Integrative Multi-Omics Reveals the Anti-Colitis Mechanisms of Polygonatum kingianum Collett & Hemsl Polysaccharides in a Mouse DSS Model
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials and Reagents
2.2. Preparation of PKPs
2.3. DSS-Induced Acute UC Mouse Model
2.4. Macroscopic Evaluation Parameters
2.5. Histopathological Scoring
2.6. Immunohistochemistry
2.7. Colon Barrier Evaluation
2.8. Inflammatory Cytokine and Oxidative Stress Measurement
2.9. Western Blot
2.10. 16S rRNA Gut Microbiota Sequencing
2.11. Short-Chain Fatty Acids (SCFAs)
2.12. Fecal Untargeted Metabolomics
2.13. Data Analysis
3. Results and Discussion
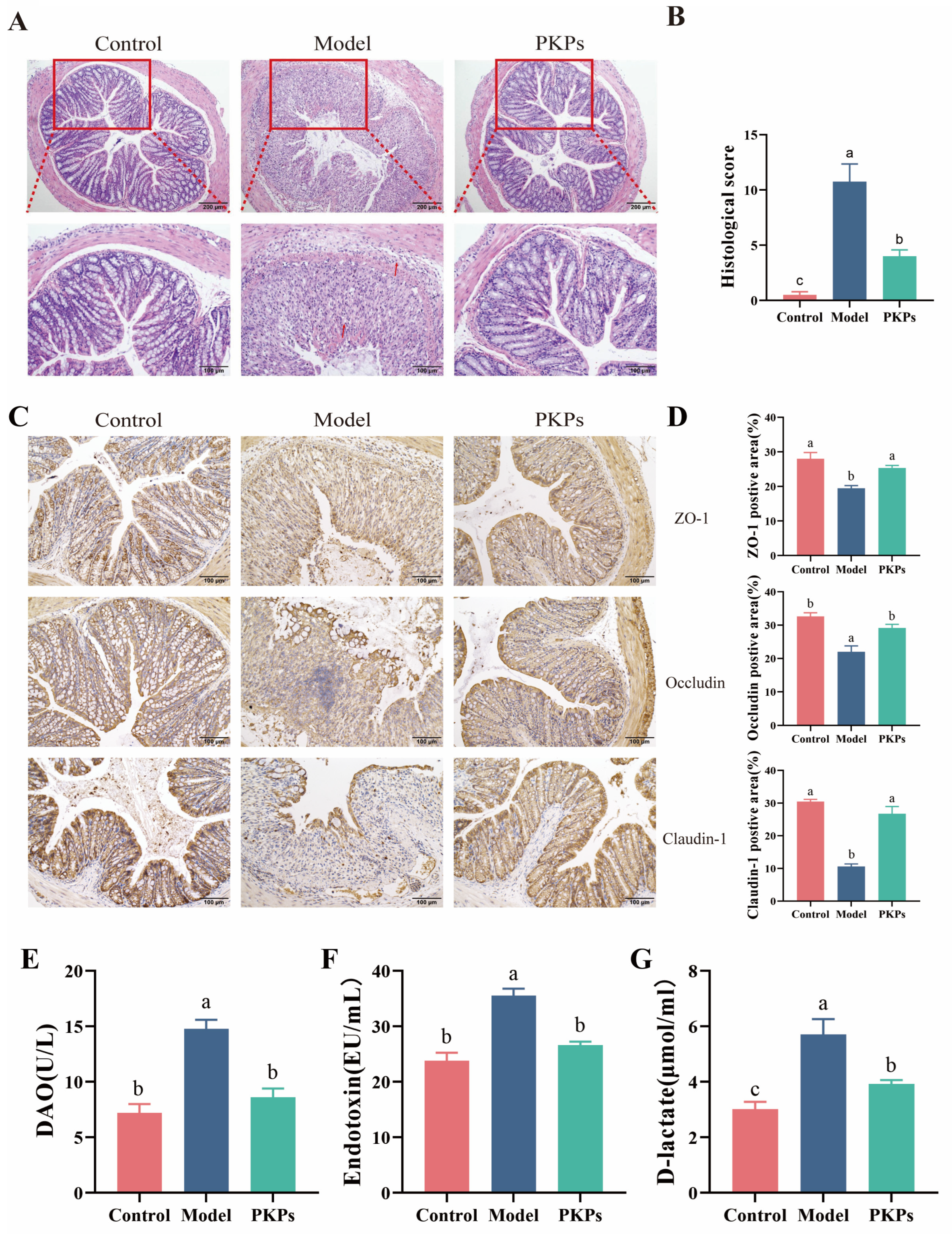
3.1. PKPs Ameliorate DSS-Induced Colitis Phenotypes in Mice
3.2. Effect of PKPs on Intestinal Barrier Function
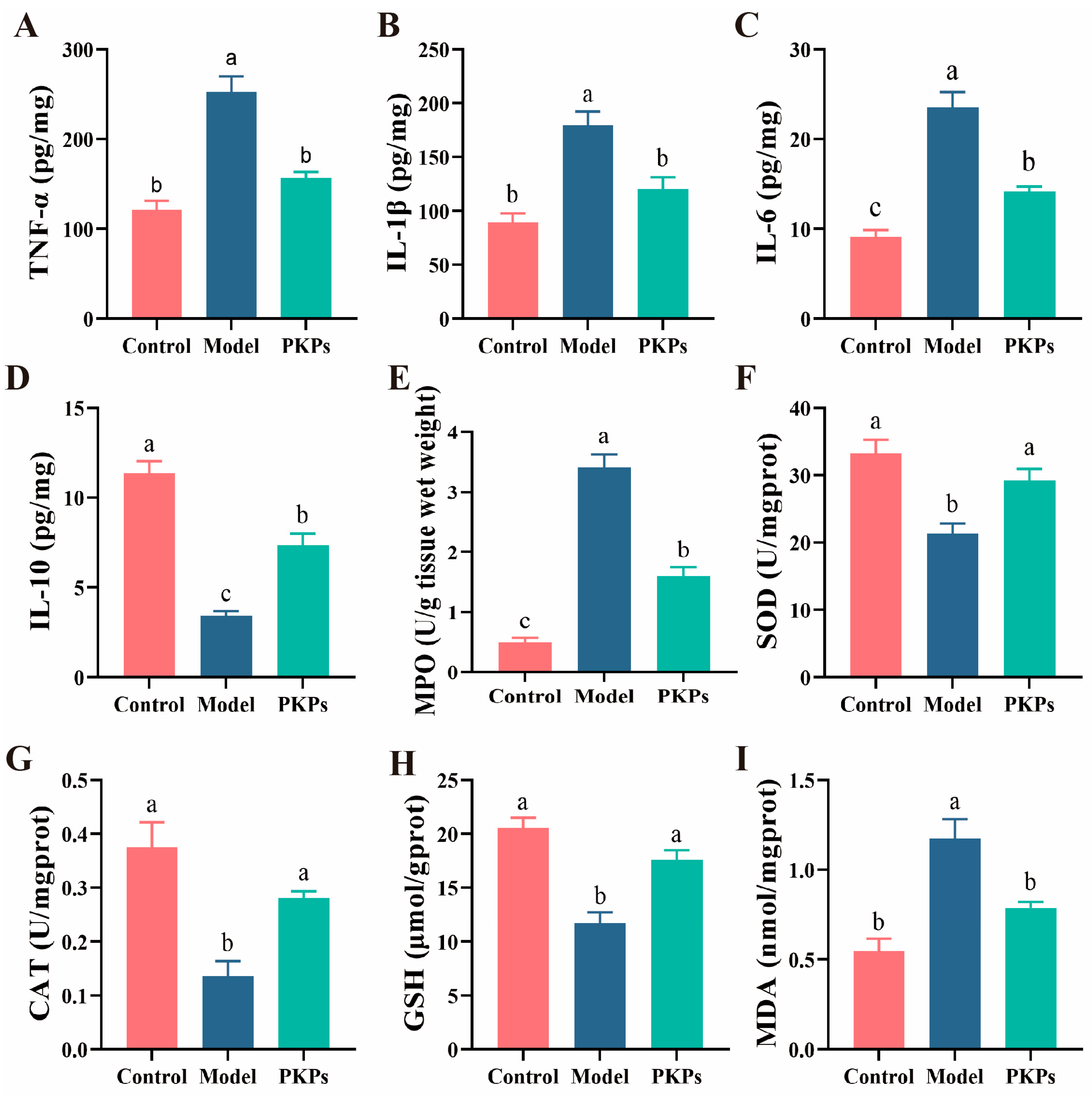
3.3. Analysis of Inflammatory Cytokines and Oxidative Dtress Markers in Colon Tissue
3.4. Effect of PKPs on the Expression of Nrf2/HO-1/NQO1 Pathway Proteins
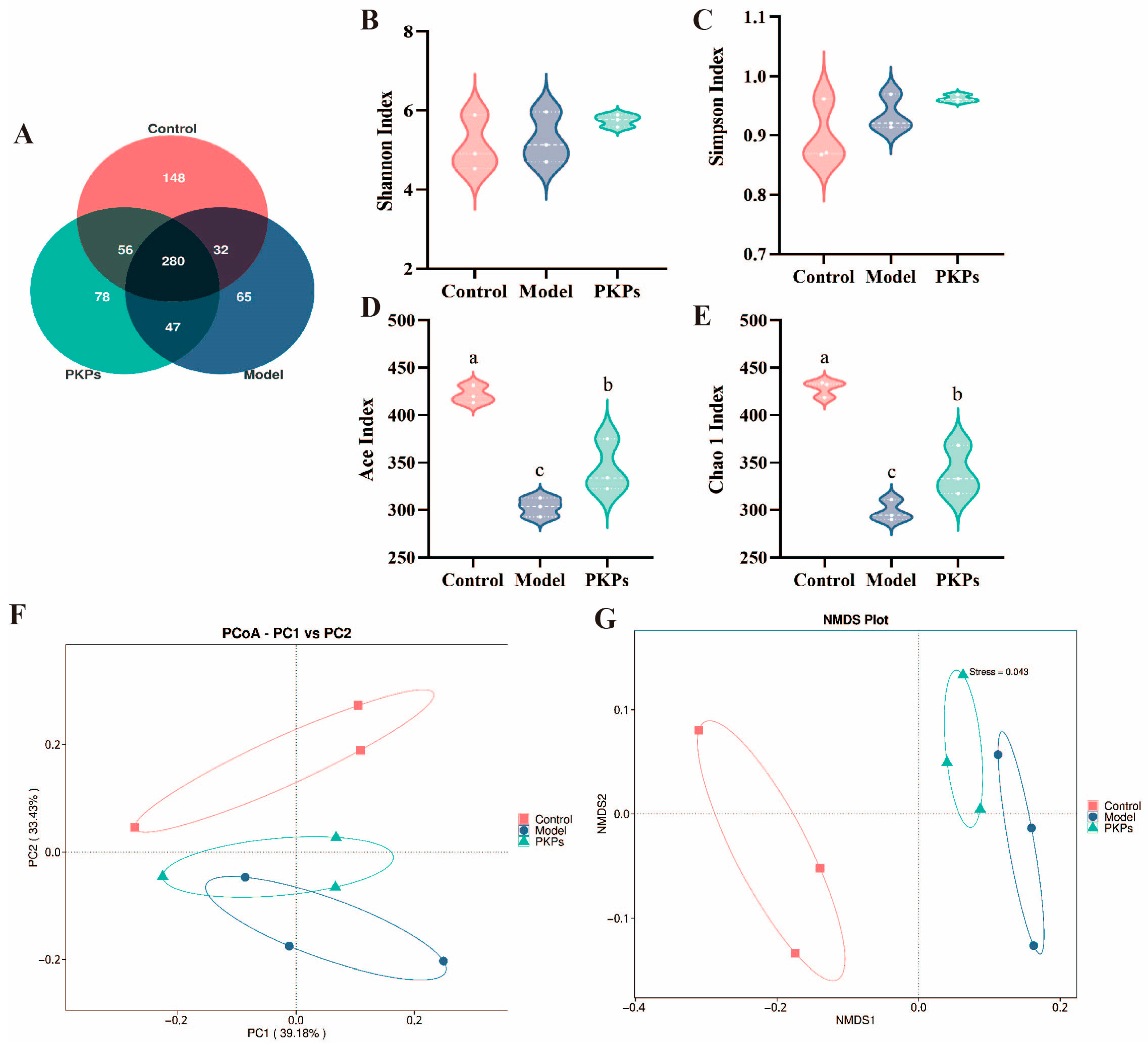
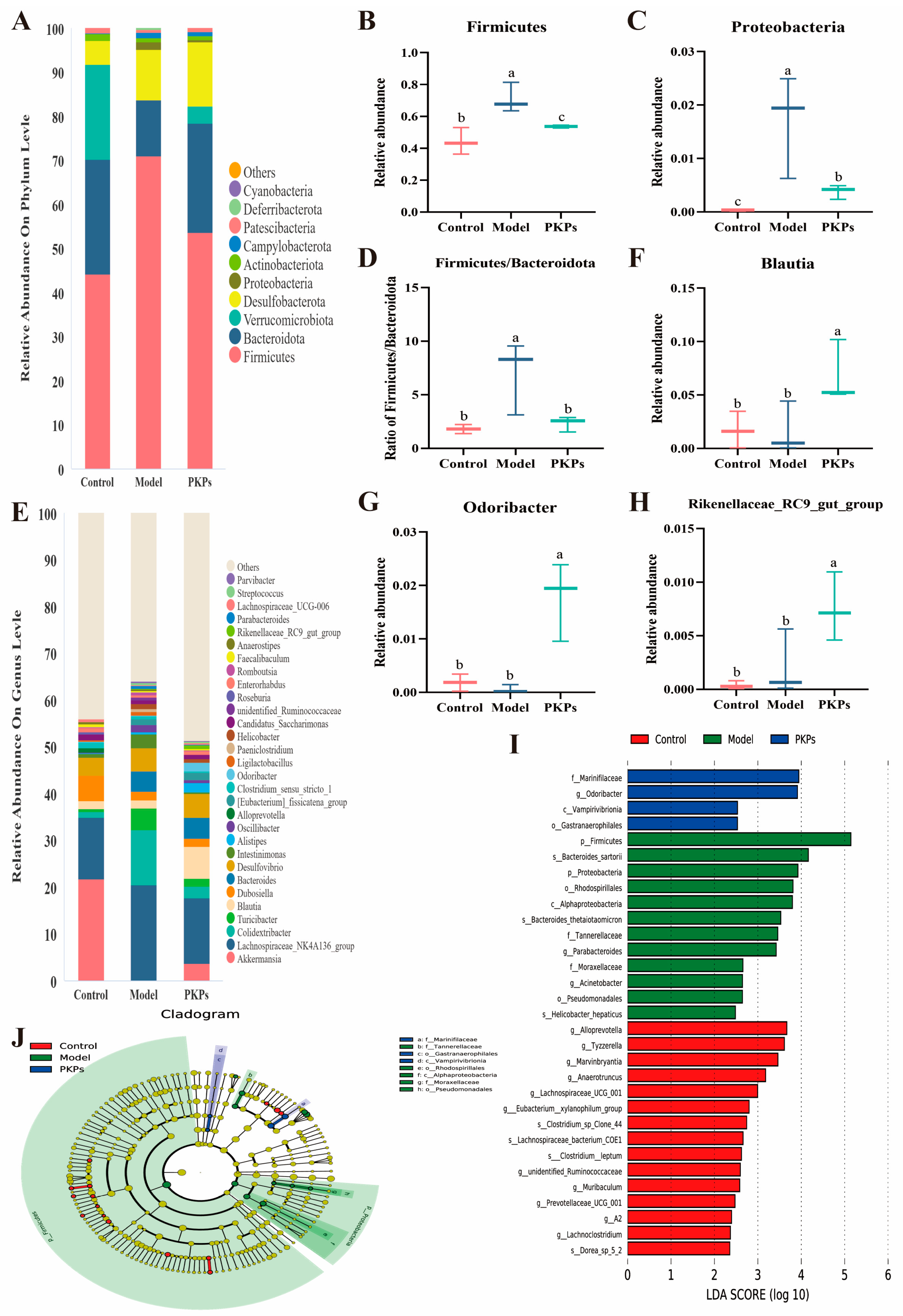
3.5. Effect of PKPs on Gut Microbiota
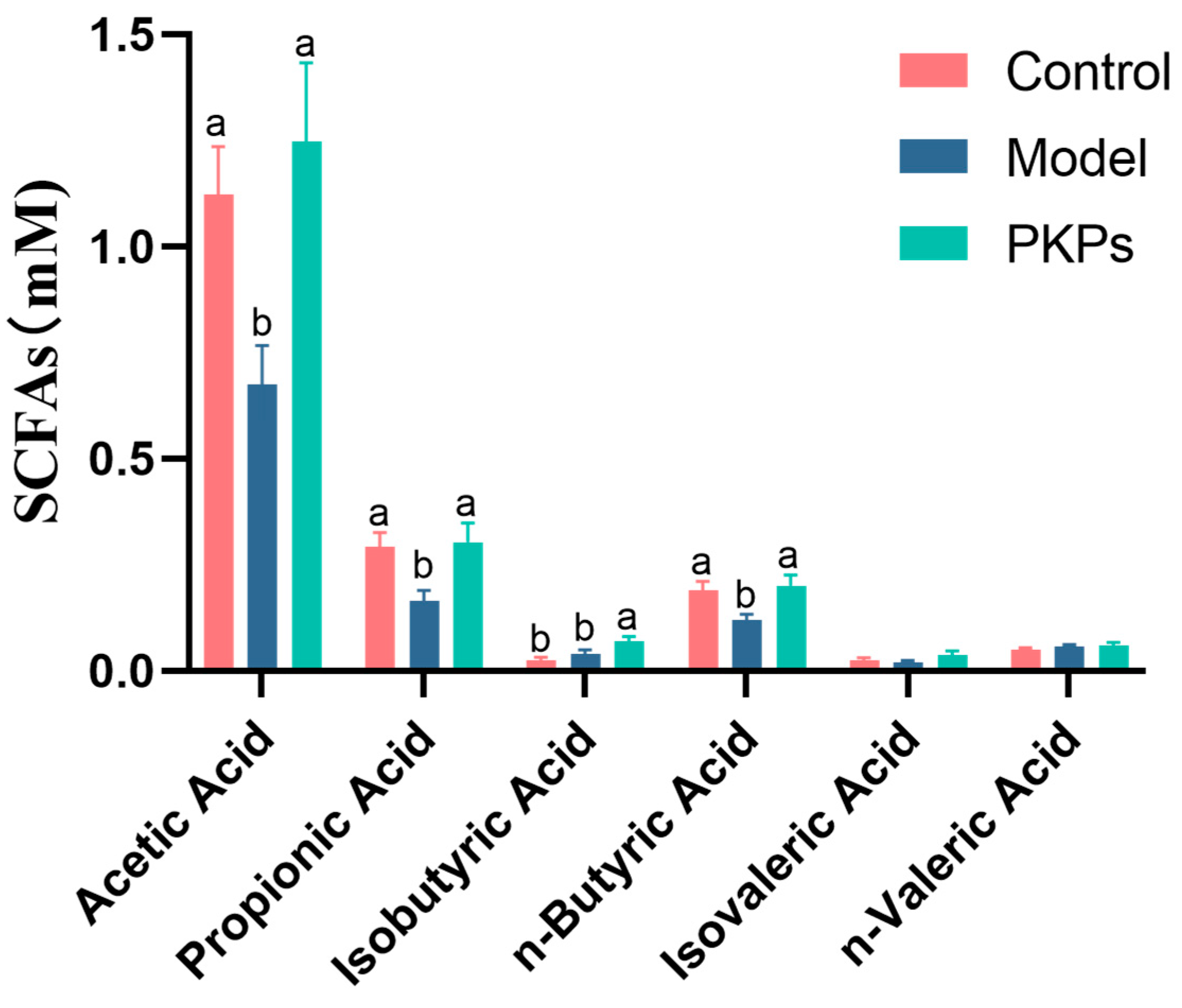
3.6. Effect of PKPs on SCFAs in Feces
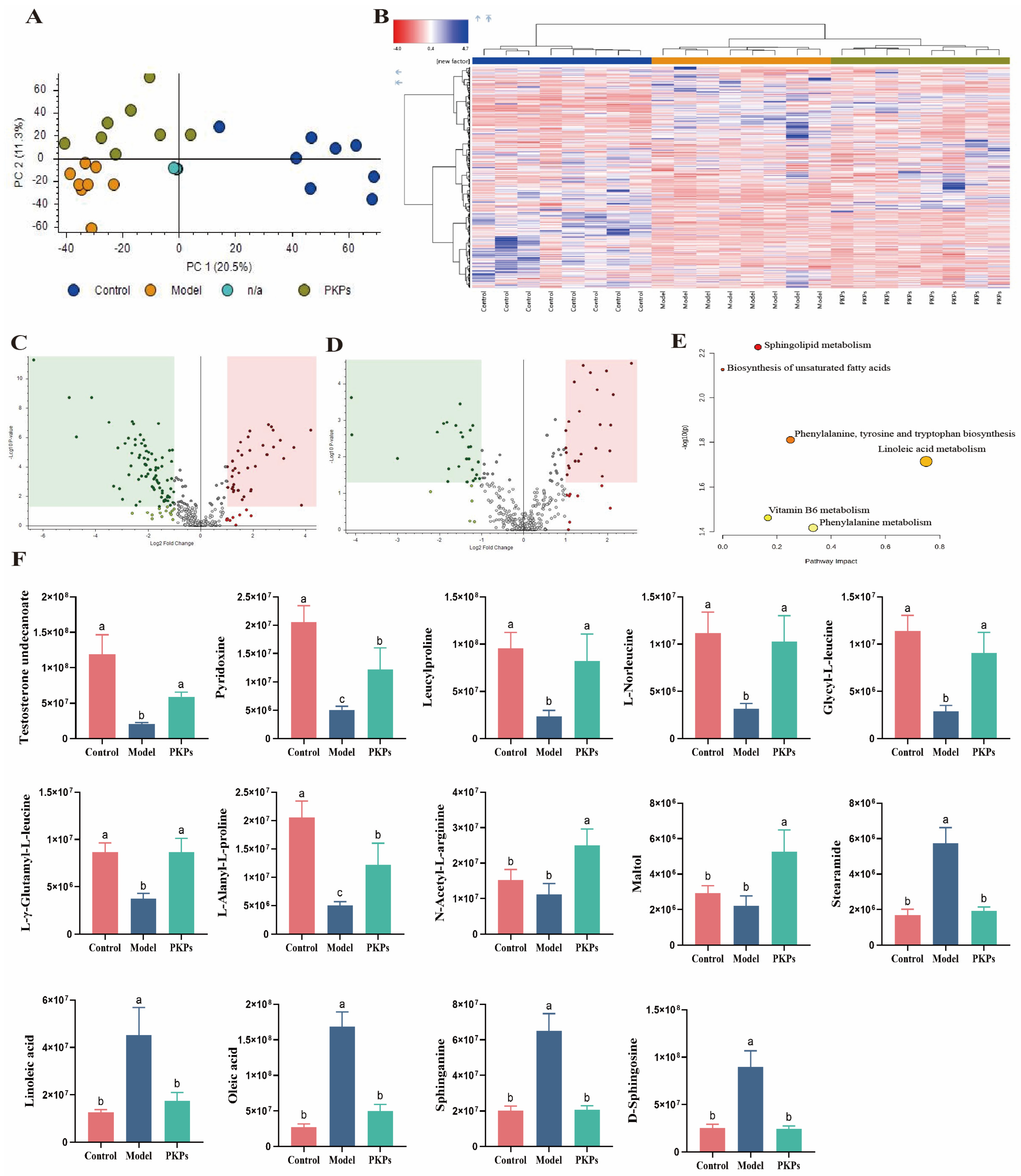
3.7. Untargeted Metabolomics Data Analysis of PKPs
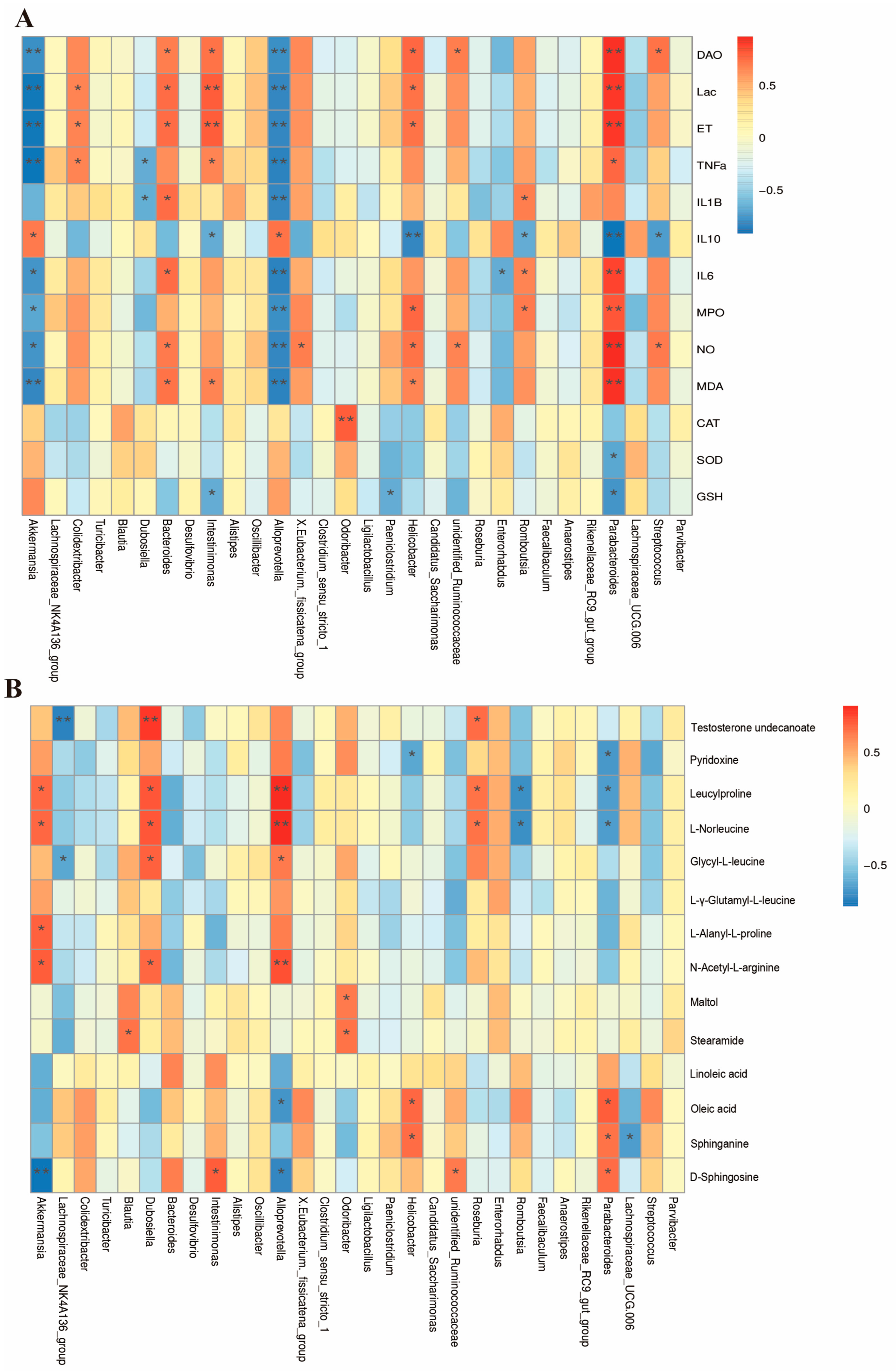
3.8. Combined Analysis of Gut Microbiota, Biochemical Indicators, and Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PKPs | Polygonatum kingianum Collett & Hemsl Polysaccharides |
| DSS | Dextran sulfate sodium |
| UC | Ulcerative colitis |
| SPF | Specific Pathogen–Free |
| DAO | Diamine Oxidase |
| D-Lac | D-lactate |
| ET | Endotoxin |
| MPO | Myeloperoxidase |
| SOD | Superoxide Dismutase |
| MDA | Malondialdehyde |
| CAT | Catalase |
| GSH | Glutathione |
| TJs | Tight junction proteins |
| IBD | Inflammatory bowel diseases |
| PCA | Principal component analysis |
| UPLC-MS | untargeted ultra-performance liquid chromatography-mass spectrometry |
References
- Li, K.; Feng, C.; Chen, H.; Feng, Y.; Li, J. Trends in Worldwide Research in Inflammatory Bowel Disease Over the Period 2012–2021: A Bibliometric Study. Front. Med. 2022, 9, 880553. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Wang, H.; Rabbi, M.F.; Bernstein, C.N.; Ghia, J.-E. Stability of Reference Genes for Messenger RNA Quantification by Real-Time PCR in Mouse Dextran Sodium Sulfate Experimental Colitis. PLoS ONE 2016, 11, e0156289. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults. JAMA 2023, 330, 951. [Google Scholar] [CrossRef]
