma. Heakthbook TIMES 2021, 3, 54–59. [Google Scholar] [CrossRef]
- Lee, I.; Piao, S.; Kim, S.; Nagar, H.; Choi, S.-J.; Jeon, B.H.; Oh, S.-H.; Irani, K.; Kim, C.-S. CRIF1 Deficiency Increased Homocysteine Production by Disrupting Dihydrofolate Reductase Expression in Vascular Endothelial Cells. Antioxidants 2021, 10, 1645. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Yu, C.; Deng, Y.; Wen, Q.; Yang, H.; Liu, H.; Luo, R. Case Report: Cerebral Folate Deficiency Caused by FOLR1 Variant. Front. Pediatr. 2024, 12, 1434209. [Google Scholar] [CrossRef]
- Skavinska, O.; Rossokha, Z.; Fishchuk, L.; Gorovenko, N. RFC and VDR -Mediated Genetic Regulation of Brain Folate Transport in Patients with Multiple Sclerosis. Hum. Gene 2025, 44, 201399. [Google Scholar] [CrossRef]
- Stefanyshyn, V.; Stetsyuk, R.; Hrebeniuk, O.; Ayoub, G.; Fishchuk, L.; Rossokha, Z.; Gorovenko, N. Analysis of the Association Between the SLC19A1 Genetic Variant (Rs1051266) and Autism Spectrum Disorders, Cerebral Folate Deficiency, and Clinical and Laboratory Parameters. J. Mol. Neurosci. MN 2025, 75, 42. [Google Scholar] [CrossRef]
- Ayoub, G. Autism Spectrum Disorder as a Multifactorial Disorder: The Interplay of Genetic Factors and Inflammation. Int. J. Mol. Sci. 2025, 26, 6483. [Google Scholar] [CrossRef] [PubMed]
- McDowell, I.F.W.; Lang, D. Homocysteine and Endothelial Dysfunction: A Link with Cardiovascular Disease. J. Nutr. 2000, 130, 369S–372S. [Google Scholar] [CrossRef]
- Moat, S.J.; Lang, D.; McDowell, I.F.W.; Clarke, Z.L.; Madhavan, A.K.; Lewis, M.J.; Goodfellow, J. Folate, Homocysteine, Endothelial Function and Cardiovascular Disease. J. Nutr. Biochem. 2004, 15, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, M.C.; Stroes, E.; Rabelink, T.J. Folates and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 6–13. [Google Scholar] [CrossRef]
- Clarke, R.; Bennett, D.A.; Parish, S.; Verhoef, P.; Dötsch-Klerk, M.; Lathrop, M.; Xu, P.; Nordestgaard, B.G.; Holm, H.; Hopewell, J.C.; et al. Homocysteine and Coronary Heart Disease: Meta-Analysis of MTHFR Case-Control Studies, Avoiding Publication Bias. PLoS Med. 2012, 9, e1001177. [Google Scholar] [CrossRef]
- McRae, M.P. High-Dose Folic Acid Supplementation Effects on Endothelial Function and Blood Pressure in Hypertensive Patients: A Meta-Analysis of Randomized Controlled Clinical Trials. J. Chiropr. Med. 2009, 8, 15–24. [Google Scholar] [CrossRef]
- Varadharaj, S.; Kelly, O.J.; Khayat, R.N.; Kumar, P.S.; Ahmed, N.; Zweier, J.L. Role of Dietary Antioxidants in the Preservation of Vascular Function and the Modulation of Health and Disease. Front. Cardiovasc. Med. 2017, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, T.; Zheng, Y.; Muka, T.; Troup, J.; Hu, F.B. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e003768. [Google Scholar] [CrossRef]
- Xu, X.; Wei, W.; Jiang, W.; Song, Q.; Chen, Y.; Li, Y.; Zhao, Y.; Sun, H.; Yang, X. Association of Folate Intake with Cardiovascular-Disease Mortality and All-Cause Mortality among People at High Risk of Cardiovascular-Disease. Clin. Nutr. Edinb. Scotl. 2022, 41, 246–254. [Google Scholar] [CrossRef]
- Otsu, Y.; Ae, R.; Kuwabara, M. Folate and Cardiovascular Disease. Hypertens. Res. 2023, 46, 1816–1818. [Google Scholar] [CrossRef]
- Loria, C.M.; Ingram, D.D.; Feldman, J.J.; Wright, J.D.; Madans, J.H. Serum Folate and Cardiovascular Disease Mortality Among US Men and Women. Arch. Intern. Med. 2000, 160, 3258–3262. [Google Scholar] [CrossRef]
- Bailey, S.W.; Ayling, J.E. The Extremely Slow and Variable Activity of Dihydrofolate Reductase in Human Liver and Its Implications for High Folic Acid Intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef]
- Fardous, A.M.; Heydari, A.R. Uncovering the Hidden Dangers and Molecular Mechanisms of Excess Folate: A Narrative Review. Nutrients 2023, 15, 4699. [Google Scholar] [CrossRef]
- Folate—Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK114318/ (accessed on 5 August 2025).
