The Neuroprotective Role of Curcumin: From Molecular Pathways to Clinical Translation—A Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
- “curcumin” AND (“neurodegeneration” OR “neurodegenerative disease” OR “Parkinson’s disease” OR “Alzheimer’s disease” OR “stroke” OR “cognitive impairment”)
- “curcumin” AND (“oxidative stress” OR “neuroinflammation” OR “BDNF” OR “NF-κB” OR “mitophagy” OR “apoptosis”)
- “curcumin” AND (“bioavailability” OR “nanoparticles” OR “clinical trial”)
2.2. Study Selection and Inclusion Criteria
- (i)
- investigated molecular or cellular mechanisms of curcumin relevant to neurodegenerative disorders;
- (ii)
- reported preclinical data from in vitro or in vivo models, or clinical evidence of neuroprotective effects;
- (iii)
- addressed pharmacokinetics, bioavailability, or delivery strategies within a neurological context.
- (i)
- non-peer-reviewed publications (unless of significant foundational value);
- (ii)
- studies focused solely on non-neurological conditions;
- (iii)
- duplicate or non-English publications.
2.3. Guiding Research Question
2.4. Data Extraction and Synthesis
- (i)
- molecular pathways modulated by curcumin (oxidative stress, neuroinflammation, autophagy, apoptosis, mitochondrial function);
- (ii)
- pharmacokinetic characteristics and bioavailability enhancement strategies;
- (iii)
- experimental models used, particularly animal models of Alzheimer’s disease, Parkinson’s disease, and ischemic stroke;
- (iv)
- clinical efficacy and safety outcomes.
3. Biological Properties of Curcumin
3.1. Chemical Structure and Physical Characteristics
3.2. Pharmacokinetics and Bioavailability
3.3. Antioxidant, Anti-Inflammatory, and Epigenetic Effects
4. Molecular Mechanisms Underlying the Neuroprotective Effects of Curcumin
4.1. Modulation of Oxidative Stress by Curcumin: Molecular Mechanisms and Neuroprotective Potential
4.1.1. Direct Free Radical-Scavenging Activity
4.1.2. Activation of Endogenous Antioxidant Enzymes
4.1.3. Activation of the Nrf2–ARE Pathway
4.1.4. Mitochondrial Protection and Inhibition of Lipid Peroxidation
4.2. Anti-Inflammatory Mechanisms of Curcumin: Inhibition of NF-κB, COX-2, and iNOS
4.2.1. Inhibition of the NF-κB Signaling Pathway
4.2.2. Inhibition of COX-2 Expression
4.2.3. Downregulation of iNOS Expression and Nitric Oxide Production
4.3. Role of Curcumin in Apoptosis Regulation: Molecular Mechanisms and Neuroprotective Relevance
4.3.1. Inhibition of Intrinsic (Mitochondrial) Apoptosis
4.3.2. Modulation of Extrinsic, Death Receptor-Mediated Apoptosis
4.3.3. Modulation of Apoptosis-Related Signaling Pathways: NF-κB, p53, and MAPK
- NF-κB pathway: Curcumin inhibits NF-κB activation by stabilizing its inhibitor IκBα, preventing NF-κB nuclear translocation. This results in decreased transcription of anti-apoptotic genes such as Bcl-2, Mcl-1, and X-linked inhibitor of apoptosis protein (XIAP). In the context of chronic neuroinflammation, this may restore apoptosis susceptibility in damaged neurons.
- p53 pathway: Curcumin upregulates p53, a key mediator of the cellular stress response. Activated p53 enhances transcription of pro-apoptotic genes such as Bax, promoting mitochondrial-mediated apoptosis in response to oxidative stress or DNA damage, thereby supporting neuronal quality control.
- MAPK pathway: Curcumin modulates multiple MAPK signaling branches. It inhibits p38 MAPK, implicated in inflammation and apoptosis, and regulates c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) pathways, exerting context-dependent effects on neuronal survival and stress response.
4.3.4. Bax/Bcl-2 Ratio as a Molecular Marker
4.4. Modulation of Pathological Protein Aggregation
4.4.1. Amyloid-β Aggregation
4.4.2. α-Synuclein Aggregation
4.4.3. Tau Aggregation and Hyperphosphorylation
4.5. Regulation of Autophagy and Mitophagy
4.6. Induction of Neurotrophic Factors
4.7. Putative Cerebrovascular Effects of Curcumin and Their Contribution to Neuroprotection
5. Preclinical Evidence
5.1. Animal Model Outcomes
5.1.1. Alzheimer’s Disease
5.1.2. Parkinson’s Disease
5.1.3. The Effects of Curcumin in Preclinical Models of Ischemic and Hemorrhagic Stroke
5.2. Dose–Response Relationships
5.3. Nanoparticle-Based Formulations
- Improved brain targeting: Nanoparticles facilitate curcumin’s transport across the blood–brain barrier, ensuring more effective delivery to brain tissues. This capability is crucial for therapeutic applications in neurodegenerative diseases, stroke, and related disorders.
- Reduced clearance: Nanoformulations prolong systemic circulation time by slowing curcumin’s elimination, thereby increasing its bioactive presence and therapeutic impact.
- Enhanced neuroprotective effects: Preclinical studies demonstrate that PLGA- or liposome-encapsulated curcumin can achieve similar or superior neuroprotective outcomes at lower doses than free curcumin, improving treatment consistency and reproducibility.
6. Neurological Effects of Curcumin: Clinical Outcomes
Human Clinical Trials
7. Neurological Effects of Curcumin: A Critical Synthesis of Clinical Evidence
7.1. The Role of Bioavailability
7.2. Characteristics of the Study Population
7.3. Sensitivity of Endpoints and Biomarkers
8. Efficacy and Safety
9. Bioavailability, Standardization
10. Future Perspectives
11. Limitations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zaib, S.; Javed, H.; Khan, I.; Jaber, F.; Sohail, A.; Zaib, Z.; Mehboob, T.; Tabassam, N.; Ogaly, H.A. Neurodegenerative diseases: Their onset, epidemiology, causes and treatment. ChemistrySelect 2023, 8, e202300225. [Google Scholar] [CrossRef]
- Lamptey, R.N.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive polyphenols and neuromodulation: Molecular mechanisms in neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.V. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef]
- Panda, P.; Mohanty, S.; Gouda, S.R.; Baral, T.C.; Mohanty, A.; Nayak, J.; Mohapatra, R. Advanced strategies for enhancing the neuroprotective potential of curcumin: Delivery systems and mechanistic insights in neurodegenerative disorders. Nutr. Neurosci. 2025, 28, 1151–1176. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and curcumin: From traditional to modern medicine. In Studies on Biomarkers and New Targets in Aging Research in Iran: Focus on Turmeric and Curcumin; Springer: Cham, Switzerland, 2021; pp. 15–39. [Google Scholar]
- Cianciulli, A.; Calvello, R.; Ruggiero, M.; Panaro, M.A. Inflammaging and brain: Curcumin and its beneficial potential as regulator of microglia activation. Molecules 2022, 27, 341. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Piovesana, R.; Salazar Intriago, M.S.; Dini, L.; Tata, A.M. Cholinergic modulation of neuroinflammation: Focus on α7 nicotinic receptor. Int. J. Mol. Sci. 2021, 22, 4912. [Google Scholar] [CrossRef] [PubMed]
- Nebrisi, E.E. Neuroprotective activities of curcumin in Parkinson’s disease: A review of the literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rauf, A.; Akter, S.; Akter, H.; Al-Imran, M.I.K.; Fakir, M.N.H.; Thufa, G.K.; Islam, M.T.; Hemeg, H.A.; Abdulmonem, W.A.; et al. Neuroprotective Potential of Curcumin in Neurodegenerative Diseases: Clinical Insights Into Cellular and Molecular Signaling Pathways. J. Biochem. Mol. Toxicol. 2025, 39, e70369. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Sarker, M.R.; Franks, S.F. Efficacy of curcumin for age-associated cognitive decline: A narrative review of preclinical and clinical studies. Geroscience 2018, 40, 73–95. [Google Scholar] [CrossRef]
- Voulgaropoulou, S.D.; Van Amelsvoort, T.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Rege, S.A.; Arya, M.; Momin, S.A. Structure activity relationship of tautomers of curcumin: A review. Ukr. Food J. 2019, 8, 45–60. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. The golden spice for life: Turmeric with the pharmacological benefits of curcuminoids components, including curcumin, bisdemethoxycurcumin, and demethoxycurcumins. Curr. Org. Synth. 2024, 21, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V. Properties, extraction methods, and delivery systems for curcumin as a natural source of beneficial health effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Stanić, Z. Curcumin, a compound from natural sources, a true scientific challenge—A review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, e2900. [Google Scholar]
- Jamil, Q.A. The Natural Compound Curcumin: Impact of Cellular Uptake and Metabolism on In Vitro Activity. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2018. [Google Scholar]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Pan-On, S.; Dilokthornsakul, P.; Tiyaboonchai, W. Trends in advanced oral drug delivery system for curcumin: A systematic review. J. Control. Release 2022, 348, 335–345. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, R.; Mir, R.H.; Wani, T.U.; Shah, A.J.; Mohi-Ud-Din, I.; Dar, M.A.; Pottoo, F.H. Novel drug delivery system for curcumin: Implementation to improve therapeutic efficacy against neurological disorders. Comb. Chem. High. Throughput Screen. 2022, 25, 607–615. [Google Scholar] [CrossRef]
- Aminnezhad, S.; Zonobian, M.A.; Moradi Douki, M.; Mohammadi, M.R.; Azarakhsh, Y. Curcumin and their derivatives with anti-inflammatory, neuroprotective, anticancer, and antimicrobial activities: A review. Micro Nano Bio Asp. 2023, 2, 25–34. [Google Scholar]
- Hunyadi, A. The mechanism (s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef]
- Wei, Q.-Y.; Chen, W.-F.; Zhou, B.; Yang, L.; Liu, Z.-L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef]
- Teiten, M.H.; Dicato, M.; Diederich, M. Curcumin as a regulator of epigenetic events. Mol. Nutr. Food Res. 2013, 57, 1619–1629. [Google Scholar] [CrossRef]
- Boyanapalli, S.S.; Kong, A.-N.T. “Curcumin, the king of spices”: Epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Peña-Corona, S.I.; Hernández-Parra, H.; Chandran, D.; Saleena, L.A.K.; Sawikr, Y.; Peluso, I.; Dhumal, S.; Kumar, M.; Leyva-Gómez, G. Neuroprotective and anti-inflammatory effects of curcumin in Alzheimer’s disease: Targeting neuroinflammation strategies. Phytother. Res. 2024, 38, 3169–3189. [Google Scholar] [CrossRef]
- Moldoveanu, C.-A.; Tomoaia-Cotisel, M.; Sevastre-Berghian, A.; Tomoaia, G.; Mocanu, A.; Pal-Racz, C.; Toma, V.-A.; Roman, I.; Ujica, M.-A.; Pop, L.-C. A Review on Current Aspects of Curcumin-Based Effects in Relation to Neurodegenerative, Neuroinflammatory and Cerebrovascular Diseases. Molecules 2024, 30, 43. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.K.; Peer, G.D.G.; Bagabir, S.A.; Haque, S.; Pandey, R.P.; Raj, V.S.; Jain, N.; Pandey, A.; Kar, S.K. The Interplay of the Unfolded Protein Response in Neurodegenerative Diseases: A Therapeutic Role of Curcumin. Front. Aging Neurosci. 2021, 13, 767493. [Google Scholar] [CrossRef]
