Evaluation of NPH Insulin Dosing Interval for Critically Ill Hyperglycemic Trauma Patients During Continuous Enteral Nutrition: A Pilot Study
Abstract
1. Introduction
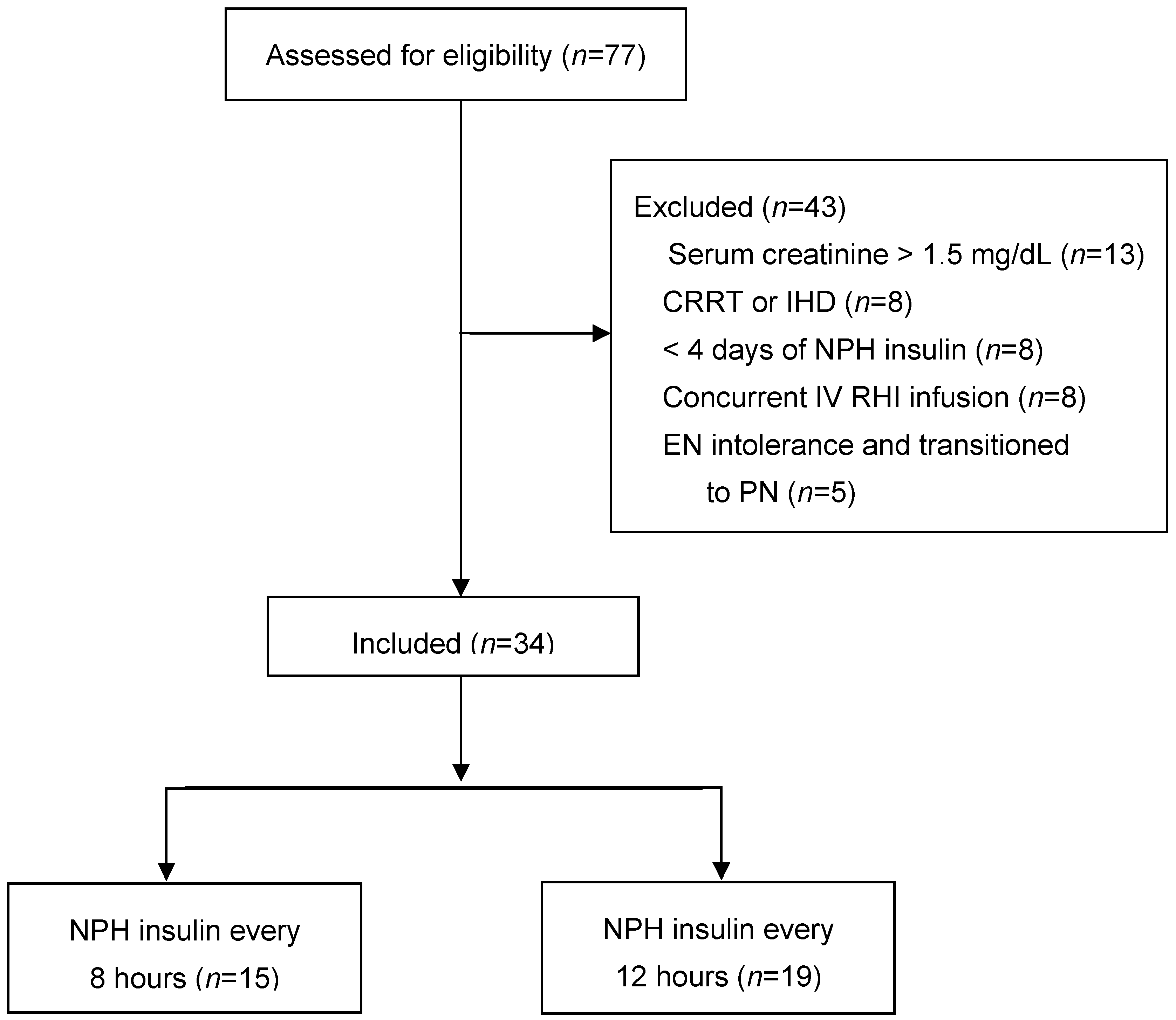
2. Materials and Methods
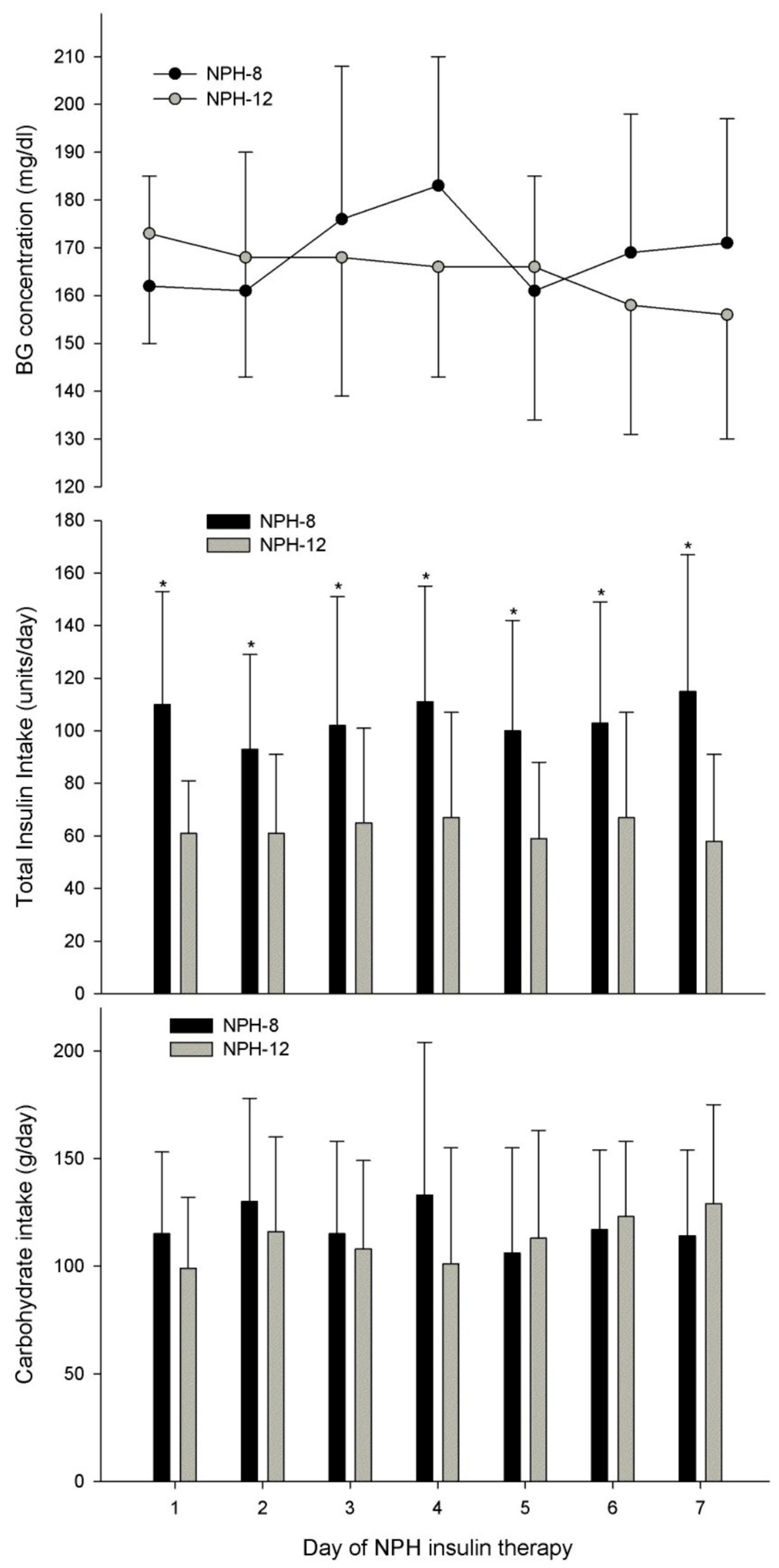
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacovides, C.L.; Skeete, D.A.; Werner, N.L.; Toschlog, E.A.; Agarwal, S.; Coopwood, B.; Crandall, M.; Tominaga, G.T. American Association for the Surgery of Trauma/American College of Surgeons—Committee on Trauma Clinical Consensus-Driven Protocol for glucose management in the post-resuscitation intensive care unit adult trauma patient. J. Trauma Acute Care Surg. 2023, 95, 951–958. [Google Scholar] [CrossRef]
- Dickerson, R.N.; McLeod, A.R.; Stonecipher, A.E.; Farrar, J.E.; Byerly, S.; Filiberto, D.M.; Fischer, P.E. Outpatient diabetes management influences glycemic control for critically ill patients during nutrition support: A retrospective observational study. Nutr. Clin. Pract. 2025, 40, 134–146. [Google Scholar] [CrossRef]
- Gale, S.C.; Sicoutris, C.; Reilly, P.M.; Schwab, C.W.; Gracias, V.H. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am. Surg. 2007, 73, 454–460. [Google Scholar] [CrossRef]
- Bochicchio, G.V.; Sung, J.; Joshi, M.; Bochicchio, K.; Johnson, S.B.; Meyer, W.; Scalea, T.M. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J. Trauma 2005, 58, 921–924. [Google Scholar] [CrossRef]
- Collier, B.; Diaz, J., Jr.; Forbes, R.; Morris, J., Jr.; May, A.; Guy, J.; Ozdas, A.; Dupont, W.; Miller, R.; Jensen, G. The impact of a normoglycemic management protocol on clinical outcomes in the trauma intensive care unit. JPEN J. Parenter. Enter. Nutr. 2005, 29, 353–358; discussion 359. [Google Scholar] [CrossRef]
- Laird, A.M.; Miller, P.R.; Kilgo, P.D.; Meredith, J.W.; Chang, M.C. Relationship of early hyperglycemia to mortality in trauma patients. J. Trauma 2004, 56, 1058–1062. [Google Scholar] [CrossRef]
- Scalea, T.M.; Bochicchio, G.V.; Bochicchio, K.M.; Johnson, S.B.; Joshi, M.; Pyle, A. Tight glycemic control in critically injured trauma patients. Ann. Surg. 2007, 246, 605–610; discussion 610–602. [Google Scholar] [CrossRef]