- Reynolds, E.H.; Sobczyńska-Malefora, A.; Green, R. Fortification, Folate and Vitamin B12 Balance, and the Nervous System. Is Folic Acid Excess Potentially Harmful? Eur. J. Clin. Nutr. 2025. [Google Scholar] [CrossRef]
- Study Details|NCT00317005|Uremic Hyperhomocysteinemia—A Folate Trial for Possible Prevention of Cardio-Vascular Events|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT00317005 (accessed on 11 September 2025).
- Tawakol, A.; Migrino, R.Q.; Aziz, K.S.; Waitkowska, J.; Holmvang, G.; Alpert, N.M.; Muller, J.E.; Fischman, A.J.; Gewirtz, H. High-Dose Folic Acid Acutely Improves Coronary Vasodilator Function in Patients with Coronary Artery Disease. JACC 2005, 45, 1580–1584. [Google Scholar] [CrossRef]
- Gori, T.; Burstein, J.M.; Ahmed, S.; Miner, S.E.S.; Al-Hesayen, A.; Kelly, S.; Parker, J.D. Folic Acid Prevents Nitroglycerin-Induced Nitric Oxide Synthase Dysfunction and Nitrate Tolerance. Circulation 2001, 104, 1119–1123. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Lolin, Y.I.; Sanderson, J.E.; Metreweli, C.; Celermajer, D.S. Folic Acid Improves Arterial Endothelial Function in Adults with Hyperhomocystinemia. J. Am. Coll. Cardiol. 1999, 34, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Tang, W.H.W. Targeting the Lymphatic System for Interstitial Decongestion. JACC Basic Transl. Sci. 2021, 6, 882–884. [Google Scholar] [CrossRef]
- Brown, S.; Campbell, A.C.; Kuonqui, K.; Sarker, A.; Park, H.J.; Shin, J.; Kataru, R.P.; Coriddi, M.; Dayan, J.H.; Mehrara, B.J. The Future of Lymphedema: Potential Therapeutic Targets for Treatment. Curr. Breast Cancer Rep. 2023, 5, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Dayan, J.H.; Coriddi, M.; Campbell, A.; Kuonqui, K.; Shin, J.; Park, H.J.; Mehrara, B.J.; Kataru, R.P. Pharmacological Treatment of Secondary Lymphedema. Front. Pharmacol. 2022, 13, 828513. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Nedergaard, M. The Glymphatic System: A Novel Component of Fundamental Neurobiology. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 7698–7711. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, K.; Zhu, J. Glymphatic System: An Emerging Therapeutic Approach for Neurological Disorders. Front. Mol. Neurosci. 2023, 16, 1138769. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System—A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, H.; Nedergaard, M. The Glymphatic System. Handb. Clin. Neurol. 2025, 209, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Beschorner, N.; Nedergaard, M. Glymphatic System Dysfunction in Neurodegenerative Diseases. Curr. Opin. Neurol. 2024, 37, 182–188. [Google Scholar] [CrossRef]
- Corbali, O.; Levey, A.I. Glymphatic System in Neurological Disorders and Implications for Brain Health. Front. Neurol. 2025, 16, 1543725. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Bagley, J.H.; Geltzeiler, M.; Sanusi, O.R.; Dogan, A.; Liu, J.J.; Piantino, J. The Perivascular Space Is a Conduit for Cerebrospinal Fluid Flow in Humans: A Proof-of-Principle Report. Proc. Natl. Acad. Sci. USA 2024, 121, e2407246121. [Google Scholar] [CrossRef]
- Baker, S.; Baker, D.; Baker, R.; Brown, C.J. Case Series of Retinal Vein Occlusions Showing Early Recovery Using Oral L-Methylfolate. Ther. Adv. Ophthalmol. 2024, 16, 25158414241240687. [Google Scholar] [CrossRef]