- Ng, T.P.; Nyunt, S.Z.; Gao, Q.; Gwee, X.; Chua, D.Q.L.; Yap, K.B. Curcumin-rich curry consumption and life expectancy: Singapore longitudinal ageing study. Geroscience 2024, 46, 969–980. [Google Scholar] [CrossRef]
- Izadi, M.; Sadri, N.; Abdi, A.; Zadeh, M.M.R.; Jalaei, D.; Ghazimoradi, M.M.; Shouri, S.; Tahmasebi, S. Longevity and anti-aging effects of curcumin supplementation. Geroscience 2024, 46, 2933–2950. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, E.; Bar, A.; Pisarek-Pacek, A.; Karas, A.; Branicki, W.; Chlopicki, S. Epigenetic clock in the aorta and age-related endothelial dysfunction in mice. Geroscience 2024, 46, 3993–4002. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Li, X.Y.; Wang, S.; Shen, L.; Ji, H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, L. The modulatory effects of curcumin on the gut microbiota: A potential strategy for disease treatment and health promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Balaji, S.; Jeyaraman, N.; Jeyaraman, M.; Ramasubramanian, S.; Muthu, S.; Santos, G.S.; da Fonseca, L.F.; Lana, J.F. Impact of curcumin on gut microbiome. World J. Exp. Med. 2025, 15, 100275. [Google Scholar] [CrossRef] [PubMed]
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef]
- Cerullo, M.; Armeli, F.; Mengoni, B.; Menin, M.; Crudeli, M.L.; Businaro, R. Curcumin Modulation of the Gut–Brain Axis for Neuroinflammation and Metabolic Disorders Prevention and Treatment. Nutrients 2025, 17, 1430. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol.-Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, gut microbiota, and neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.-J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J. Neural Transm. 2012, 119, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Tabner, B.J.; Turnbull, S.; El-Aganf, O.; Allsop, D. Production of reactive oxygen species from aggregating proteins implicated in Alzheimer’s disease, Parkinson’s disease and other neurodegenerative diseases. Curr. Top. Med. Chem. 2001, 1, 507–517. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Borra, S.K.; Mahendra, J.; Gurumurthy, P.; Iqbal, S.S.; Mahendra, L. Effect of curcumin against oxidation of biomolecules by hydroxyl radicals. J. Clin. Diagn. Res. JCDR 2014, 8, CC01. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free radicals as a double-edged sword: The cancer preventive and therapeutic roles of curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Wolnicka-Glubisz, A.; Wisniewska-Becker, A. Dual action of curcumin as an anti-and pro-oxidant from a biophysical perspective. Antioxidants 2023, 12, 1725. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.-M.; Zhang, P.-Y. Protective effects of curcumin and quercetin during benzo (a) pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1736–1743. [Google Scholar]
- Biswas, J.; Sinha, D.; Mukherjee, S.; Roy, S.; Siddiqi, M.; Roy, M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum. Exp. Toxicol. 2010, 29, 513–524. [Google Scholar] [CrossRef]
- Iqbal, M.; Sharma, S.D.; Okazaki, Y.; Fujisawa, M.; Okada, S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: Possible role in protection against chemical carcinogenesis and toxicity. Pharmacol. Toxicol. 2003, 92, 33–38. [Google Scholar] [CrossRef]
- Piper, J.T.; Singhal, S.S.; Salameh, M.S.; Torman, R.T.; Awasthi, Y.C.; Awasthi, S. Mechanisms of anticarcinogenic properties of curcumin: The effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int. J. Biochem. Cell Biol. 1998, 30, 445–456. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 regulation by curcumin: Molecular aspects for therapeutic prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Shen, G.; Xu, C.; Hu, R.; Jain, M.R.; Gopalkrishnan, A.; Nair, S.; Huang, M.T.; Chan, J.Y.; Kong, A.N. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006, 5, 39–51. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- González-Reyes, S.; Guzmán-Beltrán, S.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxidative Med. Cell. Longev. 2013, 2013, 801418. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell. Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Naserzadeh, P.; Mehr, S.N.; Sadabadi, Z.; Seydi, E.; Salimi, A.; Pourahmad, J. Curcumin protects mitochondria and cardiomyocytes from oxidative damage and apoptosis induced by hemiscorpius lepturus venom. Drug Res. 2018, 68, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Choudhury, S.T.; Ghosh, S.; Mandal, A.K.; Sarkar, S.; Ghosh, A.; Saha, K.D.; Das, N. Nanocapsulated curcumin: Oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem.-Biol. Interact. 2012, 195, 206–214. [Google Scholar] [CrossRef]
- Shishodia, S.; Potdar, P.; Gairola, C.G.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: Correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 2003, 24, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Kao, N.-J.; Hu, J.-Y.; Wu, C.-S.; Kong, Z.-L. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. Int. Immunopharmacol. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef]
- Liang, X.; Wu, L.; Wang, Q.; Hand, T.; Bilak, M.; McCullough, L.; Andreasson, K. Function of COX-2 and prostaglandins in neurological disease. J. Mol. Neurosci. 2007, 33, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Habib, S.; Ali, R. Acquired antigenicity of DNA after modification with peroxynitrite. Int. J. Biol. Macromol. 2005, 35, 221–225. [Google Scholar] [CrossRef]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef]
- Chan, M.M.-Y.; Huang, H.-I.; Fenton, M.R.; Fong, D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem. Pharmacol. 1998, 55, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006, 79, 2022–2031. [Google Scholar] [CrossRef]
- Liu, C.; Takada, K.; Zhu, D. Targeting Wnt/β-catenin pathway for drug therapy. Med. Drug Discov. 2020, 8, 100066. [Google Scholar]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, X.; He, X.; Dai, M.; Zhang, Q. Curcumin induces apoptosis involving bax/bcl-2 in human hepatoma SMMC-7721 cells. Asian Pac. J. Cancer Prev. 2011, 12, 1925–1929. [Google Scholar] [PubMed]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M. Curcumin induces apoptotic cell death via inhibition of PI3-kinase/AKT pathway in B-precursor acute lymphoblastic leukemia. Front. Oncol. 2019, 9, 484. [Google Scholar] [CrossRef]
- Bai, C.; Zhao, J.; Su, J.; Chen, J.; Cui, X.; Sun, M.; Zhang, X. Curcumin induces mitochondrial apoptosis in human hepatoma cells through BCLAF1-mediated modulation of PI3K/AKT/GSK-3β signaling. Life Sci. 2022, 306, 120804. [Google Scholar] [CrossRef]
- Chang, R.; Sun, L.; Webster, T.J. Selective cytotoxicity of curcumin on osteosarcoma cells compared to healthy osteoblasts. Int. J. Nanomed. 2014, 9, 461–465. [Google Scholar] [CrossRef][Green Version]
- Syng-Ai, C.; Kumari, A.L.; Khar, A. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Mol. Cancer Ther. 2004, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; H Lajis, N.; Naidu, R. Mechanism of apoptosis induced by curcumin in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.J.; Jiang, G.; Li, L.T.; Zheng, J.N. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol. Biol. Rep. 2015, 42, 267–275. [Google Scholar] [PubMed]
- Bush, J.A.; Cheung, K.J., Jr.; Li, G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 2001, 271, 305–314. [Google Scholar] [CrossRef]
- Wahl, H.; Tan, L.; Griffith, K.; Choi, M.; Liu, J.R. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol. Oncol. 2007, 105, 104–112. [Google Scholar] [CrossRef]
- Prakobwong, S.; Gupta, S.C.; Kim, J.H.; Sung, B.; Pinlaor, P.; Hiraku, Y.; Wongkham, S.; Sripa, B.; Pinlaor, S.; Aggarwal, B.B. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis 2011, 32, 1372–1380. [Google Scholar]
- Choudhuri, T.; Pal, S.; Agwarwal, M.L.; Das, T.; Sa, G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002, 512, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Huang, Y.H.; Chiu, L.Y.; Cherng, S.H.; Sheu, G.T.; Yang, T.Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef]
- Zhu, L.; Han, M.B.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L.X. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015, 12, 1151–1156. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Reinke, A.A.; Gestwicki, J.E. Structure–activity Relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; González, Y.; Doens, D.; Stephens, D.E.; Santamaría, R.; Murillo, E.; Gutiérrez, M.; Fernández, P.L.; Rao, K.; Larionov, O.V. Assessment of novel curcumin derivatives as potent inhibitors of inflammation and amyloid-β aggregation in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, S59–S68. [Google Scholar] [CrossRef]
- Orteca, G.; Tavanti, F.; Bednarikova, Z.; Gazova, Z.; Rigillo, G.; Imbriano, C.; Basile, V.; Asti, M.; Rigamonti, L.; Saladini, M. Curcumin derivatives and Aβ-fibrillar aggregates: An interactions’ study for diagnostic/therapeutic purposes in neurodegenerative diseases. Bioorganic Med. Chem. 2018, 26, 4288–4300. [Google Scholar] [CrossRef]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimer’s Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef]
- Ferrari, E. Curcumin derivatives as metal-chelating agents: Implications for potential therapeutic agents for neurological disorders. In Curcumin for Neurological and Psychiatric Disorders: Neurochemical and Pharmacological Properties; Elsevier: Amsterdam, The Netherlands, 2019; pp. 275–299. [Google Scholar]
- Schulz-Schaeffer, W.J. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective effects of curcumin in neurodegenerative diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Nagoor Meeran, M.F.; Azimullah, S.; Adem, A.; Sadek, B.; Ojha, S.K. Plant extracts and phytochemicals targeting α-synuclein aggregation in Parkinson’s disease models. Front. Pharmacol. 2019, 9, 1555. [Google Scholar] [CrossRef]
- Sajjad, R.; Arif, R.; Shah, A.; Manzoor, I.; Mustafa, G. Pathogenesis of Alzheimer’s Disease: Role of Amyloid-β and Hyperphosphorylated Tau Protein. Indian. J. Pharm. Sci. 2018, 80, 581–591. [Google Scholar] [CrossRef]
- Dubey, T.; Sonawane, S.K.; Mannava, M.C.; Nangia, A.K.; Chandrashekar, M.; Chinnathambi, S. The inhibitory effect of Curcumin-Artemisinin co-amorphous on Tau aggregation and Tau phosphorylation. Colloids Surf. B Biointerfaces 2023, 221, 112970. [Google Scholar] [CrossRef]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin inhibits tau aggregation and disintegrates preformed tau filaments in vitro. J. Alzheimer’s Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef]
