Gut Microbiome-Derived Short-Chain Fatty Acids in Glomerular Protection and Modulation of Chronic Kidney Disease Progression
Abstract
1. Introduction
2. Methods
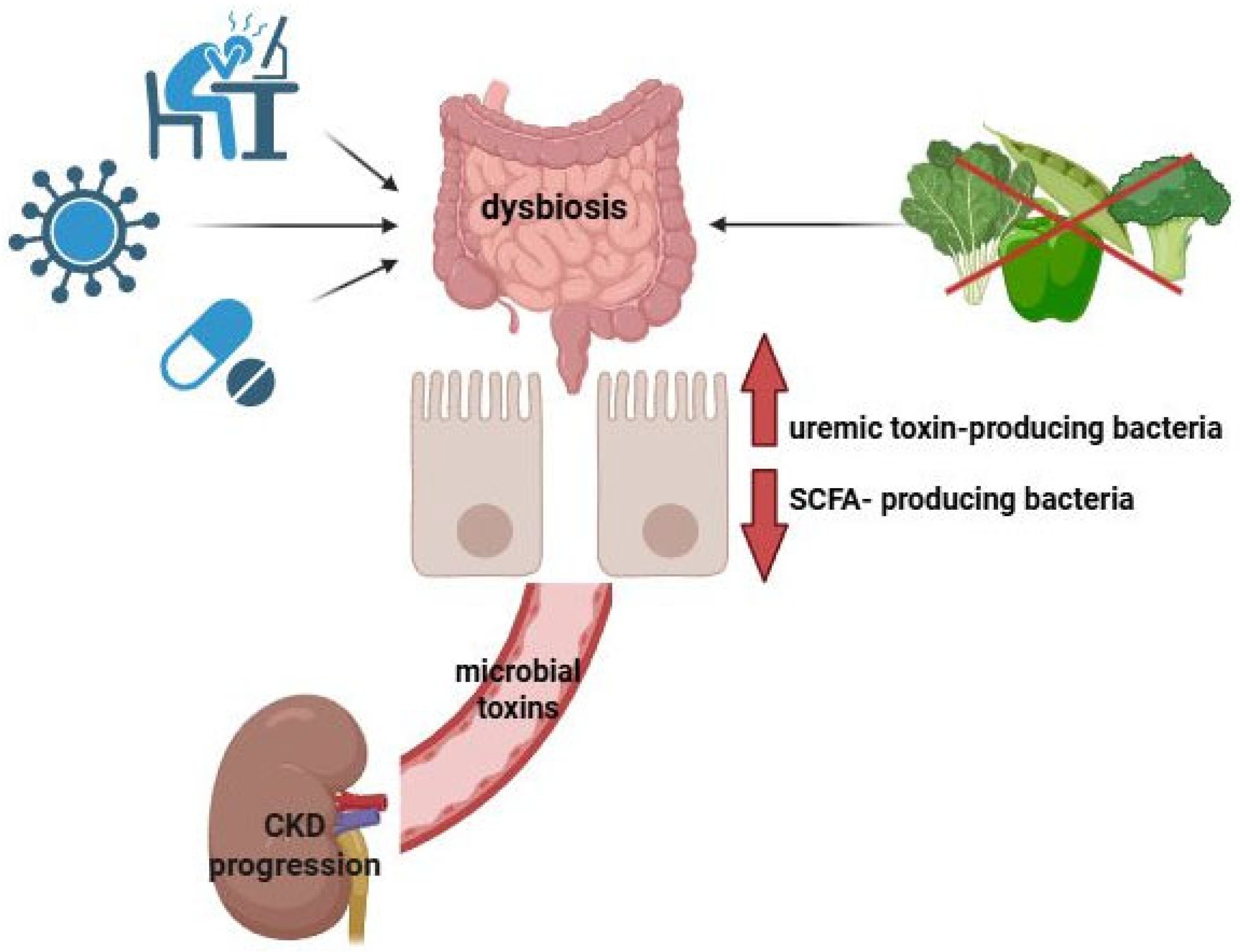
3. Gut–Kidney Axis
4. SCFAs: Overview
5. Structure of the Renal Glomerulus and Its Alterations in CKD
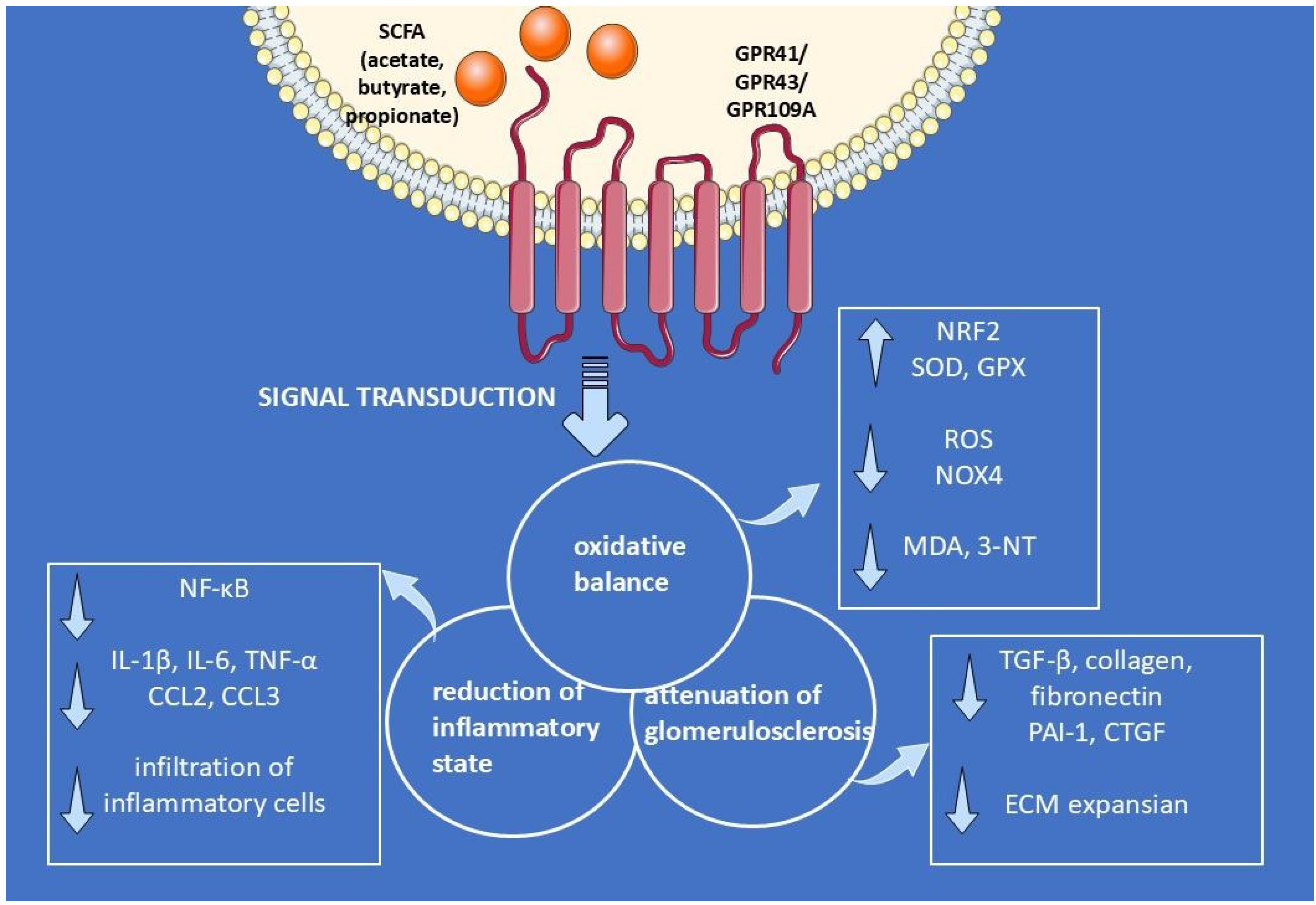
6. Multi-Targeted Mechanisms of SCFA-Mediated Renoprotection
6.1. Relationship Between Endogenous SCFAs and Renal Function in CKD
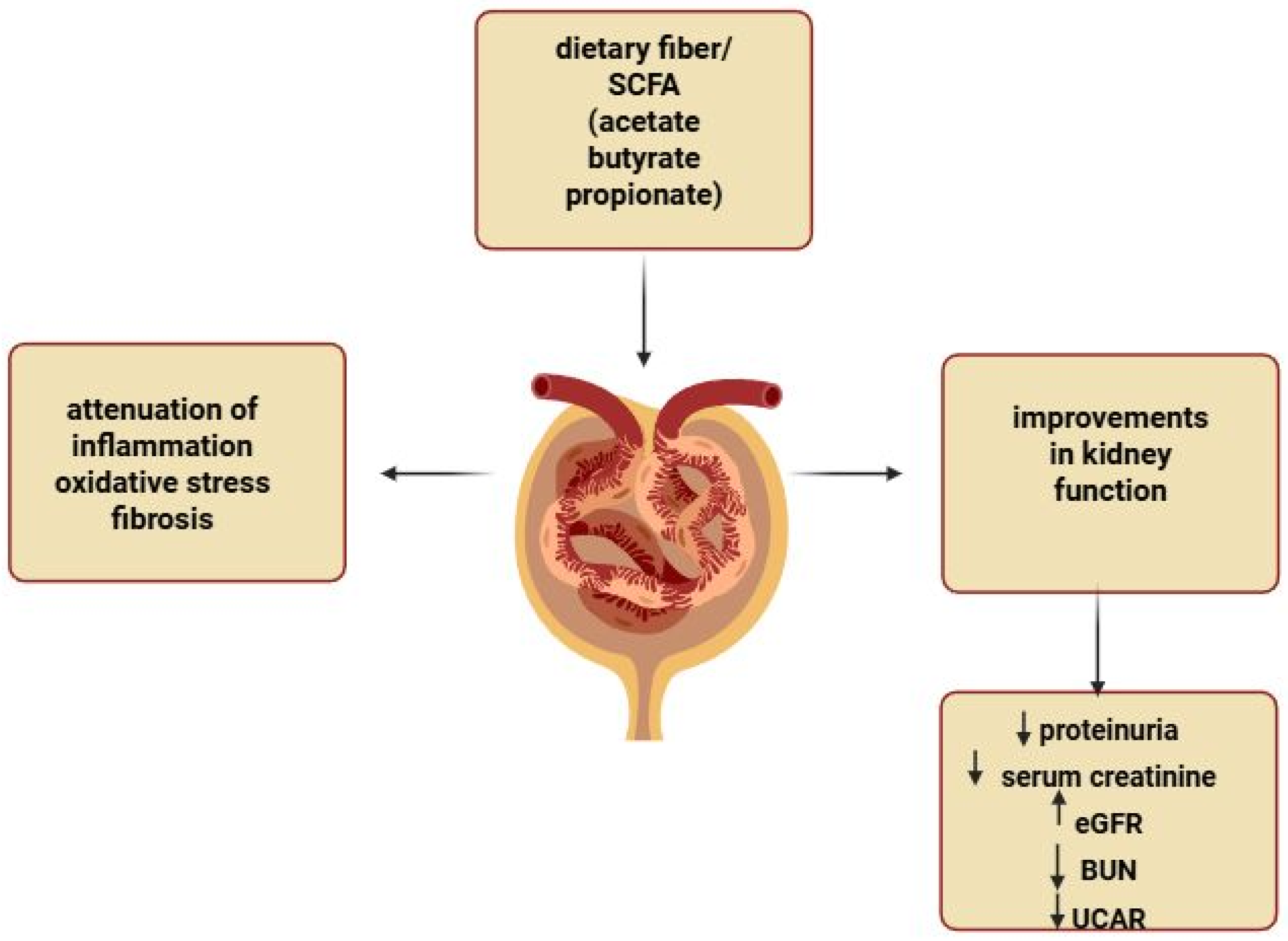
6.2. Effect of Supplemental SCFAs on Renal Function in CKD
6.3. Effect of Supplemental SCFAs on Levels of Inflammatory Cytokines in CKD
6.4. Effect of Supplemental SCFAs on Chemotaxis Inflammatory Cells
6.5. Role of Dietary Fiber in the Inflammatory Response in CKD
6.6. Oxidative Stress in CKD: Overview
6.7. Oxidative Stress: Role of SCFAs in CKD
6.8. Role of Dietary Fiber in Oxidative Stress in CKD
6.9. Role SCFAs in Mitochondrial Oxidative Stress
6.10. Role of SCFAs in Glucose Metabolism and Strengthening Barrier Integrity in CKD