- Fu, Y.-L.; Shi, L. Methods of Study on Conformation of Polysaccharides from Natural Products: A Review. Int. J. Biol. Macromol. 2024, 263, 130275. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current Advances in the Anti-Inflammatory Effects and Mechanisms of Natural Polysaccharides. Crit. Rev. Food Sci. Nutr. 2023, 63, 5890–5910. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Wang, L.; Zhao, Z.; You, L.; Pedisić, S.; Kulikouskaya, V.; Lin, Z. Polysaccharide from Gracilaria Lemaneiformis Prevents Colitis in Balb/c Mice via Enhancing Intestinal Barrier Function and Attenuating Intestinal Inflammation. Food Hydrocoll. 2020, 109, 106048. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Li, W.; He, Q.; Liang, J.; Yan, X.; Yuan, Y.; Yue, T. Selenium-Containing Tea Polysaccharides Ameliorate DSS-Induced Ulcerative Colitis via Enhancing the Intestinal Barrier and Regulating the Gut Microbiota. Int. J. Biol. Macromol. 2022, 209, 356–366. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, H.; Zhao, C.; Zhong, F.; Li, D. Oyster Polysaccharides Relieve DSS-Induced Colitis via Anti-Inflammatory and Maintaining the Physiological Hypoxia. Int. J. Biol. Macromol. 2023, 238, 124150. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Y.; Su, Y.; Yuan, W.; Peng, D.; Guan, Z.; Chen, J.; Li, P.; Du, B. Identification of Carbohydrate in Polygonatum kingianum Coll. et Hemsl and Inhibiting Oxidative Stress. Int. J. Biol. Macromol. 2024, 261, 129760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Fu, R.; Ou, J.; Wang, B. Structural Characterization and Anti-Inflammatory Activity of a Novel Polysaccharide PKP2-1 from Polygonatum kingianum. Front. Nutr. 2023, 10, 1156798. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shan, M.; Chu, C.; Bie, S.; Wang, H.; Cai, S. Polysaccharides from Polygonatum kingianum Collett & Hemsl Ameliorated Fatigue by Regulating NRF2/HO-1/NQO1 and AMPK/PGC-1α/TFAM Signaling Pathways, and Gut Microbiota. Int. J. Biol. Macromol. 2024, 266, 131440. [Google Scholar] [CrossRef]