- Sivanantharajah, L.; Mudher, A. Curcumin as a holistic treatment for tau pathology. Front. Pharmacol. 2022, 13, 903119. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.-P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Asgarov, R.; Venigalla, M.; Liang, H.; Niedermayer, G.; Münch, G.; Gyengesi, E. Effects of a solid lipid curcumin particle formulation on chronic activation of microglia and astroglia in the GFAP-IL6 mouse model. Sci. Rep. 2020, 10, 2365. [Google Scholar] [CrossRef]
- Zhou, H.; S Beevers, C.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhao, B.; Xiong, P.; Wang, C.; Zhang, J.; Tian, X.; Huang, Y. Curcumin induces autophagy via inhibition of yes-associated protein (YAP) in human colon cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7035. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, T.J.; Woo, B.H.; Choi, K.U.; Lee, C.H.; Ryu, M.H.; Park, H.R. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch. Oral. Biol. 2012, 57, 1018–1025. [Google Scholar] [CrossRef]
- Takei, Y.; Amagase, Y.; Goto, A.; Kambayashi, R.; Izumi-Nakaseko, H.; Hirasawa, A.; Sugiyama, A. Adipose chemokine ligand CX3CL1 contributes to maintaining the hippocampal BDNF level, and the effect is attenuated in advanced age. Geroscience 2025, 47, 5969–5983. [Google Scholar] [CrossRef]
- Tait, J.L.; Duckham, R.L.; Rantalainen, T.; Milte, C.M.; Main, L.C.; Nowson, C.A.; Sanders, K.M.; Taaffe, D.R.; Hill, K.D.; Abbott, G.; et al. Effects of a 6-month dual-task, power-based exercise program on cognitive function, neurological and inflammatory markers in older adults: Secondary analysis of a cluster randomised controlled trial. Geroscience 2024, 47, 1251–1268. [Google Scholar] [CrossRef]
- Bali, Z.K.; Nagy, L.V.; Bruszt, N.; Bodo, K.; Engelmann, P.; Hernadi, Z.; Gonter, K.; Tadepalli, S.A.; Hernadi, I. Increased brain cytokine level associated impairment of vigilance and memory in aged rats can be alleviated by alpha7 nicotinic acetylcholine receptor agonist treatment. Geroscience 2024, 46, 645–664. [Google Scholar] [CrossRef]
- Eroglu, B.; Isales, C.; Eroglu, A. Age and duration of obesity modulate the inflammatory response and expression of neuroprotective factors in mammalian female brain. Aging Cell 2024, 23, e14313. [Google Scholar] [CrossRef]
- Lautrup, S.; Myrup Holst, C.; Yde, A.; Asmussen, S.; Thinggaard, V.; Larsen, K.; Laursen, L.S.; Richner, M.; Vaegter, C.B.; Prieto, G.A.; et al. The role of aging and brain-derived neurotrophic factor signaling in expression of base excision repair genes in the human brain. Aging Cell 2023, 22, e13905. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Wang, J.; Ren, X.; He, J.; Wang, S.; Xing, Y.; Chen, D.; Zhang, X.; Zhou, S.; Liu, X.; et al. An enriched environment improves long-term functional outcomes in mice after intracerebral hemorrhage by mechanisms that involve the Nrf2/BDNF/glutaminase pathway. J. Cereb. Blood Flow. Metab. 2023, 43, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.-m. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.B.; Li, Y.H.; Xu, Y.; Wu, H.L.; Li, X.J. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 2008, 1210, 84–91. [Google Scholar] [CrossRef]
- Zachariou, V.; Pappas, C.; Bauer, C.E.; Shao, X.; Liu, P.; Lu, H.; Wang, D.J.J.; Gold, B.T. Regional differences in the link between water exchange rate across the blood-brain barrier and cognitive performance in normal aging. Geroscience 2024, 46, 265–282. [Google Scholar] [CrossRef] [PubMed]
- van Dinther, M.; Voorter, P.H.M.; Zhang, E.; van Kuijk, S.M.J.; Jansen, J.F.A.; van Oostenbrugge, R.J.; Backes, W.H.; Staals, J. The neurovascular unit and its correlation with cognitive performance in patients with cerebral small vessel disease: A canonical correlation analysis approach. Geroscience 2024, 46, 5061–5073. [Google Scholar] [CrossRef]
- Stankovics, L.; Ungvari, A.; Fekete, M.; Nyul-Toth, A.; Mukli, P.; Patai, R.; Csik, B.; Gulej, R.; Conley, S.; Csiszar, A.; et al. The vasoprotective role of IGF-1 signaling in the cerebral microcirculation: Prevention of cerebral microhemorrhages in aging. Geroscience 2024, 47, 445. [Google Scholar] [CrossRef]
- Salwierz, P.; Thapa, S.; Taghdiri, F.; Vasilevskaya, A.; Anastassiadis, C.; Tang-Wai, D.F.; Golas, A.C.; Tartaglia, M.C. Investigating the association between a history of depression and biomarkers of Alzheimer’s disease, cerebrovascular disease, and neurodegeneration in patients with dementia. Geroscience 2024, 46, 783–793. [Google Scholar] [CrossRef]
- Nyul-Toth, A.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Patai, R.; Petersen, B.; Negri, S.; Chandragiri, S.S.; Shanmugarama, S.; Mukli, P.; Yabluchanskiy, A.; et al. Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: Enhancing blood-brain barrier integrity and capillarization in the aged mouse brain. Geroscience 2024, 46, 4415–4442. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. Geroscience 2024, 46, 5103–5132. [Google Scholar] [CrossRef]
- Weijs, R.W.J.; Oudegeest-Sander, M.H.; Vloet, J.I.A.; Hopman, M.T.E.; Claassen, J.; Thijssen, D.H.J. A decade of aging in healthy older adults: Longitudinal findings on cerebrovascular and cognitive health. Geroscience 2023, 45, 2629–2641. [Google Scholar] [CrossRef] [PubMed]
- Gulej, R.; Nyul-Toth, A.; Ahire, C.; DelFavero, J.; Balasubramanian, P.; Kiss, T.; Tarantini, S.; Benyo, Z.; Pacher, P.; Csik, B.; et al. Elimination of senescent cells by treatment with Navitoclax/ABT263 reverses whole brain irradiation-induced blood-brain barrier disruption in the mouse brain. Geroscience 2023, 45, 2983–3002. [Google Scholar] [CrossRef]
- Zhang, H.; Roman, R.J.; Fan, F. Hippocampus is more susceptible to hypoxic injury: Has the Rosetta Stone of regional variation in neurovascular coupling been deciphered? Geroscience 2022, 44, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Nyul-Toth, A.; Fulop, G.A.; Tarantini, S.; Kiss, T.; Ahire, C.; Faakye, J.A.; Ungvari, A.; Toth, P.; Toth, A.; Csiszar, A.; et al. Cerebral venous congestion exacerbates cerebral microhemorrhages in mice. Geroscience 2022, 44, 805–816. [Google Scholar] [CrossRef]
- Sabayan, B.; Westendorp, R.G.J. Neurovascular-glymphatic dysfunction and white matter lesions. Geroscience 2021, 43, 1635–1642. [Google Scholar] [CrossRef]
- Wang, S.; Lv, W.; Zhang, H.; Liu, Y.; Li, L.; Jefferson, J.R.; Guo, Y.; Li, M.; Gao, W.; Fang, X.; et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience 2020, 42, 1387–1410. [Google Scholar] [CrossRef]
- Verheggen, I.C.M.; de Jong, J.J.A.; van Boxtel, M.P.J.; Postma, A.A.; Jansen, J.F.A.; Verhey, F.R.J.; Backes, W.H. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience 2020, 42, 1751–1764. [Google Scholar] [CrossRef]
- Sorond, F.A.; Whitehead, S.; Arai, K.; Arnold, D.; Carmichael, S.T.; De Carli, C.; Duering, M.; Fornage, M.; Flores-Obando, R.E.; Graff-Radford, J.; et al. Proceedings from the Albert Charitable Trust Inaugural Workshop on white matter and cognition in aging. Geroscience 2020, 42, 81–96. [Google Scholar] [CrossRef]
- Levit, A.; Hachinski, V.; Whitehead, S.N. Neurovascular unit dysregulation, white matter disease, and executive dysfunction: The shared triad of vascular cognitive impairment and Alzheimer disease. Geroscience 2020, 42, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Jor’dan, A.J.; Manor, B.; Iloputaife, I.; Habtemariam, D.A.; Bean, J.F.; Sorond, F.A.; Lipsitz, L.A. Diminished Locomotor Control Is Associated With Reduced Neurovascular Coupling in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Cermakova, P.; Ding, J.; Meirelles, O.; Reis, J.; Religa, D.; Schreiner, P.J.; Jacobs, D.R.; Bryan, R.N.; Launer, L.J. Carotid Intima-Media Thickness and Markers of Brain Health in a Biracial Middle-Aged Cohort: CARDIA Brain MRI Sub-study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Petersen, B.; Faakye, J.; Negri, S.; Chandragiri, S.S.; Mukli, P.; Yabluchanskiy, A.; Conley, S.; et al. Rejuvenation of cerebromicrovascular function in aged mice through heterochronic parabiosis: Insights into neurovascular coupling and the impact of young blood factors. Geroscience 2024, 46, 327–347. [Google Scholar] [CrossRef]
- Li, B.; Yabluchanskiy, A.; Tarantini, S.; Allu, S.R.; Sencan-Egilmez, I.; Leng, J.; Alfadhel, M.A.H.; Porter, J.E.; Fu, B.; Ran, C.; et al. Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: Evidence for microvascular injury in the cerebral white matter. Geroscience 2023, 45, 1491–1510. [Google Scholar]
- Fang, X.; Border, J.J.; Rivers, P.L.; Zhang, H.; Williams, J.M.; Fan, F.; Roman, R.J. Amyloid beta accumulation in TgF344-AD rats is associated with reduced cerebral capillary endothelial Kir2.1 expression and neurovascular uncoupling. Geroscience 2023, 45, 2909–2926. [Google Scholar] [CrossRef]
- Vestergaard, M.B.; Lindberg, U.; Knudsen, M.H.; Urdanibia-Centelles, O.; Bakhtiari, A.; Mortensen, E.L.; Osler, M.; Fagerlund, B.; Benedek, K.; Lauritzen, M.; et al. Subclinical cognitive deficits are associated with reduced cerebrovascular response to visual stimulation in mid-sixties men. Geroscience 2022, 44, 1905–1923. [Google Scholar] [CrossRef]
- Toth, L.; Czigler, A.; Hegedus, E.; Komaromy, H.; Amrein, K.; Czeiter, E.; Yabluchanskiy, A.; Koller, A.; Orsi, G.; Perlaki, G.; et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 2022, 44, 2771–2783. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Nation, D.A.; Kisler, K.; Toga, A.W.; Zlokovic, B.V. Imaging subtle leaks in the blood-brain barrier in the aging human brain: Potential pitfalls, challenges, and possible solutions. Geroscience 2022, 44, 1339–1351. [Google Scholar] [CrossRef]
- Tarantini, S.; Nyul-Toth, A.; Yabluchanskiy, A.; Csipo, T.; Mukli, P.; Balasubramanian, P.; Ungvari, A.; Toth, P.; Benyo, Z.; Sonntag, W.E.; et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience 2021, 43, 2387–2394. [Google Scholar] [CrossRef]
- Istvan, L.; Czako, C.; Elo, A.; Mihaly, Z.; Sotonyi, P.; Varga, A.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience 2021, 43, 1703–1723. [Google Scholar] [CrossRef]
- Fan, F.; Roman, R.J. Reversal of cerebral hypoperfusion: A novel therapeutic target for the treatment of AD/ADRD? Geroscience 2021, 43, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Nyul-Toth, A.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.D.; Garman, L.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: Transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 2020, 42, 527–546. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, W.; Liu, Q. Alterations of the blood-brain barrier during aging. J. Cereb. Blood Flow. Metab. 2024, 44, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Jeon, H.J.; Han, S.H.; Min-Young, N.; Kim, H.J.; Kwon, K.J.; Moon, W.J.; Kim, S.H. Blood-brain barrier breakdown is linked to tau pathology and neuronal injury in a differential manner according to amyloid deposition. J. Cereb. Blood Flow. Metab. 2023, 43, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, W.; Sun, Y.; Zhang, J.; Ma, C.; Jin, X. CRTC1 is a potential target to delay aging-induced cognitive deficit by protecting the integrity of the blood-brain barrier via inhibiting inflammation. J. Cereb. Blood Flow. Metab. 2023, 43, 1042–1059. [Google Scholar] [CrossRef]