6.11. Kidney Fibrosis: Overview
6.12. Kidney Fibrosis: Role of SCFAs in CKD
6.13. Role of SCFAs in Podocyte Fibrosis
6.14. Role of Dietary Fiber in Preventing Kidney Fibrosis
6.15. Epigenetic Modifications in the Context of CKD and SCFAs
7. Clinical Evidence for SCFA Supplementation in CKD
8. Future Directions and Limitations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3-NT | 3-nitrotyrosine |
| α-SMA | α-smooth muscle actin |
| ADR | Adriamycin |
| AKI | acute kidney injury |
| AMPK | adenosine 5′-monophosphate-activated protein kinase |
| ATP | adenosine 5′-triphosphate |
| BUN | blood urea nitrogen |
| CKD | chronic kidney disease |
| CTGF | connective tissue growth factor |
| DKD | diabetic kidney disease |
| DM | diabetes mellitus |
| DN | diabetic nephropathy |
| ECM | extracellular matrix |
| ESRD | end-stage renal disease |
| eGFR | estimated glomerular filtration rate |
| FPs | foot processes |
| GEnCs | glomerular endothelial cells |
| GBM | glomerular basement membrane |
| GLP-1 | glucagon-like peptide-1 |
| GPX | glutathione peroxidase |
| GMC | glomerular mesangial cells |
| GPR | G protein-coupled receptor |
| HDAC | histone deacetylase |
| HO-1 | heme oxygenase 1 |
| IFN-γ | interferon-γ |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharide |
| MDA | malondialdehyde |
| MCP-1 | monocytic chemotactic protein 1 |
| NOX4 | nicotinamide adenine dinucleotide phosphate oxidase 4 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| NF-κB | nuclear factor-κB |
| PAI-1 | plasminogen activator inhibitor type-1 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SD | slit-diaphragm |
| T2DM | type 2 diabetes mellitus |
| TGF-β | transforming growth factor-β |
| TRPC | transient receptor potential channel |
| TNF-α | tumor necrosis factor-α |
| UACR | urinary albumin-to-creatinine ratio |
| SCFAs | short-chain fatty acids |
| SOD | superoxide dismutase |
References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; Wanner, C.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 29 August 2025).
