Dual-Action Grouper Bone and Wakame Hydrolysates Supplement Enhances Exercise Performance and Modulates Gut Microbiota in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Supplement Preparation
2.2. Animals and Experimental Design
2.3. Exercise Performance Assessments
2.4. Fatigue Biochemical Markers
2.5. Body Composition and Safety Evaluations
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. General Observations and Body Weight
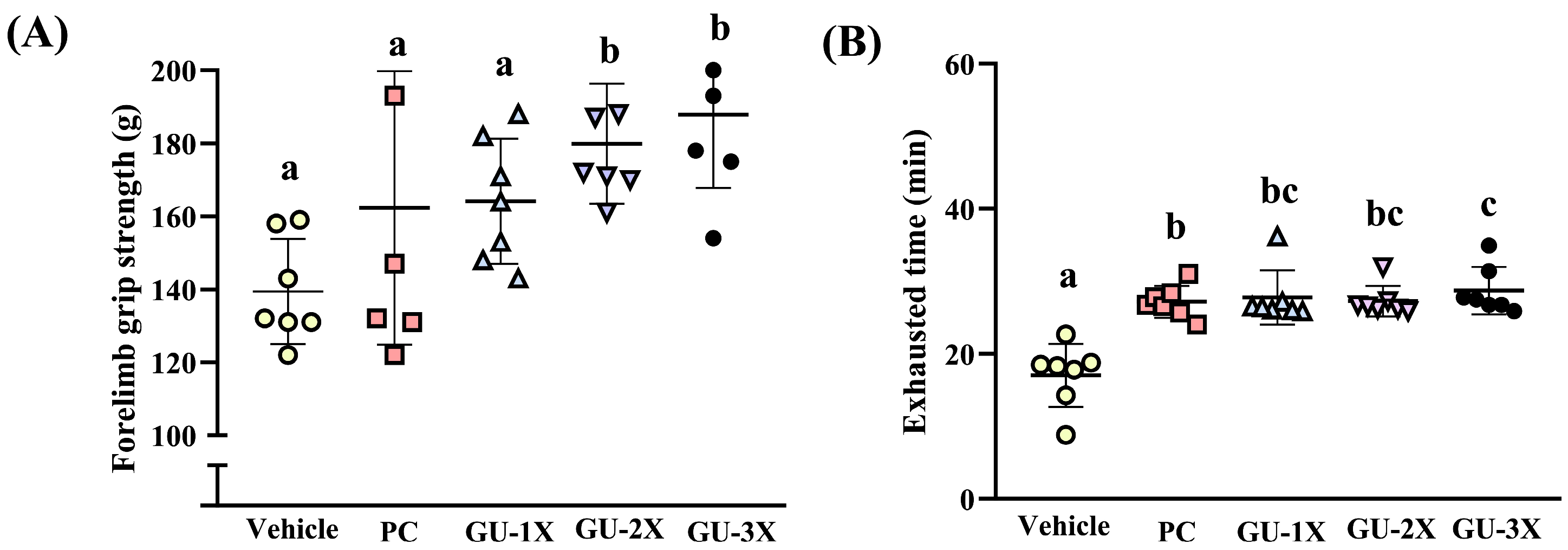
3.2. Muscle Strength and Endurance Performance
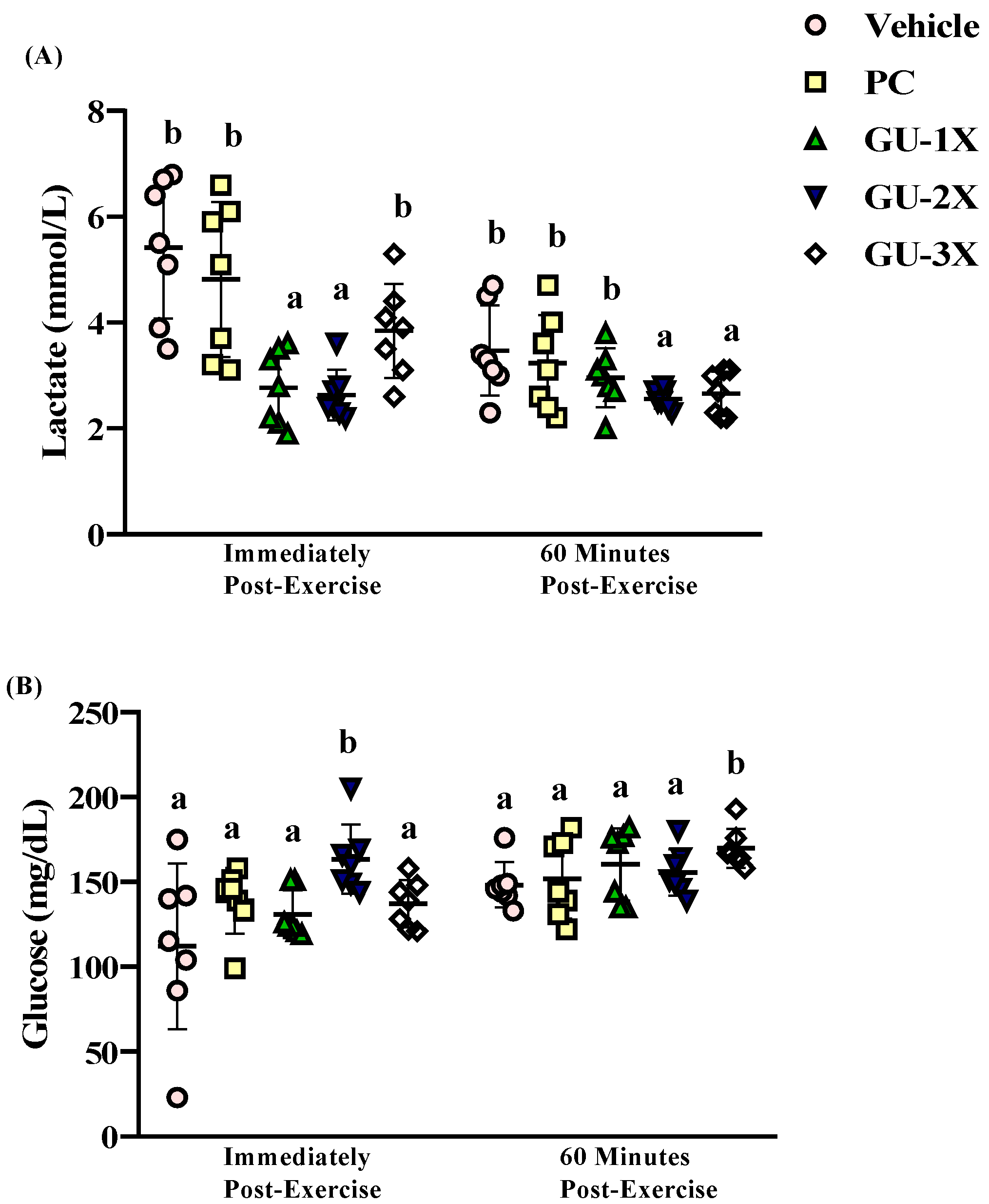
3.3. Blood Biochemical Markers of Fatigue
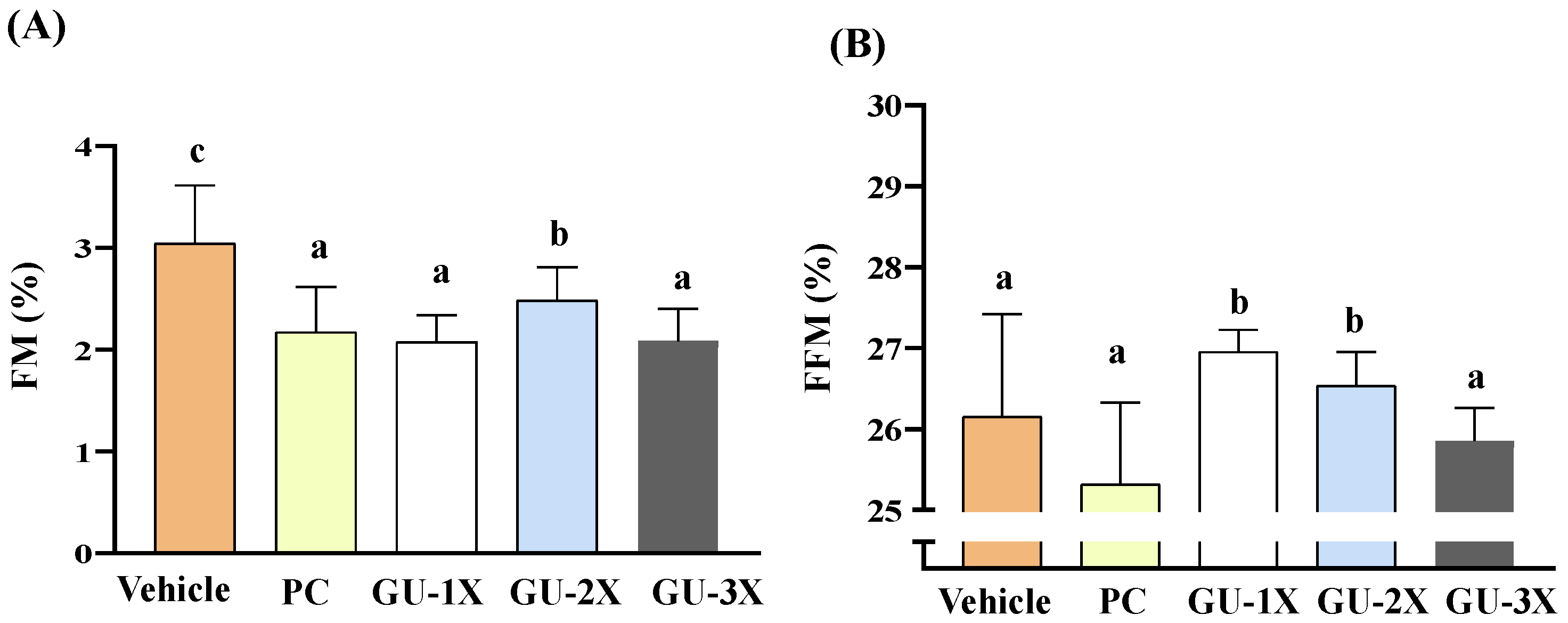
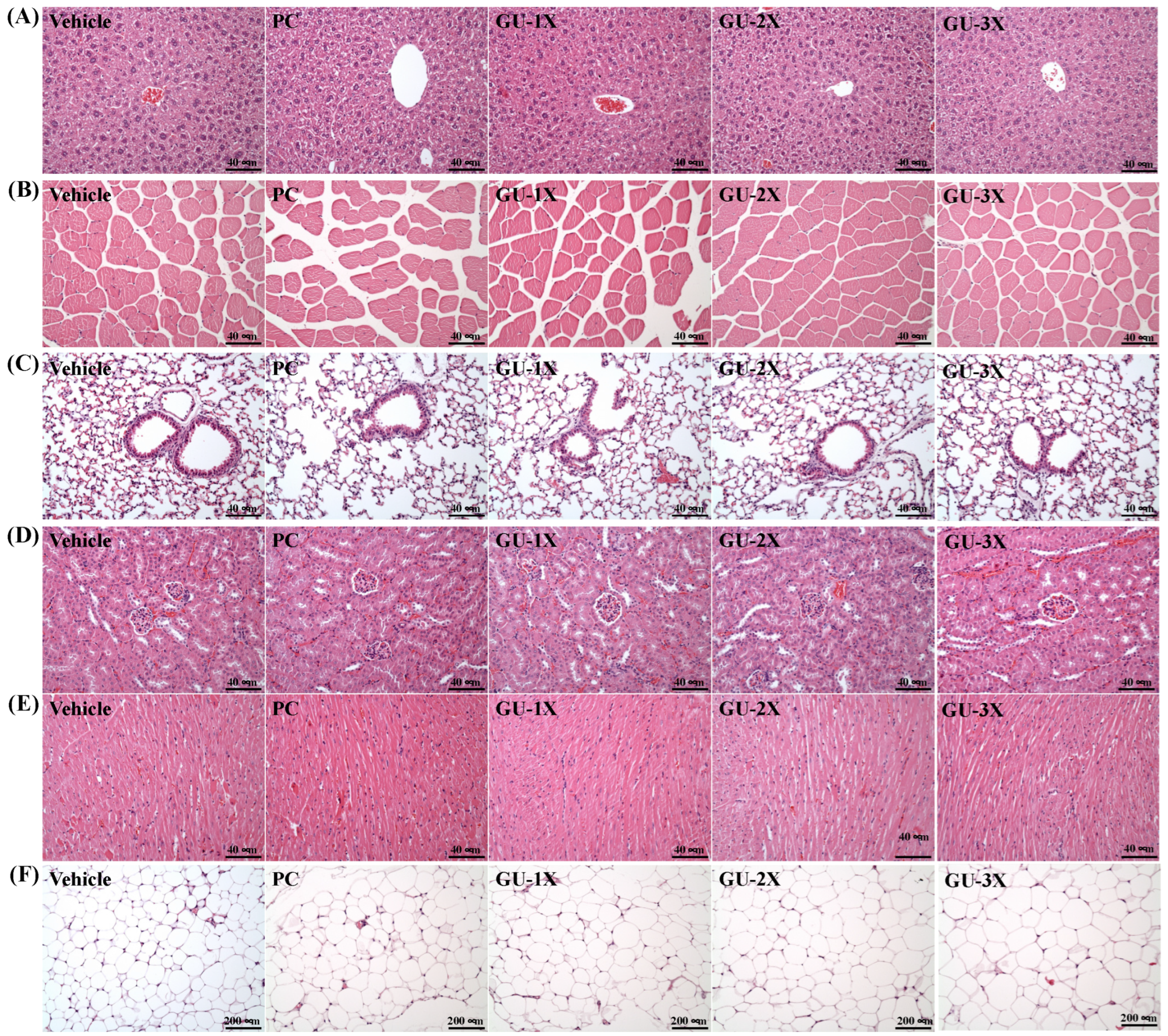
3.4. Body Composition and Organ Health
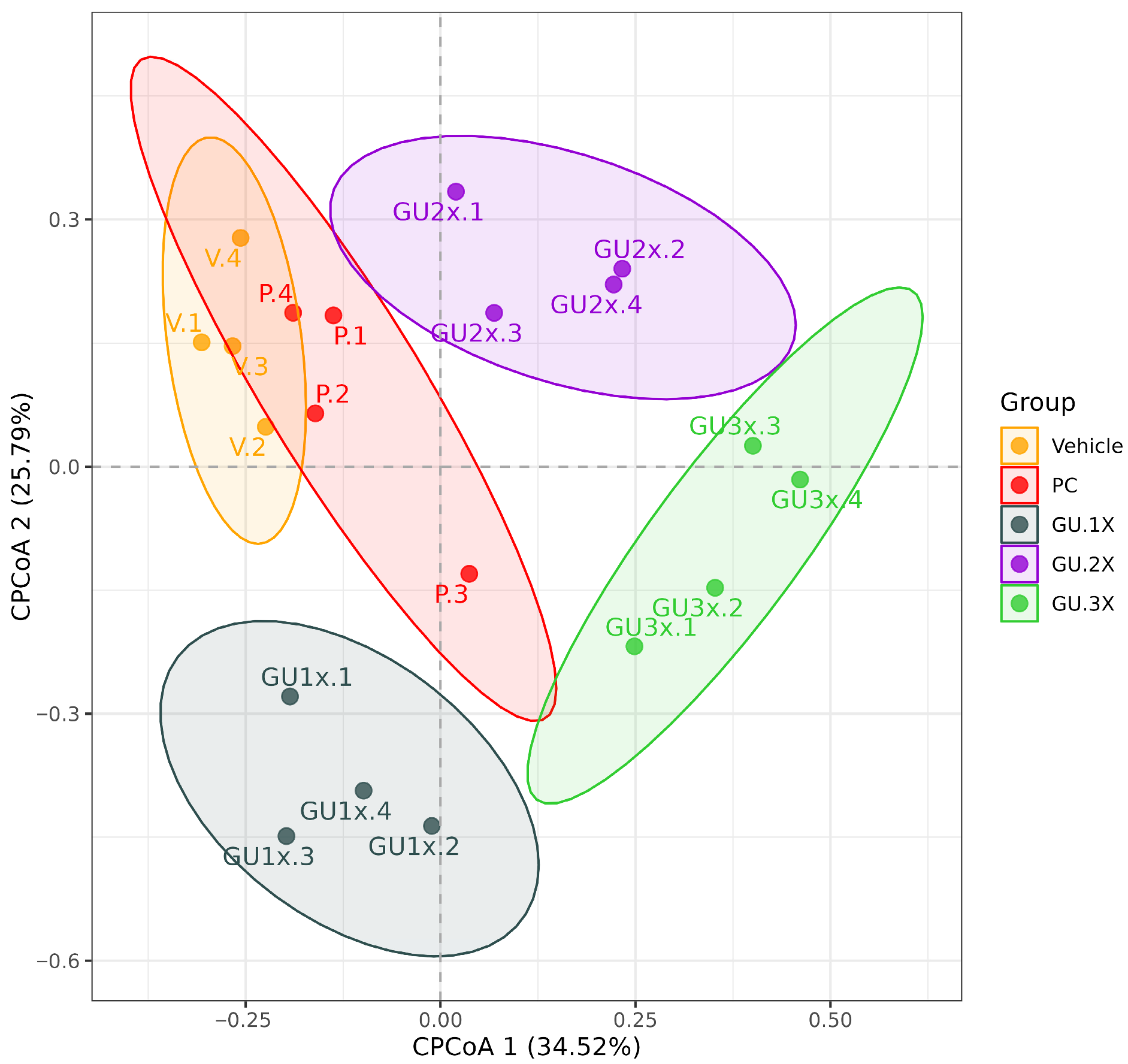
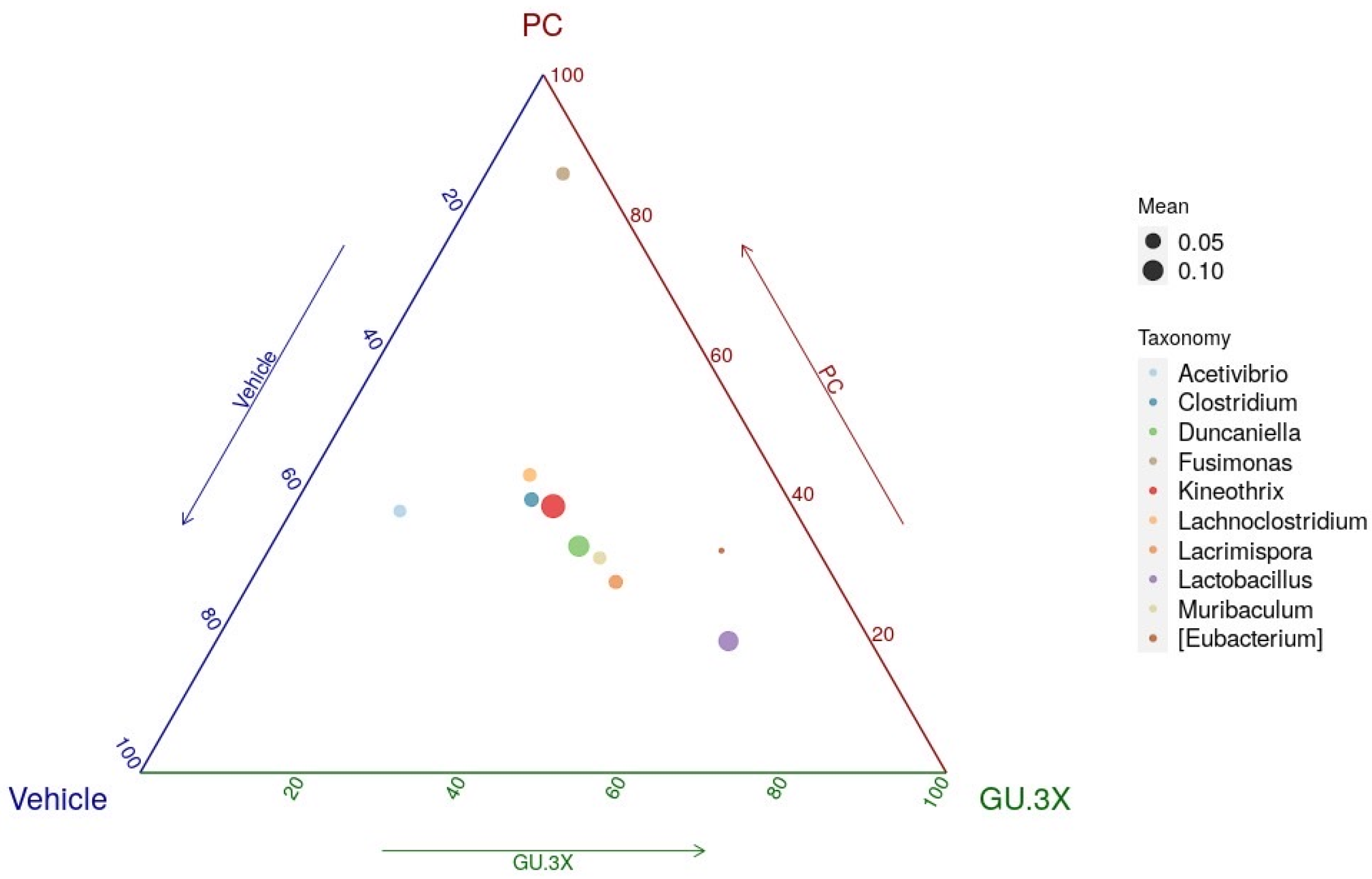
3.5. Analysis of 16S rRNA Gene Sequences Following 4-Week GU Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ASV | Amplicon sequence variants |
| BCAA | Branched-chain amino acid |
| CK | Creatine kinase |
| CPCoA | Constrained principal coordinates analysis |
| EFP | Epididymal fat pad |
| FFM | Fat-free mass |
| FM | Fat mass |
| GBH | Grouper bone hydrolysate |
| H&E | Hematoxylin and eosin |
| HDL | High-density lipoprotein |
| HED | Human-equivalent dose |
| IACUC | Institutional Animal Care and Use Committee |
| LDL | Low-density lipoprotein |
| NGS | Next generation sequencing |
| PC | Positive control |
| SCFA | Short-chain fatty acids |
| SD | Standard deviation |
| TC | Total cholesterol |