- Preininger, M.K.; Zaytseva, D.; Lin, J.M.; Kaufer, D. Blood-brain barrier dysfunction promotes astrocyte senescence through albumin-induced TGFbeta signaling activation. Aging Cell 2023, 22, e13747. [Google Scholar] [CrossRef]
- Udo, M.S.B.; Zaccarelli-Magalhaes, J.; Clemons, G.A.; Citadin, C.T.; Langman, J.; Smith, D.J.; Matuguma, L.H.; Tesic, V.; Lin, H.W. Blockade of A(2A)R improved brain perfusion and cognitive function in a mouse model of Alzheimer’s disease. Geroscience 2025, 47, 4153–4167. [Google Scholar] [CrossRef]
- Patai, R.; Csik, B.; Nyul-Toth, A.; Gulej, R.; Vali Kordestan, K.; Chandragiri, S.S.; Shanmugarama, S.; Tarantini, S.; Mukli, P.; Ungvari, A.; et al. Persisting blood-brain barrier disruption following cisplatin treatment in a mouse model of chemotherapy-associated cognitive impairment. Geroscience 2025, 47, 3835–3847. [Google Scholar] [CrossRef]
- Cummins, M.J.; Cresswell, E.T.; Bevege, R.J.; Smith, D.W. Aging disrupts blood-brain and blood-spinal cord barrier homeostasis, but does not increase paracellular permeability. Geroscience 2024, 47, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Panner Selvam, M.K.; Castorena-Gonzalez, J.A.; Rutkai, I.; Sikka, S.C.; Mostany, R.; Busija, D.W. Fibrinogen in mice cerebral microvessels induces blood-brain barrier dysregulation with aging via a dynamin-related protein 1-dependent pathway. Geroscience 2024, 46, 395–415. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, M.P.; Vijayan, M. A review on gut microbiota and miRNA crosstalk: Implications for Alzheimer’s disease. Geroscience 2024, 47, 339–385. [Google Scholar] [CrossRef]
- Ting, K.K.; Coleman, P.; Kim, H.J.; Zhao, Y.; Mulangala, J.; Cheng, N.C.; Li, W.; Gunatilake, D.; Johnstone, D.M.; Loo, L.; et al. Vascular senescence and leak are features of the early breakdown of the blood-brain barrier in Alzheimer’s disease models. Geroscience 2023, 45, 3307–3331. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.D.; Vemuri, R.; Blawas, M.; Long, M.; DeStephanis, D.; Williams, A.G.; Chen, H.; Justice, J.N.; Macauley, S.L.; Day, S.M.; et al. Long-term dasatinib plus quercetin effects on aging outcomes and inflammation in nonhuman primates: Implications for senolytic clinical trial design. Geroscience 2023, 45, 2785–2803. [Google Scholar] [CrossRef]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Bakhtiari, A.; Vestergaard, M.B.; Benedek, K.; Fagerlund, B.; Mortensen, E.L.; Osler, M.; Lauritzen, M.; Larsson, H.B.W.; Lindberg, U. Changes in hippocampal volume during a preceding 10-year period do not correlate with cognitive performance and hippocampal blood–brain barrier permeability in cognitively normal late-middle-aged men. Geroscience 2023, 45, 1161–1175. [Google Scholar] [CrossRef]
- Towner, R.A.; Gulej, R.; Zalles, M.; Saunders, D.; Smith, N.; Lerner, M.; Morton, K.A.; Richardson, A. Rapamycin restores brain vasculature, metabolism, and blood-brain barrier in an inflammaging model. Geroscience 2021, 43, 563–578. [Google Scholar] [CrossRef]
- Kerkhofs, D.; Wong, S.M.; Zhang, E.; Uiterwijk, R.; Hoff, E.I.; Jansen, J.F.A.; Staals, J.; Backes, W.H.; van Oostenbrugge, R.J. Blood-brain barrier leakage at baseline and cognitive decline in cerebral small vessel disease: A 2-year follow-up study. Geroscience 2021, 43, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, I.C.M.; de Jong, J.J.A.; van Boxtel, M.P.J.; Gronenschild, E.; Palm, W.M.; Postma, A.A.; Jansen, J.F.A.; Verhey, F.R.J.; Backes, W.H. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience 2020, 42, 1183–1193. [Google Scholar] [CrossRef]
- Islam, M.T.; Hall, S.A.; Dutson, T.; Bloom, S.I.; Bramwell, R.C.; Kim, J.; Tucker, J.R.; Machin, D.R.; Donato, A.J.; Lesniewski, L.A. Endothelial cell-specific reduction in mTOR ameliorates age-related arterial and metabolic dysfunction. Aging Cell 2024, 23, e14040. [Google Scholar] [CrossRef]
- Kiss, T.; Nyul-Toth, A.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; DelFavero, J.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wiley, G.; et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 2020, 42, 429–444. [Google Scholar] [CrossRef]
- Tarantini, S.; Balasubramanian, P.; Delfavero, J.; Csipo, T.; Yabluchanskiy, A.; Kiss, T.; Nyul-Toth, A.; Mukli, P.; Toth, P.; Ahire, C.; et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience 2021, 43, 2427–2440. [Google Scholar] [CrossRef]
- Faakye, J.; Nyul-Toth, A.; Muranyi, M.; Gulej, R.; Csik, B.; Shanmugarama, S.; Tarantini, S.; Negri, S.; Prodan, C.; Mukli, P.; et al. Preventing spontaneous cerebral microhemorrhages in aging mice: A novel approach targeting cellular senescence with ABT263/navitoclax. Geroscience 2024, 46, 21–37. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.Y.; Zaira, A.; Mau, T.; Fielding, R.A.; Atkinson, E.J.; Patel, S.; LeBrasseur, N. Biomarkers of cellular senescence and major health outcomes in older adults. Geroscience 2024, 47, 3407–3415. [Google Scholar] [CrossRef]
- Mahoney, S.A.; Venkatasubramanian, R.; Darrah, M.A.; Ludwig, K.R.; VanDongen, N.S.; Greenberg, N.T.; Longtine, A.G.; Hutton, D.A.; Brunt, V.E.; Campisi, J.; et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell 2024, 23, e14060. [Google Scholar] [CrossRef] [PubMed]
- Novo, J.P.; Gee, L.; Caetano, C.A.; Tome, I.; Vilaca, A.; von Zglinicki, T.; Moreira, I.S.; Jurk, D.; Rosa, S.; Ferreira, L. Blood-brain barrier dysfunction in aging is mediated by brain endothelial senescence. Aging Cell 2024, 23, e14270. [Google Scholar] [CrossRef] [PubMed]
- Budamagunta, V.; Kumar, A.; Rani, A.; Bean, L.; Manohar-Sindhu, S.; Yang, Y.; Zhou, D.; Foster, T.C. Effect of peripheral cellular senescence on brain aging and cognitive decline. Aging Cell 2023, 22, e13817. [Google Scholar] [CrossRef] [PubMed]
- Ahire, C.; Nyul-Toth, A.; DelFavero, J.; Gulej, R.; Faakye, J.A.; Tarantini, S.; Kiss, T.; Kuan-Celarier, A.; Balasubramanian, P.; Ungvari, A.; et al. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell 2023, 22, e13832. [Google Scholar] [CrossRef]
- Bloom, S.I.; Liu, Y.; Tucker, J.R.; Islam, M.T.; Machin, D.R.; Abdeahad, H.; Thomas, T.G.; Bramwell, R.C.; Lesniewski, L.A.; Donato, A.J. Endothelial cell telomere dysfunction induces senescence and results in vascular and metabolic impairments. Aging Cell 2023, 22, e13875. [Google Scholar] [CrossRef]
- Nyul-Toth, A.; Negri, S.; Sanford, M.; Jiang, R.; Patai, R.; Budda, M.; Petersen, B.; Pinckard, J.; Chandragiri, S.S.; Shi, H.; et al. Novel intravital approaches to quantify deep vascular structure and perfusion in the aging mouse brain using ultrasound localization microscopy (ULM). J. Cereb. Blood Flow. Metab. 2024, 44, 1378–1396. [Google Scholar] [CrossRef]
- Andonian, B.J.; Hippensteel, J.A.; Abuabara, K.; Boyle, E.M.; Colbert, J.F.; Devinney, M.J.; Faye, A.S.; Kochar, B.; Lee, J.; Litke, R.; et al. Inflammation and aging-related disease: A transdisciplinary inflammaging framework. Geroscience 2024, 47, 515–542. [Google Scholar] [CrossRef]
- Gotz, L.; Rueckschloss, U.; Reimer, A.; Bommel, H.; Beilhack, A.; Ergun, S.; Kleefeldt, F. Vascular inflammaging: Endothelial CEACAM1 expression is upregulated by TNF-alpha via independent activation of NF-kappaB and beta-catenin signaling. Aging Cell 2025, 24, e14384. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, Z.; Boyle, R.; Yau, W.W.; Coughlan, G.; Fu, J.F.; Properzi, M.J.; Buckley, R.F.; Yang, H.S.; Scanlon, C.E.; Hsieh, S.; et al. Vascular contributions to cognitive decline: Beyond amyloid and tau in the Harvard Aging Brain Study. J. Cereb. Blood Flow. Metab. 2024, 44, 1319–1328. [Google Scholar] [CrossRef]
- van Dijk, S.E.; Drenth, N.; Hafkemeijer, A.; Labadie, G.; Witjes-Ane, M.W.; Blauw, G.J.; Rombouts, S.A.; van der Grond, J.; van Rooden, S. Neurovascular coupling in early stage dementia—A case-control study. J. Cereb. Blood Flow. Metab. 2024, 44, 1013–1023. [Google Scholar] [CrossRef]
- van der Horn, H.J.; Vakhtin, A.A.; Julio, K.; Nitschke, S.; Shaff, N.; Dodd, A.B.; Erhardt, E.; Phillips, J.P.; Pirio Richardson, S.; Deligtisch, A.; et al. Parkinson’s disease cerebrovascular reactivity pattern: A feasibility study. J. Cereb. Blood Flow. Metab. 2024, 44, 1774–1786. [Google Scholar] [CrossRef]
- Jones, O.A.; Mohamed, S.; Hinz, R.; Paterson, A.; Sobowale, O.A.; Dickie, B.R.; Parkes, L.M.; Parry-Jones, A.R. Neuroinflammation and blood-brain barrier breakdown in acute, clinical intracerebral hemorrhage. J. Cereb. Blood Flow. Metab. 2025, 45, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, C.; Wang, J.; Ye, J.; Lian, Q.; Gan, L.; Deng, S.; Xu, T.; Guo, Y.; Li, W.; et al. CCL5 mediated astrocyte-T cell interaction disrupts blood-brain barrier in mice after hemorrhagic stroke. J. Cereb. Blood Flow. Metab. 2024, 44, 367–383. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Sha, W.M.; Zhang, X.P.; Mai, J.Y.; Bartlett, P.F.; Hou, S.T. Inhibition of EphA4 reduces vasogenic edema after experimental stroke in mice by protecting the blood-brain barrier integrity. J. Cereb. Blood Flow. Metab. 2024, 44, 419–433. [Google Scholar] [CrossRef]
- Wu, S.; Ren, X.S.; Shi, Y. Early and enduring: Targeting the endothelium for blood-brain barrier protection. J. Cereb. Blood Flow. Metab. 2024, 44, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Mandeville, E.T.; Buchan, A.M.; Tiedt, S. Not open and shut: Complex and prolonged blood-brain barrier responses after stroke. J. Cereb. Blood Flow. Metab. 2024, 44, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, Y.; Deng, S.; Zhou, S.; Wu, S.; Chen, T.; Shi, X.; Mamtilahun, M.; Xu, T.; Liu, Z.; et al. Hemorrhagic stroke-induced subtype of inflammatory reactive astrocytes disrupts blood-brain barrier. J. Cereb. Blood Flow. Metab. 2024, 44, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Carlsson, R.; Buizza, C.; Enstrom, A.; Paul, G. Pericyte response to ischemic stroke precedes endothelial cell death and blood-brain barrier breakdown. J. Cereb. Blood Flow. Metab. 2025, 45, 617–629. [Google Scholar] [CrossRef]
- Tudor, T.; Spinazzi, E.F.; Alexander, J.E.; Mandigo, G.K.; Lavine, S.D.; Grinband, J.; Connolly, E.S., Jr. Progressive microvascular failure in acute ischemic stroke: A systematic review, meta-analysis, and time-course analysis. J. Cereb. Blood Flow. Metab. 2024, 44, 192–208. [Google Scholar] [CrossRef]
