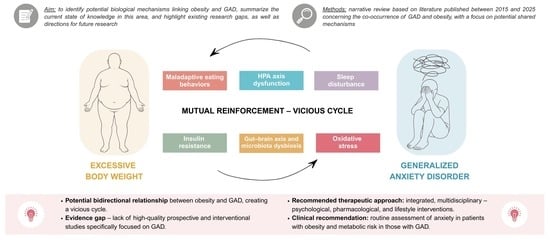
Generalized Anxiety Disorder and Obesity: Overlapping Neuroendocrine, Metabolic, and Behavioral Pathways
Abstract
1. Introduction
2. Materials and Methods
- -
- Articles published between 2015 and 2025;
- -
- Original papers (observational and randomized studies), meta-analyses, systematic and narrative reviews, as well as selected experimental studies in animal models, if they provided relevant information on potential pathophysiological mechanisms in cases of limited clinical data availability;
- -
- Articles with access to the full text;
- -
- Articles published in English;
- -
- Articles on the adult population (≥18 years).
- -
- Articles published before 2015;
- -
- Article types, such as case studies, commentaries, letters to the editor, non-peer-reviewed articles, reviews of the reviews, and books;
- -
- Articles for which the full text is not accessible;
- -
- Articles published in languages other than English;
- -
- Articles dealing exclusively with populations of children, adolescents, pregnant women, or breastfeeding women.
3. Mechanisms Linking Anxiety Disorders and Obesity
3.1. HPA Axis Dysfunction
3.2. Oxidative Stress
3.3. Insulin Resistance
3.4. Gut–Brain Axis and Microbiota Dysbiosis
3.5. Sleep Disturbance
3.6. Maladaptive Eating Behaviors
4. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Mental Disorders Collaborators. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Kirkbride, J.B.; Anglin, D.M.; Colman, I.; Dykxhoorn, J.; Jones, P.B.; Patalay, P.; Pitman, A.; Soneson, E.; Steare, T.; Wright, T.; et al. The social determinants of mental health and disorder: Evidence, prevention and recommendations. World Psychiatry 2024, 23, 58–90. [Google Scholar] [CrossRef]
- Udupa, N.S.; Twenge, J.M.; McAllister, C.; Joiner, T.E. Increases in poor mental health, mental distress, and depression symptoms among U.S. adults, 1993-2020. J. Mood Anxiety Disord. 2023, 7, 100013. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 11th ed.; World Health Organization: Geneva, Switzerland, 2025; Available online: https://icd.who.int/ (accessed on 11 March 2025).
- Szuhany, K.L.; Simon, N.M. Anxiety Disorders: A Review. JAMA 2022, 328, 2431–2445. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety disorders. Lancet 2021, 397, 880. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar] [CrossRef]
- Ruscio, A.M.; Hallion, L.S.; Lim, C.C.W.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Andrade, L.H.; Borges, G.; Bromet, E.J.; Bunting, B.; et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry 2017, 74, 465–475. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Generalised Anxiety Disorder and Panic Disorder in Adults: Management; National Institute for Health and Care Excellence: London, UK, 2019. [Google Scholar]
- Andrews, G.; Bell, C.; Boyce, P.; Gale, C.; Lampe, L.; Marwat, O.; Rapee, R.; Wilkins, G. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust. N. Z. J. Psychiatry 2018, 52, 1109–1172. [Google Scholar] [CrossRef]
- Curtiss, J.E.; Levine, D.S.; Ander, I.; Baker, A.W. Cognitive-Behavioral Treatments for Anxiety and Stress-Related Disorders. Focus. Am. Psychiatr. Publ. 2021, 19, 184–189. [Google Scholar] [CrossRef]
- World Health Organization, Obesity and Overweight. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 March 2025).