| TP | Total protein |
References
- Wimmer, B.C.; Dwan, C.; De Medts, J.; Duysburgh, C.; Rotsaert, C.; Marzorati, M. Undaria pinnatifida fucoidan enhances gut microbiome, butyrate production, and exerts anti-inflammatory effects in an in vitro short-term shime(®) coupled to a caco-2/thp-1 co-culture model. Mar. Drugs 2025, 23, 242. [Google Scholar] [CrossRef]
- Yang, C.; Dwan, C.; Wimmer, B.; Wilson, R.; Johnson, L.; Caruso, V. Fucoidan from undaria pinnatifida enhances exercise performance and increases the abundance of beneficial gut bacteria in mice. Mar. Drugs 2024, 22, 485. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Kajla, P.; Balakrishnan, G.; Phimolsiripol, Y. Strategies for upcycling food waste in the food production and supply chain. Trends Food Sci. Technol. 2024, 143, 104314. [Google Scholar]
- Vianna, G.M.S.; Zeller, D.; Pauly, D. Fisheries and policy implications for human nutrition. Curr. Environ. Health Rep. 2020, 7, 161–169. [Google Scholar] [CrossRef]
- Thorsen, M.; Skeaff, S.; Goodman-Smith, F.; Thong, B.; Bremer, P.; Mirosa, M. Upcycled foods: A nudge toward nutrition. Front. Nutr. 2022, 9, 1071829. [Google Scholar] [CrossRef] [PubMed]
- Hormoznejad, R.; Zare Javid, A.; Mansoori, A. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: A systematic review with meta-analysis. Sport Sci. Health 2019, 15, 265–279. [Google Scholar] [CrossRef]