- Schmitzer, L.; Kaczmarz, S.; Gottler, J.; Hoffmann, G.; Kallmayer, M.; Eckstein, H.H.; Hedderich, D.M.; Kufer, J.; Zimmer, C.; Preibisch, C.; et al. Macro- and microvascular contributions to cerebral structural alterations in patients with asymptomatic carotid artery stenosis. J. Cereb. Blood Flow. Metab. 2024, 44, 1629–1642. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Ip, B.; Feng, X.; Ip, H.L.; Abrigo, J.; Lan, L.; Liu, H.; Zheng, L.; Liu, Y.; et al. Cerebral hemodynamics and stroke risks in symptomatic intracranial atherosclerotic stenosis with internal versus cortical borderzone infarcts: A computational fluid dynamics study. J. Cereb. Blood Flow. Metab. 2024, 44, 516–526. [Google Scholar] [CrossRef]
- Bagi, Z.; Kroenke, C.D.; Fopiano, K.A.; Tian, Y.; Filosa, J.A.; Sherman, L.S.; Larson, E.B.; Keene, C.D.; Degener O’Brien, K.; Adeniyi, P.A.; et al. Association of cerebral microvascular dysfunction and white matter injury in Alzheimer’s disease. Geroscience 2022, 44, 1–14. [Google Scholar] [CrossRef]
- Czakó, C.; Kovács, T.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; Csipo, T.; Lipecz, A.; Horváth, H.; Sándor, G.L.; et al. Retinal biomarkers for Alzheimer’s disease and vascular cognitive impairment and dementia (VCID): Implication for early diagnosis and prognosis. Geroscience 2020, 42, 1499–1525. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Szappanos, A.; Toth, A.; Mahdi, M.; Sotonyi, P.; Benyo, Z.; Yabluchanskiy, A.; Tarantini, S.; Ungvari, Z. Cerebromicrovascular mechanisms contributing to long COVID: Implications for neurocognitive health. Geroscience 2025, 47, 745–779. [Google Scholar] [CrossRef]
- Elmansi, A.M.; Kassem, A.; Castilla, R.M.; Miller, R.A. Downregulation of the NF-kappaB protein p65 is a shared phenotype among most anti-aging interventions. Geroscience 2024, 47, 3077–3094. [Google Scholar] [CrossRef]
- Riordan, R.; Rong, W.; Yu, Z.; Ross, G.; Valerio, J.; Dimas-Munoz, J.; Heredia, V.; Magnusson, K.; Galvan, V.; Perez, V.I. Effect of Nrf2 loss on senescence and cognition of tau-based P301S mice. Geroscience 2023, 45, 1451–1469. [Google Scholar] [CrossRef]
- Shin, J.W.; Chun, K.S.; Kim, D.H.; Kim, S.J.; Kim, S.H.; Cho, N.C.; Na, H.K.; Surh, Y.J. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Huang, Y.; Wu, T.Y.; Shu, L.; Lee, J.; Kong, A.N. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011, 82, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Kobutree, P.; Tothonglor, A.; Roumwong, A.; Jindatip, D.; Agthong, S. Curcumin reduces blood-nerve barrier abnormalities and cytotoxicity to endothelial cells and pericytes induced by cisplatin. Folia Morphol. 2023, 82, 533–542. [Google Scholar] [CrossRef]
- Moore, T.L.; Bowley, B.; Shultz, P.; Calderazzo, S.; Shobin, E.; Killiany, R.J.; Rosene, D.L.; Moss, M.B. Chronic curcumin treatment improves spatial working memory but not recognition memory in middle-aged rhesus monkeys. Geroscience 2017, 39, 571–584. [Google Scholar] [CrossRef]
- Kaufmann, F.N.; Gazal, M.; Bastos, C.R.; Kaster, M.P.; Ghisleni, G. Curcumin in depressive disorders: An overview of potential mechanisms, preclinical and clinical findings. Eur. J. Pharmacol. 2016, 784, 192–198. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C.; Basu, A. Effects of Curcumin and Its Different Formulations in Preclinical and Clinical Studies of Peripheral Neuropathic and Postoperative Pain: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4666. [Google Scholar] [CrossRef]
- Marques, M.; Marinho, M.; Vian, C.; Horn, A. The action of curcumin against damage resulting from cerebral stroke: A systematic review. Pharmacol. Res. 2022, 183, 106369. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, C.; Zhang, D.; Yuan, M.; Chen, C.-h.; Li, M. Synergic effects of berberine and curcumin on improving cognitive function in an Alzheimer’s disease mouse model. Neurochem. Res. 2020, 45, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Su, I.J.; Chang, H.Y.; Wang, H.C.; Tsai, K.J. A Curcumin Analog Exhibits Multiple Biologic Effects on the Pathogenesis of Alzheimer’s Disease and Improves Behavior, Inflammation, and β-Amyloid Accumulation in a Mouse Model. Int. J. Mol. Sci. 2020, 21, 5459. [Google Scholar] [CrossRef] [PubMed]
- ELBini-Dhouib, I.; Doghri, R.; Ellefi, A.; Degrach, I.; Srairi-Abid, N.; Gati, A. Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease. Molecules 2021, 26, 3001. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Kandimalla, R.; Kuruva, C.S. Protective effects of a natural product, curcumin, against amyloid β induced mitochondrial and synaptic toxicities in Alzheimer’s disease. J. Investig. Med. 2016, 64, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, H.; Wang, L.; Shuboy, A.; Lou, J.; Han, B.; Zhang, X.; Li, J. Curcumin as a potential treatment for Alzheimer’s disease: A study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am. J. Chin. Med. 2013, 41, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. Aaps J. 2013, 15, 324–336. [Google Scholar] [CrossRef]
- Ray, B.; Bisht, S.; Maitra, A.; Maitra, A.; Lahiri, D.K. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc™) in the neuronal cell culture and animal model: Implications for Alzheimer’s disease. J. Alzheimers Dis. 2011, 23, 61–77. [Google Scholar] [CrossRef]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef]
- Xiong, Z.; Hongmei, Z.; Lu, S.; Yu, L. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s Disease. Pharmacol. Rep. 2011, 63, 1101–1108. [Google Scholar] [CrossRef]
- Ishrat, T.; Hoda, M.N.; Khan, M.B.; Yousuf, S.; Ahmad, M.; Khan, M.M.; Ahmad, A.; Islam, F. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT). Eur. Neuropsychopharmacol. 2009, 19, 636–647. [Google Scholar] [CrossRef]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Kim, C.J.; Yoon, Y.S.; et al. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J. Med. Food 2014, 17, 641–649. [Google Scholar] [CrossRef]
- Bassani, T.B.; Turnes, J.M.; Moura, E.L.R.; Bonato, J.M.; Cóppola-Segovia, V.; Zanata, S.M.; Oliveira, R.; Vital, M. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017, 335, 41–54. [Google Scholar] [CrossRef]
- McClure, R.; Ong, H.; Janve, V.; Barton, S.; Zhu, M.; Li, B.; Dawes, M.; Jerome, W.G.; Anderson, A.; Massion, P. Aerosol delivery of curcumin reduced amyloid-β deposition and improved cognitive performance in a transgenic model of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 55, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Alamro, A.A.; Alsulami, E.A.; Almutlaq, M.; Alghamedi, A.; Alokail, M.; Haq, S.H. Therapeutic Potential of Vitamin D and Curcumin in an In Vitro Model of Alzheimer Disease. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520924311. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Savall, A.S.P.; de Mello, J.D.; Fidelis, E.M.; Comis-Neto, A.A.; Nepomuceno, M.R.; Pacheco, C.d.O.; Haas, S.E.; Pinton, S. Nanoencapsulated curcumin: Enhanced efficacy in reversing memory loss in an Alzheimer disease model. Brain Sci. 2024, 14, 130. [Google Scholar] [CrossRef]
- Abbaoui, A.; Chatoui, H.; El Hiba, O.; Gamrani, H. Neuroprotective effect of curcumin-I in copper-induced dopaminergic neurotoxicity in rats: A possible link with Parkinson’s disease. Neurosci. Lett. 2017, 660, 103–108. [Google Scholar] [CrossRef]
- Rajeswari, A.; Sabesan, M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology 2008, 16, 96–99. [Google Scholar] [CrossRef]
- Pan, J.; Li, H.; Ma, J.F.; Tan, Y.Y.; Xiao, Q.; Ding, J.Q.; Chen, S.D. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl. Neurodegener. 2012, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.F.; Zhang, Y.J.; Zhou, H.Y.; Wang, H.M.; Tian, L.P.; Liu, J.; Ding, J.Q.; Chen, S.D. Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J. Neuroimmune Pharmacol. 2013, 8, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Boddapati, S.; Emadi, S.; Sierks, M.R. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 2010, 11, 57. [Google Scholar] [CrossRef]
- Ramires Júnior, O.V.; Alves, B.D.S.; Barros, P.A.B.; Rodrigues, J.L.; Ferreira, S.P.; Monteiro, L.K.S.; Araújo, G.M.S.; Fernandes, S.S.; Vaz, G.R.; Dora, C.L.; et al. Nanoemulsion Improves the Neuroprotective Effects of Curcumin in an Experimental Model of Parkinson’s Disease. Neurotox. Res. 2021, 39, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol. Res. Pract. 2014, 210, 357–362. [Google Scholar] [CrossRef]
- Fikry, H.; Saleh, L.A.; Abdel Gawad, S. Neuroprotective effects of curcumin on the cerebellum in a rotenone-induced Parkinson’s Disease Model. CNS Neurosci. Ther. 2022, 28, 732–748. [Google Scholar] [CrossRef]
- Jagatha, B.; Mythri, R.B.; Vali, S.; Bharath, M.M. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: Therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic. Biol. Med. 2008, 44, 907–917. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef]
- Wang, X.-S.; Zhang, Z.-R.; Zhang, M.-M.; Sun, M.-X.; Wang, W.-W.; Xie, C.-L. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: A systematic experiment literatures review. BMC Complement. Altern. Med. 2017, 17, 412. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Li, C.; Chen, Z.; Gao, Q.; Han, M.; Zhao, F.; Chen, D.; Chen, Q.; Hu, M. The dosage of curcumin to alleviate movement symptoms in a 6-hydroxydopamine-induced Parkinson’s disease rat model. Heliyon 2023, 9, e16921. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, H.; Hu, M.; Liu, X.; Han, M.; Xie, J.; Li, Z.; Zhao, F.; Liu, W.; Wei, S. A novel curcumin oil solution can better alleviate the motor activity defects and neuropathological damage of a Parkinson’s disease mouse model. Front. Aging Neurosci. 2022, 14, 984895. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Wang, J.; Xia, S.; Ran, H.; Gao, L.; Feng, C.; Gui, L.; Zhou, Z.; Yuan, J. Human umbilical cord-derived mesenchymal stem cell transplantation supplemented with curcumin improves the outcomes of ischemic stroke via AKT/GSK-3β/β-TrCP/Nrf2 axis. J. Neuroinflamm. 2023, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Tan, B.; Ma, J.; Zhang, L.; Jin, X.; Li, C. Prdx6 Upregulation by Curcumin Attenuates Ischemic Oxidative Damage via SP1 in Rats after Stroke. Biomed. Res. Int. 2017, 2017, 6597401. [Google Scholar] [CrossRef] [PubMed]
- Altinay, S.; Cabalar, M.; Isler, C.; Yildirim, F.; Celik, D.S.; Zengi, O.; Tas, A.; Gulcubuk, A. Is Chronic Curcumin Supplementation Neuroprotective Against Ischemia for Antioxidant Activity, Neurological Deficit, or Neuronal Apoptosis in an Experimental Stroke Model? Turk. Neurosurg. 2017, 27, 537–545. [Google Scholar] [PubMed][Green Version]
- Marques, M.S.; Cordeiro, M.F.; Marinho, M.A.G.; Vian, C.O.; Vaz, G.R.; Alves, B.S.; Jardim, R.D.; Hort, M.A.; Dora, C.L.; Horn, A.P. Curcumin-loaded nanoemulsion improves haemorrhagic stroke recovery in wistar rats. Brain Res. 2020, 1746, 147007. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Su, W.; Gao, F.; Ding, Z.; Yang, S.; Ye, L.; Chen, X.; Tian, G.; Xi, J.; Liu, Z. Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-κB Suppression and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell Longev. 2021, 2021, 1552127. [Google Scholar] [CrossRef]
