The Role of Dietary Supplements in Modulating Menopause Onset: A Comprehensive Analysis of Nutritional and Lifestyle Influences on Menopause Timing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
- Documented natural menopause, characterized as a minimum of 12 uninterrupted months of amenorrhea that cannot be linked to surgical procedures or medical interventions.
- Provided complete data on age at natural menopause, dietary supplement use, and relevant covariates.
- Reported plausible energy intakes between 500 and 3500 kcal/day to exclude implausible dietary reports [15].
2.3. Dietary and Supplement Assessment
2.4. Outcome Assessment
2.5. Covariates
2.6. Statistical Analysis
2.6.1. Cox Proportional Hazards Models
2.6.2. Gradient Boosting Machine
2.7. Sensitivity Analyses
3. Results
3.1. Participant Characteristics
3.2. Cox Model Results
3.3. GBM Findings
4. Discussion
4.1. Principal Findings
4.2. Comparison with Previous Studies
4.3. Strengths and Limitations
4.4. Public Health Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CI | Confidence Interval |
| DAG | Directed Acyclic Graph |
| FFQ | Food Frequency Questionnaire |
| GBM | Gradient Boosting Machine |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| SD | Standard Deviation |
| SES | Socioeconomic Status |
| UKWCS | UK Women’s Cohort Study |
| WCRF | World Cancer Research Fund |
Appendix A

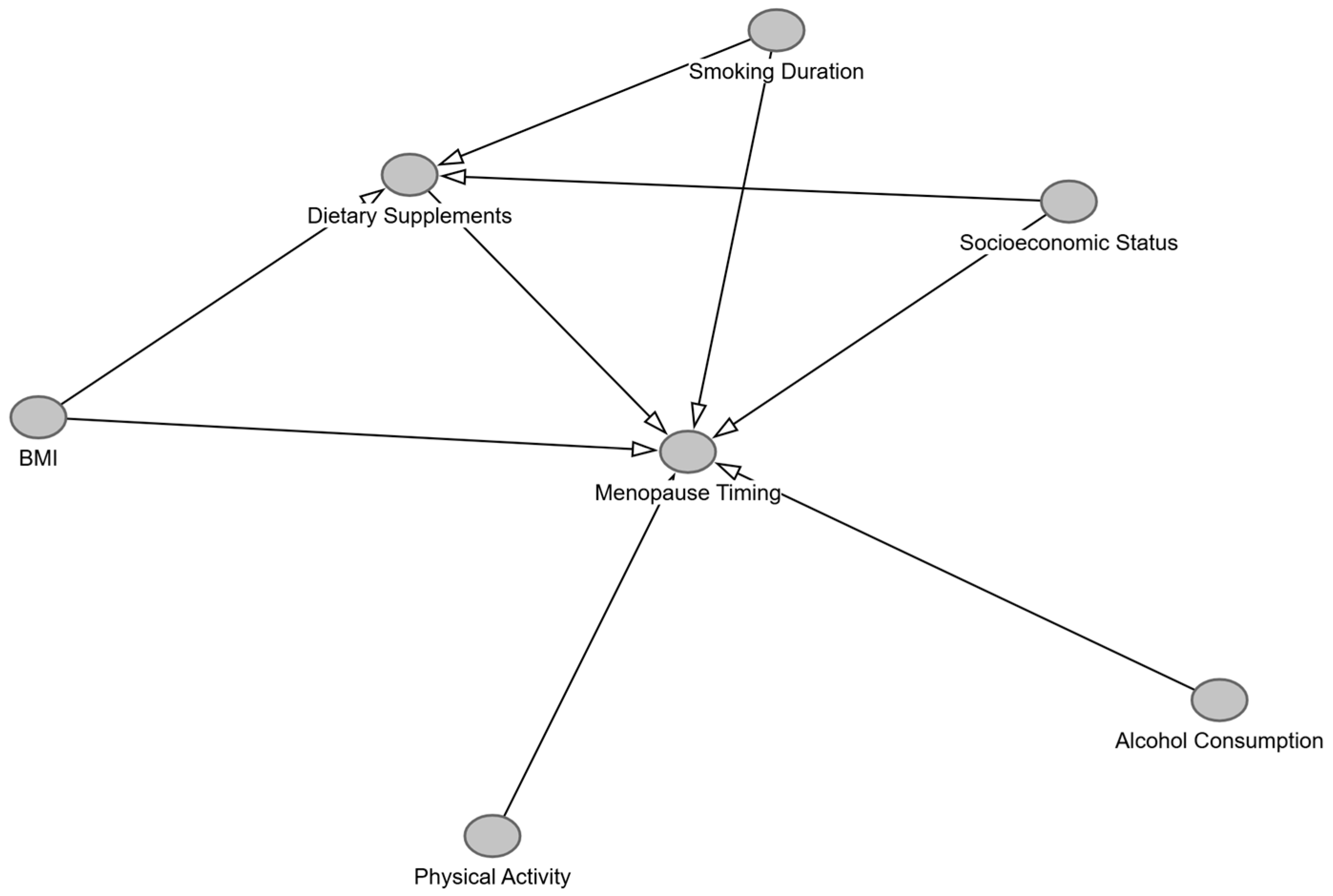
References
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J. Executive Summary of the Stages of Reproductive Aging Workshop + 10. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef]
- Schoenaker, D.A.; Jackson, C.A.; Rowlands, J.V.; Mishra, G.D. Socioeconomic Position, Lifestyle Factors and Age at Natural. Menopause: A Systematic Review and Meta-Analyses of Studies Across Six Continents. Int. J. Epidemiol. 2014, 43, 1542–1562. [Google Scholar] [CrossRef]
- Svejme, O.; Ahlborg, H.G.; Nilsson, J.-Å.; Karlsson, M.K. Early Menopause and Risk of Osteoporosis, Fracture, and Mortality: A 34-Year Prospective Observational Study in 390 Women. BJOG 2012, 119, 810–816. [Google Scholar] [CrossRef]
- Appiah, D.; Winters, S.J.; Hornung, C.A.; Racial and Ethnic Disparities in Menopause Timing Study Group. Age at natural menopause and risk of incident cardiovascular disease: A pooled analysis of prospective cohort studies. J. Am. Heart Assoc. 2016, 5, e003801. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; van der Schouw, Y.T.; Onland-Moret, N.C.; Sharp, S.J.; Ong, K.K.; Khaw, K.T.; Ardanaz, E.; Amiano, P.; Boeing, H.; Chirlaque, M.D.; et al. Age at Menopause, Reproductive Life Span, and Type 2 Diabetes Risk. Diabetes Care 2013, 36, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, J.T.; Matthews, K.A.; Kuller, L.H.; Wing, R.R.; Meilahn, E.N.; Plantinga, P. Prospective Study of the Determinants of Age at Menopause. Am. J. Epidemiol. 2001, 153, 760–770. [Google Scholar] [CrossRef]
- Wellons, M.; Ouyang, P.; Schreiner, P.J.; Herrington, D.M.; Vaidya, D. Early Menopause Predicts Future Coronary Heart Disease and Stroke: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 2012, 97, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, Menopause, and Breast Cancer Risk: Re-analysis of 34 Studies. Lancet 2019, 393, 2203–2211. [Google Scholar]