- Bikbov, B.; Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transplant. 2019, 34, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xu, H.; Cheng, X.; He, Y.; Ren, Q.; Li, D.; Xie, Y.; Gao, C.; Zhang, Y.; Sun, X.; et al. Sodium butyrate attenuates diabetic kidney disease partially via histone butyrylation modification. Mediat. Inflamm. 2022, 2022, 7643322. [Google Scholar] [CrossRef]
- Marzocco, S.; Fazeli, G.; Di Micco, L.; Autore, G.; Adesso, S.; Piaz, F.D.; Heidland, A.; Di Iorio, B. Supplementation of short-chain fatty acid, sodium propionate, in patients on maintenance hemodialysis: Beneficial effects on inflammatory parameters and gut-derived uremic toxins, a pilot study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, X.; Wang, W.; Chen, Y.; Cai, Z.; Wang, Q.; Wang, J.; Shi, Y. Promotion of astrocyte-neuron glutamate-glutamine shuttle by SCFA contributes to the alleviation of Alzheimer’s disease. Redox Biol. 2023, 62, 102690. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, X.; Maruyama, D.; Arjomandi, M.; Prakash, A. Lung immune tone via gut-lung axis: Gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L65–L78. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Felizardo, R.J.F.; de Almeida, D.C.; Pereira, R.L.; Watanabe, I.K.M.; Doimo, N.T.S.; Ribeiro, W.R.; Cenedeze, M.A.; Hiyane, M.I.; Amano, M.T.; Braga, T.T.; et al. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J. 2019, 33, 11894–11908. [Google Scholar] [CrossRef]
- Kawabata, C.; Hirakawa, Y.; Inagi, R.; Nangaku, M. Acetate attenuates kidney fibrosis in an oxidative stress-dependent manner. Physiol Rep. 2023, 11, e15774. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Man, Y.; Gao, C.; Zhou, L.; Gu, J.; Xu, H.; Wan, Q.; Long, Y.; Chai, L.; Xu, Y.; et al. Short-shain fatty acids ameliorate diabetic nephropathy via GPR43-mediated inhibition of oxidative stress and NF-κB signaling. Oxid. Med. Cell. Longev. 2020, 2020, 4074832. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Nigam, S.K.; Vanholder, R.; Verbeke, F. Role of the microbiome in gut-heart-kidney cross talk. Circ. Res. 2023, 132, 1064–1083. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Bartochowski, P.; Gayrard, N.; Bornes, S.; Druart, C.; Argilés, A.; Cordaillat-Simmons, M.; Duranton, F. Gut-kidney axis investigations in animal models of chronic kidney disease. Toxins 2022, 14, 626. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Kovesdy, C.P. The gut-kidney-heart axis in chronic kidney disease. Physiol. Int. 2019, 106, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Dai, Z.; Chai, L.; Wu, L.; Li, J.; Guo, W.; Zhang, J.; Zhang, Q.; Xue, C.; Lin, H.; et al. The change of gut microbiota-derived short-chain fatty acids in diabetic kidney disease. J. Clin. Lab. Anal. 2021, 35, e24062. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; Szabo, K.; Varvara, R.A.; Uifãlean, A.; Cozma, A.; Vulturar, R.; Sitar-Taut, A.V.; Gabbianelli, R.; Myhrstad, M.C.W.; Telle-Hansen, V.H.; et al. Resistant Starch and Microbiota-Derived Secondary Metabolites: A Focus on Postbiotic Pathways in Gut Health and Irritable Bowel Syndrome. Int. J. Mol. Sci. 2025, 26, 7753. [Google Scholar] [CrossRef]
- Roediger, W.E.W. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 1982, 83, 424–429. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-chain fatty acid transporters: Role in colonic homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef]
- Luo, P.; Lednovich, K.; Xu, K.; Nnyamah, C.; Layden, B.T.; Xu, P. Central and peripheral regulations mediated by short-chain fatty acids on energy homeostasis. Transl. Res. 2022, 248, 128–150. [Google Scholar] [CrossRef]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, M.T.; Han, J.H. GPR41 and GPR43: From development to metabolic regulation. Biomed. Pharmacother. 2024, 175, 116735. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Zhou, H.; Niu, B.; Liu, J.; Li, Y.; Mi, Y.; Li, P. ACT001 alleviates chronic kidney injury induced by a high-fat diet in mice through the GPR43/AMPK pathway. Lipids Health Dis. 2023, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hang, X.; Wei, Y.; Wang, H.; Zhang, L.; Zhao, L. Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: An updated review. Cell Commun. Signal. 2024, 22, 136. [Google Scholar] [CrossRef] [PubMed]