- Xie, C.J.; Gu, A.P.; Cai, J.; Wu, Y.; Chen, R.C. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018, 8, e00921. [Google Scholar] [CrossRef]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3 expression. Mol. Med. Rep. 2017, 16, 4710–4720. [Google Scholar] [CrossRef]
- Liu, S.; Cao, Y.; Qu, M.; Zhang, Z.; Feng, L.; Ye, Z.; Xiao, M.; Hou, S.T.; Zheng, R.; Han, Z. Curcumin protects against stroke and increases levels of Notch intracellular domain. Neurol. Res. 2016, 38, 553–559. [Google Scholar] [CrossRef]
- Wu, S.; Guo, T.; Qi, W.; Li, Y.; Gu, J.; Liu, C.; Sha, Y.; Yang, B.; Hu, S.; Zong, X. Curcumin ameliorates ischemic stroke injury in rats by protecting the integrity of the blood-brain barrier. Exp. Ther. Med. 2021, 22, 783. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Y.; Huang, S.; Wen, S.; Zhang, W.; Liu, X.; Ji, Z.; Geng, X.; Ji, X.; Du, H.; et al. Curcumin Protects against Ischemic Stroke by Titrating Microglia/Macrophage Polarization. Front. Aging Neurosci. 2017, 9, 233. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, W.; Sun, Y.J.; Hu, M.; Li, F.; Zhu, D.Y. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur. J. Pharmacol. 2007, 561, 54–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Cao, Y.; Yang, Y.; Zhao, Q.; Jing, R.; Hu, J.; Bao, J. Potential therapeutic and protective effect of curcumin against stroke in the male albino stroke-induced model rats. Life Sci. 2017, 183, 45–49. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Zhang, M. The beneficial effects of curcumin on aging and age-related diseases: From oxidative stress to antioxidant mechanisms, brain health and apoptosis. Front. Aging Neurosci. 2025, 17, 1533963. [Google Scholar] [CrossRef]
- Dutta, A.; Patil, R.; Pati, H.l.L. Curcumin: Its bioavailability and nanoparticle formulation: A review. Int. J. Health Sci. Res. 2021, 11, 228–238. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, S.; Morasso, C.; Stivaktakis, P.; Pandini, C.; Tinelli, V.; Tsatsakis, A.; Prosperi, D.; Hickey, M.; Corsi, F.; Cereda, C. Curcumin formulations and trials: What’s new in neurological diseases. Molecules 2020, 25, 5389. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, R.; Liu, B.; Li, K. The effect of curcumin supplementation on cognitive function: An updated systematic review and meta-analysis. Front. Nutr. 2025, 12, 1549509. [Google Scholar] [CrossRef]
- Francis, A.J.; Sreenivasan, C.; Parikh, A.; AlQassab, O.; Kanthajan, T.; Pandey, M.; Nwosu, M. Curcumin and Cognitive Function: A Systematic Review of the Effects of Curcumin on Adults With and Without Neurocognitive Disorders. Cureus 2024, 16, e67706. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic. Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Ghodsi, H.; Rahimi, H.R.; Aghili, S.M.; Saberi, A.; Shoeibi, A. Evaluation of curcumin as add-on therapy in patients with Parkinson’s disease: A pilot randomized, triple-blind, placebo-controlled trial. Clin. Neurol. Neurosurg. 2022, 218, 107300. [Google Scholar] [CrossRef]
- Maghbooli, M.; Safarnejad, B.; Mostafavi, H.; Mazloomzadeh, S.; Ghoreishi, A. Effect of nanomicelle curcumin on quality of life and sleep in patients with Parkinson’s disease: A double-blind, randomized, and PlaceboControlled trial. Int. Clin. Neurosci. J. 2019, 6, 140–145. [Google Scholar] [CrossRef]
- Donadio, V.; Incensi, A.; Rizzo, G.; Fileccia, E.; Ventruto, F.; Riva, A.; Tiso, D.; Recchia, M.; Vacchiano, V.; Infante, R.; et al. The Effect of Curcumin on Idiopathic Parkinson Disease: A Clinical and Skin Biopsy Study. J. Neuropathol. Exp. Neurol. 2022, 81, 545–552. [Google Scholar] [CrossRef]
- Boshagh, K.; Khorvash, F.; Sahebkar, A.; Majeed, M.; Bahreini, N.; Askari, G.; Bagherniya, M. The effects of curcumin-piperine supplementation on inflammatory, oxidative stress and metabolic indices in patients with ischemic stroke in the rehabilitation phase: A randomized controlled trial. Nutr. J. 2023, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, J.; Pandey, A.V. Innovative delivery systems for curcumin: Exploring nanosized and conventional formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Nahar, P.P.; Slitt, A.L.; Seeram, N.P. Anti-Inflammatory Effects of Novel Standardized Solid Lipid Curcumin Formulations. J. Med. Food 2015, 18, 786–792. [Google Scholar] [CrossRef]
- Gupte, P.A.; Giramkar, S.A.; Harke, S.M.; Kulkarni, S.K.; Deshmukh, A.P.; Hingorani, L.L.; Mahajan, M.P.; Bhalerao, S.S. Evaluation of the efficacy and safety of Capsule Longvida(®) Optimized Curcumin (solid lipid curcumin particles) in knee osteoarthritis: A pilot clinical study. J. Inflamm. Res. 2019, 12, 145–152. [Google Scholar] [CrossRef]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Murata, S.; Yabumoto, H.; Maeda, T.; Akamatsu, S. The Efficacy and Safety of Highly-Bioavailable Curcumin for Treating Knee Osteoarthritis: A 6-Month Open-Labeled Prospective Study. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 1179544120948471. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Miyazaki, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Nurmila, S.; Katanasaka, Y.; Ito, M.; Ogawa, T.; Ozawa-Umeta, H. A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: A single-dose, double-blind, two-way crossover study. J. Funct. Foods 2021, 81, 104443. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Jaiswal, P.; Mishra, A. Role of curcumin and its nanoformulations in neurotherapeutics: A comprehensive review. J. Biochem. Mol. Toxicol. 2020, 34, e22478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ciallella, H.L.; Aleksunes, L.M.; Zhu, H. Advancing computer-aided drug discovery (CADD) by big data and data-driven machine learning modeling. Drug Discov. Today 2020, 25, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Vinicki, M.; Pribic, T.; Vuckovic, F.; Frkatovic-Hodzic, A.; Plaza-Andrades, I.; Tinahones, F.; Raffaele, J.; Fernandez-Garcia, J.C.; Lauc, G. Effects of testosterone and metformin on the GlycanAge index of biological age and the composition of the IgG glycome. Geroscience 2024, 47, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M. Anti-aging interventions in geriatric mice: Insights into the timing of treatment, benefits, and limitations. Geroscience 2024, 47, 109–119. [Google Scholar] [CrossRef]
- Landsberger, T.; Amit, I.; Alon, U. Geroprotective interventions converge on gene expression programs of reduced inflammation and restored fatty acid metabolism. Geroscience 2024, 46, 1627–1639. [Google Scholar] [CrossRef]
- Shintani, T.; Shintani, H.; Sato, M.; Ashida, H. Calorie restriction mimetic drugs could favorably influence gut microbiota leading to lifespan extension. Geroscience 2023, 45, 3475–3490. [Google Scholar] [CrossRef]
- Li, X.; McPherson, M.; Hager, M.; Lee, M.; Chang, P.; Miller, R.A. Four anti-aging drugs and calorie-restricted diet produce parallel effects in fat, brain, muscle, macrophages, and plasma of young mice. Geroscience 2023, 45, 2495–2510. [Google Scholar] [CrossRef]
- Katsube, M.; Ishimoto, T.; Fukushima, Y.; Kagami, A.; Shuto, T.; Kato, Y. Ergothioneine promotes longevity and healthy aging in male mice. Geroscience 2024, 46, 3889–3909. [Google Scholar] [CrossRef]
- Sonsalla, M.M.; Lamming, D.W. Geroprotective interventions in the 3xTg mouse model of Alzheimer’s disease. Geroscience 2023, 45, 1343–1381. [Google Scholar] [CrossRef] [PubMed]
- Sandalova, E.; Goh, J.; Lim, Z.X.; Lim, Z.M.; Barardo, D.; Dorajoo, R.; Kennedy, B.K.; Maier, A.B. Alpha-ketoglutarate supplementation and BiologicaL agE in middle-aged adults (ABLE)-intervention study protocol. Geroscience 2023, 45, 2897–2907. [Google Scholar] [CrossRef]
- McGee, K.C.; Sullivan, J.; Hazeldine, J.; Schmunk, L.J.; Martin-Herranz, D.E.; Jackson, T.; Lord, J.M. A combination nutritional supplement reduces DNA methylation age only in older adults with a raised epigenetic age. Geroscience 2024, 46, 4333–4347. [Google Scholar] [CrossRef]
- Dai, Z.; Lee, S.Y.; Sharma, S.; Ullah, S.; Tan, E.C.K.; Brodaty, H.; Schutte, A.E.; Sachdev, P.S. A systematic review of diet and medication use among centenarians and near-centenarians worldwide. Geroscience 2024, 46, 6625–6639. [Google Scholar] [CrossRef] [PubMed]
- Bizzozero-Peroni, B.; Diaz-Goni, V.; Beneit, N.; Oliveira, A.; Jimenez-Lopez, E.; Martinez-Vizcaino, V.; Mesas, A.E. Nut consumption is associated with a lower risk of all-cause dementia in adults: A community-based cohort study from the UK Biobank. Geroscience 2024, 47, 1721–1733. [Google Scholar] [CrossRef]
- Zupo, R.; Donghia, R.; Castellana, F.; Bortone, I.; De Nucci, S.; Sila, A.; Tatoli, R.; Lampignano, L.; Sborgia, G.; Panza, F.; et al. Ultra-processed food consumption and nutritional frailty in older age. Geroscience 2023, 45, 2229–2243. [Google Scholar] [CrossRef]
- Zhou, Z.; Kim, K.; Ramsey, J.J.; Rutkowsky, J.M. Ketogenic diets initiated in late mid-life improved measures of spatial memory in male mice. Geroscience 2023, 45, 2481–2494. [Google Scholar] [CrossRef]
- Aversa, Z.; White, T.A.; Heeren, A.A.; Hulshizer, C.A.; Saul, D.; Zhang, X.; Molina, A.J.A.; Redman, L.M.; Martin, C.K.; Racette, S.B.; et al. Calorie restriction reduces biomarkers of cellular senescence in humans. Aging Cell 2024, 23, e14038. [Google Scholar] [CrossRef]
- Das, S.K.; Silver, R.E.; Senior, A.; Gilhooly, C.H.; Bhapkar, M.; Le Couteur, D. Diet composition, adherence to calorie restriction, and cardiometabolic disease risk modification. Aging Cell 2023, 22, e14018. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, G.; Tosti, V.; Polidoro, S.; Bertozzi, B.; Veronese, N.; Cava, E.; Spelta, F.; Piccio, L.; Early, D.S.; Raftery, D.; et al. Multi-omic analysis of biological aging biomarkers in long-term calorie restriction and endurance exercise practitioners: A cross-sectional study. Aging Cell 2025, 24, e14442. [Google Scholar] [CrossRef]
- Han, W.; Zhang, B.; Zhao, W.; Zhao, W.; He, J.; Qiu, X.; Zhang, L.; Wang, X.; Wang, Y.; Lu, H.; et al. Ketogenic beta-hydroxybutyrate regulates beta-hydroxybutyrylation of TCA cycle-associated enzymes and attenuates disease-associated pathologies in Alzheimer’s mice. Aging Cell 2025, 24, e14368. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.V.; Senior, A.M.; Simpson, S.J.; Tan, J.; Raubenheimer, D.; Le Couteur, D.; Macia, L.; Holmes, A.; Eberhard, J.; O’Sullivan, J.; et al. Rapid benefits in older age from transition to whole food diet regardless of protein source or fat to carbohydrate ratio: Arandomised control trial. Aging Cell 2024, 23, e14276. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fazekas-Pongor, V.; Csiszar, A.; Kunutsor, S.K. The multifaceted benefits of walking for healthy aging: From Blue Zones to molecular mechanisms. Geroscience 2023, 45, 3211–3239. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Fekete, J.T.; Buda, A.; Szappanos, A.; Lehoczki, A.; Mozes, N.; Grosso, G.; Menyhart, O.; et al. Impact of adherence to the Mediterranean diet on stroke risk. Geroscience 2025, 47, 3565–3581. [Google Scholar] [CrossRef]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, A.; Lehoczki, A.; Mozes, N.; Grosso, G.; Godos, J.; et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. Geroscience 2025, 47, 3111–3130. [Google Scholar] [CrossRef]
