Associations Between Nutrient Intake and Vascular Inflammation Among Healthy Adults Living in Rural and Peri-Urban Particulate Matter 2.5-Affected Areas: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
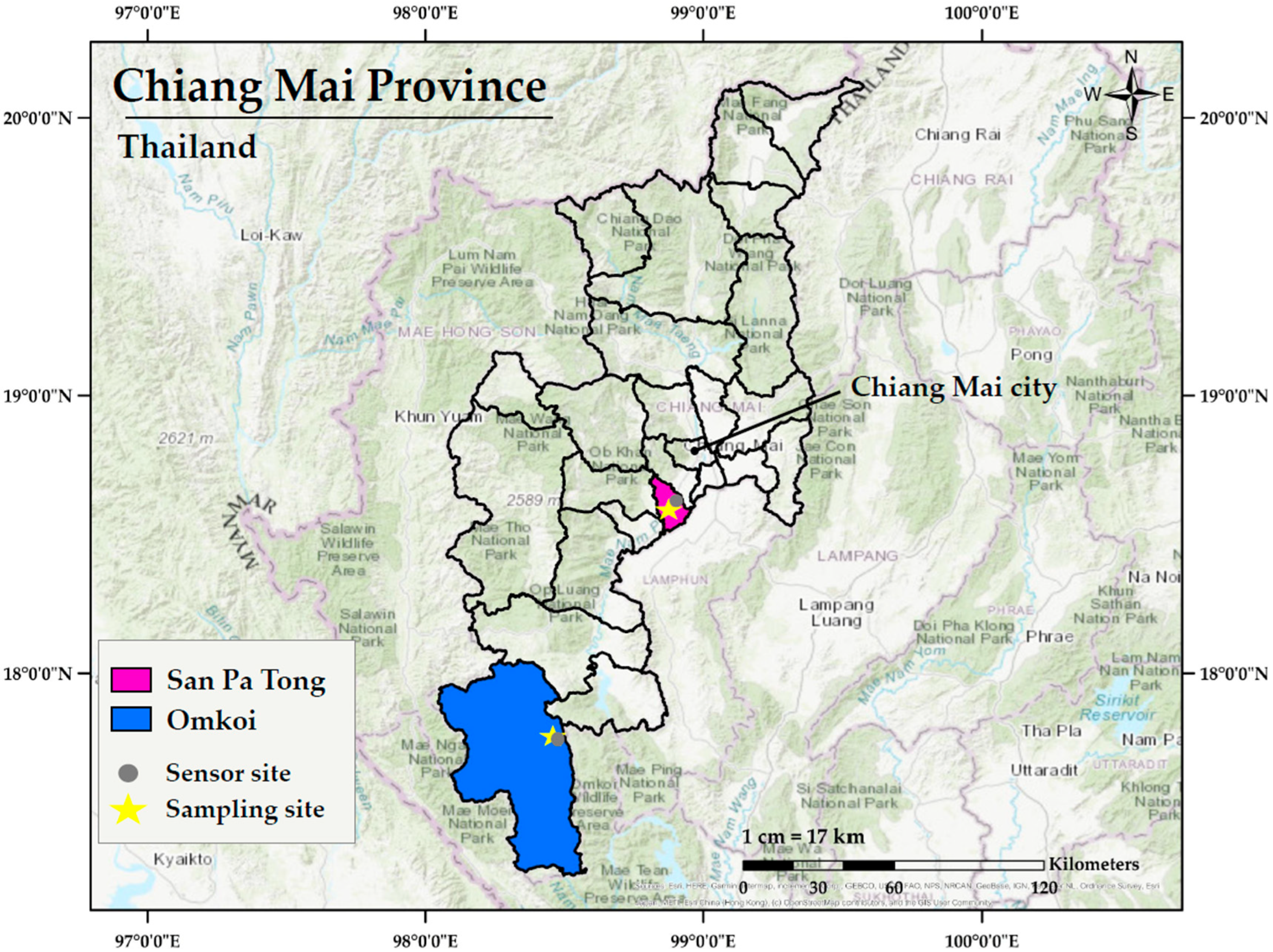
2.1. Study Locations
2.2. Ethical Considerations
2.3. Study Subjects
- Aged 35 years or older;
- Had resided continuously in either Omkoi or San Pa Tong District for at least three years without relocation during that period;
- In generally good health;
- Free from any diagnosed serious medical conditions, including but not limited to cardiovascular disease, chronic kidney disease, liver disease, gout, cancer, or hereditary disorders such as thalassemia;
- Free from any ongoing infections at the time of enrollment;
- Not pregnant or breastfeeding;
- Not currently dependent on illicit substances or chronically addicted to alcohol;
- Without psychiatric disorders that could interfere with their ability to participate in the study.