- Lingvay, I.; Cohen, R.V.; le Roux, C.W.; Sumithran, P. Obesity in Adults. Lancet 2024, 404, 972–987. [Google Scholar] [CrossRef]
- Han, J.C.; Rasmussen, M.C.; Forte, A.R.; Schrage, S.B.; Zafar, S.K.; Haqq, A.M. Management of Monogenic and Syndromic Obesity. Gastroenterol. Clin. N. Am. 2023, 52, 733–750. [Google Scholar] [CrossRef]
- Leutner, M.; Dervic, E.; Bellach, L.; Klimek, P.; Thurner, S.; Kautzky, A. Obesity as Pleiotropic Risk State for Metabolic and Mental Health throughout Life. Transl. Psychiatry 2023, 13, 175. [Google Scholar] [CrossRef]
- Herhaus, B.; Kersting, A.; Brähler, E.; Petrowski, K. Depression, Anxiety and Health Status across Different BMI Classes: A Representative Study in Germany. J. Affect. Disord. 2020, 276, 45–52. [Google Scholar] [CrossRef] [PubMed]
- de Wit, L.; Have, M.T.; Cuijpers, P.; de Graaf, R. Body Mass Index and risk for onset of mood and anxiety disorders in the general population: Results from the Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2). BMC Psychiatry 2022, 22, 522. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, K.L.; Zhang, X.C.; Schneider, S.; Margraf, J. Obesity and Mental Health: A Longitudinal, Cross-Cultural Examination in Germany and China. Front. Psychol. 2021, 12, 712567. [Google Scholar] [CrossRef]
- Amiri, S.; Behnezhad, S. Obesity and Anxiety Symptoms: A Systematic Review and Meta-Analysis. Neuropsychiatry 2019, 33, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Nameni, G.; Jazayeri, S.; Salehi, M.; Esrafili, A.; Hajebi, A.; Motevalian, S.A. Association between visceral adiposity and generalized anxiety disorder (GAD). BMC Psychol. 2024, 12, 49. [Google Scholar] [CrossRef]
- Casanova, F.; O’Loughlin, J.; Martin, S.; Beaumont, R.N.; Wood, A.R.; Watkins, E.R.; Freathy, R.M.; Hagenaars, S.P.; Frayling, T.M.; Yaghootkar, H.; et al. Higher adiposity and mental health: Causal inference using Mendelian randomization. Hum. Mol. Genet. 2021, 30, 2371–2382. [Google Scholar] [CrossRef]
- Duarte-Guerra, L.S.; Coêlho, B.M.; Santo, M.A.; Wang, Y.-P. Psychiatric disorders among obese patients seeking bariatric surgery: Results of structured clinical interviews. Obes. Surg. 2015, 25, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Witaszek, T.; Kłoda, K.; Mastalerz-Migas, A.; Babicki, M. Association between Symptoms of Depression and Generalised Anxiety Disorder Evaluated through PHQ-9 and GAD-7 and Anti-Obesity Treatment in Polish Adult Women. Nutrients 2024, 16, 2438. [Google Scholar] [CrossRef]
- Incollingo Rodriguez, A.C.; Epel, E.S.; White, M.L.; Standen, E.C.; Seckl, J.R.; Tomiyama, A.J. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 2015, 62, 301–318. [Google Scholar] [CrossRef]
- Reeves, J.W.; Fisher, A.J.; Newman, M.G.; Granger, D.A. Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder. Psychophysiology 2016, 53, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Costello, H.; Gould, R.L.; Abrol, E.; Howard, R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalized anxiety disorder. BMJ Open 2019, 9, e027925. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. (Lausanne) 2023, 14, 1134025. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bonifacio, K.L.; Morelli, N.R.; Vargas, H.O.; Moreira, E.G.; St Stoyanov, D.; Barbosa, D.S.; Carvalho, A.F.; Nunes, S.O.V. Generalized Anxiety Disorder (GAD) and Comorbid Major Depression with GAD Are Characterized by Enhanced Nitro-oxidative Stress, Increased Lipid Peroxidation, and Lowered Lipid-Associated Antioxidant Defenses. Neurotox. Res. 2018, 34, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, H.E.; Bekhet, M.M.M.; Saleh, A.M.M.; Tawfik, F.A.; Elias, D.G. Study of the relationship between insulin resistance and risk of developing depression and anxiety disorders. QJM 2024, 117, 373. [Google Scholar] [CrossRef]