- Lin, C.; Song, D.; Wang, S.; Chu, Y.; Chi, C.; Jia, S.; Lin, M.; He, C.; Jiang, C.; Gong, F.; et al. Polygonatum Cyrtonema Polysaccharides Reshape the Gut Microbiota to Ameliorate Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. Front. Pharmacol. 2024, 15, 1424328. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Mi, H.; Cai, S. The Preventive Effect and Underlying Mechanism of Rhus Chinensis Mill. Fruits on Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The Protective Role of Phloretin against Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Tang, S.; Liu, W.; Zhao, Q.; Li, K.; Zhu, J.; Yao, W.; Gao, X. Combination of Polysaccharides from Astragalus Membranaceus and Codonopsis Pilosula Ameliorated Mice Colitis and Underlying Mechanisms. J. Ethnopharmacol. 2021, 264, 113280. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, E.; Kang, J.; Kwon, S.; Sung, M.; Kim, M.; Cho, H.; Lee, G. Untargeted Metabolomic Analysis Using High-Resolution Orbitrap Mass Spectrometry for the Comparison of Volatile and Non-Volatile Compounds in Hot and Cold Brew Coffee. Beverages 2025, 11, 10. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, N.; Wu, X.; Cao, G.; Qiao, H.; Wang, J.; Hao, R. The Preventive Effect of Glycyrrhiza Polysaccharide on Lipopolysaccharide-Induced Acute Colitis in Mice by Modulating Gut Microbial Communities. Int. J. Biol. Macromol. 2023, 239, 124199. [Google Scholar] [CrossRef]
- Weiss, F.; Lee, I.F.M.; Fromm, A.; Hu, J.C.E.; Breiderhoff, T.; Fromm, M.; Schulzke, J.D.; Krug, S.M. P015 Tricellulin Overexpression Protects against Elevated Macromolecule Uptake in DSS Colitis Mice. J. Crohns Colitis 2020, 14, S137. [Google Scholar] [CrossRef]
- Schroder, A.L.; Chami, B.; Liu, Y.; Doyle, C.M.; El Kazzi, M.; Ahlenstiel, G.; Ahmad, G.; Pathma-Nathan, N.; Collins, G.; Toh, J.; et al. Neutrophil Extracellular Trap Density Increases with Increasing Histopathological Severity of Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 586–598. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Song, J.; Cao, L.; Tang, L.; Qi, C. Sinomenine Alleviates Dextran Sulfate Sodium-Induced Colitis via the Nrf2/NQO-1 Signaling Pathway. Mol. Med. Rep. 2018, 18, 3691–3698. [Google Scholar] [CrossRef]