2.4. Study Design
2.5. Assessment of Serum Vascular Inflammatory Biomarkers
2.6. Dietary Intake Assessment
2.7. Statistical Analysis
3. Results
3.1. Sociodemographic and Behavioral Characteristics of Participants
3.2. Physical Examination Parameters of Participants
3.3. Clinical Biochemistry Profiles of Participants
3.4. Vascular Inflammatory Biomarkers of Participants
3.5. Energy and Nutrient Intakes of Participants
3.6. Associations Between Nutrient Intakes and Vascular Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, T.; Qi, W.; Yang, P.; Yang, L.; Shi, Y.; Zhou, L.; Ye, L. Mechanisms of cardiovascular toxicity induced by PM2.5: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 65033–65051. [Google Scholar] [CrossRef]
- Xie, W.; You, J.; Zhi, C.; Li, L. The toxicity of ambient fine particulate matter (PM2.5) to vascular endothelial cells. J. Appl. Toxicol. 2021, 41, 713–723. [Google Scholar] [CrossRef]
- Ding, R.; Huang, L.; Yan, K.; Sun, Z.; Duan, J. New insight into air pollution-related cardiovascular disease: An adverse outcome pathway framework of PM2.5-associated vascular calcification. Cardiovasc. Res. 2024, 120, 699–707. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Sergeev, A.K. The Role of Fine Particles (PM2.5) in the Genesis of Atherosclerosis and Myocardial Damage: Emphasis on Clinical and Epidemiological Data, and Pathophysiological Mechanisms. Cardiol. Res. 2022, 13, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, C.; Wolf, S.D.; Bode, J.G. Acute-phase protein synthesis: A key feature of innate immune functions of the liver. Biol. Chem. 2021, 402, 1129–1145. [Google Scholar] [CrossRef]
- Mehta, N.N.; deGoma, E.; Shapiro, M.D. IL-6 and Cardiovascular Risk: A Narrative Review. Curr. Atheroscler. Rep. 2024, 27, 12. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta. 2023, 548, 117487. [Google Scholar] [CrossRef]
- Wang, K.; Lei, L.; Li, G.; Lan, Y.; Wang, W.; Zhu, J.; Liu, Q.; Ren, L.; Wu, S. Association between Ambient Particulate Air Pollution and Soluble Biomarkers of Endothelial Function: A Meta-Analysis. Toxics 2024, 12, 76. [Google Scholar] [CrossRef]
- Iyer, H.S.; Hart, J.E.; Fiffer, M.R.; Elliott, E.G.; Yanosky, J.D.; Kaufman, J.D.; Puett, R.C.; Laden, F. Impacts of long-term ambient particulate matter and gaseous pollutants on circulating biomarkers of inflammation in male and female health professionals. Environ. Res. 2022, 214, 113810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, Y.; Kuang, X.; Liu, H.; Guo, Z.; Qian, J.; Wang, D.; Wang, M.; Chu, H.; Gong, W.; et al. Effect of PM2.5 exposure on circulating fibrinogen and IL-6 levels: A systematic review and meta-analysis. Chemosphere 2021, 271, 129565. [Google Scholar] [CrossRef]
- Kumar, V.; S, H.; Huligowda, L.K.D.; Umesh, M.; Chakraborty, P.; Thazeem, B.; Singh, A.P. Environmental Pollutants as Emerging Concerns for Cardiac Diseases: A Review on Their Impacts on Cardiac Health. Biomedicines 2025, 13, 241. [Google Scholar] [CrossRef]