- Fulton, S.; Décarie-Spain, L.; Fioramonti, X.; Guiard, B.; Nakajima, S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol. Metab. 2022, 33, 18–35. [Google Scholar] [CrossRef]
- Baske, M.M.; Timmerman, K.C.; Garmo, L.G.; Freitas, M.N.; McCollum, K.A.; Ren, T.Y. Fecal Microbiota Transplant on Escherichia-Shigella Gut Composition and Its Potential Role in the Treatment of Generalized Anxiety Disorder: A Systematic Review. J. Affect. Disord. 2024, 354, 309–317. [Google Scholar] [CrossRef]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Morkl, S.; Berding, K.; Ritz, N.L.; Strain, C.; Patangia, D.; Patel, S.; Stanton, C.; et al. The gut microbiome in social anxiety disorder: Evidence of altered composition and function. Transl. Psychiatry 2023, 13, 95. [Google Scholar] [CrossRef]
- Li, C.; Xia, L.; Ma, J.; Li, S.; Liang, S.; Ma, X.; Wang, T.; Li, M.; Wen, H.; Jiang, G. Dynamic functional abnormalities in generalized anxiety disorders and their increased network segregation of a hyperarousal brain state modulated by insomnia. J. Affect. Disord. 2019, 246, 338–345. [Google Scholar] [CrossRef]
- Blanchard, A.W.; Rufino, K.A.; Nadorff, M.R.; Patriquin, M.A. Nighttime sleep quality & daytime sleepiness across inpatient psychiatric treatment is associated with clinical outcomes. Sleep Med. 2023, 110, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.C.; Olatunji, B.O. A systematic review of sleep disturbance in anxiety and related disorders. J. Anxiety Disord. 2016, 37, 104–129. [Google Scholar] [CrossRef] [PubMed]
- Benbaibeche, H.; Saidi, H.; Bounihi, A.; Koceir, E.A. Emotional and external eating styles associated with obesity. J. Eat. Disord. 2023, 11, 67. [Google Scholar] [CrossRef]
- Gonçalves, I.D.S.A.; Filgueiras, M.S.; Moreira, T.R.; Thomé, M.S.; Paiva, G.L.D.; Almeida, G.P.; Cotta, R.M.M.; Campos, T.D.N.; Freitas, D.M.O.; Novaes, J.F.; et al. Interrelation of Stress, Eating Behavior, and Body Adiposity in Women with Obesity: Do Emotions Matter? Nutrients 2024, 16, 4133. [Google Scholar] [CrossRef]
- Vakkalagadda, S.G.; Darshan, C.M.; Chaithra, G.C.; Nischitha, S.; Anupama, H.B. A review on hypothalamo-pituitary-adrenal axis. J. Multidiscip. Res. 2022, 2, 19–22. [Google Scholar] [CrossRef]
- Speer, K.E.; Semple, S.; Naumovski, N.; D’Cunha, N.M.; McKune, A.J. HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiol. Stress 2019, 11, 100180. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, S.; Luo, R.; Wu, L.M.; Wang, W. Inhibition of acid-sensing ion channels reduces the hypothalamus-pituitary-adrenal axis activity and ameliorates depression-like behavior in rats. RSC Adv. 2019, 9, 8707–8713. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, L.; Streit, F.; Zhu, G.; McAloney, K.; Frank, J.; Couvy-Duchesne, B.; Witt, S.H.; Binz, T.M.; CORtisolNETwork (CORNET) Consortium; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (PGC); et al. Hair Cortisol in Twins: Heritability and Genetic Overlap with Psychological Variables and Stress-System Genes. Sci. Rep. 2017, 7, 15351. [Google Scholar] [CrossRef]
- Lei, A.A.; Phang, V.W.X.; Lee, Y.Z.; Kow, A.S.F.; Tham, C.L.; Ho, Y.-C.; Lee, M.T. Chronic Stress-Associated Depressive Disorders: The Impact of HPA Axis Dysregulation and Neuroinflammation on the Hippocampus—A Mini Review. Int. J. Mol. Sci. 2025, 26, 2940. [Google Scholar] [CrossRef] [PubMed]
- Călinescu, A. Stomach’s always going to catch up with you: An interdisciplinary approach to emotional eating. Eur. J. Interdiscip. Stud. 2020, 12, 14–40. [Google Scholar] [CrossRef]
- Packard, A.E.; Egan, A.E.; Ulrich-Lai, Y.M. HPA axis-Interaction with Behavioral Systems. Compr. Physiol. 2016, 6, 1897. [Google Scholar] [CrossRef]
- Pringle, J.; Laird, Y.; Sivaramakrishnan, D. Obesity and Mental Health: Evidence Overview. Obesity Action Scotland. 2019. Available online: https://www.obesityactionscotland.org/media/iyeoaxyl/obesity-and-mental-health-final-report-with-cover.pdf (accessed on 24 March 2025).
