Synergistic Effects of Green Tea Extract and Ginger Supplementation on Endurance Performance and Thermal Perception in Normothermic and Cold Environments: A Randomized, Placebo-Controlled, Double-Blind Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TTE | Time-to-exhaustion |
| RER | Respiratory exchange ratio |
| RPE | Perceived exertion |
| TSS | Thermal sensation |
| VAS | Visual analog scale |
| VO2 | Oxygen consumption |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| VO2 max | Maximum oxygen consumption |
| CO2 | Carbon dioxide |
| SD | Standard deviation |
| EGCG | Epigallocatechin gallate |
| AMPK | AMP-activated protein kinase |
| TRPV1 | Transient receptor potential vanilloid 1 |
| PGC-1α | Proliferator-activated receptor gamma coactivator 1-alpha |
| COX-2 | Cyclooxygenase-2 |
References
- Riera, F.; Bellenoue, S.; Fischer, S.; Méric, H. Impact of a cold environment on the performance of professional cyclists: A pilot study. Life 2021, 11, 1326. [Google Scholar] [CrossRef]
- Wallace, P.J.; Hartley, G.L.; Nowlan, J.G.; Ljubanovich, J.; Sieh, N.; Taber, M.J.; Gagnon, D.D.; Cheung, S.S. Endurance capacity impairment in cold air ranging from skin cooling to mild hypothermia. J. Appl. Physiol. 2024, 136, 58–69. [Google Scholar] [CrossRef]
- Ulupinar, S.; Özbay, S.; Gençoğlu, C.; Altinkaynak, K.; Şebin, E.; Oymak, B. Exercise in the cold causes greater irisin release but may not be enough for adropin. J. Physiol. Investig. 2021, 64, 129–134. [Google Scholar]
- Ozbay, S.; Ulupınar, S.; Şebin, E.; Altınkaynak, K. Acute and chronic effects of aerobic exercise on serum irisin, adropin, and cholesterol levels in the winter season: Indoor training versus outdoor training. J. Physiol. Investig. 2020, 63, 21–26. [Google Scholar]
- Sawka, M.N.; Young, A.J. Physiologic systems and their responses to conditions of heat and cold. In ACSM’s Advanced Exercise Physiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 567–602. [Google Scholar]
- Gatterer, H.; Dünnwald, T.; Turner, R.; Csapo, R.; Schobersberger, W.; Burtscher, M.; Faulhaber, M.; Kennedy, M.D. Practicing sport in cold environments: Practical recommendations to improve sport performance and reduce negative health outcomes. Int. J. Environ. Res. Public Health 2021, 18, 9700. [Google Scholar]
- González-Gross, M.; Quesada-González, C.; Rueda, J.; Sillero-Quintana, M.; Issaly, N.; Díaz, A.E.; Gesteiro, E.; Escobar-Toledo, D.; Torres-Peralta, R.; Roller, M.; et al. Analysis of effectiveness of a supplement combining Harpagophytum procumbens, Zingiber officinale and Bixa orellana in healthy recreational runners with self-reported knee pain: A pilot, randomized, triple-blind, placebo-controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 5538. [Google Scholar]