- Weber, M.G.; Dias, S.S.; de Angelis, T.R.; Fernandes, E.V.; Bernardes, A.G.; Milanez, V.F.; Jussiani, E.I.; de Paula Ramos, S. The use of bcaa to decrease delayed-onset muscle soreness after a single bout of exercise: A systematic review and me-ta-analysis. Amino Acids 2021, 53, 1663–1678. [Google Scholar] [CrossRef]
- Salem, A.; Ben Maaoui, K.; Jahrami, H.; AlMarzooqi, M.A.; Boukhris, O.; Messai, B.; Clark, C.C.T.; Glenn, J.M.; Ghazzaoui, H.A.; Bragazzi, N.L. Attenuating muscle damage biomarkers and muscle soreness after an exercise-induced muscle damage with branched-chain amino acid (bcaa) supplementation: A systematic review and meta-analysis with meta-regression. Sports Med. Open 2024, 10, 42. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chai, H.-J.; Tsai, C.-J.; Tsai, T.-Y.; Liu, T.-H.; Yi, T.-K.; Chen, Y.-M. Development of functional foods from grouper fish-bone residues to enhance muscle strength and exercise endurance in mice. Front. Sustain. Food Syst. 2024, 8, 1483028. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Maruya, R.; Koikeda, T.; Nakano, T. Effects of undaria pinnatifida (wakame) on the human intestinal environment. Funct. Foods Health Dis. 2018, 8, 478. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Chiu, W.-C.; Chiu, Y.-S. Effect of inonotus obliquus extract supplementation on endurance exercise and ener-gy-consuming processes through lipid transport in mice. Nutrients 2022, 14, 5007. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16s rrna diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Youssef, N.; Sheik, C.S.; Krumholz, L.R.; Najar, F.Z.; Roe, B.A.; Elshahed, M.S. Comparison of species richness estimates obtained using nearly complete fragments and simulated pyrosequencing-generated fragments in 16s rrna gene-based environmental surveys. Appl. Environ. Microbiol. 2009, 75, 5227–5236. [Google Scholar] [CrossRef]