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef]
- Raut, S.K.; Khullar, M. Oxidative stress in metabolic diseases: Current scenario and therapeutic relevance. Mol. Cell. Biochem. 2023, 478, 185–196. [Google Scholar] [CrossRef]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Bouayed, J.; Soulimani, R. Evidence that hydrogen peroxide, a component of oxidative stress, induces high-anxiety-related behaviour in mice. Behav. Brain Res. 2019, 359, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Fat Cell and Fatty Acid Turnover in Obesity. Adv. Exp. Med. Biol. 2017, 960, 135–160. [Google Scholar] [CrossRef]
- Jankovic, A.; Korac, A.; Buzadzic, B.; Otasevic, V.; Stancic, A.; Daiber, A.; Korac, B. Redox implications in adipose tissue (dys)function--A new look at old acquaintances. Redox Biol. 2015, 6, 19–32. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef]
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef]
- Monteiro, L.; Pereira, J.A.D.S.; Palhinha, L.; Moraes-Vieira, P.M.M. Leptin in the regulation of the immunometabolism of adipose tissue-macrophages. J. Leukoc. Biol. 2019, 106, 703–716. [Google Scholar] [CrossRef]
- Lin, H.Y.; Weng, S.W.; Shen, F.C.; Chang, Y.H.; Lian, W.S.; Hsieh, C.H.; Chuang, J.H.; Lin, T.K.; Liou, C.W.; Chang, C.S.; et al. Abrogation of Toll-Like Receptor 4 Mitigates Obesity-Induced Oxidative Stress, Proinflammation, and Insulin Resistance Through Metabolic Reprogramming of Mitochondria in Adipose Tissue. Antioxid. Redox Signal. 2020, 33, 66–86. [Google Scholar] [CrossRef]
- Qiu, H.; Schlegel, V. Impact of nutrient overload on metabolic homeostasis. Nutr. Rev. 2018, 76, 693–707. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Drougard, A.; Fournel, A.; Valet, P.; Knauf, C. Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake. Front. Neurosci. 2015, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Szlachta, B.; Birková, A.; Čižmárová, B.; Głogowska-Gruszka, A.; Zalejska-Fiolka, P.; Dydoń, M.; Zalejska-Fiolka, J. Erythrocyte Oxidative Status in People with Obesity: Relation to Tissue Losses, Glucose Levels, and Weight Reduction. Antioxidants 2024, 13, 960. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Kanikowska, D.; Kanikowska, A.; Swora-Cwynar, E.; Grzymisławski, M.; Sato, M.; Bręborowicz, A.; Witowski, J.; Korybalska, K. Moderate Caloric Restriction Partially Improved Oxidative Stress Markers in Obese Humans. Antioxidants 2021, 10, 1018. [Google Scholar] [CrossRef]
- Fedoce, A.D.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Beltrán González, A.N.; López Pazos, M.I.; Calvo, D.J. Reactive Oxygen Species in the Regulation of the GABA Mediated Inhibitory Neurotransmission. Neuroscience. 2020, 439, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. Neuroinflammation-Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int. J. Mol. Sci. 2020, 21, 6546. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Chen, H.J.; Spiers, J.G.; Sernia, C.; Lavidis, N.A. Response of the nitrergic system to activation of the neuroendocrine stress axis. Front. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Chen, H.J.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Fındıklı, E.; Camkurt, M.A.; İzci, F.; Karaaslan, M.F.; Fındıklı, H.A.; Sümer, P.; Kurutaş, E.B. The Diagnostic Value of Malondialdehyde, Superoxide Dismutase and Catalase Activity in Drug Naïve, First Episode, Non-Smoker Generalized Anxiety Disorder Patients. Clin. Psychopharmacol. Neurosci. 2018, 16, 88–94. [Google Scholar] [CrossRef]
- Oktay, M.; Asoğlu, M.; Taskin, S.; Kirmit, A. Biological Markers in Newly Diagnosed Generalized Anxiety Disorder Patients: 8-OHdG, S100B and Oxidative Stress. Neuropsychiatr. Dis. Treat. 2024, 20, 19–24. [Google Scholar] [CrossRef]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The impact of obesity on neurodegenerative diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Gomoll, B.P.; Kumar, A. Managing anxiety associated with neurodegenerative disorders. F1000Prime Rep. 2015, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Cunarro, J.; Casado, S.; Lugilde, J.; Tovar, S. Hypothalamic Mitochondrial Dysfunction as a Target in Obesity and Metabolic Disease. Front. Endocrinol. 2018, 9, 283. [Google Scholar] [CrossRef]