- Talavera-Rodriguez, I.; Banegas, J.R.; de la Cruz, J.J.; Martinez-Gomez, D.; Ruiz-Canela, M.; Ortola, R.; Hershey, M.S.; Artalejo, F.R.; Sotos-Prieto, M. Mediterranean lifestyle index and 24-h systolic blood pressure and heart rate in community-dwelling older adults. Geroscience 2024, 46, 1357–1369. [Google Scholar] [CrossRef]
- Dobreva, I.; Marston, L.; Mukadam, N. Which components of the Mediterranean diet are associated with dementia? A UK Biobank cohort study. Geroscience 2022, 44, 2541–2554. [Google Scholar] [CrossRef]
- Gensous, N.; Garagnani, P.; Santoro, A.; Giuliani, C.; Ostan, R.; Fabbri, C.; Milazzo, M.; Gentilini, D.; di Blasio, A.M.; Pietruszka, B.; et al. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the NU-AGE project. Geroscience 2020, 42, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience 2020, 42, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Armenta, J.L.; Bergstrom, J.; Lee, J.; Furdui, C.M.; Nicklas, B.J.; Molina, A.J.A. Serum factors mediate changes in mitochondrial bioenergetics associated with diet and exercise interventions. Geroscience 2024, 46, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Takacs, B.; Szekeres, R.; Tarjanyi, V.; Nagy, D.; Priksz, D.; Bombicz, M.; Kiss, R.; Szabo, A.M.; Lehoczki, A.; et al. Effects of voluntary and forced physical exercise on the retinal health of aging Wistar rats. Geroscience 2024, 46, 4707–4728. [Google Scholar] [CrossRef]
- Roslund, K.J.; Ramsey, J.J.; Rutkowsky, J.M.; Zhou, Z.; Slupsky, C.M. Two-month ketogenic diet alters systemic and brain metabolism in middle-aged female mice. Geroscience 2024, 47, 935–952. [Google Scholar] [CrossRef]
- Frohlich, S.; Kutz, D.F.; Muller, K.; Voelcker-Rehage, C. Cardiorespiratory fitness is associated with cognitive performance in 80+-year-olds: Detangling processing levels. Geroscience 2024, 46, 3297–3310. [Google Scholar] [CrossRef]
- Chauquet, S.; Willis, E.F.; Grice, L.; Harley, S.B.R.; Powell, J.E.; Wray, N.R.; Nguyen, Q.; Ruitenberg, M.J.; Shah, S.; Vukovic, J. Exercise rejuvenates microglia and reverses T cell accumulation in the aged female mouse brain. Aging Cell 2024, 23, e14172. [Google Scholar] [CrossRef]
- Fox, F.A.U.; Liu, D.; Breteler, M.M.B.; Aziz, N.A. Physical activity is associated with slower epigenetic ageing-Findings from the Rhineland study. Aging Cell 2023, 22, e13828. [Google Scholar] [CrossRef]
- Kawamura, T.; Radak, Z.; Tabata, H.; Akiyama, H.; Nakamura, N.; Kawakami, R.; Ito, T.; Usui, C.; Jokai, M.; Torma, F.; et al. Associations between cardiorespiratory fitness and lifestyle-related factors with DNA methylation-based ageing clocks in older men: WASEDA’S Health Study. Aging Cell 2024, 23, e13960. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.; Bains, G.; Cole, S.; Gharibvand, L.; Berk, L.; Lohman, E. High-Intensity interval training reduces transcriptomic age: A randomized controlled trial. Aging Cell 2023, 22, e13841. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Battillo, D.J.; Beeri, M.S.; Mustapic, M.; Delgado-Peraza, F.; Kapogiannis, D. Two weeks of exercise alters neuronal extracellular vesicle insulin signaling proteins and pro-BDNF in older adults with prediabetes. Aging Cell 2025, 24, e14369. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.M.; Hall, K.S.; Sloane, R.; Magistro, D.; Rabaglietti, E.; Lee, C.C.; Castle, S.; Kopp, T.; Giffuni, J.; Katzel, L.; et al. Longitudinal analysis of physical function in older adults: The effects of physical inactivity and exercise training. Aging Cell 2024, 23, e13987. [Google Scholar] [CrossRef]
- Moore, A.Z.; Simonsick, E.M.; Landman, B.; Schrack, J.; Wanigatunga, A.A.; Ferrucci, L. Correlates of life course physical activity in participants of the Baltimore longitudinal study of aging. Aging Cell 2024, 23, e14078. [Google Scholar] [CrossRef]
- Voisin, S.; Seale, K.; Jacques, M.; Landen, S.; Harvey, N.R.; Haupt, L.M.; Griffiths, L.R.; Ashton, K.J.; Coffey, V.G.; Thompson, J.M.; et al. Exercise is associated with younger methylome and transcriptome profiles in human skeletal muscle. Aging Cell 2024, 23, e13859. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Far, B.F.; Safaei, M.; Pourmolaei, A.; Adibamini, S.; Shirdel, S.; Shirdel, S.; Emadi, R.; Kaushik, A.K. Exploring curcumin-loaded lipid-based nanomedicine as efficient targeted therapy for Alzheimer’s diseases. ACS Appl. Bio Mater. 2024, 7, 3535–3555. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Disease-modifying treatment of Parkinson’s disease by phytochemicals: Targeting multiple pathogenic factors. J. Neural Transm. 2022, 129, 737–753. [Google Scholar] [CrossRef] [PubMed]
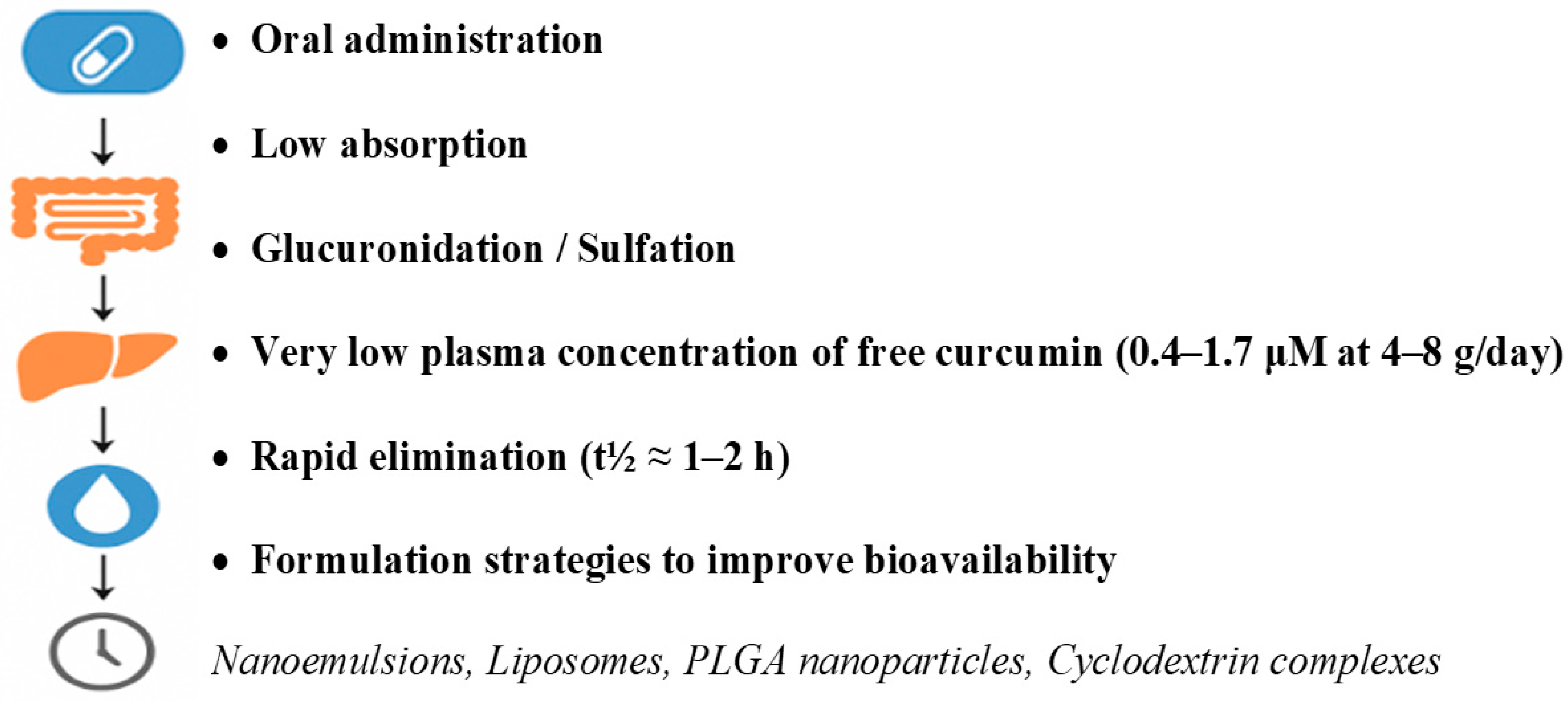

| Ref. | Model | Dose | Duration | Route | Key Effects | Mechanisms |
|---|---|---|---|---|---|---|
| Sun et al. [45] | APP/PS1 male mice | 50 or 200 mg/kg | 3 months | Oral gavage (daily) | Improved spatial memory; reduced hippocampal Aβ plaques | Modulated gut microbiota (↑ Lactobacillaceae, ↓ Bacteroidaceae); curcumin biotransformation to neuroprotective metabolites |
| Lin et al. [206] | APPswe/PSEN1dE9 mice (both sexes) | Curcumin 200 mg/kg; Berberine 100 mg/kg | 3 months | Oral gavage (daily) | Improved cognition; ↓ Aβ1–42, inflammation (IL-1β, TNF-α, IL-6), oxidative stress; ↑ AMPKα phosphorylation and autophagy | Synergistic anti-amyloid, anti-inflammatory, antioxidative effects; modulation of APP processing and AMPK–autophagy pathway |
| Su et al. [207] | Triple-transgenic Alzheimer’s disease mice | 150 mg/kg (TML-6) | 4 months | Oral | Improved learning; reduced brain Aβ and microglial activation | Enhanced bioavailability; reduced Aβ synthesis; ↑ ApoE; NF-κB/mTOR suppression; Nrf2 pathway activation |
| ELBini-Dhouib et al. [208] | AlCl3-induced sporadic AD rats | 100 mg/kg (CUR1 co-treatment; CUR2 post-treatment) | 90 days | Oral | Improved cognition; reduced anxiety; restored AChE activity; improved oxidative markers; ↓ apoptosis and inflammation | Antioxidant and anti-inflammatory actions; neuroprotection; restored cholinergic function |
| Reddy et al. [209] | SH-SY5Y cells + Aβ25–35 | 66.3 μM | 24 h (pretreatment) | In vitro | Improved cell viability; ↑ MFN1/2, OPA1; ↓ DRP1, FIS1; ↑ PGC1α, Nrf1/2, TFAM; ↑ synaptic markers | Enhanced mitochondrial biogenesis and dynamics; synaptic protection; reduced oxidative stress |
| Wang et al. [210] | Aβ1–40-induced AD in Sprague–Dawley rats | 300 mg/kg | 7 days | Intraperitoneal | Improved spatial memory; reduced hippocampal GFAP mRNA; less reactive astrocytosis | Suppression of astrogliosis and neuroinflammation via GFAP downregulation |
| Cheng et al. [211] | Tg2576 AD mice | 100 mg/kg | 3 months | Oral gavage (weekly) | Improved cue memory; trend to improved working memory; ↓ amyloid plaques; ↑ brain/plasma curcumin | Nanoparticle formulation enhanced BBB penetration, pharmacokinetics, and bioavailability |
| Ray et al. [212] | SK-N-SH cells and athymic mice | 25 mg/kg NanoCurc™ i.p. twice daily; 1 nM–5 μM in vitro | In vitro: 24 h; in vivo: ≤16 h post-dose | In vitro; i.p. | Neuroprotection from ROS; rescued injured cells; ↑ brain curcumin; ↓ ROS and caspase 3/7; ↑ GSH | Polymeric nanoparticles improved bioavailability; enhanced redox balance; antioxidant and anti-apoptotic effects |
| Begum et al. [213] | Tg2576 APPsw mice; LPS-injected WT mice; neuron/microglia cultures | Chronic: ~83 mg/kg diet (~500 ppm); Acute: 0.4 μmol | Chronic: ~4 months; Acute: 2 days pre-LPS | Oral (diet/gavage), i.p., i.m. | Both regimens reduced neuroinflammation (iNOS, IL-1β); Cur reduced plaques/insoluble Aβ, TC reduced soluble Aβ and JNK phosphorylation | Cur dienone bridge essential for plaque reduction; TC selectively inhibits JNK phosphorylation; both antioxidant/anti-inflammatory |
| Xiong et al. [214] | SH-SY5Y neuroblastoma cells (APP-transfected) | 0–20 μM (24 h), or 5 μM (12–48 h) | 24–48 h | In vitro | Dose- and time-dependent reduction in extracellular Aβ40/42 | Inhibition of Aβ generation via PS1 and GSK-3β suppression; ↑ GSK-3β Ser9 phosphorylation; downregulation of APP-processing enzymes |
| Ishrat et al. [215] | ICV-STZ-infused male Wistar rats | 80 mg/kg | 3 weeks | Oral gavage | Improved cognition; ↓ oxidative stress (↓ 4-HNE, MDA, TBARS, H2O2, PC, GSSG; ↑ GSH, GPx, GR); ↑ choline acetyltransferase | Antioxidant activity; restored glutathione system; reduced lipid peroxidation; enhanced cholinergic function |
| Nam et al. [216] | Adult and D-galactose-induced aged male C57BL/6 mice | 300 mg/kg Curcuma longa extract; 100 mg/kg D-galactose | 10 weeks D-gal; 3 weeks C. longa | Oral gavage; subcutaneous (D-gal) | Reduced escape latency; ↑ cell proliferation (Ki67), neuroblast differentiation (DCX); ↑ pCREB, ↑ BDNF in hippocampus | CREB pathway activation; enhanced neurogenesis; increased neurotrophic factors |
| Bassani et al. [217] | Male Wistar rats | 25, 50, or 100 mg/kg | 30 days | Oral gavage | Improved object recognition (50/100 mg/kg) in ICV-STZ dementia; mild anti-inflammatory effect | Memory improvement unrelated to hippocampal neurogenesis; mild anti-inflammatory action |
| Ma et al. [108] | hTau transgenic mice (15–16 months) and controls | 500 ppm curcumin (Longvida SLN) | 4–5 months | Diet (ad libitum) | Reduced behavioral, synaptic, and chaperone deficits; ↓ soluble Tau dimers; ↑ HSP90/HSC70 in synaptic fractions | Increased HSP proteins; modulation of HSP90 client kinases; enhanced Tau clearance |
| McClure et al. [218] | 5XFAD transgenic mice | 5 mg/kg | 18 weeks | Inhalation (aerosol) | Reduced Aβ plaques in hippocampus/subiculum; improved memory; no toxicity | ↓ Aβ and COX-2; prevention of neuritic dystrophy and autophagic vesicle formation |
| Alamro et al. [219] | Primary cortical neurons (Wistar rat pups) | 1 μM Aβ1–42 + 1 nM vitamin D3 + 5 μM curcumin | ≤72 h | In vitro | Reduced lipid peroxidation; ↑ GST, catalase, SOD; improved viability; ↑ NGF | Reduced oxidative stress; enhanced antioxidant defense; increased NGF |
| Lim et al. [220] | APPSw Tg+ mice | 160 or 5000 ppm curcumin | 6 months | Diet | ↓ oxidized proteins, IL-1β, Aβ plaques; ↓ GFAP (low dose); ↓ microglial activation | Anti-inflammatory (IL-1β); reduced oxidative stress and plaque formation; microglial suppression |