- Patrakka, J.; Tryggvason, K. Molecular make-up of the glomerular filtration barrier. Biochem. Biophys. Res. Commun. 2010, 396, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Daehn, I.S.; Duffield, J.S. The glomerular filtration barrier: A structural target for novel kidney therapies. Nat. Rev. Drug Discov. 2021, 20, 770–788. [Google Scholar] [CrossRef]
- Doshi, S.M.; Friedman, A.N. Diagnosis and management of type 2 diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1366–1373. [Google Scholar] [CrossRef]
- Tomino, Y. Pathogenesis and treatment of chronic kidney disease: A review of our recent basic and clinical data. Kidney Blood Press. Res. 2014, 39, 450–489. [Google Scholar] [CrossRef]
- Li, F.X.; Wang, M.H.; Wang, J.P.; Li, R.S.; Zhang, Y.Q. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front. Cell. Infect. Microbiol. 2019, 9, 206. [Google Scholar] [CrossRef]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef]
- Corte-Iglesias, V.; Saiz, M.L.; Andrade-Lopez, A.C.; Salazar, N.; Bernet, C.R.; Martin-Martin, C.; Borra, J.M.; Lozano, J.-J.; Aransay, A.M.; Diaz-Corte, C.; et al. Propionate and butyrate counteract renal damage and progression to chronic kidney disease. Nephrol. Dial. Transplant. 2024, 16, 518–524. [Google Scholar] [CrossRef]
- Chai, L.; Luo, Q.; Cai, K.; Wang, K.; Xu, B. Reduced fecal short-chain fatty acids levels and the relationship with gut microbiota in IgA nephropathy. BMC Nephrol. 2021, 22, 209. [Google Scholar] [CrossRef]
- Tang, G.; Du, Y.; Guan, H.; Jia, J.; Zhu, N.; Shi, Y.; Rong, S.; Yuan, W. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br. J. Pharmacol. 2022, 179, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Ma, Y.; Cai, F.; Huang, X.; Xiao, L.; Zhong, C.; Ren, P.; Luo, Q.; Chen, J.; Han, F. Changes of gut microbiota in diabetic nephropathy and its effect on the progression of kidney injury. Endocrine 2022, 76, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, Q.-H.; Cui, Z.; Zhao, M.-H.; Jia, X.-Y. Short-chain fatty acids ameliorate experimental anti-glomerular basement membrane disease. Clin. Immunol. 2024, 259, 109903. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.F.; Hsu, C.N. Perinatal propionate supplementation protects adult male offspring from maternal chronic kidney disease-induced hypertension. Nutrients 2022, 14, 3435. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The role of inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Dou, X.; Yan, D.; Ma, Z.; Gao, N.; Shan, A. Sodium butyrate alleviates LPS-induced kidney injury via inhibiting TLR2/4 to regulate rBD2 expression. J. Food Biochem. 2022, 46, e14126. [Google Scholar] [CrossRef]
- Dong, W.; Jia, Y.; Liu, X.; Zhang, H.; Li, T.; Huang, W.; Chen, X.; Wang, F.; Sun, W.; Wu, H. Sodium butyrate activates NRF2 to ameliorate diabetic nephropathy possibly via inhibition of HDAC. J. Endocrinol. 2017, 232, 71–83. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Sarkar, A.; Mitra, P.; Lahiri, A.; Das, T.; Sarkar, J.; Paul, S.; Chakrabarti, P. Butyrate limits inflammatory macrophage niche in NASH. Cell Death Dis. 2023, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Uguccioni, M.; D’Apuzzo, M.; Loetscher, M.; Dewald, B.; Baggiolini, M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1 alpha and MIP-1 beta on human monocytes. Eur. J. Immunol. 1995, 25, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.-T.; Xiong, J.-F.; Xu, Y.-H.; Xu, Y. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, T.; Liu, S.; Wang, C.; Zhao, M.; Feng, Y.; Ma, L.; Lu, Y.; Fu, P.; Liu, J. Complement C5 activation promotes type 2 diabetic kidney disease via activating STAT3 pathway and disrupting the gut-kidney axis. J. Cell. Mol. Med. 2021, 25, 960–974. [Google Scholar] [CrossRef]