- Cui, L.; Lu, J.; Shen, Z.; Zhu, J.; Chen, H.; Yang, S.; Wang, S.; Shen, X. Oxidative Stress Markers Predict Treatment Outcomes in Patients with Generalized Anxiety Disorder Treated with Selective Serotonin Reuptake Inhibitors. Neuropsychobiology 2025, 84, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Signorello, M.G.; Ravera, S.; Leoncini, G. Oxidative Stress Induced by Cortisol in Human Platelets. Int. J. Mol. Sci. 2024, 25, 3776. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, J.M.; Fadel, J.R.; Reagan, L.P. Peripheral versus central insulin and leptin resistance: Role in metabolic disorders, cognition, and neuropsychiatric diseases. Neuropharmacology 2022, 203, 108877. [Google Scholar] [CrossRef]
- Arneth, B. Mechanisms of Insulin Resistance in Patients with Obesity. Endocrines 2024, 5, 153–165. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, T.; Zhang, S.; Zhou, L. Associations of Different Adipose Tissue Depots with Insulin Resistance: A Systematic Review and Meta-analysis of Observational Studies. Sci. Rep. 2015, 5, 18495. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Cen, M.; Song, L.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Associations between metabolic syndrome and anxiety, and the mediating role of inflammation: Findings from the UK Biobank. Brain Behav. Immun. 2024, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L. New Insights into the Role of Insulin and Hypothalamic-Pituitary-Adrenal (HPA) Axis in the Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. Pharmacological treatment of anxiety disorders: The role of the HPA Axis. Front. Psychiatry 2020, 11, 443. [Google Scholar] [CrossRef]
- Fiksdal, A.; Hanlin, L.; Kuras, Y.; Gianferante, D.; Chen, X.; Thoma, M.V.; Rohleder, N. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology 2019, 102, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Lightman, S. The Human Stress Response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef]
- Jaremka, L.M.; Pacanowski, C.R. Social anxiety symptoms moderate the link between obesity and metabolic function. Psychoneuroendocrinology 2019, 110, 104425. [Google Scholar] [CrossRef]
- Díaz-Carías, J.P.; Morilla Romero de la Osa, R.; Cano-Rodríguez, M. Relationship between insulin-biochemical resistance levels and the degree of depression and anxiety in patients from Honduras. Int. J. Diabetes Dev. Ctries. 2023, 43, 750–757. [Google Scholar] [CrossRef]
- Kalva, L.T.; Jannabhatla, V.B.K.; Anusha, N.; Tirupathe, S. Insulin resistance and metabolic syndrome in generalized anxiety disorder: A cross-sectional study. Int. J. Res. Med. Sci. 2024, 12, 3703–3707. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Zhou, Y.; Cao, Y.; Li, X.; Ding, G.; Tang, F. Association between anxiety and metabolic syndrome: An updated systematic review and meta-analysis. Front. Psychiatry 2023, 14, 1118836. [Google Scholar] [CrossRef] [PubMed]
- Genchi, V.A.; D’Oria, R.; Palma, G.; Caccioppoli, C.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. Impaired Leptin Signalling in Obesity: Is Leptin a New Thermolipokine? Int. J. Mol. Sci. 2021, 22, 6445. [Google Scholar] [CrossRef]
- Park, H.-K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism 2016, 64, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Bouillon-Minois, J.-B.; Trousselard, M.; Thivel, D.; Benson, A.C.; Schmidt, J.; Moustafa, F.; Bouvier, D.; Dutheil, F. Leptin as a Biomarker of Stress: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3350. [Google Scholar] [CrossRef]