- Xiang, X.; Jiang, Q.; Shao, W.; Li, J.; Zhou, Y.; Chen, L.; Deng, S.; Zheng, B.; Chen, Y. Protective Effects of Shrimp Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Nutr. 2021, 8, 773064. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, C.; Li, Y.; Yang, Y.; Luo, J.; Zhang, C.; Wang, L. Moutai Distiller’s Grains Polyphenol Extracts and Rutin Alleviate DSS-Induced Colitis in Mice: Modulation of Gut Microbiota and Intestinal Barrier Function (R2). Heliyon 2023, 9, e22186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Luo, J.; Liu, Y.; Yu, S.; Liu, J. Rheum Tanguticum Polysaccharide Alleviates DSS-Induced Ulcerative Colitis and Regulates Intestinal Microbiota in Mice. Food Biosci. 2023, 53, 102788. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Riboflavin Ameliorates Intestinal Inflammation via Immune Modulation and Alterations of Gut Microbiota Homeostasis in DSS-Colitis C57BL/6 Mice. Food Funct. 2024, 15, 4109–4121. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, T.; Tian, Z.; Shi, C.; Yan, C.; Li, H.; Du, Y.; Li, G. Progress in the Investigation of the Firmicutes/Bacteroidetes Ratio as a Potential Pathogenic Factor in Ulcerative Colitis. J. Med. Microbiol. 2025, 74, 001966. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, Z.; Wu, G.; Zhang, R.; Dong, L.; Huang, F.; Zhang, M.; Su, D. Lychee (Litchi Chinensis Sonn.) Pulp Phenolics Activate the Short-Chain Fatty Acid-Free Fatty Acid Receptor Anti-Inflammatory Pathway by Regulating Microbiota and Mitigate Intestinal Barrier Damage in Dextran Sulfate Sodium-Induced Colitis in Mice. J. Agric. Food Chem. 2021, 69, 3326–3339. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, H.; Hong, N.; Lv, C.; Zhu, Q.; Feng, Y.; Wang, B.; Tian, J.; Yu, Y. Gut Microbiome and Metabonomic Profile Predict Early Remission to Anti-Integrin Therapy in Patients with Moderate to Severe Ulcerative Colitis. Microbiol. Spectr. 2023, 11, e01457-23. [Google Scholar] [CrossRef]
- Chen, C.; Lin, X.; Xie, Y.; Xiong, S.; Hou, S.; Huang, S.; Jian, H.; Wen, Y.; Jiang, X.; Liang, J. Shengjiang Xiexin Decoction Ameliorates DSS-Induced Ulcerative Colitis via Activating Wnt/β-Catenin Signaling to Enhance Epithelium Renovation and Modulating Intestinal Flora. Phytomedicine 2025, 139, 156456. [Google Scholar] [CrossRef]
- Mao, B.; Guo, W.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Zhang, H. Blautia Producta Displays Potential Probiotic Properties against Dextran Sulfate Sodium-Induced Colitis in Mice. Food Sci. Hum. Wellness 2024, 13, 709–720. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Fernández-García, J.C.; Gutiérrez-Repiso, C.; Bernal-López, M.R.; Ocaña-Wilhelmi, L.; García-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Incidental Prophylactic Appendectomy Is Associated with a Profound Microbial Dysbiosis in the Long-Term. Microorganisms 2020, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Huber-Ruano, I.; Calvo, E.; Mayneris-Perxachs, J.; Rodríguez-Peña, M.-M.; Ceperuelo-Mallafré, V.; Cedó, L.; Núñez-Roa, C.; Miro-Blanch, J.; Arnoriaga-Rodríguez, M.; Balvay, A.; et al. Orally Administered Odoribacter Laneus Improves Glucose Control and Inflammatory Profile in Obese Mice by Depleting Circulating Succinate. Microbiome 2022, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.-B.; Pires, S.; Guo, C.-J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable Immunoglobulin A–Coated Odoribacter Splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology 2022, 162, 166–178. [Google Scholar] [CrossRef]
- Nagayama, M.; Lima, S.; Khan, Z.; Shetty, M.; Santambrogio, L.; Oguntunmibi, S.; Longman, R. Ddietary Fiber-Induced Odoribacter Metabolites Promote Mucosal Healing in Colitis. Gastroenterology 2024, 166, S-33. [Google Scholar] [CrossRef]
- Teofani, A.; Marafini, I.; Laudisi, F.; Pietrucci, D.; Salvatori, S.; Unida, V.; Biocca, S.; Monteleone, G.; Desideri, A. Intestinal Taxa Abundance and Diversity in Inflammatory Bowel Disease Patients: An Analysis Including Covariates and Confounders. Nutrients 2022, 14, 260. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Lu, D.; Zhang, R.; Zheng, X.; Xu, B.; Zhao, Y.; Ji, S.; Guo, M.; Wang, L.; et al. Morus Alba L. Water Extract Changes Gut Microbiota and Fecal Metabolome in Mice Induced by High-fat and High-sucrose Diet plus Low-dose Streptozotocin. Phytother. Res. 2022, 36, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, X.; Deng, N.; Peng, X.; Tan, Z. Diarrhea with Deficiency Kidney-Yang Syndrome Caused by Adenine Combined with Folium Senna Was Associated with Gut Mucosal Microbiota. Front. Microbiol. 2022, 13, 1007609. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wang, J.; Guo, Y.; Gu, T.; Shen, Z.; Zhou, C.; Li, B.; Xu, X.; Li, F.; Zhang, Q.; et al. Dextran Sulfate Sodium Salt-Induced Colitis Aggravates Gut Microbiota Dysbiosis and Liver Injury in Mice with Non-Alcoholic Steatohepatitis. Front. Microbiol. 2021, 12, 756299. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.L.; Wang, Q.; Mirza, A.; Dwyer, D.; Wu, Q.; Dowling, C.A.; Martens, J.W.; Yang, J.; Krementsov, D.N.; Mao-Draayer, Y. Identification of Commensal Gut Microbiota Signatures as Predictors of Clinical Severity and Disease Progression in Multiple Sclerosis. Sci. Rep. 2024, 14, 15292. [Google Scholar] [CrossRef]