- Yu, D.; Nguyen, S.M.; Yang, Y.; Xu, W.; Cai, H.; Wu, J.; Cai, Q.; Long, J.; Zheng, W.; Shu, X.-O. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am. J. Clin. Nutr. 2021, 113, 684–694. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- Lin, Z.; Qin, X.; Yang, Y.; Huang, Y.; Wang, J.; Kong, Y.; Li, Y.; Yang, S.; Lu, Y.; Zhao, Y.; et al. Higher dietary fibre intake is associated with lower CVD mortality risk among maintenance haemodialysis patients: A multicentre prospective cohort study. Br. J. Nutr. 2021, 126, 1510–1518. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.J.; Loh, Y.W.; Singer, J.; Zhu, W.; Macia, L.; Mackay, C.R.; Wang, W.; Chadban, S.J.; Wu, H. Fiber derived microbial metabolites prevent acute kidney injury through G-protein coupled receptors and HDAC inhibition. Front. Cell Dev. Biol. 2021, 9, 648639. [Google Scholar] [CrossRef]
- Tayebi Khosroshahi, H.; Vaziri, N.D.; Abedi, B.; Asl, B.H.; Ghojazadeh, M.; Jing, W.; Vatankhah, A.M. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: A randomized clinical trial. Hemodial. Int. 2018, 22, 492–500. [Google Scholar] [CrossRef]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Renal. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular basis of the KEAP1-NRF2 signaling pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The impact of CKD on uremic toxins and gut microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, D.Q.; Chen, L.; Liu, J.-R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Zhao, Y.; Huang, W.; Zhu, Y. Sodium butyrate improves renal injury in diabetic nephropathy through AMPK/SIRT1/PGC-1α signaling pathway. Sci. Rep. 2024, 14, 17867. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Xu, X.-D.; Tang, Y.-Y.; Jiang, H.-L.; Li, L.; Xia, W.-J.; Cui, N.; Bai, J.; Dai, Z.-M.; et al. Faecalibacterium prausnitzii attenuates CKD via butyrate-renal GPR43 axis. Circ. Res. 2022, 131, E120–E134. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Liu, S.M.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J.; Sands, J.M. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 2014, 9, e114881. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H. Oxidative stress and mitochondrial dysfunction in chronic kidney disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Nicese, M.N.; Bijkerk, R.; Van Zonneveld, A.J.; Van den Berg, B.M.; Rotmans, J.I. Sodium butyrate as key regulator of mitochondrial function and barrier integrity of human glomerular endothelial cells. Int. J. Mol. Sci. 2023, 24, 13090. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jia, Y.Y.; Li, H.J. Sodium butyrate improves mitochondrial function and kidney tissue injury in diabetic kidney disease via the AMPK/PGC-1α pathway. Ren. Fail. 2023, 45, 2287129. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; You, Q.; He, S.; Meng, Q.; Gao, J.; Wu, X.; Shen, Y.; Sun, Y.; Wu, X.; et al. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front. Pharmacol. 2018, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Szrejder, M.; Topolewska, A.; Rychłowski, M.; Piwkowska, A. The Role of PKGIα and AMPK signaling interplay in the regulation of albumin permeability in cultured rat podocytes. Int. J. Mol. Sci. 2023, 24, 3952. [Google Scholar] [CrossRef]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Kreft, E.; Angielski, S.; Piwkowska, A. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165610. [Google Scholar] [CrossRef]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity. Theranostics 2021, 11, 4728–4742. [Google Scholar] [CrossRef]