- Xu, H.; Guo, B.; Qian, W.; Ciren, Z.; Guo, W.; Zeng, Q.; Mao, D.; Xiao, X.; Wu, J.; Wang, X.; et al. Dietary Pattern and Long-Term Effects of Particulate Matter on Blood Pressure: A Large Cross-Sectional Study in Chinese Adults. Hypertension 2021, 78, 184–194. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, T.; Song, Q.; Ma, H.; Hu, Y.; Heianza, Y.; Qi, L. Ambient air pollution, healthy diet and vegetable intakes, and mortality: A prospective UK Biobank study. Int. J. Epidemiol. 2022, 51, 1243–1253. [Google Scholar] [CrossRef]
- Tehrani, S.D.; Ahmadi, A.R.; Sadeghi, N.; Keshani, M. The effects of the mediterranean diet supplemented with olive oils on pro-inflammatory biomarkers and soluble adhesion molecules: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. 2025, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Dahiya, R.; Buttar, H.S.; Wilson, D.W.; De Meester, F.; Telessy, I.G. Antiatherogenic Effects of Vitamins, Mediterranean Diet and DASH Diet: An Overview for the Prevention of Cardiovascular Diseases. In Hydrophilic Vitamins in Health and Disease. Advances in Biochemistry in Health and Disease; Shah, A.K., Tappia, P.S., Dhalla, N.S., Eds.; Springer: Cham, Switzerland, 2024; Volume 29, pp. 45–66. [Google Scholar] [CrossRef]
- Caiati, C.; Stanca, A.; Lepera, M.E. Free Radicals and Obesity-Related Chronic Inflammation Contrasted by Antioxidants: A New Perspective in Coronary Artery Disease. Metabolites 2023, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Aasuri, N.; Shaikh, M.A. Impact of Modern Food Style on Cardiovascular Health in Young Adults. Arch. Med. Rep. 2024, 1, 14–20. [Google Scholar]
- Angelico, F.; Baratta, F.; Coronati, M.; Ferro, D.; Del Ben, M. Diet and metabolic syndrome: A narrative review. Intern. Emerg. Med. 2023, 18, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.; Byrne, N.M.; Hills, A.P. Cultural influences on dietary choices. Prog. Cardiovasc. Dis. 2025, 90, 22–26. [Google Scholar] [CrossRef]
- Sabir, S.; Hongsibsong, S.; Chuljerm, H.; Parklak, W.; Ounjaijean, S.; Fakfum, P.; Kausar, S.; Kulprachakarn, K. Assessment of urinary oxidative stress biomarkers associated with fine particulate matter (PM2.5) exposure in Chiang Mai, Thailand. PeerJ. 2025, 13, e19047. [Google Scholar] [CrossRef]
- Parklak, W.; Chuljerm, H.; Kawichai, S.; Fakfum, P.; Jiraya, P.; Kijkuokool, P.; Khiaolaongam, W.; Ngamsang, P.; Ounjaijean, S.; Rerkasem, K.; et al. The Impact of Nutrition and Fine Particulate Matter (PM2.5) on Inflammatory Responses in Individuals with Metabolic Syndrome: A Paired Case Study from Chiang Mai, Thailand. Toxics 2025, 13, 325. [Google Scholar] [CrossRef]
- Parasin, N.; Amnuaylojaroen, T. Effect of PM2.5 on burden of mortality from non-communicable diseases in northern Thailand. PeerJ 2024, 12, e18055. [Google Scholar] [CrossRef] [PubMed]