- Ge, X.; Wang, C.; Chen, H.; Liu, T.; Chen, L.; Huang, Y.; Zeng, F.; Liu, B. Luteolin Cooperated with Metformin Hydrochloride Alleviates Lipid Metabolism Disorders and Optimizes Intestinal Flora Compositions of High-Fat Diet Mice. Food Funct. 2020, 11, 10033–10046. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; García-Sancho, M.; Villaescusa, A.; Sainz, Á.; Agulla, B.; Reyes-Prieto, M.; Rodríguez-Bertos, A.; Rodríguez-Franco, F. Characterization of the Fecal and Mucosa-Associated Microbiota in Dogs with Chronic Inflammatory Enteropathy. Animals 2023, 13, 326. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Liu, C.; Hua, H.; Zhu, H.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Aloe Polysaccharides Ameliorate Acute Colitis in Mice via Nrf2/HO-1 Signaling Pathway and Short-Chain Fatty Acids Metabolism. Int. J. Biol. Macromol. 2021, 185, 804–812. [Google Scholar] [CrossRef]
- Han, D.; Guan, X.; Zhu, F.; Yang, Q.; Su, D. Oral Aged Garlic (Allium sativum) Alleviates Ulcerative Colitis in Mice by Improving Gut Homeostasis. Food Funct. 2024, 15, 8935–8951. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Yoshida, T.; Hanada, H.; Takeuchi, I.; et al. Short Chain Fatty Acids-Producing and Mucin-Degrading Intestinal Bacteria Predict the Progression of Early Parkinson’s Disease. npj Park. Dis. 2022, 8, 65. [Google Scholar] [CrossRef]
- Nasser, M.; Haider, A.; Saad, F.; Kurtz, W.; Doros, G.; Fijak, M.; Vignozzi, L.; Gooren, L. Testosterone Therapy in Men with Crohn’s Disease Improves the Clinical Course of the Disease: Data from Long-Term Observational Registry Study. Horm. Mol. Biol. Clin. Investig. 2015, 22, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Mun, E.C.; Chung, J.-W.; Ha, M.; Ahn, S.-M.; Han, M.-D.; Han, S.-H.; Yun, S.-C.; Kim, J.H.; Kim, K.O.; et al. Increased Genomic Damage and Vitamin B Status in Inflammatory Bowel Disease Patients: A Case-Control, Prospective, Pilot Study. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 837, 42–47. [Google Scholar] [CrossRef]