- Gu, J.; Lei, C.; Zhang, M. Folate and Retinal Vascular Diseases. BMC Ophthalmol. 2023, 23, 413. [Google Scholar] [CrossRef]
- Mohamed, R.; Sharma, I.; Ibrahim, A.S.; Saleh, H.; Elsherbiny, N.M.; Fulzele, S.; Elmasry, K.; Smith, S.B.; Al-Shabrawey, M.; Tawfik, A. Hyperhomocysteinemia Alters Retinal Endothelial Cells Barrier Function and Angiogenic Potential via Activation of Oxidative Stress. Sci. Rep. 2017, 7, 11952. [Google Scholar] [CrossRef]
- Mysona, B.A.; Jiang, G.; Martin-Studdard, A.; Roon, P.; Moister, B.; Ganapathy, V.; Smith, S.B. Effects of Folate Supplementation on Homocysteine-Induced Retinopathy in Cbs Mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 633. [Google Scholar]
- Flammer, J.; Konieczka, K. Retinal Venous Pressure: The Role of Endothelin. EPMA J. 2015, 6, 21. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Sharma, I.; Kira, D.; Alhusban, S.; Samra, Y.A.; Jadeja, R.; Martin, P.; Al-Shabrawey, M.; Tawfik, A. Homocysteine Induces Inflammation in Retina and Brain. Biomolecules 2020, 10, 393. [Google Scholar] [CrossRef]
- George, A. Managing Normal Tension Glaucoma with Dietary Folate. J. Clin. Res. Ophthalmol. 2024, 11, 017–023. [Google Scholar] [CrossRef]
- Navneet, S.; Cui, X.; Zhao, J.; Wang, J.; Kaidery, N.A.; Thomas, B.; Bollinger, K.E.; Yoon, Y.; Smith, S.B. Excess Homocysteine Upregulates the NRF2-Antioxidant Pathway in Retinal Müller Glial Cells. Exp. Eye Res. 2019, 178, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.R.B.; Suganeswari, G.; Sharma, T.; Thennarasu, M.; Angayarkanni, N. Homocysteine Induces Oxidative Stress in Young Adult Central Retinal Vein Occlusion. Br. J. Ophthalmol. 2012, 96, 1122–1126. [Google Scholar] [CrossRef]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative Stress in the Eye and Its Role in the Pathophysiology of Ocular Diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Samra, Y.A.; Elsherbiny, N.M.; Al-Shabrawey, M. Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules 2020, 10, 1119. [Google Scholar] [CrossRef]
- Brown, C.; Wang, J.; Jiang, H.; Elias, M.F. Homocysteine Reduction for Stroke Prevention: Regarding the Recent AHA/ASA 2021 Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack. Pharmacogenomics Pers. Med. 2023, 16, 895–900. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimer’s Dis. JAD 2018, 62, 561–570. [Google Scholar] [CrossRef]
- Kronenberg, G.; Harms, C.; Sobol, R.W.; Cardozo-Pelaez, F.; Linhart, H.; Winter, B.; Balkaya, M.; Gertz, K.; Gay, S.B.; Cox, D.; et al. Folate Deficiency Induces Neurodegeneration and Brain Dysfunction in Mice Lacking Uracil DNA Glycosylase. J. Neurosci. 2008, 28, 7219–7230. [Google Scholar] [CrossRef]
- Rotstein, A.; Kodesh, A.; Goldberg, Y.; Reichenberg, A.; Levine, S.Z. Serum Folate Deficiency and the Risks of Dementia and All-Cause Mortality: A National Study of Old Age. Evid. Based Ment. Health 2022, 25, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, T.; Zhao, J.; Song, A.; Liu, H.; Xu, W.; Huang, G. Folic Acid Supplementation Improves Cognitive Function by Reducing the Levels of Peripheral Inflammatory Cytokines in Elderly Chinese Subjects with MCI. Sci. Rep. 2016, 6, 37486. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.M.A.; Scarlett, S.; De Looze, C.; O’Halloran, A.M.; Laird, E.; Molloy, A.M.; Clarke, R.; McGarrigle, C.A.; Kenny, R.A. Low Folate Predicts Accelerated Cognitive Decline: 8-Year Follow-up of 3140 Older Adults in Ireland. Eur. J. Clin. Nutr. 2022, 76, 950–957. [Google Scholar] [CrossRef]