- Sooktawee, S.; Kanchanasuta, S.; Bunplod, N. Assessment of 24-h moving average PM2.5 concentrations in Bangkok, Thailand against WHO guidelines. Sustain. Environ. Res. 2023, 33, 3. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Jin, S.; Kang, P.M. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants 2024, 13, 923. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, S.; Lichtenstein, A.H.; Chen, S.; Na, M.; Veldheer, S.; Xing, A.; Wang, Y.; Wu, S.; et al. Alcohol consumption and risk of cardiovascular disease, cancer and mortality: A prospective cohort study. Nutr. J. 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jaramillo, P.; Joseph, P.; Lopez-Lopez, J.P.; Lanas, F.; Avezum, A.; Diaz, R.; Camacho, P.A.; Seron, P.; Oliveira, G.; Orlandini, A.; et al. Risk factors, cardiovascular disease, and mortality in South America: A PURE substudy. Eur. Heart J. 2022, 43, 2841–2851. [Google Scholar] [CrossRef]
- Nyawo, T.A.; Pheiffer, C.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Nyambuya, T.M.; Nkambule, B.B.; Sadie-Van Gijsen, H.; Strijdom, H.; Tiano, L.; Dludla, P.V. Physical Exercise Potentially Targets Epicardial Adipose Tissue to Reduce Cardiovascular Disease Risk in Patients with Metabolic Diseases: Oxidative Stress and Inflammation Emerge as Major Therapeutic Targets. Antioxidants 2021, 10, 1758. [Google Scholar] [CrossRef]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M.; et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002495. [Google Scholar] [CrossRef]
- Soheilipour, F.; Hatami, M.; Salehiniya, H.; Alaei, M. Indicators of Obesity and Cardio-metabolic Risks: Important Consideration in Adults and Children. Curr. Diabetes Rev. 2022, 18, e160721194839. [Google Scholar] [CrossRef]
- Tham, K.W.; Abdul Ghani, R.; Cua, S.C.; Deerochanawong, C.; Fojas, M.; Hocking, S.; Lee, J.; Nam, T.Q.; Pathan, F.; Saboo, B.; et al. Obesity in South and Southeast Asia-A new consensus on care and management. Obes. Rev. 2023, 24, e13520. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Liu, Y.; Lyu, Y.; Bian, W.; Song, Z.; Liu, Y.; Ma, G.; Liu, Y.; Chen, T.; et al. Time in target range of fasting blood glucose ranges defined by WHO and ADA guidelines and cardiorenal Risk: Insights from two cohorts. Diabetes Res. Clin. Pract. 2025, 226, 112323. [Google Scholar] [CrossRef]
- Yamaji, T.; Harada, T.; Hashimoto, Y.; Takaeko, Y.; Kajikawa, M.; Kihara, Y.; Hida, E.; Chayama, K.; Goto, C.; Han, Y.; et al. Pre-impaired fasting glucose state is a risk factor for endothelial dysfunction: Flow-mediated Dilation Japan (FMD-J) study. BMJ Open Diabetes Res. Care. 2020, 8, e001610. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Chakravarthi, S.; Jegasothy, R.; Seng, W.Y.; Fuloria, N.K.; Fuloria, S.; Hazarika, I.; Das, A. Alcohol-associated liver disease: A review on its pathophysiology, diagnosis and drug therapy. Toxicol. Rep. 2021, 8, 376–385. [Google Scholar] [CrossRef]
- Dmitrieva, N.I.; Liu, D.; Wu, C.O.; Boehm, M. Middle age serum sodium levels in the upper part of normal range and risk of heart failure. Eur. Heart J. 2022, 43, 3335–3348. [Google Scholar] [CrossRef]