- Huang, J.; Tagawa, T.; Ma, S.; Suzuki, K. Black ginger (Kaempferia parviflora) extract enhances endurance capacity by improving energy metabolism and substrate utilization in mice. Nutrients 2022, 14, 3845. [Google Scholar] [CrossRef] [PubMed]
- Zalakiyan, P.; Naghibi, M. The Effect of Eight Weeks of Interval Aerobic Training with Green Tea and Ginger Consumption on Lipid Profiles of Overweight Women. Rep. Health Care 2019, 5, 52–59. [Google Scholar]
- Nobari, H.; Saedmocheshi, S.; Chung, L.H.; Suzuki, K.; Maynar-Mariño, M.; Pérez-Gómez, J. An overview on how exercise with green tea consumption can prevent the production of reactive oxygen species and improve sports performance. Int. J. Environ. Res. Public Health 2021, 19, 218. [Google Scholar] [PubMed]
- Wilson, P.B. A randomized double-blind trial of ginger root for reducing muscle soreness and improving physical performance recovery among experienced recreational distance runners. J. Diet. Suppl. 2020, 17, 121–132. [Google Scholar]
- Heck, A.M.; DeWitt, B.A.; Lukes, A.L. Potential interactions between alternative therapies and warfarin. Am. J. Health-Syst. Pharm. 2000, 57, 1221–1227. [Google Scholar]
- Kondori, B.J.; Ghaleh, H.E.G.; Hosseini, S.M. Effect of green tea extract on exercise-induced inflammatory markers. J. Mil. Med. 2021, 23, 69–74. [Google Scholar]
- Mashhadi, M.R.; Hosseini, S.R.A. The interaction effect of green tea consumption and exercise training on fat oxidation, body composition and blood lipids in humans: A review of the literature. Sport Sci. Health 2023, 19, 461–477. [Google Scholar]
- Wilson, P.B. Ginger (Zingiber officinale) as an analgesic and ergogenic aid in sport: A systemic review. J. Strength Cond. Res. 2015, 29, 2980–2995. [Google Scholar]
- Hodgson, A.B.; Randell, R.K.; Jeukendrup, A.E. The effect of green tea extract on fat oxidation at rest and during exercise: Evidence of efficacy and proposed mechanisms. Adv. Nutr. 2013, 4, 129–140. [Google Scholar]
- Mazyed, E.A.; Helal, D.A.; Elkhoudary, M.M.; Elhameed, A.G.A.; Yasser, M. Formulation and optimization of nanospanlastics for improving the bioavailability of green tea epigallocatechin gallate. Pharmaceuticals 2021, 14, 68. [Google Scholar] [CrossRef]
- Tritsch, N.; Steger, M.C.; Segatz, V.; Blumenthal, P.; Rigling, M.; Schwarz, S.; Zhang, Y.; Franke, H.; Lachenmeier, D.W. Risk assessment of caffeine and epigallocatechin gallate in coffee leaf tea. Foods 2022, 11, 263. [Google Scholar] [CrossRef]
- Unno, K.; Ikka, T.; Yamashita, H.; Kameoka, Y.; Nakamura, Y. Stress-Relieving Effects of Japanese Green Tea: Evaluation Using the Molar Ratio of Caffeine and Epigallocatechin Gallate to Theanine and Arginine as an Indicator. Foods 2025, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Feizi, A.; Hariri, M.; Darvishi, L.; Barani, A.; Taghiyar, M.; Shiranian, A.; Hajishafiee, M. Influence of ginger and cinnamon intake on inflammation and muscle soreness endued by exercise in Iranian female athletes. Int. J. Prev. Med. 2013, 4 (Suppl. 1), S11. [Google Scholar]