- Valladolid-Acebes, I. Hippocampal Leptin Resistance and Cognitive Decline: Mechanisms, Therapeutic Strategies and Clinical Implications. Biomedicines 2024, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Both, E.; Huţanu, A.; Şular, F.L.; Roiban, A.L. Correlations of serum leptin and leptin resistance with depression and anxiety in patients with type 2 diabetes. Psychiatry Clin. Neurosci. 2019, 73, 745–753. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Cryan, J.F.; Schellekens, H. Gut peptides and the microbiome: Focus on ghrelin. Curr. Opin. Endocrinol. Diabetes 2021, 28, 243–252. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Panda, S.S.; Nayak, A.; Shah, S.; Aich, P. A Systematic Review on the Association between Obesity and Mood Disorders and the Role of Gut Microbiota. Metabolites 2023, 13, 488. [Google Scholar] [CrossRef]
- Muheyati, D.; Han, J.; Lv, M.; Jielili, M.; Jing, Z.; Zaibibuli, K.; Aisike, G.; Aihemaiti, A.; Yu, Y.; Kaliszewski, C.R. Composition of gut microbiota in obese and normal-weight Uygur adults and its association with adipocyte-related factors. Sci. Rep. 2024, 14, 24649. [Google Scholar] [CrossRef]
- Bouter, K.E.; Van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate Systems in DSM-5 Anxiety Disorders: Their Role and a Review of Glutamate and GABA Psychopharmacology. Front. Psychiatry 2020, 11, 548505. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Helton, S.G.; Lohoff, F.W. Serotonin Pathway Polymorphisms and the Treatment of Major Depressive Disorder and Anxiety Disorders. Pharmacogenomics 2015, 16, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Role of Probiotics and Diet in the Management of Neurological Diseases and Mood States: A Review. Microorganisms 2022, 10, 2268. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef]
- Nikel, K.; Stojko, M.; Smolarczyk, J.; Piegza, M. The Impact of Gut Microbiota on the Development of Anxiety Symptoms—A Narrative Review. Nutrients 2025, 17, 933. [Google Scholar] [CrossRef]
- Molska, M.; Mruczyk, K.; Cisek-Woźniak, A.; Prokopowicz, W.; Szydełko, P.; Jakuszewska, Z.; Marzec, K.; Trocholepsza, M. The Influence of Intestinal Microbiota on BDNF Levels. Nutrients 2024, 16, 2891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Zhang, X.; Yu, Z.H.; Zhang, Z.; Deng, M.; Zhao, J.H.; Ruan, B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, J.; Wu, D.; Yu, S.; Qiang, X.; Bai, H.; Wang, H.; Peng, Z. Association between Fecal Microbiota and Generalized Anxiety Disorder: Severity and Early Treatment Response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, C.; Wang, J.; Tang, B.; Cao, J.; Hu, X.; Zhao, X.; Feng, C. Association between Gut Microbiota and Anxiety Disorders: A Bidirectional Two-Sample Mendelian Randomization Study. BMC Psychiatry 2024, 24, 398. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.M.; Zhang, W.M.; Yin, J.M.; Dong, J.M.; Liu, J.M. Relationship between Intestinal Flora, Inflammation, BDNF Gene Polymorphism and Generalized Anxiety Disorder: A Clinical Investigation. Medicine 2022, 101, e28910. [Google Scholar] [CrossRef]
- Allison, K.C.; Parnarouskis, L.; Moore, M.D.; Minnick, A.M. Insomnia, short sleep, and their treatments: Review of their associations with weight. Curr. Obes. Rep. 2024, 13, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Consensus Conference Panel; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; et al. Joint consensus statement of the American Academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Figorilli, M.; Velluzzi, F.; Redolfi, S. Obesity and sleep disorders: A bidirectional relationship. Nutr. Metab. Cardiovasc. Dis. 2025, 15, 104014. [Google Scholar] [CrossRef]
- Kohanmoo, A.; Akhlaghi, M.; Sasani, N.; Nouripour, F.; Lombardo, C.; Kazemi, A. Short Sleep Duration Is Associated with Higher Risk of Central Obesity in Adults: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Obes. Sci. Pract. 2024, 10, e772. [Google Scholar] [CrossRef]