- Office for National Statistics. Life Expectancy at Birth and at Age 65 by Local Areas in England and Wales: 2017 to 2019. 2021. Available online: https://www.ons.gov.uk (accessed on 1 March 2025).
- Denison, H.J.; Jameson, K.A.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Patterns of dietary supplement use among older men and women in the UK: Findings from the Hertfordshire Cohort Study. J. Nutr. Health Aging 2012, 16, 307–311. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.; Kim, H. B-Vitamins and Ovarian Reserve: A Review. Nutrients 2021, 13, 3443. [Google Scholar]
- Manokaran, K.; Bhat, P.; Nayak, D.; Baskaran, R.; Paramasivam, P.; Ahmed, S.F.; Priya, K.; Pai, K.S.R.; Balaji, V.E. Oxidative stress and female reproductive disorder: A review. Asian Pac. J. Reprod. 2022, 11, 107–116. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Dietary Patterns and Age at Natural Menopause: Evidence from the UK Women’s Cohort Study. Maturitas 2020, 143, 165–170. [Google Scholar] [CrossRef]
- Cade, J.E.; Burley, V.J.; Alwan, N.A.; Greenwood, D.C.; Collins, E.; Harvey, I.M. Cohort Profile: The UK Women’s Cohort Study (UKWCS). Int. J. Epidemiol. 2017, 46, e11. [Google Scholar] [CrossRef]
- Food Standards Agency. Food Portion Sizes, 3rd ed.; The Stationery Office: London, UK, 1994. [Google Scholar]
- FSA. McCance & Widdowson’s the Composition of Foods, 6th ed.; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Willett, W.C.; Stampfer, M.J. Total Energy Intake: Implications for Epidemiologic Analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- Torgerson, D.J.; Thomas, R.E.; Reid, D.M. Mothers and Daughters Menopause Study: A Prospective Study of Recall Bias in Age at Menopause. Maturitas 1994, 19, 169–175. [Google Scholar]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Lis’kiewicz, M.; Ellison, G.T. Robust Causal Inference Using Directed Acyclic Graphs: The R Package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.A.; Appleby, P.N.; Davey, G.K.; Key, T.J. Validity of Self-Reported Height and Weight in 4808 EPIC-Oxford Participants. Public Health Nutr. 2002, 5, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Galobardes, B.; Shaw, M.; Lawlor, D.A.; Lynch, J.W.; Davey Smith, G. Indicators of Socioeconomic Position (Part 1). J. Epidemiol. Community Health 2006, 60, 7–12. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Pizzorno, J. Mitochondria and omega-3. Integr. Med. 2014, 13, 14–22. [Google Scholar]
- Zhu, D.; Chung, H.F.; Pandeya, N.; Dobson, A.J.; Kuh, D.; Crawford, S.L.; Mishra, G.D. Body mass index and age at natural menopause: An international pooled analysis of 11 prospective studies. Eur. J. Epidemiol. 2018, 33, 699–710. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E.; Burley, V.J. Dietary intake and age at natural menopause: Results from the UK Women’s Cohort Study. J. Epidemiol. Community Health 2021, 75, 87–94. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Association of diet with the onset of menopause in Japanese women. Am. J. Epidemiol. 2000, 152, 863–867. [Google Scholar] [CrossRef]