- Duan, S.; Ma, Y.; Lu, F.; Zhang, C.; Guo, H.; Zeng, M.; Sun, B.; Yuan, Y.; Xing, C.; Mao, H.; et al. High sodium intake and fluid overhydration predict cardiac structural and functional impairments in chronic kidney disease. Front. Nutr. 2024, 11, 1388591. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gowri, B.S.; Lakshmi, A.J.; Prakash, J. Retention of nutrients in green leafy vegetables on dehydration. J. Food Sci. Technol. 2013, 50, 918–925. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and Microgreens-Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Penkert, L.P.; Li, R.; Huang, J.; Gurcan, A.; Chung, M.C.; Wallace, T.C. Pork Consumption and Its Relationship to Human Nutrition and Health: A Scoping Review. Meat Muscle Biol. 2021, 5, 43. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [PubMed]
- Cherian, G. Chapter 16—Eggs and Health: Nutrient Sources and Supplement Carriers. In Complementary and Alternative Therapies and the Aging Population; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 333–346. [Google Scholar] [CrossRef]
- Wongwaiwech, D.; Kamchonemenukool, S.; Ho, C.T.; Li, S.; Thongsook, T.; Majai, N.; Premjet, D.; Sujipuli, K.; Weerawatanakorn, M. Nutraceutical Difference between Two Popular Thai Namwa Cultivars Used for Sun Dried Banana Products. Molecules 2022, 27, 5675. [Google Scholar] [CrossRef]
- Lin, H.L.; Tseng, Y.H.; Yeh, C.C.; Chen, Y.M.; Shen, K.P. Effect Evaluation of Banana on Improving Hyperglycemia and Hypertension in Diabetic Spontaneously Hypertensive Rats. Nat. Prod. Commun. 2023, 18, 1934578X231213225. [Google Scholar] [CrossRef]
- Carlos Kusano Bucalen, F. Antioxidant and anti-atherosclerotic potential of Banana (Musa spp.): A review of biological mechanisms for prevention and protection against atherosclerosis. Avicenna J. Phytomed. 2023, 13, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef]
- Ebadi, S.; Azlan, A. The Effect of Unrefined Sugar on Inflammation: A Systematic Review of Intervention Studies. Int. J. Prev. Med. 2023, 14, 121. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Liang, D.; Liu, C.; Zhang, X. Association between dietary selenium intake and the risk of cardiovascular disease in US adults: A population-based study. Sci. Rep. 2025, 15, 13427. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Yan, S.M.; Guo, Y.M.; Zhang, B.Q.; Guo, X.Y.; Shi, B.L. Vitamin A pretreatment protects NO-induced bovine mammary epithelial cells from oxidative stress by modulating Nrf2 and NF-κB signaling pathways. J. Anim. Sci. 2018, 96, 1305–1316. [Google Scholar] [CrossRef]
- Gatica, L.V.; Oliveros, L.B.; Pérez Díaz, M.F.; Domínguez, N.S.; Fornes, M.W.; Gimenez, M.S. Implication of vitamin A deficiency on vascular injury related to inflammation and oxidative stress. Effects on the ultrastructure of rat aorta. Eur. J. Nutr. 2012, 51, 97–106. [Google Scholar] [CrossRef]
- Martin, K.R.; Wu, D.; Meydani, M. The effect of carotenoids on the expression of cell surface adhesion molecules and binding of monocytes to human aortic endothelial cells. Atherosclerosis 2000, 150, 265–274. [Google Scholar] [CrossRef]