| Savall et al. [221] | STZ-induced AD male Wistar rats | 6 mg/kg | 14 days treatment (total 36 days) | Oral gavage; STZ i.c.v. | Nanoencapsulated curcumin improved memory; both forms ↓ AChE activity; NC reduced oxidative stress | Reduced neuroinflammation (GFAP); antioxidant effects; cognitive improvement |
| Reference | Model | Dose | Duration | Route | Key Effects | Proposed Mechanisms |
|---|---|---|---|---|---|---|
| Abbaoui et al. [222] | Wistar rats (copper-induced Parkinson’s disease) | Cu: 10 mg/kg i.p.; Curcumin: 30 mg/kg oral | 3 days | i.p., oral | Restored tyrosine hydroxylase (TH) expression and locomotor function | Antioxidant and anti-inflammatory activity; protection of TH+ neurons from copper-induced neurotoxicity |
| Rajeswari et al. [223] | Mice (MPTP model) | MPTP: 40 mg/kg; Curcumin: 80 mg/kg; ThC: 60 mg/kg | 7 days | i.p. | Restored dopamine (DA) and DOPAC levels; reduced MAO-B activity | Dopaminergic neuron preservation via MAO-B inhibition and dopamine metabolism protection |
| Pan et al. [224] | C57BL/6 mice (MPTP model) | 50 mg/kg | 5 days | i.p. | Reduced dopaminergic neuron loss; improved TH levels | Inhibition of JNK-mediated mitochondrial apoptosis |
| Jiang et al. [225] | SH-SY5Y cells (A53T α-syn overexpression) | 6 μM | 24–48 h | In vitro | Reduced α-synuclein accumulation; restored autophagic flux; improved neuronal viability | Downregulation of mTOR/p70S6K signaling; increased LC3-II; enhanced autophagosome formation |
| Ramires Júnior et al. [227] | Mice (rotenone model) | 25–50 mg/kg | 30 days | Oral | Improved motor function; preserved mitochondrial complex I activity | Oxidative stress reduction; mitochondrial protection; enhanced efficacy via nanoemulsion formulation |
| Yang et al. [228] | Rats (6-OHDA model) | 5–20 mg/kg | 37 days | Oral | Improved behavior, cognition, DA and NE levels | Activation of BDNF/TrkB/PI3K pathway; neurogenesis; monoaminergic system support |
| Fikry et al. [229] | Rats (rotenone model) | 30 mg/kg | 60 days | i.p. | Preserved cerebellar structure and motor function; reduced oxidative stress | Antioxidant defense restoration (↑GSH, ↑SOD, ↓MDA); reduced gliosis; improved acetylcholinesterase (AChE) activity |
| Wang et al. [226] | SH-SY5Y cells (α-syn oligomers) | 4 μM | 48–72 h | In vitro | Reduced ROS and apoptosis; stabilized α-syn aggregates | Caspase-3 inhibition; ROS suppression; attenuation of α-syn toxicity |
| Jagatha et al. [230] | N27 cells (in vitro) and GSH-deficient mice (BSO-induced) | 10 μM (in vitro); 50 mg/kg i.p. (in vivo) | 1–3 days | In vitro, i.p. | Restored GSH; protected mitochondrial complex I; reduced protein oxidation | γ-GCL activation; mitochondrial stabilization; redox balance normalization |
| Sharma et al. [231] | Rats (LPS-induced PD) | 40 mg/kg | 21 days | i.p. | Reduced astrocytosis, cytokines, iron deposition, and α-syn aggregation | NF-κB inhibition; antioxidant effects; apoptosis regulation; iron metabolism normalization |
| Liu et al. [233] | Wistar rats (6-OHDA model) | 40, 80, or 160 mg/kg/day | 14 days | Oral (gavage) | High dose improved motor performance; preserved TH+ neurons in substantia nigra | Dose- and time-dependent neuroprotection; dopaminergic preservation; reduced oxidative stress and inflammation |
| Geng et al. [234] | Male C57BL/6J mice (MPTP model) | MPTP: 30 mg/kg/day i.p.; Curcumin or Curoil: 120 mg/kg/day oral | 7 days | i.p., oral | Curoil improved motor function; protected TH+ neurons in substantia nigra | Enhanced curcumin bioavailability; antioxidant and neuroprotective effects; TH+ neuron preservation |
| Ref. | Model | Dose | Duration | Route | Key Effects | Mechanisms |
|---|---|---|---|---|---|---|
| Li et al. [235] | MCAO in mice; neuronal cultures | 100 mg/kg i.p. (in vivo); 4 μM (in vitro) | 3 days (in vivo); 24 h (in vitro) | i.p., i.v. | Improved neurological function; reduced brain edema and infarct volume | Activation of AKT/GSK-3β/Nrf2 pathway; M2 microglia polarization; reduced oxidative stress |
| Jia et al. [236] | Transient MCAO in rats + MTM | 300 mg/kg i.p. + 250 μg/kg MTM i.c.v. | 24 h | i.p., i.c.v. | Reduced infarct size and oxidative stress; improved neurological outcomes | SP1-mediated upregulation of Prdx6; antioxidant protection |
| Altinay et al. [237] | Global ischemia (BCCAO) | 300 mg/kg oral or i.p. | 21 days (oral); 72 h (i.p.) | Oral, i.p. | Increased antioxidant enzymes; reduced inflammation; improved outcome | Increased SOD, CAT, GPx; reduced IL-6, TNF-α, apoptosis |
| Marques et al. [238] | ICH in rats | 30 mg/kg | 3 doses/48 h | i.p. | Improved motor recovery; reduced hematoma volume | Enhanced antioxidant activity; improved bioavailability |
| Ran et al. [239] | MCAO in mice | 150 mg/kg | 7 days | i.p. | Reduced white matter damage; improved sensorimotor function | Inhibition of NF-κB/NLRP3; reduced pyroptosis |
| Xie et al. [240] | MCAO in mice; OGD/R in cells | 100–400 mg/kg (in vivo); 5–35 μM (in vitro) | Single dose | i.p., in vitro | Reduced apoptosis; improved mitochondrial function | Downregulation of Bax, upregulation of Bcl-2; preserved mitochondrial membrane potential |
| Li et al. [241] | MCAO/reperfusion in rats | 300 mg/kg | Single dose, pre-reperfusion | i.p. | Reduced infarct size, brain edema, and inflammation | Inhibition of NF-κB, MMP-9, and caspase-3; BBB protection |
| Liu et al. [242] | MCAO in rats | 300 mg/kg | 7 days | i.p. | Increased neurogenesis; reduced neurological deficits | Activation of Notch signaling |
| Wu et al. [243] | MCAO/reperfusion in rats | 300 mg/kg | Single dose, pre-MCAO | i.p. | Reduced BBB leakage; increased tight junction protein expression | Upregulation of ZO-1 and occludin; inhibition of NF-κB, MMP-9 |
| Liu et al. [244] | Distal MCAO in mice | 150 mg/kg | 10 days | i.p. | Reduced infarct size; improved sensorimotor recovery; M2 microglia shift | Anti-inflammatory activity; enhanced M2 polarization |
| Wu et al. [245] | Primary cortical neurons (OGD/R); MCAO in rats | 2.5–25 μM (in vitro); dose not specified (in vivo) | 1 h OGD + 24 h reoxygenation (in vitro); 60 min MCAO (in vivo) | In vitro; likely i.p. (in vivo) | Reduced neuronal injury and oxidative stress; improved cell viability; reduced infarct volume | Activation of Akt/Nrf2 pathway; NQO1 upregulation; effect blocked by PI3K inhibitor LY294002 |
| Jiang et al. [246] | MCAO in rats; astrocytes; brain capillary endothelial cells (BCECs) | 0.5–2.0 mg/kg | Single i.v. injection, 30 min post-reperfusion (effects at 48 h) | i.v. | Reduced infarct size, BBB permeability, brain edema, and mortality; protected BCECs | BBB preservation via ONOO− inhibition; suppression of iNOS/NO pathway |
| Zhang et al. [247] | MCAO in rats | 25 mg/kg | Single dose | i.p. | Reduced brain edema and infarct size; decreased TNF-α, IL-6, p53, Bax; increased Bcl-2 and Sirt1; improved mitochondrial function | Anti-inflammatory and anti-apoptotic activity; mitochondrial protection |
| Ref. | Population | Dose | Duration | Route | Key Effects | Mechanisms |
|---|---|---|---|---|---|---|
| Small et al. [255] | 40 non-demented adults (51–84 y) | 180 mg/day Theracurmin® (90 mg BID) | 18 months | Oral | Improved verbal and visual memory; enhanced attention; reduced amyloid and tau deposition (PET) | Anti-inflammatory; anti-amyloidogenic; Akt/Nrf2 activation |
| Cox et al. [256] | 60 healthy adults (60–85 y) | 400 mg/day Longvida® | 4 weeks | Oral | Acute: improved attention and working memory (1 h); Chronic: improved mood, reduced fatigue and LDL | Antioxidant; anti-inflammatory; modulation of lipid metabolism |
| Baum et al. [257] | 34 probable/possible AD patients | 1 g or 4 g/day | 6 months | Oral | No cognitive improvement (MMSE); safe; curcuminoids detected in plasma | Possible Aβ disaggregation; antioxidant; low bioavailability; improved capsule absorption |
| Ringman et al. [258] | 36 mild-to-moderate AD patients | 2 g or 4 g/day Curcumin C3 Complex® | 24 weeks double-blind + 24 weeks open-label | Oral | No cognitive benefit (ADAS-Cog); well tolerated | Anti-inflammatory; antioxidant; inhibition of amyloid-β aggregation |
| Rainey-Smith et al. [259] | 96 older adults (40–90 y) | 1500 mg/day Biocurcumax™ (BCM-95®) | 12 months | Oral | Prevention of cognitive decline (MoCA) at 6 months; no other changes | Antioxidant; anti-inflammatory |
| Ghodsi et al. [260] | 60 idiopathic PD patients (≥30 y) | 80 mg/day nanomicellar curcumin | 9 months | Oral | No motor or QoL improvement; mild GI adverse effects | Anti-inflammatory; anti-apoptotic |
| Maghbooli et al. [261] | 50 idiopathic PD patients (≥35 y) | 160 mg/day nanomicellar curcumin (80 mg BID) | 3 months | Oral | Improved sleep quality and QoL (PDQ-39); no effect on fatigue | Neuroprotective; antioxidant; anti-inflammatory |
| Donadio et al. [262] | 19 PD patients + 14 PD controls | 2 g/day curcumin–phospholipid (Meriva®) | 12 months | Oral | Reduced autonomic and non-motor symptoms; slowed progression; reduced phosphorylated α-synuclein deposition | BBB penetration; anti-amyloidogenic; antioxidant; anti-inflammatory |
| Boshagh et al. [263] | 56 ischemic stroke patients in rehabilitation | 500 mg curcumin + 5 mg piperine/day | 12 weeks | Oral | Reduced hs-CRP, cholesterol, TG, CIMT, BP, weight, waist circumference; increased TAC | Anti-inflammatory; antioxidant; vascular plaque reduction; BP lowering; lipid profile improvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehoczki, A.; Fekete, M.; Jarecsny, T.; Zábó, V.; Szappanos, Á.; Csípő, T.; Lipécz, Á.; Major, D.; Fazekas-Pongor, V.; Varga, P.; et al. The Neuroprotective Role of Curcumin: From Molecular Pathways to Clinical Translation—A Narrative Review. Nutrients 2025, 17, 2884. https://doi.org/10.3390/nu17172884
Lehoczki A, Fekete M, Jarecsny T, Zábó V, Szappanos Á, Csípő T, Lipécz Á, Major D, Fazekas-Pongor V, Varga P, et al. The Neuroprotective Role of Curcumin: From Molecular Pathways to Clinical Translation—A Narrative Review. Nutrients. 2025; 17(17):2884. https://doi.org/10.3390/nu17172884
Chicago/Turabian StyleLehoczki, Andrea, Mónika Fekete, Tamás Jarecsny, Virág Zábó, Ágnes Szappanos, Tamás Csípő, Ágnes Lipécz, Dávid Major, Vince Fazekas-Pongor, Péter Varga, and et al. 2025. "The Neuroprotective Role of Curcumin: From Molecular Pathways to Clinical Translation—A Narrative Review" Nutrients 17, no. 17: 2884. https://doi.org/10.3390/nu17172884
APA StyleLehoczki, A., Fekete, M., Jarecsny, T., Zábó, V., Szappanos, Á., Csípő, T., Lipécz, Á., Major, D., Fazekas-Pongor, V., Varga, P., & Varga, J. T. (2025). The Neuroprotective Role of Curcumin: From Molecular Pathways to Clinical Translation—A Narrative Review. Nutrients, 17(17), 2884. https://doi.org/10.3390/nu17172884