- Song, G.; Gan, Q.; Qi, W.; Wang, Y.; Xu, M.; Li, Y. Fructose Stimulated Colonic Arginine and Proline Metabolism Dysbiosis, Altered Microbiota and Aggravated Intestinal Barrier Dysfunction in DSS-Induced Colitis Rats. Nutrients 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, J.; Liu, X.; Dong, Z.; Tang, J.; Zhang, C.; Jiao, J.; Chen, J.; Yin, F.; Qiu, S.; et al. Chaihu-Guizhi-Ganjiang Decoction Is More Efficacious in Treating Irritable Bowel Syndrome than Dicetel According to Metabolomics Analysis. Chin. Med. 2022, 17, 139. [Google Scholar] [CrossRef]
- Xiao, H.; Yin, D.; Du, L.; Li, G.; Lin, J.; Fang, C.; Shen, S.; Xiao, G.; Fang, R. Effects of Pork Sausage on Intestinal Microecology and Metabolism in Mice. J. Sci. Food Agric. 2024, 104, 3413–3427. [Google Scholar] [CrossRef]
- Budhathoki, S.; Iwasaki, M.; Yamaji, T.; Yamamoto, H.; Kato, Y.; Tsugane, S. Association of Plasma Concentrations of Branched-Chain Amino Acids with Risk of Colorectal Adenoma in a Large Japanese Population. Ann. Oncol. 2017, 28, 818–823. [Google Scholar] [CrossRef]
- Sudan, S.; Zhan, X.; Li, J. A Novel Probiotic Bacillus subtilis Strain Confers Cytoprotection to Host Pig Intestinal Epithelial Cells during Enterotoxic Escherichia coli Infection. Microbiol. Spectr. 2022, 10, e01257-21. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Yu, L.; Li, Y.; Guo, H.; Zhai, Q.; Chen, W.; Tian, F. Lactobacillus Plantarum CCFM405 against Rotenone-Induced Parkinson’s Disease Mice via Regulating Gut Microbiota and Branched-Chain Amino Acids Biosynthesis. Nutrients 2023, 15, 1737. [Google Scholar] [CrossRef]
- Hao, X.; Guo, W.; Li, F.; Cui, L.; Kang, W. Analysis of the Liver–Gut Axis Including Metabolomics and Intestinal Flora to Determine the Protective Effects of Kiwifruit Seed Oil on CCl4− Induced Acute Liver Injury. Food Funct. 2024, 15, 9149–9164. [Google Scholar] [CrossRef]
- Dawkins, J.J.; Gerber, G.K. MMETHANE: Interpretable AI for Predicting Host Status from Microbial Composition and Metabolomics Data. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Minaiyan, M.; Mostaghel, E.; Mahzouni, P. Preventive Therapy of Experimental Colitis with Selected Iron Chelators and Anti-Oxidants. Int. J. Prev. Med. 2012, 3, S162–S169. [Google Scholar]
- Han, L.; Zhao, L.; Zhou, Y.; Yang, C.; Xiong, T.; Lu, L.; Deng, Y.; Luo, W.; Chen, Y.; Qiu, Q.; et al. Altered Metabolome and Microbiome Features Provide Clues in Understanding Irritable Bowel Syndrome and Depression Comorbidity. ISME J. 2022, 16, 983–996. [Google Scholar] [CrossRef]