- Ramakodi, M.P. Influence of 16s rrna reference databases in amplicon-based environmental microbiome research. Biotechnol. Lett. 2022, 44, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-T.; Peng, X.; Deng, G.-H.; Sheng, H.-F.; Wang, Y.; Zhou, H.-W.; Tam, N.F.-Y. Illumina sequencing of 16s rrna tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb. Ecol. 2013, 66, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zgadzaj, R.; Garrido-Oter, R.; Jensen, D.B.; Koprivova, A.; Schulze-Lefert, P.; Radutoiu, S. Root nodule symbiosis in lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc. Natl. Acad. Sci. USA 2016, 113, E7996–E8005. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Rasmussen, B.B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, R.; Zhang, H.; Zafar, M.H.; Wang, M. Research progress in the role and mechanism of leucine in regulating animal growth and development. Front. Physiol. 2023, 14, 1252089. [Google Scholar] [CrossRef]
- Li, F.; Yin, Y.; Tan, B.; Kong, X.; Wu, G. Leucine nutrition in animals and humans: Mtor signaling and beyond. Amino Acids 2011, 41, 1185–1193. [Google Scholar] [CrossRef]
- Lees, M.; Carson, B. The potential role of fish-derived protein hydrolysates on metabolic health, skeletal muscle mass and function in ageing. Nutrients 2020, 12, 2434. [Google Scholar] [CrossRef]