- Satheannoppakao, W.; Kasemsup, R.; Inthawong, R.; Chariyalertsak, S.; Sangthong, R.; Taneepanichskul, S.; Putwatana, P.; Kessomboon, P.; Aekplakorn, W. Sodium intake and socio-demographic determinants of the non-compliance with daily sodium intake recommendations: Thai NHES IV. J. Med. Assoc. 2013, 96, S161–S170. [Google Scholar]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Naska, A.; Orsini, N.; Vinceti, M. Blood Pressure Effects of Sodium Reduction: Dose-Response Meta-Analysis of Experimental Studies. Circulation 2021, 143, 1542–1567. [Google Scholar] [CrossRef] [PubMed]
- Chailimpamontree, W.; Kantachuvesiri, S.; Aekplakorn, W.; Lappichetpaiboon, R.; Sripaiboonkij Thokanit, N.; Vathesatogkit, P.; Kunjang, A.; Boonyagarn, N.; Sukhonthachit, P.; Chuaykarn, N.; et al. Estimated dietary sodium intake in Thailand: A nationwide population survey with 24-hour urine collections. J. Clin. Hypertens. 2021, 23, 744–754. [Google Scholar] [CrossRef] [PubMed]

| Districts | Annual Average Particulate Matter 2.5 Concentration (2021–2023) (µg/m3) ns |
|---|---|
| Omkoi District | 15.58 ± 5.60 |
| San Pa Tong District | 19.27 ± 7.32 |
| Characteristics | Rural (n = 27) | Peri-Urban (n = 26) | Total (n = 53) | p |
|---|---|---|---|---|
| Sex | <0.001 ** | |||
| Male | 13 (48.15%) | 1 (3.85%) | 14 (26.42%) | |
| Female | 14 (51.85%) | 25 (96.15%) | 39 (73.58%) | |
| Age (years) | 50.04 ± 7.58 | 56.04 ± 7.12 | 52.92 ± 7.89 | 0.005 ** |
| Alcohol consumption | 0.009 ** | |||
| Never drank | 7 (25.93%) | 16 (61.54%) | 23 (43.40%) | |
| Drank | 20 (74.07%) | 10 (38.46%) | 30 (56.60%) | |
| Smoking status | 0.002 ** | |||
| Never smoked | 16 (59.26%) | 23 (88.46%) | 39 (73.58%) | |
| Current smoker | 9 (33.33%) | 0 | 9 (17.98%) | |
| Former smoker | 2 (7.41%) | 3 (11.54%) | 5 (9.43%) | |
| Total physical activity (MET-min/week) 1 | 9492.00 (2772.00–17,598.00) | 2451.00 (1339.50–8671.50) | 6612.00 (1812.00–11,838.00) | 0.020 * |
| Vigorous activity (MET-min/week) 1 | 3360.00 (0.00–13,440.00) | 720.00 (0.00–4800.00) | 1200.00 (0.00–6720.00) | 0.082 |
| Moderate activity (MET-min/week) 1 | 480.00 (0.00–720.00) | 1320.00 (360.00–1680.00) | 720.00 (0.00–1680.00) | 0.001 ** |
| Walking (MET-min/week) 1 | 1584.00 (594.00–4158.00) | 297 (0.00–1386.00) | 1386.00 (132.00–2772.00) | 0.019 * |
| Parameters | Rural (n = 27) | Peri-Urban (n = 26) | p |
|---|---|---|---|
| Body height (cm) | 158.27 ± 9.80 | 153.68 ± 6.66 | 0.082 |
| Body weight (kg) | 58.32 ± 12.04 | 61.00 ± 10.93 | 0.464 |
| Body mass index (kg/m2) | 23.33 ± 4.92 | 25.76 ± 3.76 | 0.004 ** |
| Waist circumference (cm) | 79.94 ± 12.22 | 84.17 ± 9.54 | 0.058 |
| Male | 80.77 ± 7.42 | 93.50 | 0.171 |
| Female | 79.04 ± 15.7 | 83.76 ± 9.55 | 0.056 |
| Waist-to-hip ratio | 0.85 ± 0.07 | 0.85 ± 0.06 | 0.756 |
| Male | 0.87 ± 0.06 | 0.93 | 0.385 |
| Female | 0.84 ± 0.07 | 0.84 ± 0.05 | 0.570 |
| Systolic blood pressure (mmHg) | 120.81 ± 8.47 | 121.23 ± 10.82 | 0.877 |
| Diastolic blood pressure (mmHg) | 75.37 ± 8.99 | 75.23 ± 8.74 | 0.955 |
| Heart rate (bpm) | 74.93 ± 14.33 | 76.08 ± 8.36 | 0.790 |
| Parameters | Rural (n = 27) | Peri-Urban (n = 26) | p |
|---|---|---|---|