- Standing, J.F. Understanding and applying pharmacometric modelling and simulation in clinical practice and research. Br. J. Clin. Pharmacol. 2017, 83, 247–254. [Google Scholar] [PubMed]
- Samota, M.K.; Rawat, M.; Kaur, M.; Garg, D. Gingerol: Extraction methods, health implications, bioavailability and signaling pathways. Sustain. Food Technol. 2024, 2, 1652–1669. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, X.-L.; Xu, Y.-C.; Chen, S.; Cai, M.; Deng, S.-H.; Ding, W.-J.; Xu, H.-L.; Zhang, S.-W.; Tan, Z.-B.; et al. 10-Gingerol, a natural AMPK agonist, suppresses neointimal hyperplasia and inhibits vascular smooth muscle cell proliferation. Food Funct. 2022, 13, 3234–3246. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2021, 17, 317–331. [Google Scholar]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott williams & wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Huggett, D.L.; Connelly, D.M.; Overend, T.J. Maximal aerobic capacity testing of older adults: A critical review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 57–66. [Google Scholar]
- Faulkner, J.; Parfitt, G.; Eston, R. Prediction of maximal oxygen uptake from the ratings of perceived exertion and heart rate during a perceptually-regulated sub-maximal exercise test in active and sedentary participants. Eur. J. Appl. Physiol. 2007, 101, 397–407. [Google Scholar]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Gagge, A.P.; Stolwijk, J.A.J.; Hardy, J.D. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ. Res. 1967, 1, 1–20. [Google Scholar] [CrossRef]
- Huskisson, E.C. Measurement of pain. Lancet 1974, 304, 1127–1131. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, B.; Ji, W.; Zhu, Y. Study on clothing insulation distribution between half-bodies and its effects on thermal comfort in cold environments. Energy Build. 2020, 211, 109796. [Google Scholar] [CrossRef]
- Ioannou, L.G.; Tsoutsoubi, L.; Gkiata, P.; Brown, H.A.; Periard, J.D.; Mekjavic, I.B.; Kenny, G.P.; Nybo, L.; Flouris, A.D. Effect of sportswear on performance and physiological heat strain during prolonged running in moderately hot conditions. Scand. J. Med. Sci. Sports 2024, 34, e14520. [Google Scholar]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar]
- Espinosa-Salas, S.; Gonzalez-Arias, M. Nutrition: Micronutrient intake, imbalances, and interventions. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: England, UK, 2013. [Google Scholar]