- Freeman, J.R.; Whitcomb, B.W.; Purdue-Smithe, A.C.; Manson, J.E.; Langton, C.R.; Hankinson, S.E.; Rosner, B.A. Bertone-Johnson ER. Is Alcohol Consumption Associated with Risk of Early Menopause? Am. J. Epidemiol. 2021, 190, 2612–2617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stabile, G.; Topouzova, G.A.; De Seta, F. The role of microbiota in the management of genitourinary syndrome of menopause. Climacteric 2023, 26, 353–360. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, Economic Research Service. Trends in U.S. Production and Supply of High-Concentration Omega-3 Supplements. 15 November 2021. Available online: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=103078 (accessed on 8 September 2025).
- Purdue-Smithe, A.C.; Whitcomb, B.W.; Szegda, K.L.; Boutot, M.E.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Troy, L.M.; Michels, K.B.; Bertone-Johnson, E.R. Vitamin D and calcium intake and risk of early menopause. Am. J. Clin. Nutr. 2017, 105, 1493–1501. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Yong, Z.; Yang, L.; Zhao, Y.; Yan, M.; Zheng, R.; Luo, X. Association Between Protein-Rich Foods, Nutritional Supplements, and Age of Natural Menopause and Its Symptoms. Nutrients 2025, 17, 356. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Non-Users (n = 1205) | Users (n = 2361) |
|---|---|---|
| Age at menopause (y, median) | 50 | 51 |
| BMI (kg/m2, mean ± SD) | 25.4 ± 4.2 | 24.7 ± 3.9 |
| Smoking duration (y, median) | 20 | 18 |
| Alcohol (units/week, median) | 5 | 4 |
| Physical activity (h/week, median) | 3.0 | 3.2 |
| University degree (%) | 20 | 40 |
| Supplement | HR (95% CI) | p-Value |
|---|---|---|
| Fish oil | 0.05 (0.02–0.09) | <0.001 |
| Vitamin B-complex | 0.48 (0.38–0.62) | <0.001 |
| Antioxidants | 0.54 (0.38–0.69) | 0.017 |
| Vitamin C | 0.75 (0.56–0.93) | 0.041 |
| Folic acid | 0.81 (0.65–1.01) | 0.059 |
| Multivitamin | 0.97 (0.87–1.08) | 0.58 |
| Supplement | Variable Importance (%) |
|---|---|
| Red-meat servings | 34.5 |
| BMI | 28.7 |
| Education level | 15.2 |
| Smoking | 11.9 |
| Fish serving | 8.3 |
| Walking hours/week | 6.5 |
| Alcohol frequency | 5.7 |
| Sleep hours | 4.8 |
| White-meat servings | 3.4 |
| Vegetarian diet | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebaraj, S.; Nlebedim, V. The Role of Dietary Supplements in Modulating Menopause Onset: A Comprehensive Analysis of Nutritional and Lifestyle Influences on Menopause Timing. Nutrients 2025, 17, 2921. https://doi.org/10.3390/nu17182921
Jebaraj S, Nlebedim V. The Role of Dietary Supplements in Modulating Menopause Onset: A Comprehensive Analysis of Nutritional and Lifestyle Influences on Menopause Timing. Nutrients. 2025; 17(18):2921. https://doi.org/10.3390/nu17182921
Chicago/Turabian StyleJebaraj, Shekhinamary, and Valentine Nlebedim. 2025. "The Role of Dietary Supplements in Modulating Menopause Onset: A Comprehensive Analysis of Nutritional and Lifestyle Influences on Menopause Timing" Nutrients 17, no. 18: 2921. https://doi.org/10.3390/nu17182921
APA StyleJebaraj, S., & Nlebedim, V. (2025). The Role of Dietary Supplements in Modulating Menopause Onset: A Comprehensive Analysis of Nutritional and Lifestyle Influences on Menopause Timing. Nutrients, 17(18), 2921. https://doi.org/10.3390/nu17182921