| Fasting blood glucose (mg/dL) | 97.19 ± 18.59 | 100.85 ± 14.03 | 0.050 * |
| Lipid profiles | |||
| Total cholesterol (mg/dL) | 202.81 ± 41.93 | 194.38 ± 43.51 | 0.476 |
| Triglyceride (mg/dL) | 109.41 ± 58.59 | 103.88 ± 39.94 | 0.972 |
| High-density lipoprotein cholesterol (mg/dL) | 44.45 ± 8.44 | 42.80 ± 11.79 | 0.559 |
| Low-density lipoprotein cholesterol (mg/dL) | 150.59 ± 48.64 | 142.23 ± 53.50 | 0.554 |
| Liver function tests | |||
| Alanine aminotransferase (U/L) | 13.44 ± 5.86 | 9.19 ± 4.20 | 0.004 ** |
| Aspartate aminotransferase (U/L) | 22.04 ± 6.62 | 18.65 ± 3.75 | 0.027 * |
| Alkaline phosphatase (U/L) | 101.56 ± 24.53 | 91.38 ± 26.22 | 0.150 |
| Kidney function tests | |||
| Creatinine (mg/dL) | 0.80 ± 0.41 | 0.67 ± 0.27 | 0.226 |
| Blood urea nitrogen (mg/dL) | 12.25 ± 5.08 | 12.24 ± 2.99 | 0.996 |
| Serum electrolytes | |||
| Calcium (mg/dL) | 9.07 ± 0.34 | 8.90 ± 0.31 | 0.067 |
| Sodium (mmol/L) | 143.09 ± 1.79 | 135.32 ± 26.85 | 0.007 ** |
| Potassium (mmol/L) | 4.02 ± 0.44 | 4.04 ± 0.29 | 0.857 |
| Chloride (mmol/L) | 106.77 ± 1.90 | 106.21 ± 2.15 | 0.317 |
| Carbon dioxide (mmol/L) | 24.56 ± 2.99 | 25.45 ± 1.66 | 0.188 |
| Macronutrients | Rural (n = 27) | Peri-Urban (n = 26) | p ns |
|---|---|---|---|
| Carbohydrate (%) | 54.65 (44.92–67.52) | 59.39 (46.99–65.81) | 0.957 |
| Protein (%) | 14.55 (12.43–21.97) | 14.12 (11.75–20.91) | 0.355 |
| Fat (%) | 28.05 (13.64–34.71) | 27.80 (19.88–34.06) | 0.644 |
| Variables | Rural (n = 27) | Peri-Urban (n = 26) | p |
|---|---|---|---|
| Energy (kcal) | 1734.12 (1394.09–2058.34) | 1808.27 (1368.30–2107.04) | 0.735 |
| Carbohydrates (g) | 234.13 (190.15–272.34) | 231.77 (204.40–280.56) | 0.510 |
| Sugars (g) | 27.09 (14.50–53.05) | 49.57 (26.20–68.60) | 0.030 * |
| Proteins (g) | 64.34 (52.86–93.75) | 57.37 (46.93–85.56) | 0.594 |
| Animal protein (g) | 38.28 (21.96–59.42) | 33.09 (16.34–53.69) | 0.702 |
| Vegetable protein (g) | 18.85 (15.90–23.70) | 18.02 (15.55–20.56) | 0.270 |
| Fats (g) | 48.67 (31.85–82.09) | 50.34 (32.06–80.59) | 0.557 |
| Total saturated fatty acids (g) | 6.44 (2.21–12.35) | 19.04 (8.69–25.45) | 0.002 ** |
| Cholesterol (mg) | 470.49 (107.12–759.93) | 174.57 (90.86–302.19) | 0.013 * |
| Calcium (mg) | 496.56 (278.99–728.75) | 314.33 (252.88–453.21) | 0.048 * |
| Phosphorus (mg) | 819.02 (678.84–1289.22) | 685.92 (516.69–819.95) | 0.031 * |
| Iron (mg) | 13.05 (10.49–21.40) | 10.27 (7.97–16.27) | 0.046 * |
| Potassium (mg) | 1897.38 (1520.51–2619.09) | 1560.36 (1259.69–2167.93) | 0.039 * |
| Sodium (mg) | 3174.42 (2578.65–4108.96) | 2944.25 (2085.47–3999.52) | 0.302 |
| Magnesium (mg) | 47.05 (26.28–100.62) | 34.30 (19.61–58.14) | 0.130 |
| Copper (mg) | 0.84 (0.65–1.30) | 0.75 (0.61–1.09) | 0.294 |
| Selenium (μg) | 55.54 (6.78–131.34) | 34.41 (24.96–53.31) | 0.045 * |
| Zinc (mg) | 6.24 (4.32–7.10) | 4.70 (3.70–5.69) | 0.055 |
| Vitamin A (μg RAE) | 473.09 (288.40–811.75) | 212.29 (126.34–513.62) | 0.024 * |
| Retinol (μg) | 185.25 (6.71–297.65) | 87.84 (23.15–184.42) | 0.824 |
| β-carotene (μg) | 1575.86 (362.14–6680.85) | 1076.78 (392.89–2312.90) | 0.160 |