- Calónico, S.; Galiani, S. Beyond Bonferroni: Hierarchical Multiple Testing in Empirical Research; National Bureau of Economic Research: Cambridge, MA, USA, 2025. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.R. Effect size guidelines, sample size calculations, and statistical power in gerontology. Innov. Aging 2019, 3, igz036. [Google Scholar] [CrossRef]
- Ulupınar, S.; İnce, İ. Effect size and alternative statistical approaches in sports sciences. Spormetre J. Phys. Educ. Sport Sci. 2021, 19, 1–17. [Google Scholar]
- van der Lans, A.A.; Hoeks, J.; Brans, B.; Vijgen, G.H.; Visser, M.G.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef]
- Xu, J.; Cui, L.; Wang, J.; Zheng, S.; Zhang, H.; Ke, S.; Cao, X.; Shi, Y.; Li, J.; Zen, K.; et al. Cold-activated brown fat-derived extracellular vesicle-miR-378a-3p stimulates hepatic gluconeogenesis in male mice. Nat. Commun. 2023, 14, 5480. [Google Scholar] [PubMed]
- Gholami, F.; Antonio, J.; Iranpour, M.; Curtis, J.; Pereira, F. Does green tea catechin enhance weight-loss effect of exercise training in overweight and obese individuals? A systematic review and meta-analysis of randomized trials. J. Int. Soc. Sports Nutr. 2024, 21, 2411029. [Google Scholar]
- Mika, M.; Wikiera, A.; Antończyk, A.; Grabacka, M. The impact of catechins included in high fat diet on AMP-dependent protein kinase in apoE knock-out mice. Int. J. Food Sci. Nutr. 2021, 72, 348–356. [Google Scholar]
- Sharma, V.K.; Sharma, A.; Verma, K.K.; Gaur, P.K.; Kaushik, R.; Abdali, B. A comprehensive review on pharmacological potentials of caffeine. J. Appl. Pharm. Sci. Res. 2023, 6, 16–26. [Google Scholar] [CrossRef]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of ingesting both catechins and chlorogenic acids on glucose, incretin, and insulin sensitivity in healthy men: A randomized, double-blinded, placebo-controlled crossover trial. Nutrients 2022, 14, 5063. [Google Scholar]
- Matsuzaki, R.; Matsuoka, T.; Nakanishi, K.; Tani, A.; Kakimoto, S.; Kato, Y.; Kawatani, T.; Nakagawa, S.; Baba, Y.; Kobayashi, M.; et al. Effects of green tea catechins and exercise on age-related muscle atrophy and satellite cell functions in a mouse model of sarcopenia. Exp. Gerontol. 2025, 202, 112720. [Google Scholar] [CrossRef]
- Alsabri, S.G.; Mari, W.O.; Younes, S.; Elsadawi, M.A.; Oroszi, T.L. Kinetic and dynamic description of caffeine. J. Caffeine Adenosine Res. 2018, 8, 3–9. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Zhang, N.; Udenigwe, C.C.; Zhang, Y.; Fu, Y. Preparation, pungency and bioactivity of gingerols from ginger (Zingiber officinale Roscoe): A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2708–2733. [Google Scholar] [PubMed]
- Liu, X.; Meng, X.; Su, X.; Ren, K.; Ning, C.; Qi, X.; Zhang, S. The mechanism of ginger and its processed products in the treatment of estradiol valerate coupled with oxytocin-induced dysmenorrhea in mice via regulating the TRP ion channel-mediated ERK 1/2/NF-κB signaling pathway. Food Funct. 2022, 13, 11236–11248. [Google Scholar]