- Ramos, M.I.; Allen, L.H.; Mungas, D.M.; Jagust, W.J.; Haan, M.N.; Green, R.; Miller, J.W. Low Folate Status Is Associated with Impaired Cognitive Function and Dementia in the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2005, 82, 1346–1352. [Google Scholar] [CrossRef]
- Jang, S.; Han, J.W.; Shin, J.; Kim, T.H.; Kwak, K.P.; Kim, K.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Kim, T.H.; et al. Normal-But-Low Serum Folate Levels and the Risks for Cognitive Impairment. Psychiatry Investig. 2019, 16, 532–538. [Google Scholar] [CrossRef]
- Reynolds, E.H. Folic Acid, Ageing, Depression, and Dementia. BMJ 2002, 324, 1512–1515. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, D.; Zhang, H. Cardiovascular Disease Attenuates the Protective Effect of Folate on Global Cognitive Function in an Elderly Population: A Cross-Sectional Study. Sci. Rep. 2025, 15, 3327. [Google Scholar] [CrossRef]
- Yukawa, M.; Naka, H.; Murata, Y.; Katayama, S.; Kohriyama, T.; Mimori, Y.; Nakamura, S. Folic Acid-Responsive Neurological Diseases in Japan. J. Nutr. Sci. Vitaminol. 2001, 47, 181–187. [Google Scholar] [CrossRef]
- Puga, A.M.; Ruperto, M.; Samaniego-Vaesken, M.d.L.; Montero-Bravo, A.; Partearroyo, T.; Varela-Moreiras, G. Effects of Supplementation with Folic Acid and Its Combinations with Other Nutrients on Cognitive Impairment and Alzheimer’s Disease: A Narrative Review. Nutrients 2021, 13, 2966. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fang, M.; Zang, W. Effects of Folic Acid Supplementation on Cognitive Function and Inflammation in Elderly Patients with Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Gerontol. Geriatr. 2024, 126, 105540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Chen, Y.; Yan, J.; Huang, G.; Li, W. A Comparative Study Evaluating the Effectiveness of Folate-Based B Vitamin Intervention on Cognitive Function of Older Adults under Mandatory Folic Acid Fortification Policy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 2199. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Zhao, J.; Han, F.; Marseglia, A.; Liu, H.; Huang, G. Effects of 6-Month Folic Acid Supplementation on Cognitive Function and Blood Biomarkers in Mild Cognitive Impairment: A Randomized Controlled Trial in China. J. Gerontol. Ser. A 2016, 71, 1376–1383. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine—From Disease Biomarker to Disease Prevention. J. Intern. Med. 2021, 290, 826–854. [Google Scholar] [CrossRef]
- Wu, Y.; Smith, A.D.; Bastani, N.E.; Refsum, H.; Kwok, T. The Dihydrofolate Reductase 19-Bp Deletion Modifies the Beneficial Effect of B-Vitamin Therapy in Mild Cognitive Impairment: Pooled Study of Two Randomized Placebo-Controlled Trials. Hum. Mol. Genet. 2022, 31, 1151–1158. [Google Scholar] [CrossRef]
- Ling, Y.; Yuan, S.; Huang, X.; Tan, S.; Cheng, H.; Xu, A.; Lyu, J. Associations of Folate/Folic Acid Supplementation Alone and in Combination with Other B Vitamins on Dementia Risk and Brain Structure: Evidence From 466 224 UK Biobank Participants. J. Gerontol. Ser. A 2024, 79, glad266. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, H.; You, J.; Chen, S.-D.; Fu, Y.; Zhang, W.; Huang, L.; Feng, J.-F.; Gao, X.; Cheng, W.; et al. Machine Learning-Assisted Optimization of Dietary Intervention against Dementia Risk. Nat. Hum. Behav. 2025. [Google Scholar] [CrossRef]
