Discovery of Anti-Aging Effects of Wheat Bran Extract in a D-Galactose-Induced Rat Model of Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Bran Extract (WBE) Preparation and Handling
2.2. Experimental Approach
2.3. Sample Collection and Biochemical Analyses
2.4. Analysis of SOD in Erythrocytes
2.5. Analysis of Catalase, SOD2, and TERT in Skin and Liver Tissues
2.6. Analysis of β-Galactosidase
2.7. Statistical Analysis
3. Results
3.1. Change in Body Weights and Food Intake
3.2. Effect of WBE Supplements on Body Fat Percentage in Aging Rats
3.3. Effect of WBE Supplements on SOD in Aging Rats
3.4. Effect of WBE Supplements of β-Galactosidase in Aging Rats
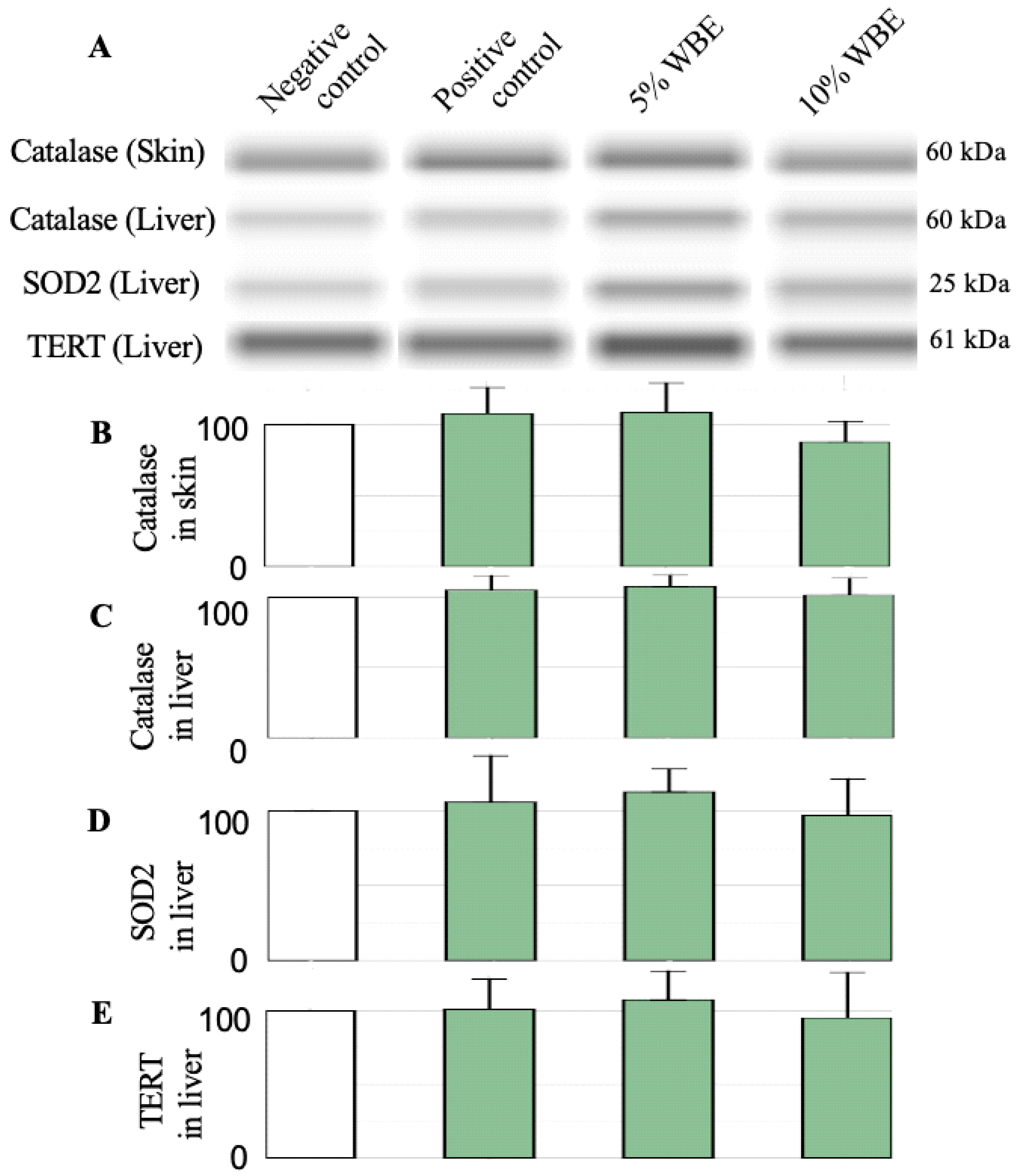
3.5. Protein Expression in Skin and Liver Tissues
4. Discussion
| Characteristics | Erythrocyte | Liver |
|---|---|---|
| ROS generation source | Systemic oxidative stress due to galactose loading [43]. | ROS (predominantly of mitochondrial origin) resulting from galactose metabolism [32]. |
| Type of SOD | Mainly Cu/Zn-SOD (cytosolic type) [44]. | Mn-SOD (mitochondrial type) and Cu/Zn-SOD [45]. |
| Antioxidant effect | The direct action of antioxidants is easily observed [27]. | Antioxidant effects may be gradual or sub-marked [46]. |
| The time scale of impact | Short-term changes in oxidative stress are likely to be reflected [47]. | Long-term metabolic compensatory effects may weaken the impact [48]. |
| Changes in SOD activity | Often significantly increased or stabilized by antioxidants [49]. | Baseline SOD activity is high, and changes may be small [30]. |