- Cogle, S.V.; Smith, S.E.; Maish, G.O., 3rd; Minard, G.; Croce, M.A.; Dickerson, R.N. Sliding scale regular human insulin for identifying critically ill trauma patients who require intensive insulin therapy and for glycemic control in those with mild to moderate hyperglycemia. J. Pharm. Nutr. Sci. 2017, 7, 106–115. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Swiggart, C.E.; Morgan, L.M.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Brown, R.O. Safety and efficacy of a graduated intravenous insulin infusion protocol in critically ill trauma patients receiving specialized nutritional support. Nutrition 2008, 24, 536–545. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Wilson, V.C.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Brown, R.O. Transitional NPH insulin therapy for critically ill patients receiving continuous enteral nutrition and intravenous regular human insulin. JPEN J. Parenter. Enter. Nutr. 2013, 37, 506–516. [Google Scholar] [CrossRef]
- Mulder, M.B.; Eidelson, S.A.; Sussman, M.S.; Schulman, C.I.; Lineen, E.B.; Iyenger, R.S.; Namias, N.; Proctor, K.G. Risk factors and clinical outcomes associated with augmented renal clearance in trauma patients. J. Surg. Res. 2019, 244, 477–483. [Google Scholar] [CrossRef]
- Krinsley, J.S.; Egi, M.; Kiss, A.; Devendra, A.N.; Schuetz, P.; Maurer, P.M.; Schultz, M.J.; van Hooijdonk, R.T.; Kiyoshi, M.; Mackenzie, I.M.; et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: An international multicenter cohort study. Crit. Care 2013, 17, R37. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Tamim, H.M.; Arabi, Y.M. Glycaemic fluctuation predicts mortality in critically ill patients. Anaesth. Intensive Care 2010, 38, 695–702. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Maish, G.O., 3rd; Minard, G.; Brown, R.O. Nutrition support team-led glycemic control program for critically ill patients. Nutr. Clin. Pract. 2014, 29, 534–541. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S321–S334. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S111–S125. [Google Scholar] [CrossRef]