- Barwood, M.J.; Gibson, O.R.; Gillis, D.J.; Jeffries, O.; Morris, N.B.; Pearce, J.; Ross, M.L.; Stevens, C.; Rinaldi, K.; Kounalakis, S.N.; et al. Menthol as an Ergogenic aid for the Tokyo 2021 Olympic games: An Expert-Led consensus statement using the modified Delphi method. Sports Med. 2020, 50, 1709–1727. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zeng, Y.; Zeng, X.; Tan, Q.; He, Q.; Wang, S.; Wang, J. 6-Gingerol improves lipid metabolism disorders in skeletal muscle by regulating AdipoR1/AMPK signaling pathway. Biomed. Pharmacother. 2024, 180, 117462. [Google Scholar]
- Yahyazadeh, R.; Rahimi, V.B.; Yahyazadeh, A.; Mohajeri, S.A.; Askari, V.R. Promising effects of gingerol against toxins: A review article. Biofactors 2021, 47, 885–913. [Google Scholar]
- Yücel, Ç.; Karatoprak, G.Ş.; Açıkara, Ö.B.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E.; Aschner, M.; Shirooie, S. Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations. Front. Pharmacol. 2022, 13, 902551. [Google Scholar] [CrossRef] [PubMed]
- Black, C.D.; Herring, M.P.; Hurley, D.J.; O’Connor, P.J. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. J. Pain 2010, 11, 894–903. [Google Scholar] [CrossRef]
- Rocha, A.; Bolin, A.P.; Cardoso, C.A.L.; Otton, R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur. J. Nutr. 2016, 55, 2231–2244. [Google Scholar]
- Nishimura, T.; Motoi, M.; Egashira, Y.; Choi, D.; Aoyagi, K.; Watanuki, S. Seasonal variation of non-shivering thermogenesis (NST) during mild cold exposure. J. Physiol. Anthropol. 2015, 34, 11. [Google Scholar]
- Hursel, R.; Westerterp-Plantenga, M.S. Thermogenic ingredients and body weight regulation. Int. J. Obes. 2010, 34, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Sheikhhossein, F.; Borazjani, M.; Jafari, A.; Vataniyan, E.; Gholami, F.; Amini, M.R. Effects of ginger supplementation on biomarkers of oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 111–119. [Google Scholar] [CrossRef] [PubMed]
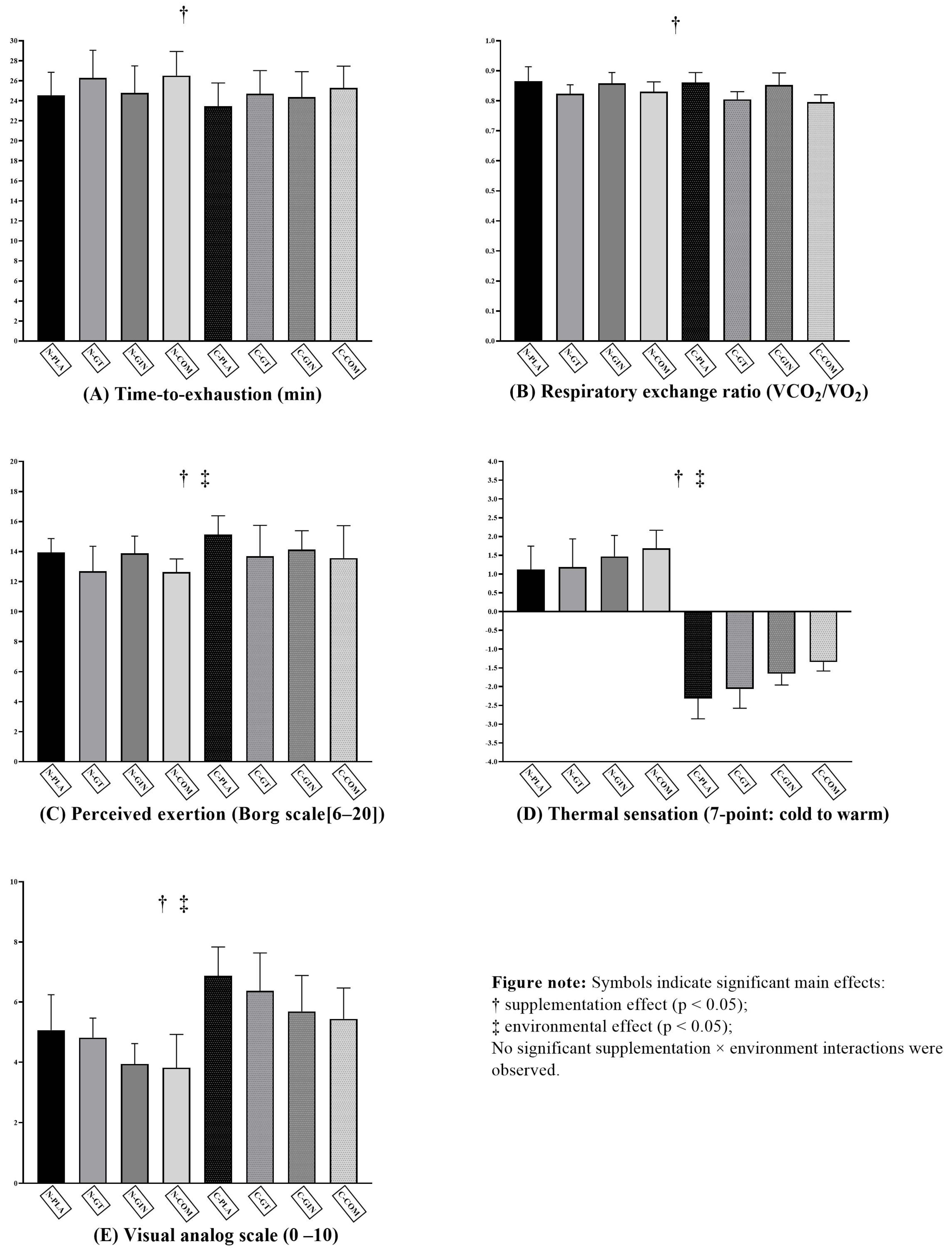
| Variable | Placebo | Green Tea | Ginger | Combined | Statistics (F, p, ηp2) |
|---|---|---|---|---|---|
| TTE (Normothermic) | 24.5 ± 2.3 | 26.3 ± 2.8 a | 24.8 ± 2.7 | 26.5 ± 2.4 a | Supplementation effect: F = 27.274; p < 0.001; ηp2 = 0.476 Environmental condition effect: F = 1.741; p = 0.197; ηp2 = 0.055 Interaction effect: F = 2.316; p = 0.081; ηp2 = 0.072 |
| TTE (s, Cold) | 23.5 ± 2.3 abd | 24.7 ± 2.3 d | 24.4 ± 2.5 bd | 25.3 ± 2.2 aeg | |
| RER (Normothermic) | 0.86 ± 0.05 | 0.82 ± 0.03 a | 0.86 ± 0.04 | 0.83 ± 0.03 | Supplementation effect: F = 29.976; p < 0.001; ηp2 = 0.500 Environmental condition effect: F = 3.142; p = 0.086; ηp2 = 0.095 Interaction effect: F = 2.200; p = 0.093; ηp2 = 0.068 |