| Effect of damage | Oxidative stress-induced membrane damage and shortened erythrocyte lifespan are reduced [47]. | Limited effect of antioxidants if oxidative stress is too high [50]. |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WBE | Wheat Bran Extract |
| SOD | Superoxide Dismutase |
| TERT | Telomerase Reverse Transcriptase |
| SA-β-gal | Senescence-Associated β-Galactosidase |
| ROS | Reactive Oxygen Species |
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2023, 15, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; van der Laan, L.J.W.; Pan, Q.; Verstegen, M.M.A. Mitochondrial Dysfunction and Oxidative Stress in Liver Transplantation and Underlying Diseases: New Insights and Therapeutics. Transplantation 2021, 105, 2362–2373. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere Dysfunction in Ageing and Age-Related Diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Yu, L.; de Jager, P.L.; Levey, A.; Seyfried, N.T.; Bennett, D.A. Exploring Cortical Proteins Underlying the Relation of Neuroticism to Cognitive Resilience. Aging Brain 2022, 2, 100031. [Google Scholar] [CrossRef]
- Dutta, C. BIOMARKERS OF AGING. Innov. Aging 2024, 8, 301. [Google Scholar] [CrossRef]
- Tao, W.; Yu, Z.; Han, J.-D.J. Single-Cell Senescence Identification Reveals Senescence Heterogeneity, Trajectory, and Modulators. Cell Metab. 2024, 36, 1126–1143.e5. [Google Scholar] [CrossRef]
- Arbatskiy, M.S.; Balandin, D.E. Universal Markers of Cellular and Replicative Senescence. Adv. Gerontol. 2024, 14, 127–134. [Google Scholar] [CrossRef]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial Superoxide Dismutase: What the Established, the Intriguing, and the Novel Reveal About a Key Cellular Redox Switch. Antioxid. Redox Signal 2020, 32, 701–714. [Google Scholar] [CrossRef]