- Tatucu-Babet, O.A.; King, S.J.; Zhang, A.Y.; Lambell, K.J.; Tierney, A.C.; Nyulasi, I.B.; McGloughlin, S.; Pilcher, D.; Bailey, M.; Paul, E.; et al. Measured energy expenditure according to the phases of critical illness: A descriptive cohort study. JPEN J. Parenter. Enter. Nutr. 2025, 49, 314–323. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Pitts, S.L.; Maish, G.O., 3rd; Schroeppel, T.J.; Magnotti, L.J.; Croce, M.A.; Minard, G.; Brown, R.O. A reappraisal of nitrogen requirements for patients with critical illness and trauma. J. Trauma Acute Care Surg. 2012, 73, 549–557. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Charriere, M.; Ridley, E.; Hastings, J.; Bianchet, O.; Scheinkestel, C.; Berger, M.M. Propofol sedation substantially increases the caloric and lipid intake in critically ill patients. Nutrition 2017, 42, 64–68. [Google Scholar] [CrossRef]
- Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, W.R.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Bochicchio, G.V.; Joshi, M.; Bochicchio, K.M.; Pyle, A.; Johnson, S.B.; Meyer, W.; Lumpkins, K.; Scalea, T.M. Early hyperglycemic control is important in critically injured trauma patients. J. Trauma 2007, 63, 1353–1358; discussion 1358–1359. [Google Scholar] [CrossRef]
- Egi, M.; Bellomo, R.; Stachowski, E.; French, C.J.; Hart, G.K.; Taori, G.; Hegarty, C.; Bailey, M. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit. Care Med. 2011, 39, 105–111. [Google Scholar] [CrossRef]
- Kotagal, M.; Symons, R.G.; Hirsch, I.B.; Umpierrez, G.E.; Dellinger, E.P.; Farrokhi, E.T.; Flum, D.R.; Collaborative, S.-C. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann. Surg. 2015, 261, 97–103. [Google Scholar] [CrossRef]
- Krinsley, J.S.; Rule, P.; Pappy, L.; Ahmed, A.; Huley-Rodrigues, C.; Prevedello, D.; Preiser, J.C. The interaction of acute and chronic glycemia on the relationship of hyperglycemia, hypoglycemia, and glucose variability to mortality in the critically ill. Crit. Care Med. 2020, 48, 1744–1751. [Google Scholar] [CrossRef]
- Luethi, N.; Cioccari, L.; Biesenbach, P.; Lucchetta, L.; Kagaya, H.; Morgan, R.; Di Muzio, F.; Presello, B.; Gaafar, D.; Hay, A.; et al. Liberal glucose control in ICU patients with diabetes: A before-and-after study. Crit. Care Med. 2018, 46, 935–942. [Google Scholar] [CrossRef]