- Isaka, Y. Targeting TGF-β signaling in kidney fibrosis. Int. J. Mol. Sci. 2018, 19, 2532. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New insights into fibrosis from the ECM degradation perspective: The macrophage-MMP-ECM interaction. Cell Biosci. 2022, 12, 117. [Google Scholar] [CrossRef]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Li, Y.J.; Ma, J.; Loh, Y.W.; Chadban, S.J.; Wu, H. Short-chain fatty acids directly exert anti-inflammatory responses in podocytes and tubular epithelial cells exposed to high glucose. Front. Cell Dev. Biol. 2023, 11, 1182570. [Google Scholar] [CrossRef]
- Lu, C.C.; Wang, G.H.; Lu, J.; Chen, P.-P.; Zhang, Y.; Hu, Z.-B.; Ma, K.-L. Role of podocyte injury in glomerulosclerosis. Adv. Exp. Med. Biol. 2019, 1165, 195–232. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, X.Y.; Quan, X.-Y.; Yang, C.; Liu, Z.-J.; Su, H.-Y.; An, N.; Liu, H.-F. The role of parietal epithelial cells in the pathogenesis of podocytopathy. Front. Physiol. 2022, 13, 832772. [Google Scholar] [CrossRef]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Angielski, S.; Piwkowska, A. Extracellular ATP modulates podocyte function through P2Y purinergic receptors and pleiotropic effects on AMPK and cAMP/PKA signaling pathways. Arch. Biochem. Biophys. 2020, 695, 108649. [Google Scholar] [CrossRef] [PubMed]
- Rachubik, P.; Szrejder, M.; Rogacka, D.; Typiak, M.; Audzeyenka, I.; Kasztan, M.; Pollock, D.M.; Angielski, S.; Piwkowska, A. Insulin controls cytoskeleton reorganization and filtration barrier permeability via the PKGIα-Rac1-RhoA crosstalk in cultured rat podocytes. Biochim. Biophys. Acta-Mol. Cell Res. 2022, 1869, 119301. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, Y.T.; Tang, G.; Jia, J.S.; Zhu, N.; Yuan, W.J. Butyrate alleviates diabetic kidney disease by mediating the miR-7a-5p/P311/TGF-β1 pathway. FASEB J. 2020, 34, 10462–10475. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jena, G. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem. Toxicol. 2014, 73, 127–139. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhen, J.; Zhang, C.; Wan, Q.; Liu, G.; Wei, X.; Zhang, Y.; Wang, Z.; Han, H.; et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014, 86, 712–725. [Google Scholar] [CrossRef]
- Meyer, F.; Seibert, F.S.; Nienen, M.; Welzel, M.; Beisser, D.; Bauer, F.; Rohn, B.; Westhoff, T.H.; Stervbo, U.; Babel, N. Propionate supplementation promotes the expansion of peripheral regulatory T-Cells in patients with end-stage renal disease. J. Nephrol. 2020, 33, 817–827. [Google Scholar] [CrossRef]
- Anft, M.; Meyer, F.; Czygan, S.; Seibert, F.S.; Rohn, B.J.; Tsimas, F.; Viebahn, R.; Westhoff, T.H.; Stervbo, U.; Babel, N.; et al. Propionic acid supplementation promotes the expansion of regulatory T cells in patients with end-stage renal disease but not in renal transplant patients. Front. Transplant. 2024, 3, 1404740. [Google Scholar] [CrossRef]
- Tougaard, N.H.; Frimodt-Møller, M.; Salmenkari, H.; Stougaard, E.B.; Zawadzki, A.D.; Mattila, I.M.; Hansen, T.W.; Legido-Quigley, C.; Hörkkö, S.; Forsblom, C.; et al. Effects of Butyrate Supplementation on Inflammation and Kidney Parameters in Type 1 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 3573. [Google Scholar] [CrossRef] [PubMed]
- Study Details|NCT07024238|Impact of SCFA Supplementation on Gut Microbiome Composition of Kidney Transplant Recipients|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT07024238 (accessed on 29 August 2025).
- Study Details|NCT06951581|Impact of SCFA Supplementation on Metabolic Profiles in Serum and Urine of Kidney Transplant Recipients|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06951581 (accessed on 29 August 2025).
- Study Details|NCT05858437|Impact of Propionic Acid on Regulatory T Cell Function in Children with CKD|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05858437 (accessed on 29 August 2025).