- Weyemi, U.; Parekh, P.R.; Redon, C.E.; Bonner, W.M. SOD2 Deficiency Promotes Aging Phenotypes in Mouse Skin. Aging 2012, 4, 116–118. [Google Scholar] [CrossRef]
- Rasheed, Z. Therapeutic Potentials of Catalase: Mechanisms, Applications, and Future Perspectives. Int. J. Health Sci. 2024, 18, 1–6. [Google Scholar]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Qu, H.; Madl, R.L.; Takemoto, D.J.; Baybutt, R.; Wang, W. Phytochemical Lignans Associated with the Cancer Prevention by Wheat Bran. J. Nutr. 2005, 135, 598–602. [Google Scholar] [CrossRef]
- Rahman, M.S.; Qi, G.; Li, C.; Li, Y.; Wang, W.; Atala, A.; Sun, X.S. Differential Effects of Wheat Bran Antioxidants on the Growth Dynamics of Human Cancer Cells. Foods 2025, 14, 1633. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, W.; Tilley, M.; Chen, R.; Sun, X.S.; Wang, W.; Li, Y. In Vitro Antioxidant Properties of Wheat Bran Extracts and Their Inhibitory Effects on Collagenase, Elastase, and Hyaluronidase. ACS Food Sci. Technol. 2024, 4, 1960–1966. [Google Scholar] [CrossRef]
- Kobayashi, K.; Suzauddula, M.; Bender, R.; Li, C.; Li, Y.; Sun, X.S.; Wang, W. Functional Properties and Potential Applications of Wheat Bran Extracts in Food and Cosmetics: A Review of Antioxidant, Enzyme-Inhibitory, and Anti-Aging Benefits. Foods 2025, 14, 515. [Google Scholar] [CrossRef]
- Pantiya, P.; Thonusin, C.; Ongnok, B.; Chunchai, T.; Kongkaew, A.; Nawara, W.; Arunsak, B.; Chattipakorn, N.; Chattipakorn, S.C. Chronic D-Galactose Administration Induces Natural Aging Characteristics, in Rat’s Brain and Heart. Toxicology 2023, 492, 153553. [Google Scholar] [CrossRef]