- Magee, F.; Bailey, M.; Pilcher, D.V.; Martensson, J.; Bellomo, R. Early glycemia and mortality in critically ill septic patients: Interaction with insulin-treated diabetes. J. Crit. Care 2018, 45, 170–177. [Google Scholar] [CrossRef]
- Deane, A.M.; Chapman, M.J.; Reintam Blaser, A.; McClave, S.A.; Emmanuel, A. Pathophysiology and treatment of gastrointestinal motility disorders in the acutely ill. Nutr. Clin. Pract. 2019, 34, 23–36. [Google Scholar] [CrossRef]
- Kasti, A.N.; Theodorakopoulou, M.; Katsas, K.; Synodinou, K.D.; Nikolaki, M.D.; Zouridaki, A.E.; Fotiou, S.; Kapetani, A.; Armaganidis, A. Factors associated with interruptions of enteral nutrition and the impact on macro- and micronutrient deficits in ICU patients. Nutrients 2023, 15, 917. [Google Scholar] [CrossRef]
- Masse, J.; Giuliano, C.A.; Brown, S.; Paxton, R.A. Association between the use of long-acting insulin and hypoglycemia in nondiabetic patients in the surgical intensive care unit. J. Intensive Care Med. 2018, 33, 317–321. [Google Scholar] [CrossRef]
- Nader, N.D.; Hamishehkar, H.; Naghizadeh, A.; Shadvar, K.; Iranpour, A.; Sanaie, S.; Chang, F.; Mahmoodpoor, A. Effect of adding insulin glargine on glycemic control in critically ill patients admitted to intensive care units: A prospective randomized controlled study. Diabetes Metab. Syndr. Obes. 2020, 13, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Perez, M.I.; Florencio Ojeda, L.; Garcia-Luna, P.P.; Irles Rocamora, J.A.; Olveira, G.; Lacalle Remigio, J.R.; Arraiza Irigoyen, C.; Calanas Continente, A.; Campos Martin, C.; Fernandez Soto, M.L.; et al. Standards for the use of enteral nutrition in patients with diabetes or stress hyperglycaemia: Expert consensus. Nutrients 2023, 15, 4976. [Google Scholar] [CrossRef] [PubMed]
- Mesejo, A.; Montejo-Gonzalez, J.C.; Vaquerizo-Alonso, C.; Lobo-Tamer, G.; Zabarte-Martinez, M.; Herrero-Meseguer, J.I.; Acosta-Escribano, J.; Blesa-Malpica, A.; Martinez-Lozano, F. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: A prospective, open-label, blind-randomized, multicenter study. Crit. Care 2015, 19, 390. [Google Scholar] [CrossRef] [PubMed]

| BG, mg/dL (mmol/L) | Lower-Intensity SSI Intervention | Higher-Intensity SSI Intervention |