- Zhao, Y.; Cholewa, J.; Shang, H.; Yang, Y.; Ding, X.; Wang, Q.; Su, Q.; Zanchi, N.E.; Xia, Z. Advances in the role of leu-cine-sensing in the regulation of protein synthesis in aging skeletal muscle. Front. Cell Dev. Biol. 2021, 9, 646482. [Google Scholar] [CrossRef]
- McBean, S.E.; Church, J.E.; Thompson, B.K.; Taylor, C.J.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y.; Poel, C. Oral fucoidan improves muscle size and strength in mice. Physiol. Rep. 2021, 9, e14730. [Google Scholar] [CrossRef]
- Pérez, A.; Ruz, M.; García, P.; Jiménez, P.; Valencia, P.; Ramírez, C.; Pinto, M.; Nuñez, S.M.; Park, J.W.; Almonacid, S. Nutritional properties of fish bones: Potential applications in the food industry. Food Rev. Int. 2024, 40, 79–91. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Jeong, W.-S.; Lee, H.-Y. Effect of bcaa intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J. Exerc. Nutr. Biochem. 2013, 17, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lim, S.Y. Fucoidans and bowel health. Mar. Drugs 2021, 19, 436. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C. Microbiota-derived short-chain fatty acids promote th1 cell il-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.-C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry polyphenols and fibers modulate distinct microbial metabolic functions and gut microbiota enterotype-like clustering in obese mice. Front. Microbiol. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food. Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Peng, F.; Yang, H.; Luo, J.; Zhang, L.; Chen, X.; Liao, H.; Lei, H.; Liu, S.; Yang, T. Probiotics and muscle health: The impact of lactobacillus on sarcopenia through the gut-muscle axis. Front. Microbiol. 2025, 16, 1559119. [Google Scholar] [CrossRef]
- Sales, K.M.; Reimer, R.A. Unlocking a novel determinant of athletic performance: The role of the gut microbiota, short-chain fatty acids, and “biotics” in exercise. J. Sport Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023, 35, 361–375.e9. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]

| Characteristics | Vehicle | PC | GU-1X | GU-2X | GU-3X |
|---|---|---|---|---|---|
| Initial BW (g) | 32.44 ± 0.51 | 32.23 ± 1.30 | 32.30 ± 0.58 | 32.35 ± 0.38 | 32.42 ± 0.31 |
| Final BW (g) | 36.59 ± 1.40 | 35.09 ± 1.60 | 36.58 ± 1.19 | 36.44 ± 1.45 | 36.27 ± 0.53 |
| Food intake (g/day) | 7.96 ± 1.05 | 8.02 ± 0.27 | 8.05 ± 0.31 | 7.98 ± 0.70 | 8.02 ± 0.56 |
| Water intake (mL/day) | 5.92 ± 0.26 | 6.13 ± 0.15 | 6.16 ± 0.37 | 5.97 ± 0.37 | 5.99 ± 0.26 |
| Liver (g) | 1.46 ± 0.06 | 1.29 ± 0.10 | 1.32 ± 0.15 | 1.38 ± 0.11 | 1.34 ± 0.14 |
| Kidney (g) | 0.66 ± 0.05 | 0.68 ± 0.03 | 0.67 ± 0.04 | 0.67 ± 0.04 | 0.67 ± 0.04 |
| EFP (g) | 0.49 ± 0.08 | 0.43 ± 0.10 | 0.42 ± 0.06 | 0.42 ± 0.10 | 0.41 ± 0.07 |
| Heart (g) | 0.20 ± 0.01 | 0.18 ± 0.02 | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.01 |
| Lung (g) | 0.27 ± 0.01 | 0.28 ± 0.04 | 0.28 ± 0.01 | 0.26 ± 0.01 | 0.27 ± 0.01 |
| Muscle (g) | 0.30 ± 0.02 a | 0.35 ± 0.04 b | 0.38 ± 0.04 b | 0.39 ± 0.04 b | 0.40 ± 0.04 b |