- Sauer, J.; Mason, J.B.; Choi, S.-W. Too Much Folate—A Risk Factor for Cancer and Cardiovascular Disease? Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 30–36. [Google Scholar] [CrossRef]
- Fallah, M.; Karim Dehnavi, M.; Lotfi, K.; Aminianfar, A.; Azadbakht, L.; Esmaillzadeh, A. Folate Biomarkers, Folate Intake, and Risk of Death from All Causes, Cardiovascular Disease, and Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutr. Rev. 2025, 83, e801–e813. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.-C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef]
- Chen, P.; Tang, L.; Song, Y.; Wang, B.; Qin, X.; Zhang, N.; Wei, Y.; Xu, X.; Zhou, Z.; He, Q.; et al. Association of Folic Acid Dosage with Circulating Unmetabolized Folic Acid in Chinese Adults with H-Type Hypertension: A Multicenter, Double-Blind, Randomized Controlled Trial. Front. Nutr. 2023, 10, 1191610. [Google Scholar] [CrossRef]
- Husebye, E.S.N.; Wendel, A.W.K.; Gilhus, N.E.; Riedel, B.; Bjørk, M.H. Plasma Unmetabolized Folic Acid in Pregnancy and Risk of Autistic Traits and Language Impairment in Antiseizure Medication-Exposed Children of Women with Epilepsy. Am. J. Clin. Nutr. 2022, 115, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.M.; Elango, R.; Devlin, A.M.; Hutcheon, J.A.; Karakochuk, C.D. Human Milk Unmetabolized Folic Acid Is Increased Following Supplementation with Synthetic Folic Acid as Compared to (6S)-5-Methyltetrahydrofolic Acid. Sci. Rep. 2023, 13, 11298. [Google Scholar] [CrossRef] [PubMed]
- Jankovic-Karasoulos, T.; Smith, M.D.; Leemaqz, S.; Mittinty, M.; Williamson, J.; McCullough, D.; Arthurs, A.L.; Dekker, G.A.; Roberts, C.T. Maternal Folate Excess, Placental Hormones, and Gestational Diabetes Mellitus: Findings from Prospective Cohorts Before and After Mandatory Folic Acid Food Fortification. Nutrients 2025, 17, 2863. [Google Scholar] [CrossRef]
- Schöber, C.; Papageorgiou, E.; Harstrick, A.; Bokemeyer, C.; Mügge, A.; Stahl, M.; Wilke, H.; Poliwoda, H.; Hiddemann, W.; Köhne-Wömpner, C.H.; et al. Cardiotoxicity of 5-Fluorouracil in Combination with Folinic Acid in Patients with Gastrointestinal Cancer. Cancer 1993, 72, 2242–2247. [Google Scholar] [CrossRef]
- Ayoub, G. Neurodevelopment of Autism: Critical Periods, Stress and Nutrition. Cells 2024, 13, 1968. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Leheup, B.; Guéant-Rodriguez, R.-M.; Oussalah, A.; Quadros, E.V.; Guéant, J.-L. Folinic Acid Improves the Score of Autism in the EFFET Placebo-Controlled Randomized Trial. Biochimie 2020, 173, 57–61. [Google Scholar] [CrossRef]
- Panda, P.K.; Sharawat, I.K.; Saha, S.; Gupta, D.; Palayullakandi, A.; Meena, K. Efficacy of Oral Folinic Acid Supplementation in Children with Autism Spectrum Disorder: A Randomized Double-Blind, Placebo-Controlled Trial. Eur. J. Pediatr. 2024, 183, 4827–4835. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Hou, F.; Li, Y.; Wang, W.; Guo, L.; Zhang, C.; Li, L.; Lu, C. Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms. Nutrients 2025, 17, 1602. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Modell, B.; Lawn, J. Folic Acid to Reduce Neonatal Mortality from Neural Tube Disorders. Int. J. Epidemiol. 2010, 39 (Suppl. S1), i110–i121. [Google Scholar] [CrossRef]