|---|---|---|
| <40 (2.2) | Give 25 g D50W IV; call primary service | Give 25 g D50W IV; call primary service |
| 40–70 (2.2–3.8) | Give 12.5 g D50W IV; call primary service | Give 12.5 g D50W IV; call primary service |
| 71–125 (3.9–6.9) | 0 units RHI | 0 units RHI |
| 126–150 (7.0–8.3) | 2 units RHI | 3 units RHI |
| 151–175 (8.4–9.7) | 4 units RHI | 6 units RHI |
| 176–200 (9.8–11.1) | 6 units RHI | 9 units RHI |
| 201–225 (11.2–12.5) | 8 units RHI | 12 units RHI |
| 226–250 (12.6–13.8) | 10 units RHI | 15 units RHI |
| 251–275 (13.9–15.2) | 12 units RHI | 18 units RHI |
| 276–300 (15.3–16.6) | 14 units RHI | 21 units RHI |
| >300 (16.6) | 16 units RHI; call primary service | 24 units RHI; call primary service |
| Variable * | NPH-8 | NPH-12 | p |
|---|---|---|---|
| n | 15 | 19 | - |
| Age, y | 54 ± 12 | 61 ± 14 | 0.127 |
| Sex, male/female | 11/4 | 13/6 | 1.000 |
| Race White Black Hispanic | 8 7 0 | 14 4 1 | 0.220 |
| Admission diagnosis MVC, n GSW, n Fall/Assault, n Other, n | 13 0 1 1 | 14 1 4 0 | 0.332 |
| Injury severity score | 22.7 ± 10.3 | 22.7 ± 9.3 | 0.996 |
| Ventilator-dependent, n (%) | 14 (93%) | 18 (95%) | 1.000 |
| TBI, n (%) | 11 (67%) | 7 (37%) | 0.077 |
| Vasopressor therapy, n (%) | 1 (7%) | 1 (5%) | 1.000 |
| Corticosteroid therapy, n (%) | 1 (7%) | 3 (16%) | 0.613 |
| Weight, kg | 119 ± 24 | 100 ± 33 | 0.065 |
| BMI, kg/ht2 | 38.1 ± 5.8 | 34.8 ± 11.0 | 0.048 |
| Prealbumin, mg/dL | 9.5 ± 4.2 | 7.7 ± 3.4 | 0.189 |
| C-reactive protein, mg/dL | 24.1 ± 6.6 | 24.5 ± 9.1 | 0.873 |
| WBC, cells/µm3 | 12.2 ± 3.9 | 12.9 ± 6.5 | 0.703 |
| Tmax, °C | 37.7 ± 0.5 | 37.7 ± 0.5 | 0.854 |
| Serum creatinine, mg/dL | 1.1 ± 0.3 | 0.9 ± 0.3 | 0.092 |
| Home insulin therapy, n (%) | 5 (33%) | 6 (32%) | 0.616 |
| Diabetes, n (%) | 12 (80%) | 17 (89%) | 0.634 |
| Hgb A1c, % | 8.6 ± 2.3 | 7.6 ± 1.4 | 0.159 |
| Hospital day of Hgb A1c, d | 3 ± 3 | 5 ± 5 | 0.284 |
| Survived, n (%) | 14 (97%) | 18 (95%) | 1.000 |
| Infection, n (%) | 7 (47%) | 10 (53%) | 1.000 |
| TICU LOS, d | 30 ± 29 | 22 ± 15 | 0.340 |
| Hospital LOS, d | 42 ± 31 | 33 ± 17 | 0.445 |
| Variable * | NPH-8 | NPH-12 | p |
|---|---|---|---|
| n | 15 | 19 | - |