| Relative liver weight (%) | 3.91 ± 0.23 | 3.65 ± 0.22 | 3.62 ± 0.32 | 3.69 ± 0.21 | 3.72 ± 0.33 |
| Relative kidney weight (%) | 1.94 ± 0.16 | 1.92 ± 0.08 | 1.87 ± 0.13 | 1.89 ± 0.07 | 1.95 ± 0.09 |
| Relative EFP weight (%) | 1.32 ± 0.19 | 1.21 ± 0.29 | 1.14 ± 0.18 | 1.13 ± 0.28 | 1.14 ± 0.19 |
| Relative heart weight (%) | 0.52 ± 0.04 | 0.50 ± 0.04 | 0.51 ± 0.03 | 0.51 ± 0.04 | 0.51 ± 0.04 |
| Relative Lung weight (%) | 0.76 ± 0.04 | 0.78 ± 0.10 | 0.78 ± 0.01 | 0.74 ± 0.01 | 0.75 ± 0.04 |
| Relative Muscle weight (%) | 0.79 ± 0.03 a | 1.00 ± 0.11 b | 1.04 ± 0.13 b | 1.07 ± 0.10 b | 1.11 ± 0.33 b |
| Parameter | Vehicle | PC | GU-1X | GU-2X | GU-3X |
|---|---|---|---|---|---|
| AST (U/L) | 157 ± 61 b | 118 ± 47 b | 86 ± 22 a | 90 ± 11 a | 89 ± 20 a |
| ALT (U/L) | 108 ± 23 | 97 ± 37 | 95 ± 30 | 84 ± 24 | 75 ± 20 |
| LDH (U/L) | 633 ± 151 b | 436 ± 127 a | 434 ± 74 a | 445 ± 67 a | 455 ± 89 a |
| Creatinine (mg/dL) | 0.14 ± 0.03 b | 0.09 ± 0.02 a | 0.10 ± 0.02 a | 0.10 ± 0.02 a | 0.10 ± 0.02 a |
| TC (mg/dL) | 138 ± 24 | 140 ± 21 | 146 ± 21 | 144 ± 19 | 137 ± 22 |
| HDL (mg/dL) | 109 ± 13 | 105 ± 18 | 110 ± 14 | 110 ± 10 | 102 ± 15 |
| LDL (mg/dL) | 2.48 ± 0.71 | 2.20 ± 0.61 | 2.53 ± 1.02 | 2.60 ± 0.58 | 2.54 ± 0.30 |
| TG (mg/dL) | 185 ± 44 | 137 ± 32 | 179 ± 32 | 191 ± 31 | 196 ± 23 |
| TP (g/dL) | 5.07 ± 0.26 | 4.94 ± 0.15 | 5.02 ± 0.08 | 5.08 ± 0.09 | 5.04 ± 0.16 |
| Albumin (g/dL) | 2.80 ± 0.19 | 2.75 ± 0.16 | 2.82 ± 0.08 | 2.80 ± 0.07 | 2.80 ± 0.06 |