- Wu, J.; Deng, X.; Sun, Y.; Li, J.; Dai, H.; Qi, S.; Huang, Y.; Sun, W. Aged Oolong Tea Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating the Gut Microbiota and Its Metabolites. Food Chem. X 2024, 21, 101102. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, J.; Ren, G.; Chen, X.; Cai, H.; Hong, J.; Kan, J.; Jin, C.; Niu, F.; Zhang, W. Impact of Purple Sweet Potato (Ipomoea batatas L.) Polysaccharides on the Fecal Metabolome in a Murine Colitis Model. RSC Adv. 2022, 12, 11376–11390. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, F.; Yang, M.; Chen, C.; Cui, J.; Xing, M.; Dai, Y.; Li, M.; Cao, Y.; Lu, L.; et al. Gastrointestinal Dysmotility Predisposes to Colitis through Regulation of Gut Microbial Composition and Linoleic Acid Metabolism. Adv. Sci. 2024, 11, 2306297. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, J.; Zhu, F.; Wu, J.; Wu, P.; Liu, Y. Gancao Xiexin Decoction Ameliorates Ulcerative Colitis in Mice via Modulating Gut Microbiota and Metabolites. Drug Des. Dev. Ther. 2022, 16, 1383–1405. [Google Scholar] [CrossRef]
- Duan, R.-D.; Nilsson, Å. Metabolism of Sphingolipids in the Gut and Its Relation to Inflammation and Cancer Development. Prog. Lipid Res. 2009, 48, 62–72. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xu, X.; Pan, Y.; Chen, Y.; Wu, Z.; Cai, S. Integrative Multi-Omics Reveals the Anti-Colitis Mechanisms of Polygonatum kingianum Collett & Hemsl Polysaccharides in a Mouse DSS Model. Nutrients 2025, 17, 2895. https://doi.org/10.3390/nu17172895
Li S, Xu X, Pan Y, Chen Y, Wu Z, Cai S. Integrative Multi-Omics Reveals the Anti-Colitis Mechanisms of Polygonatum kingianum Collett & Hemsl Polysaccharides in a Mouse DSS Model. Nutrients. 2025; 17(17):2895. https://doi.org/10.3390/nu17172895
Chicago/Turabian StyleLi, Siyu, Xingrui Xu, Yuezhi Pan, Yu Chen, Zihuan Wu, and Shengbao Cai. 2025. "Integrative Multi-Omics Reveals the Anti-Colitis Mechanisms of Polygonatum kingianum Collett & Hemsl Polysaccharides in a Mouse DSS Model" Nutrients 17, no. 17: 2895. https://doi.org/10.3390/nu17172895
APA StyleLi, S., Xu, X., Pan, Y., Chen, Y., Wu, Z., & Cai, S. (2025). Integrative Multi-Omics Reveals the Anti-Colitis Mechanisms of Polygonatum kingianum Collett & Hemsl Polysaccharides in a Mouse DSS Model. Nutrients, 17(17), 2895. https://doi.org/10.3390/nu17172895