- Guo, B.; Guo, Q.; Wang, Z.; Shao, J.-B.; Liu, K.; Du, Z.-D.; Gong, S.-S. D-Galactose-Induced Oxidative Stress and Mitochondrial Dysfunction in the Cochlear Basilar Membrane: An in Vitro Aging Model. Biogerontology 2020, 21, 311–323. [Google Scholar] [CrossRef]
- Bruce, C.L.; Juszczak, E.; Ogollah, R.; Partlett, C.; Montgomery, A. A Systematic Review of Randomisation Method Use in RCTs and Association of Trial Design Characteristics with Method Selection. BMC Med. Res. Methodol. 2022, 22, 314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, C.; Liu, Y.; Chen, W.; Qi, J.; Xu, Y.; Ren, L.; Yang, G.; Min, D.; Liu, Z.; et al. D-Galactose Causes Sinoatrial Node Dysfunction: From Phenotype to Mechanism. Aging 2023, 15, 12551–12569. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular Senescence: The Good, the Bad and the Unknown. Nat. Reviews. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Ghaffari, S. Oxidative Stress in the Regulation of Normal and Neoplastic Hematopoiesis. Antioxid. Redox Signal 2008, 10, 1923–1940. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Shi, Y.-C. Distribution of Antioxidants and Phenolic Compounds in Flour Milling Fractions from Hard Red Winter Wheat. Grain Oil Sci. Technol. 2024, 7, 71–78. [Google Scholar] [CrossRef]
- Homolak, J.; Varvaras, K.; Sciacca, V.; Babic Perhoc, A.; Virag, D.; Knezovic, A.; Osmanovic Barilar, J.; Salkovic-Petrisic, M. Insights into Gastrointestinal Redox Dysregulation in a Rat Model of Alzheimer’s Disease and the Assessment of the Protective Potential of D-Galactose. ACS Omega 2024, 9, 11288–11304. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Orrico, F.; Villar, S.F.; López, A.C.; Silva, N.; Donzé, M.; Thomson, L.; Denicola, A. Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells. ACS Omega 2023, 8, 147–168. [Google Scholar] [CrossRef]