| RER (Cold) | 0.86 ± 0.03 a | 0.80 ± 0.03 abcde | 0.85 ± 0.04 f | 0.80 ± 0.02 abcdeg | |
| RPE (Normothermic) | 13.94 ± 0.93 | 12.69 ± 1.66 a | 13.88 ± 1.15 | 12.62 ± 0.89 ac | Supplementation effect: F = 13.572; p < 0.001; ηp2 = 0.311 Environmental condition effect: F = 4.094; p = 0.052; ηp2 = 0.120 Interaction effect: F = 1.212; p = 0.310; ηp2 = 0.039 |
| RPE (Cold) | 15.12 ± 1.26 abcd | 13.69 ± 2.06 | 14.12 ± 1.26 d | 13.56 ± 2.16 e | |
| TSS (Normothermic) | 1.12 ± 0.62 | 1.19 ± 0.75 | 1.47 ± 0.56 | 1.69 ± 0.48 ab | Supplementation effect: F = 39.729; p < 0.001; ηp2 = 0.570 Environmental condition effect: F = 406.125; p < 0.001; ηp2 = 0.931 Interaction effect: F = 2.588; p = 0.058; ηp2 = 0.079 |
| TSS (Cold) | −2.31 ± 0.54 abcd | −2.06 ± 0.51 abcde | −1.66 ± 0.30 abcdef | −1.34 ± 0.24 abcdefg | |
| VAS (Normothermic) | 5.06 ± 1.18 | 4.81 ± 0.66 | 3.94 ± 0.68 | 3.81 ± 1.11 | Supplementation effect: F = 24.463; p < 0.001; ηp2 = 0.449 Environmental condition effect: F = 34.225; p < 0.001; ηp2 = 0.533 Interaction effect: F = 0.196; p = 0.899; ηp2 = 0.007 |
| VAS (Cold) | 6.88 ± 0.96 abcd | 6.38 ± 1.26 bcde | 5.09 ± 1.20 cde | 5.44 ± 1.03 cdef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirli, A.; Ulupınar, S.; Terzi, M.; Özbay, S.; Özkara, A.B.; Gençoğlu, C.; Ouergui, I.; Ardigò, L.P. Synergistic Effects of Green Tea Extract and Ginger Supplementation on Endurance Performance and Thermal Perception in Normothermic and Cold Environments: A Randomized, Placebo-Controlled, Double-Blind Crossover Trial. Nutrients 2025, 17, 2949. https://doi.org/10.3390/nu17182949
Demirli A, Ulupınar S, Terzi M, Özbay S, Özkara AB, Gençoğlu C, Ouergui I, Ardigò LP. Synergistic Effects of Green Tea Extract and Ginger Supplementation on Endurance Performance and Thermal Perception in Normothermic and Cold Environments: A Randomized, Placebo-Controlled, Double-Blind Crossover Trial. Nutrients. 2025; 17(18):2949. https://doi.org/10.3390/nu17182949
Chicago/Turabian StyleDemirli, Abdullah, Süleyman Ulupınar, Merve Terzi, Serhat Özbay, Abdullah Bora Özkara, Cebrail Gençoğlu, Ibrahim Ouergui, and Luca Paolo Ardigò. 2025. "Synergistic Effects of Green Tea Extract and Ginger Supplementation on Endurance Performance and Thermal Perception in Normothermic and Cold Environments: A Randomized, Placebo-Controlled, Double-Blind Crossover Trial" Nutrients 17, no. 18: 2949. https://doi.org/10.3390/nu17182949
APA StyleDemirli, A., Ulupınar, S., Terzi, M., Özbay, S., Özkara, A. B., Gençoğlu, C., Ouergui, I., & Ardigò, L. P. (2025). Synergistic Effects of Green Tea Extract and Ginger Supplementation on Endurance Performance and Thermal Perception in Normothermic and Cold Environments: A Randomized, Placebo-Controlled, Double-Blind Crossover Trial. Nutrients, 17(18), 2949. https://doi.org/10.3390/nu17182949