- Naninck, E.F.G.; Stijger, P.C.; Brouwer-Brolsma, E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019, 10, 502–519. [Google Scholar] [CrossRef]
- Barua, S.; Chadman, K.K.; Kuizon, S.; Buenaventura, D.; Stapley, N.W.; Ruocco, F.; Begum, U.; Guariglia, S.R.; Brown, W.T.; Junaid, M.A. Increasing Maternal or Post-Weaning Folic Acid Alters Gene Expression and Moderately Changes Behavior in the Offspring. PLoS ONE 2014, 9, e101674. [Google Scholar] [CrossRef]
- Surén, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children. JAMA 2013, 309, 570–577. [Google Scholar] [CrossRef]
- Wang, M.; Li, K.; Zhao, D.; Li, L. The Association between Maternal Use of Folic Acid Supplements during Pregnancy and Risk of Autism Spectrum Disorders in Children: A Meta-Analysis. Mol. Autism 2017, 8, 51. [Google Scholar] [CrossRef]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic Biomarkers of Increased Oxidative Stress and Impaired Methylation Capacity in Children with Autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral Folate Receptor Autoantibodies in Autism Spectrum Disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Stoccoro, A.; Cagiano, R.; Nicolì, V.; Ricciardi, R.; Tancredi, R.; Trovato, R.; Santorelli, F.M.; Calderoni, S.; Muratori, F.; et al. Correlation among Maternal Risk Factors, Gene Methylation and Disease Severity in Females with Autism Spectrum Disorder. Epigenomics 2022, 14, 175–185. [Google Scholar] [CrossRef]
- Viswanathan, M.; Urrutia, R.P.; Hudson, K.N.; Middleton, J.C.; Kahwati, L.C. Folic Acid Supplementation to Prevent Neural Tube Defects: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 330, 460. [Google Scholar] [CrossRef]
- Bahous, R.H.; Jadavji, N.M.; Deng, L.; Cosín-Tomás, M.; Lu, J.; Malysheva, O.; Leung, K.-Y.; Ho, M.-K.; Pallàs, M.; Kaliman, P.; et al. High Dietary Folate in Pregnant Mice Leads to Pseudo-MTHFR Deficiency and Altered Methyl Metabolism, with Embryonic Growth Delay and Short-Term Memory Impairment in Offspring. Hum. Mol. Genet. 2017, 26, 888–900. [Google Scholar] [CrossRef]
- Jennings, L.; Basiri, R. Amino Acids, B Vitamins, and Choline May Independently and Collaboratively Influence the Incidence and Core Symptoms of Autism Spectrum Disorder. Nutrients 2022, 14, 2896. [Google Scholar] [CrossRef]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Sequeira, J.M.; Blau, N.; Quadros, E.V. A Milk-Free Diet Downregulates Folate Receptor Autoimmunity in Cerebral Folate Deficiency Syndrome. Dev. Med. Child. Neurol. 2008, 50, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, B.; Hoxha, M.; Domi, E.; Gervasoni, J.; Persichilli, S.; Malaj, V.; Zappacosta, B. Folic Acid and Autism: A Systematic Review of the Current State of Knowledge. Cells 2021, 10, 1976. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, C.; Kuang, M.; Lin, L.; Xu, G.; Pan, N.; Weng, X.; Jing, J.; Shi, L.; Yi, Q.; et al. Examining Associations of Folic Acid Supplements Administered to Mothers during Pre-Conceptional and Prenatal Periods with Autism Spectrum Disorders in Their Offspring: Insights from a Multi-Center Study in China. Front. Public Health 2024, 12, 1321046. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal Periconceptional Folic Acid Intake and Risk of Autism Spectrum Disorders and Developmental Delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) Case-Control Study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Giorlandino, C.; Margiotti, K.; Fabiani, M.; Mesoraca, A. Folinic Acid Supplementation During Pregnancy in Two Women with Folate Receptor Alpha Autoantibodies: Potential Prevention of Autism Spectrum Disorder in Offspring. Clin. Transl. Neurosci. 2025, 9, 30. [Google Scholar] [CrossRef]