- Eigenschink, M.; Savran, D.; Zitterer, C.P.; Granitzer, S.; Fritz, M.; Baron, D.M.; Müllner, E.W.; Salzer, U. Redox Properties of Human Erythrocytes Are Adapted for Vitamin C Recycling. Front. Physiol. 2021, 12, 767439. [Google Scholar] [CrossRef]
- Zhu, J.; Lian, J.; Wang, X.; Wang, R.; Pang, X.; Xu, B.; Wang, X.; Li, C.; Ji, S.; Lu, H. Role of Endogenous and Exogenous Antioxidants in Risk of Six Cancers: Evidence from the Mendelian Randomization Study. Front. Pharmacol. 2023, 14, 1185850. [Google Scholar] [CrossRef]
- Trist, D.B.G.; Hilton, D.J.B.; Hare, P.D.J.; Crouch, P.P.J.; Double, P.K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. (Int. Ed. Engl.) 2020, 60, 9215. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Dossena, S.; Marino, A. D-Galactose Decreases Anion Exchange Capability through Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Ross, K.S.; Smith, C. D-Galactose: A Model of Accelerated Ageing Sufficiently Sensitive to Reflect Preventative Efficacy of an Antioxidant Treatment. Biogerontology 2020, 21, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Homolak, J.; Babic Perhoc, A.; Knezovic, A.; Osmanovic Barilar, J.; Virag, D.; Joja, M.; Salkovic-Petrisic, M. The Effect of Acute Oral Galactose Administration on the Redox System of the Rat Small Intestine. Antioxidants 2021, 11, 37. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Zhao, S.; Li, Z.; Qin, X. 1H NMR-Based Fecal Metabolomics Reveals Changes in Gastrointestinal Function of Aging Rats Induced by d-Galactose. Rejuvenation Res. 2021, 24, 86–96. [Google Scholar] [CrossRef]