| CK (U/L) | 216 ± 93 | 257 ± 112 | 160 ± 49 | 198 ± 31 | 222 ± 92 |
| Glucose (mg/dL) | 196 ± 17 | 201 ± 22 | 194 ± 19 | 197 ± 19 | 195 ± 15 |
| Comparison | F | R2 | p-Value |
|---|---|---|---|
| Vehicle vs. PC | 0.7531 | 0.1115 | 1.0000 |
| Vehicle vs. GU-1X | 1.1679 | 0.1629 | 0.1880 |
| Vehicle vs. GU-2X | 1.0627 | 0.1505 | 0.3660 |
| Vehicle vs. GU-3X | 0.7506 | 0.2259 | 0.0210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, H.-J.; Yi, T.-K.; Kao, Y.-F.; Liu, T.-H.; Tsai, T.-Y.; Chen, Y.-M. Dual-Action Grouper Bone and Wakame Hydrolysates Supplement Enhances Exercise Performance and Modulates Gut Microbiota in Mice. Nutrients 2025, 17, 2933. https://doi.org/10.3390/nu17182933
Chai H-J, Yi T-K, Kao Y-F, Liu T-H, Tsai T-Y, Chen Y-M. Dual-Action Grouper Bone and Wakame Hydrolysates Supplement Enhances Exercise Performance and Modulates Gut Microbiota in Mice. Nutrients. 2025; 17(18):2933. https://doi.org/10.3390/nu17182933
Chicago/Turabian StyleChai, Huey-Jine, Tsung-Kai Yi, Yi-Feng Kao, Te-Hua Liu, Tsung-Yu Tsai, and Yi-Ming Chen. 2025. "Dual-Action Grouper Bone and Wakame Hydrolysates Supplement Enhances Exercise Performance and Modulates Gut Microbiota in Mice" Nutrients 17, no. 18: 2933. https://doi.org/10.3390/nu17182933
APA StyleChai, H.-J., Yi, T.-K., Kao, Y.-F., Liu, T.-H., Tsai, T.-Y., & Chen, Y.-M. (2025). Dual-Action Grouper Bone and Wakame Hydrolysates Supplement Enhances Exercise Performance and Modulates Gut Microbiota in Mice. Nutrients, 17(18), 2933. https://doi.org/10.3390/nu17182933