- Caffrey, A.; McNulty, H.; Irwin, R.E.; Walsh, C.P.; Pentieva, K. Maternal Folate Nutrition and Offspring Health: Evidence and Current Controversies. Proc. Nutr. Soc. 2019, 78, 208–220. [Google Scholar] [CrossRef]
- Irvine, N.; England-Mason, G.; Field, C.J.; Dewey, D.; Aghajafari, F. Prenatal Folate and Choline Levels and Brain and Cognitive Development in Children: A Critical Narrative Review. Nutrients 2022, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Virdi, S.; Jadavji, N.M. The Impact of Maternal Folates on Brain Development and Function after Birth. Metabolites 2022, 12, 876. [Google Scholar] [CrossRef]
- Chen, H.; Qin, L.; Gao, R.; Jin, X.; Cheng, K.; Zhang, S.; Hu, X.; Xu, W.; Wang, H. Neurodevelopmental Effects of Maternal Folic Acid Supplementation: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 3771–3787. [Google Scholar] [CrossRef] [PubMed]
- Batebi, N.; Moghaddam, H.S.; Hasanzadeh, A.; Mohammadi, M.R.; Akhondzadeh, S. Folinic Acid as Adjunctive Therapy in Treatment of Inappropriate Speech in Children with Autism: A Double-Blind and Placebo-Controlled Randomized Trial. Child. Psychiatry Hum. Dev. 2021, 52, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Zauche, L.H.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef] [PubMed]
| Food Product | Example Foods |
|---|---|
| Dark green leafy vegetables | Spinach, kale, collard greens, and romaine lettuce |
| Legumes | Beans, lentils, and peas |
| Fruits | Oranges, avocados, bananas, and melons |
| Nuts and seeds | Peanuts and sunflower seeds |
| Other vegetables | Asparagus, Brussels sprouts, broccoli, and beets |
| Animal products | Liver and eggs (liver being especially high) |
| Whole grains | Fortified and unfortified whole grain products |
| Outcome | Dose/Duration/Ref. | Population | Notes |
|---|---|---|---|
| Improved FMD *, lowered homocysteine | 5 mg folic acid, 6 wks [6,12] | CAD * patients | Benefits independent of homocysteine |
| Improved FMD * | 5–10 mg, 6–8 wks [49] | Hyperhomocysteinemia | |
| Reduced plaque progression | 2.5–5 mg, 4 y [49] | Premature atherosclerosis | With B6, B12 |
| J-shaped risk curve; benefit to modest folate intake | Various (food/suppl) [54] | US adults at CVD * risk | High intake possibly adverse |
| ↓ CVD * events (RR~0.96), ↓ stroke (RR~0.90) | Various [53] | General and high-risk adults | Greater benefit with low baseline |
| No effect on CVD risk | Folic acid, 2 y [61] | CVD * patients |
| Risk Factor | Evidence |
|---|---|
| Masking B12 deficiency | Well-established, especially in elderly |
| Increased CVD/all-cause mortality | U-shaped association in epidemiological studies |
| Attenuated cognitive benefit | Seen in CVD/diabetes populations with high folate intake |
| Unmetabolized folic acid | Potential immune/cancer risk, more research needed |
| Mechanism | Effect of Folate Deficiency | Link to Autism Risk |