- Nagode, A.; Vanbeselaere, J.; Dutkiewicz, Z.; Kaltenbrunner, S.; Wilson, I.B.H.; Duchêne, M. Molecular Characterisation of Entamoeba Histolytica UDP-Glucose 4-Epimerase, an Enzyme Able to Provide Building Blocks for Cyst Wall Formation. PLoS Neglected Trop. Dis. 2023, 17, e0011574. [Google Scholar] [CrossRef]
- Vargas, E.; Podder, V.; Carrillo Sepulveda, M.A. Physiology, Glucose Transporter Type 4. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Chadt, A.; Al-Hasani, H. Glucose Transporters in Adipose Tissue, Liver, and Skeletal Muscle in Metabolic Health and Disease. Pflug. Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of D-Galactose-Induced Brain Aging and Its Potential Used for Therapeutic Interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Yang, X.; Ding, Z.; Hu, M.; Huang, X.; Zhang, Q.; Yu, Y. NMN Reverses D-Galactose-Induced Neurodegeneration and Enhances the Intestinal Barrier of Mice by Activating the Sirt1 Pathway. Front. Pharmacol. 2025, 16, 1545585. [Google Scholar] [CrossRef]
- Cardoso, A.; Magano, S.; Marrana, F.; Andrade, J.P. D-Galactose High-Dose Administration Failed to Induce Accelerated Aging Changes in Neurogenesis, Anxiety, and Spatial Memory on Young Male Wistar Rats. Rejuvenation Res. 2015, 18, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Pantiya, P.; Thonusin, C.; Ongnok, B.; Chunchai, T.; Kongkaew, A.; Nawara, W.; Arunsak, B.; Chattipakorn, N.; Chattipakorn, S.C. Long-Term D-Galactose Administration Mimics Natural Aging in Rat’s Hippocampus. Alzheimer’s Dement. 2023, 19, e073540. [Google Scholar] [CrossRef]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte Oxidative Stress and Thrombosis. Expert. Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef]