- Wu, I.W.; Lee, C.C.; Hsu, H.J.; Sun, C.-Y.; Chen, Y.-C.; Yang, K.-J.; Yang, C.-W.; Chung, W.-H.; Lai, H.-C.; Chang, L.-C.; et al. Compositional and functional adaptations of intestinal microbiota and related metabolites in CKD patients receiving dietary protein restriction. Nutrients 2020, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Muller, G.Y.; Flores-Treviño, S.; Bocanegra-Ibarias, P.; Robles-Espino, D.; Garza-González, E.; Fabela-Valdez, G.C.; Camacho-Ortiz, A. Changes in the Progression of Chronic Kidney Disease in Patients Undergoing Fecal Microbiota Transplantation. Nutrients 2024, 16, 1109. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Liebert, A.; Bicknell, B.; Chen, X.M.; Huang, C.; Pollock, C.A. Faecal Microbiota Transplantation and Chronic Kidney Disease. Nutrients 2022, 14, 2528. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, M.; Yang, X.; Wang, Y.; Li, R.; Sun, S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: The first case reports. Ren. Fail. 2021, 43, 928. [Google Scholar] [CrossRef]
- Piteková, B.; Hric, I.; Baranovičová, E.; Zieg, J.; Planet, P.J.; Bielik, V. The effect of fecal microbial transplantation in a pediatric patient after 28 episodes of febrile urinary tract infection. Pediatr. Nephrol. 2025, 40, 3085–3088. [Google Scholar] [CrossRef]
- Zhou, G.; Zeng, J.; Peng, L.; Wang, L.; Zheng, W.; Wu, D.; Yang, Y. Fecal microbiota transplantation for membranous nephropathy. CEN Case Rep. 2021, 10, 261–264. [Google Scholar] [CrossRef]
| Study (Year/ID) | Population/Demographics | Intervention and Route | Dose | Duration | Outcomes Measured | Key Findings/Conclusions | Type of Study |
|---|---|---|---|---|---|---|---|
| Completed/Published Interventions | |||||||
| Marzocco et al., 2018 (PLAN) [6] | Adults on maintenance hemodialysis (n = 20) | Sodium propionate (oral capsules) | 2 × 500 mg (1 g/day) | 12 weeks (+4-week follow-up) | Markers of inflammation, oxidative stress and gut-derived toxins, biochemical parameters | Reduced inflammation/oxidative stress and uremic toxins; improved insulin resistance | Single-center non-randomized pilot study |
| Meyer et al., 2020 [90] | Hemodialysis (n = 10) and healthy volunteers (n = 7) | Sodium propionate (oral) | 2 × 500 mg | 30 days (+60-day follow-up) | Circulating Tregs, CRP, electrolytes, renal parameters | ↑ Tregs, ↓ CRP | Prospective study |
| Anft et al., 2024 [91] | Hemodialysis (n = 10) and kidney transplant recipients (n = 16) | Propionic acid (oral) | 2 × 500 mg | 30 days (+60-day follow-up) | Tregs, immune cells analysis | Treg expansion in HD; absent in transplant recipients on triple immunosuppression | Prospective study |
| Tougaard et al., 2022 (RCT) [92] | Adults with T1D and albuminuria (n = 53; early DKD) | Sodium butyrate (oral) vs. placebo | 3.6 g/day | 12 weeks | Fecal calprotectin and SCFAs, CRP, UACR, eGFR, HbA1c | No significant differences vs. placebo for inflammatory or kidney endpoints | Randomized, double-blind, placebo-controlled trial |
| Ongoing/Registered Trials | |||||||
| RENOBIOME (NCT07024238) [93] | Stable kidney transplant recipients (adults) n = 41 | Sodium butyrate (oral) vs. placebo | 1000 mg/day | 12 weeks | Gut microbiome composition, serum and urinary metabolome profiling | Status: Enrolling by invitation | Randomized, double-blind, placebo-controlled clinical trial |
| METAKID (NCT06951581) [94] | Stable kidney transplant recipients, n = 41 | SCFA oral formulation vs. placebo | 200 mg/day | 12 weeks + 12-week washout period | Serum/urine metabolomics; inflammatory and immunologic markers, eGFR/UACR, tacrolimus | Status: Primary data collection completed, results pending publication | Randomized, double-blind, placebo-controlled clinical trial |
| Pro-Kids (NCT05858437) [95] | Children/adolescents with CKD on hemodialysis (ages 12–20; n = 16) | Sodium propionate (oral) vs. placebo | 2 × 500 mg | 28 days | Tregs analysis, propionic acid serum levels, targeted metabolomics, fecal microbiome analysis, intestinal barrier function | Status: Recruiting | Multi-center, double-blind, randomized and placebo-controlled intervention study (pediatric) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szrejder, M.; Piwkowska, A. Gut Microbiome-Derived Short-Chain Fatty Acids in Glomerular Protection and Modulation of Chronic Kidney Disease Progression. Nutrients 2025, 17, 2904. https://doi.org/10.3390/nu17172904
Szrejder M, Piwkowska A. Gut Microbiome-Derived Short-Chain Fatty Acids in Glomerular Protection and Modulation of Chronic Kidney Disease Progression. Nutrients. 2025; 17(17):2904. https://doi.org/10.3390/nu17172904
Chicago/Turabian StyleSzrejder, Maria, and Agnieszka Piwkowska. 2025. "Gut Microbiome-Derived Short-Chain Fatty Acids in Glomerular Protection and Modulation of Chronic Kidney Disease Progression" Nutrients 17, no. 17: 2904. https://doi.org/10.3390/nu17172904
APA StyleSzrejder, M., & Piwkowska, A. (2025). Gut Microbiome-Derived Short-Chain Fatty Acids in Glomerular Protection and Modulation of Chronic Kidney Disease Progression. Nutrients, 17(17), 2904. https://doi.org/10.3390/nu17172904