|---|---|---|
| DNA synthesis and repair | Impaired neuronal development | Structural/functional brain defects |
| Homocysteine metabolism | Homocysteine accumulation, neurotoxicity | Disrupted neurodevelopment |
| DNA methylation (epigenetics) | Hypomethylation, gene dysregulation | Aberrant gene expression in brain |
| Neurotransmitter synthesis | Imbalanced serotonin/ dopamine/norepinephrine | ASD-related behavioral changes |
| Oxidative stress/ inflammation | Increased neuronal damage | Neurodevelopmental risk |
| Factor | Timing/Context | Outcome/Association | Supporting Evidence |
|---|---|---|---|
| Maternal leucovorin in women with FRAA | Periconceptional (before and just after conception), through gestation | ASD risk reduced; small study of 2 cases, no ASD outcomes | [135] |
| Maternal folic acid supplementation | Periconceptional (before and just after conception), 1st trimester | ASD risk decreased by 50% or more | [121,132,133,134] |
| Maternal folic acid supplementation | No supplementation during pregnancy | Potential increased ASD risk; fetal brain may be sensitive to excess micronutrients | [133] |
| Maternal plasma/whole-blood folate concentration | Early pregnancy | Mixed findings; some studies show no direct association with autistic traits | [132] |
| Maternal plasma folate and B12 (very high levels) | At delivery, 3rd trimester | Potential increased ASD risk; fetal brain may be sensitive to excess micronutrients | [132] |
| Maternal MTHFR gene variant (C677T) | With low folic acid intake | Stronger association between low maternal folate and increased ASD risk | [132,134] |
| Prenatal multivitamin use (with folic acid) | Preconception and 1st month of pregnancy | Reduced ASD diagnosis and symptom severity in children | [121,132] |
| Lack of prenatal vitamin/folic acid use | Preconception and 1st month of pregnancy | Higher risk of ASD and more severe symptoms | [121,132] |
| Disorder | Folate/B9 | B12 | B6 | C | D |
|---|---|---|---|---|---|
| Cardiovascular Disease | Deficiency, Excess† | Deficiency | Deficiency | Deficiency | Deficiency |
| Cerebrovascular Disease | Deficiency | Deficiency | Deficiency | Deficiency | Deficiency |
| Peripheral Artery Disease | Deficiency | Deficiency | Deficiency | Deficiency [133] | Deficiency |
| Lymphedema | Deficiency | Deficiency | Deficiency | Deficiency | Deficiency [132] |
| Age-Related Macular Degeneration | Deficiency | Deficiency | Deficiency | Deficiency [132] | |
| Diabetic Retinopathy | Deficiency | Deficiency | Deficiency | ||
| Glaucoma | Deficiency | Deficiency | Deficiency | [121,132] | |
| Dementia/Cognitive Decline | Deficiency, Excess † | Deficiency | Deficiency | [121,132] | |
| Autism Spectrum Disorder | Deficiency, Excess † | Deficiency | Deficiency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, G. Vitamins, Vascular Health and Disease. Nutrients 2025, 17, 2955. https://doi.org/10.3390/nu17182955
Ayoub G. Vitamins, Vascular Health and Disease. Nutrients. 2025; 17(18):2955. https://doi.org/10.3390/nu17182955
Chicago/Turabian StyleAyoub, George. 2025. "Vitamins, Vascular Health and Disease" Nutrients 17, no. 18: 2955. https://doi.org/10.3390/nu17182955
APA StyleAyoub, G. (2025). Vitamins, Vascular Health and Disease. Nutrients, 17(18), 2955. https://doi.org/10.3390/nu17182955