- Toro-Román, V.; Siquier-Coll, J.; Bartolomé, I.; Grijota, F.J.; Muñoz, D.; Maynar-Mariño, M. Copper Concentration in Erythrocytes, Platelets, Plasma, Serum and Urine: Influence of Physical Training. J. Int. Soc. Sports Nutr. 2021, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front. Physiol. 2020, 11, 755. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Brankovic, M.; Djuretić, J.; Grujic-Milanovic, J.; Kovacic, M.; Jevtic, J.; Stankovic, S.; Samardzic, J.; Vucevic, D.; Jakovljevic, V. Alpinetin Exhibits Antioxidant and Anti-Inflammatory Effects in C57BL/6 Mice with Alcoholic Liver Disease Induced by the Lieber–DeCarli Ethanol Liquid Diet. Int. J. Mol. Sci. 2025, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Igwe, M.C.; Obeagu, G.U. Oxidative Stress’s Impact on Red Blood Cells: Unveiling Implications for Health and Disease. Medicine 2024, 103, e37360. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Ściskalska, M.; Ołdakowska, M.; Marek, G.; Milnerowicz, H. Changes in the Activity and Concentration of Superoxide Dismutase Isoenzymes (Cu/Zn SOD, MnSOD) in the Blood of Healthy Subjects and Patients with Acute Pancreatitis. Antioxidants 2020, 9, 948. [Google Scholar] [CrossRef]
- Vascotto, C.; Tiribelli, C. Oxidative Stress, Antioxidant Defenses, and the Liver. In Studies on Hepatic Disorders; Albano, E., Parola, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 41–64. ISBN 978-3-319-15539-5. [Google Scholar]
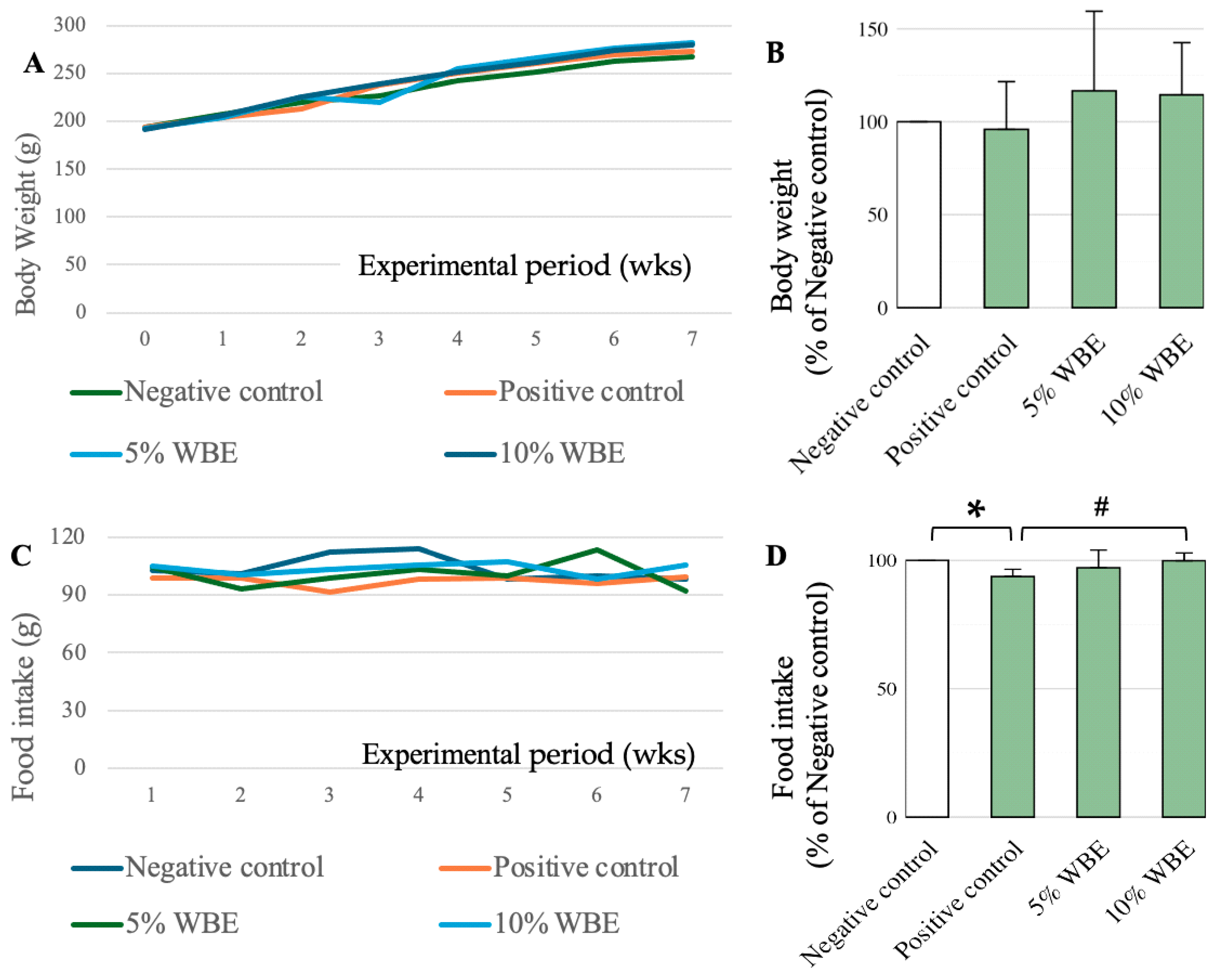
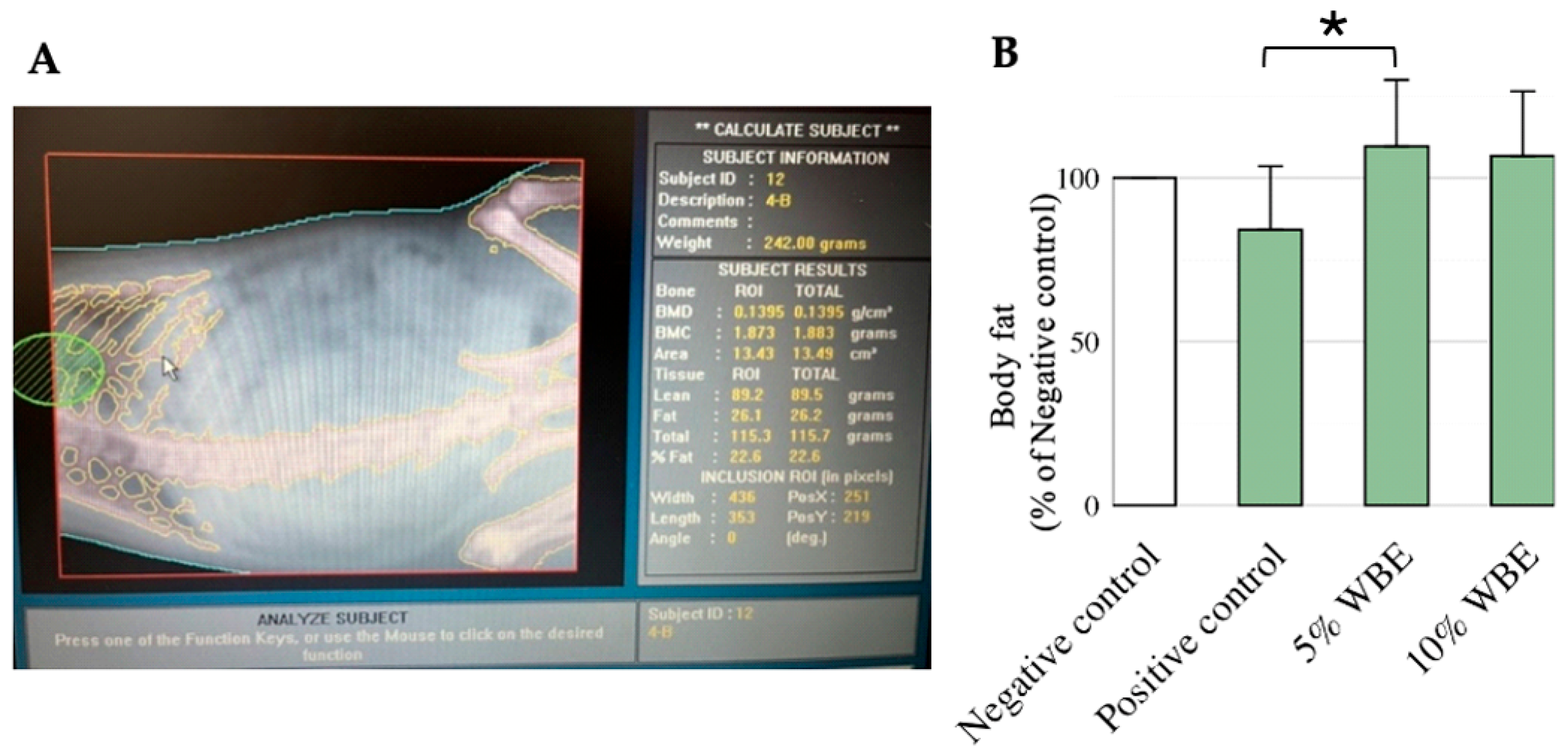
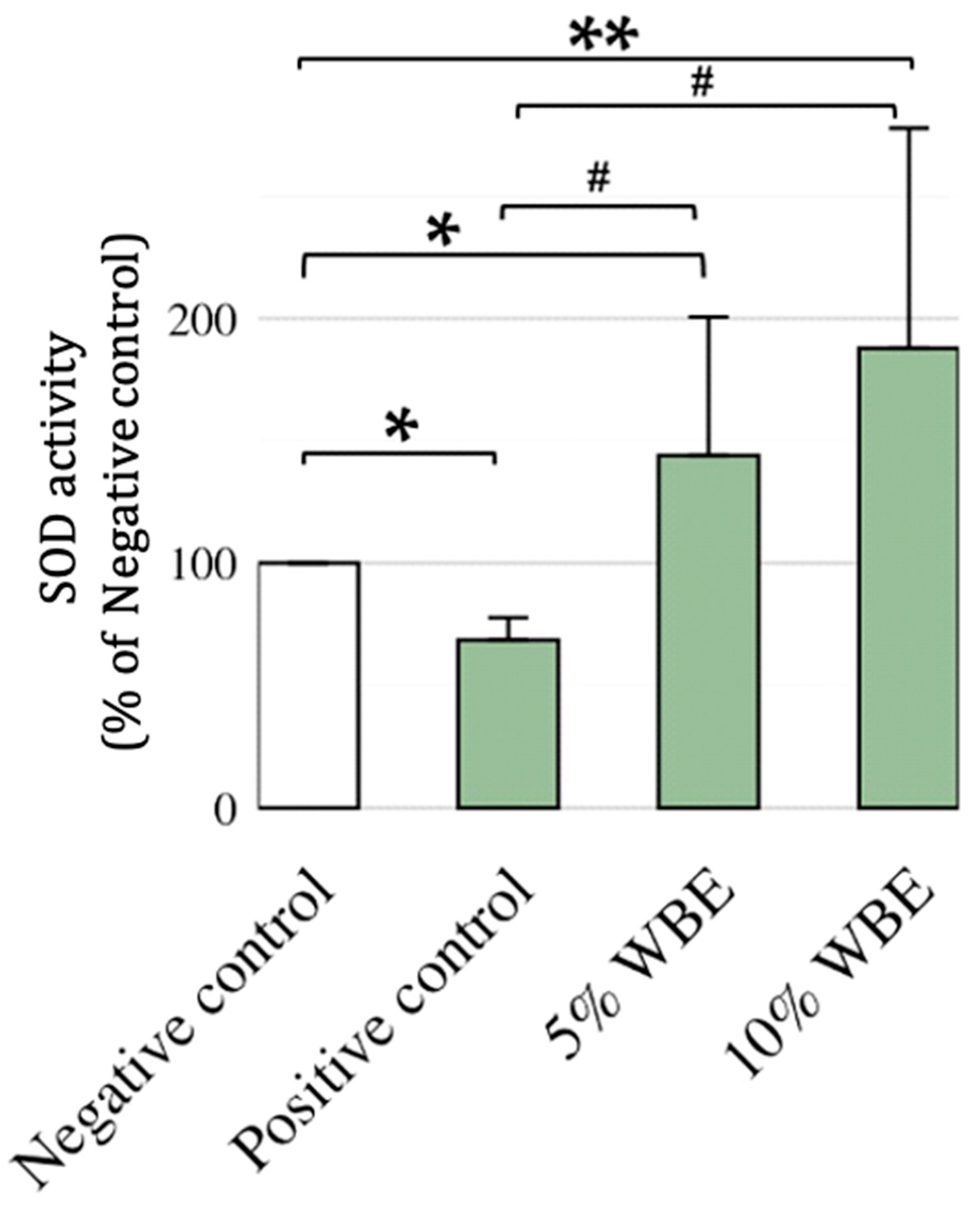
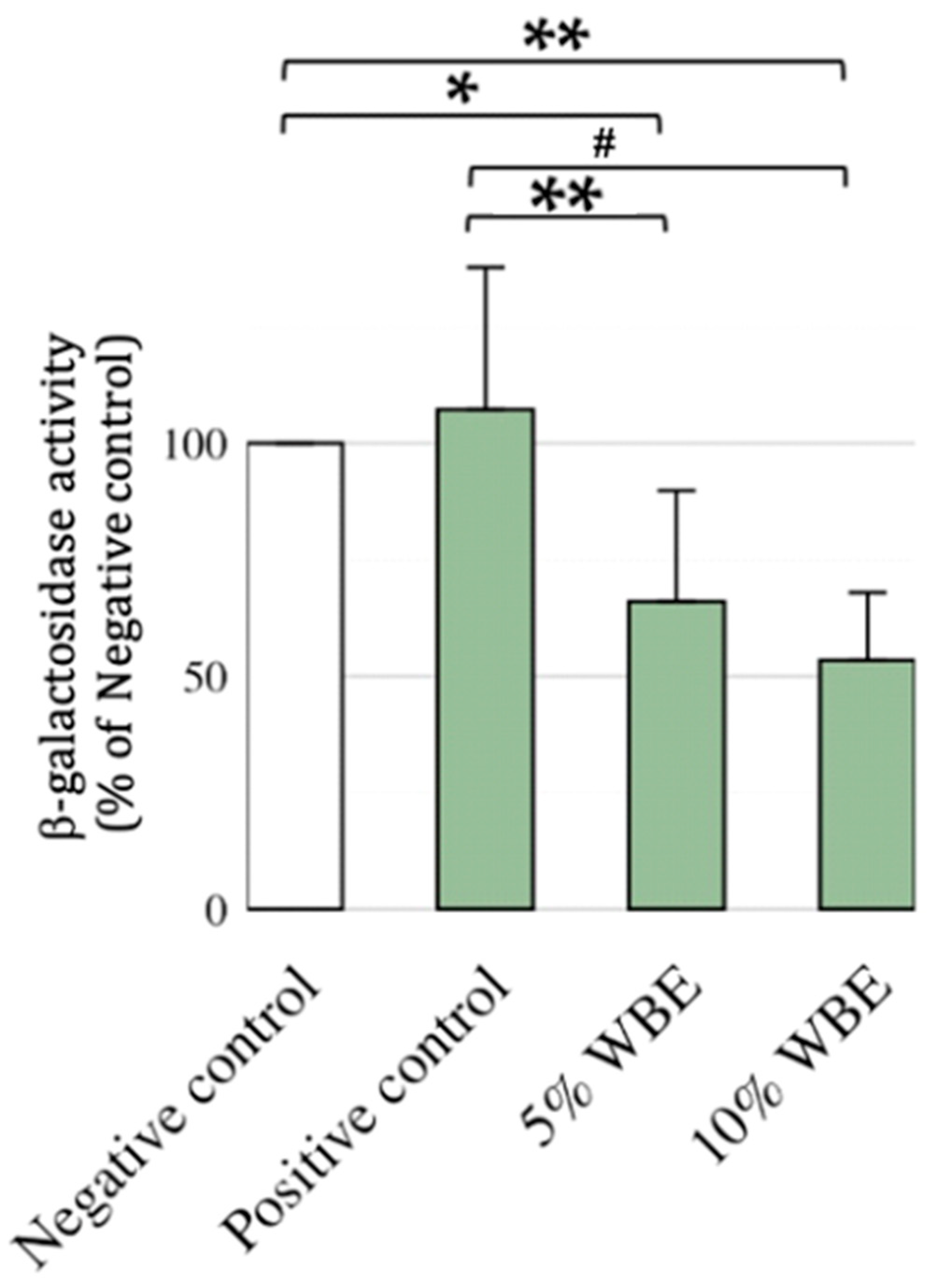
| AIN-93G | 5% WBE | 10% WBE | |
|---|---|---|---|
| Composition (%) | |||