| Vitamin B1 (mg) | 0.87 (0.51–1.46) | 1.33 (0.77–2.54) | 0.091 |
| Vitamin B2 (mg) | 1.29 (0.85–1.63) | 1.08 (0.64–1.50) | 0.328 |
| Vitamin B6 (mg) | 0.56 (0.06–1.04) | 0.48 (0.23–0.86) | 0.790 |
| Vitamin B12 (mg) | 1.82 (0.00–3.17) | 0.46 (0.00–2.20) | 0.688 |
| Niacin (mg) | 14.99 (12.48–22.36) | 15.17 (11.78–19.01) | 0.581 |
| Vitamin C (mg) | 70.23 (40.99–118.86) | 40.44 (16.81–68.81) | 0.017 * |
| Vitamin E (mg) | 1.77 (0.21–4.89) | 0.42 (0.25–0.72) | 0.027 * |
| Dietary fiber (g) | 13.06 (8.47–17.96) | 7.25 (5.53–16.51) | 0.052 |
| Nutrients | ICAM-1 | VCAM-1 | IL-6 | |||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Sugars | 0.467 | <0.001 3 | 0.481 | <0.001 2 | 0.557 | <0.001 1 |
| Total saturated fatty acids | 0.444 | 0.002 5 | 0.212 | 0.148 | 0.127 | 0.726 |
| Cholesterol | −0.119 | 0.363 | −0.096 | 0.514 | −0.055 | 0.857 |
| Calcium | −0.171 | 0.180 | −0.043 | 0.755 | −0.319 | 0.287 |
| Phosphorus | −0.129 | 0.439 | −0.101 | 0.537 | 0.096 | 0.621 |
| Iron | −0.221 | 0.131 | −0.078 | 0.579 | 0.132 | 0.594 |
| Potassium | −0.352 | 0.014 | −0.281 | 0.053 | 0.419 | 0.178 |
| Selenium | −0.097 | 0.150 | −0.406 | 0.002 6 | −0.218 | 0.319 |
| Vitamin A | −0.262 | 0.072 | −0.447 | 0.001 4 | −0.223 | 0.417 |
| Vitamin C | −0.293 | 0.014 | −0.187 | 0.204 | −0.271 | 0.319 |
| Vitamin E | −0.136 | 0.256 | −0.231 | 0.097 | −0.339 | 0.266 |
| Dietary fiber | −0.246 | 0.045 | −0.332 | 0.010 | −0.453 | 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parklak, W.; Chuljerm, H.; Kawichai, S.; Fakfum, P.; Jiraya, P.; Kijkuokool, P.; Khiaolaongam, W.; Somnuk, S.; Kulprachakarn, K. Associations Between Nutrient Intake and Vascular Inflammation Among Healthy Adults Living in Rural and Peri-Urban Particulate Matter 2.5-Affected Areas: An Exploratory Study. Nutrients 2025, 17, 2867. https://doi.org/10.3390/nu17172867
Parklak W, Chuljerm H, Kawichai S, Fakfum P, Jiraya P, Kijkuokool P, Khiaolaongam W, Somnuk S, Kulprachakarn K. Associations Between Nutrient Intake and Vascular Inflammation Among Healthy Adults Living in Rural and Peri-Urban Particulate Matter 2.5-Affected Areas: An Exploratory Study. Nutrients. 2025; 17(17):2867. https://doi.org/10.3390/nu17172867
Chicago/Turabian StyleParklak, Wason, Hataichanok Chuljerm, Sawaeng Kawichai, Puriwat Fakfum, Putita Jiraya, Praporn Kijkuokool, Wiritphon Khiaolaongam, Surasawadee Somnuk, and Kanokwan Kulprachakarn. 2025. "Associations Between Nutrient Intake and Vascular Inflammation Among Healthy Adults Living in Rural and Peri-Urban Particulate Matter 2.5-Affected Areas: An Exploratory Study" Nutrients 17, no. 17: 2867. https://doi.org/10.3390/nu17172867
APA StyleParklak, W., Chuljerm, H., Kawichai, S., Fakfum, P., Jiraya, P., Kijkuokool, P., Khiaolaongam, W., Somnuk, S., & Kulprachakarn, K. (2025). Associations Between Nutrient Intake and Vascular Inflammation Among Healthy Adults Living in Rural and Peri-Urban Particulate Matter 2.5-Affected Areas: An Exploratory Study. Nutrients, 17(17), 2867. https://doi.org/10.3390/nu17172867