| Max BG prior to RHI, mg/dL mmol/L | 293 ± 82 16.3 ± 4.6 | 248 ± 97 13.8 ± 5.4 | 0.046 |
| IV RHI infusion prior to NPH, n (%) | 11 (73%) | 6 (32%) | 0.038 |
| NPH intake, units/d | 62 ± 30 | 26 ± 15 | 0.026 |
| NPH duration, d | 13 ± 7 | 8 ± 5 | 0.006 |
| SSI intake, units/d | 42 ± 18 | 35 ± 15 | 0.206 |
| Total insulin intake, units/d | 115 ± 52 | 58 ± 33 | 0.004 |
| CHO intake, g/d | 115 ± 35 | 108 ± 37 | 0.584 |
| RHI received prior to NPH, units/d | 97 ± 35 | 44 ± 25 | 0.001 |
| BG 70–149 mg/dL, h/d 3.9–8.3 mmol/L | 7.5 ± 4.7 | 8.1 ± 5.0 | 0.678 |
| BG > 149 mg/dL, h/d >8.3 mmol/L | 16.5 ± 4.7 | 15.8 ± 5.0 | 0.703 |
| BG 70–179 mg/dL, h/d 3.9–9.9 mmol/L | 14.5 ± 5.0 | 16.0 ± 5.6 | 0.419 |
| BG > 179 mg/dL, h/d >9.9 mmol/L | 9.4 ± 5.0 | 8.0 ± 5.6 | 0.436 |
| Level 1 hypoglycemia, n (%) | 2 (13%) | 2 (11%) | 1.000 |
| Level 2 hypoglycemia, n (%) | 1 (5%) | 0 (0%) | 0.441 |
| BG variability (CV), % | 14.2 ± 3.4 | 13.9 ± 3.8 | 0.848 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, D.S.; Conaway, B.D.; Farrar, J.E.; Byerly, S.; Filiberto, D.M.; Dickerson, R.N. Evaluation of NPH Insulin Dosing Interval for Critically Ill Hyperglycemic Trauma Patients During Continuous Enteral Nutrition: A Pilot Study. Nutrients 2025, 17, 2880. https://doi.org/10.3390/nu17172880
Adams DS, Conaway BD, Farrar JE, Byerly S, Filiberto DM, Dickerson RN. Evaluation of NPH Insulin Dosing Interval for Critically Ill Hyperglycemic Trauma Patients During Continuous Enteral Nutrition: A Pilot Study. Nutrients. 2025; 17(17):2880. https://doi.org/10.3390/nu17172880
Chicago/Turabian StyleAdams, Delaney S., Brandon D. Conaway, Julie E. Farrar, Saskya Byerly, Dina M. Filiberto, and Roland N. Dickerson. 2025. "Evaluation of NPH Insulin Dosing Interval for Critically Ill Hyperglycemic Trauma Patients During Continuous Enteral Nutrition: A Pilot Study" Nutrients 17, no. 17: 2880. https://doi.org/10.3390/nu17172880
APA StyleAdams, D. S., Conaway, B. D., Farrar, J. E., Byerly, S., Filiberto, D. M., & Dickerson, R. N. (2025). Evaluation of NPH Insulin Dosing Interval for Critically Ill Hyperglycemic Trauma Patients During Continuous Enteral Nutrition: A Pilot Study. Nutrients, 17(17), 2880. https://doi.org/10.3390/nu17172880