| Carbohydrate | 60.1 | 59.2 | 58.8 |
| Protein | 17.7 | 17.5 | 17.4 |
| Fat | 7.2 | 7.1 | 7.0 |
| Ingredients (g/kg) | |||
| Casein | 200 | 187 | 176 |
| L-Cystine | 3 | 3 | 3 |
| Corn Starch | 397.5 | 397.5 | 361.0 |
| Maltodextrin | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 |
| Soybean Oil | 70 | 69 | 68.5 |
| Cellulose | 50 | 35 | 12 |
| Mineral Mix S10022G | 35 | 35 | 35 |
| Vitamin Mix V10037 | 10 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 |
| TBHQ, an antioxidant | 0.014 | 0.014 | 0.014 |
| Wheat Bran Extracts | 0 | 50 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, K.; Sudasinghe, K.; Bender, R.; Suzauddula, M.; Li, C.; Wu, C.; Li, Y.; Wang, W. Discovery of Anti-Aging Effects of Wheat Bran Extract in a D-Galactose-Induced Rat Model of Oxidative Stress. Nutrients 2025, 17, 2954. https://doi.org/10.3390/nu17182954
Kobayashi K, Sudasinghe K, Bender R, Suzauddula M, Li C, Wu C, Li Y, Wang W. Discovery of Anti-Aging Effects of Wheat Bran Extract in a D-Galactose-Induced Rat Model of Oxidative Stress. Nutrients. 2025; 17(18):2954. https://doi.org/10.3390/nu17182954
Chicago/Turabian StyleKobayashi, Kaori, Keshari Sudasinghe, Ryan Bender, Md Suzauddula, Cheng Li, Cen Wu, Yonghui Li, and Weiqun Wang. 2025. "Discovery of Anti-Aging Effects of Wheat Bran Extract in a D-Galactose-Induced Rat Model of Oxidative Stress" Nutrients 17, no. 18: 2954. https://doi.org/10.3390/nu17182954
APA StyleKobayashi, K., Sudasinghe, K., Bender, R., Suzauddula, M., Li, C., Wu, C., Li, Y., & Wang, W. (2025). Discovery of Anti-Aging Effects of Wheat Bran Extract in a D-Galactose-Induced Rat Model of Oxidative Stress. Nutrients, 17(18), 2954. https://doi.org/10.3390/nu17182954