- Bacaro, V.; Ballesio, A.; Cerolini, S.; Vacca, M.; Poggiogalle, E.; Donini, L.M.; Lucidi, F.; Lombardo, C. Sleep duration and obesity in adulthood: An updated systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 301–309. [Google Scholar] [CrossRef]
- Cox, R.C.; Olatunji, B.O. Sleep in the Anxiety-Related Disorders: A Meta-Analysis of Subjective and Objective Research. Sleep. Med. Rev. 2020, 51, 101282. [Google Scholar] [CrossRef]
- Hetkamp, M.; Schweda, A.; Bäuerle, A.; Weismüller, B.; Kohler, H.; Musche, V.; Dörrie, N.; Schöbel, C.; Teufel, M.; Skoda, E.-M. Sleep disturbances, fear, and generalized anxiety during the COVID-19 shut down phase in Germany: Relation to infection rates, deaths, and German stock index DAX. Sleep Med. 2020, 75, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, W.D.; Liu, Y.J.; Wang, J.; Walters, A.S. Sleep disturbances in generalized anxiety Disorder: The central role of insomnia. Sleep Med. 2025, 132, 106545. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P., Jr. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef]
- Lengton, R.; Schoenmakers, M.; Penninx, B.W.J.H.; Boon, M.R.; van Rossum, E.F.C. Glucocorticoids and HPA axis regulation in the stress–obesity connection: A comprehensive overview of biological, physiological and behavioural dimensions. Clin. Obes. 2024, 15, e12725. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Zheng, Q.; Gao, L.; Sun, Q. Sleep Deprivation and Central Appetite Regulation. Nutrients 2022, 14, 5196. [Google Scholar] [CrossRef]
- van Egmond, L.T.; Meth, E.M.S.; Engström, J.; Ilemosoglou, M.; Keller, J.A.; Vogel, H.; Benedict, C. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: A laboratory study. Obesity 2023, 31, 635–641. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, Y.; Wang, G.; Meng, M.; Zhu, Q.; Mei, H.; Liu, S.; Jiang, F. Associations of short sleep duration with appetite-regulating hormones and adipokines: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13051. [Google Scholar] [CrossRef]
- Gresser, D.; McLimans, K.; Lee, S.; Morgan-Bathke, M. The Impact of Sleep Deprivation on Hunger-Related Hormones: A Meta-Analysis and Systematic Review. Obesities 2025, 5, 48. [Google Scholar] [CrossRef]
- Ballesio, A.; Fiori, V.; Lombardo, C. Effects of Experimental Sleep Deprivation on Peripheral Inflammation: An Updated Meta-Analysis of Human Studies. J. Sleep Res. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, Y.; Zhu, M. The Relationship between Global Sleep Score and Inflammatory Markers in Obese Adults from the United States. Nat. Sci. Sleep. 2019, 11, 317–324. [Google Scholar] [CrossRef]
- Al-Sharif, F.M.; El-Kader, S.M.A. Inflammatory cytokines and sleep parameters response to life style intervention in subjects with obese chronic insomnia syndrome. Afr. Health Sci. 2021, 21, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Zinna, L.; Verde, L.; Tolla, M.F.D.; Barrea, L.; Parascandolo, A.; D’Alterio, F.; Colao, A.; Formisano, P.; D’Esposito, V.; Muscogiuri, G. Chronodisruption enhances inflammatory cytokine release from visceral adipose tissue in obesity. J. Transl. Med. 2025, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Papatriantafyllou, E.; Efthymiou, D.; Zoumbaneas, E.; Popescu, C.A.; Vassilopoulou, E. Sleep Deprivation: Effects on Weight Loss and Weight Loss Maintenance. Nutrients 2022, 14, 1549. [Google Scholar] [CrossRef]
- Peng, A.; Ji, S.; Lai, W.; Hu, D.; Wang, M.; Zhao, X.; Chen, L. The Bidirectional Relationship between Sleep Disturbance and Anxiety: Sleep Disturbance Is a Stronger Predictor of Anxiety. Sleep. Med. 2024, 121, 63–68. [Google Scholar] [CrossRef]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016, 3, 67–104. [Google Scholar] [CrossRef]
- Harris, A.; Reme, S.E.; Tangen, T.; Hansen, A.M.; Garde, A.H.; Eriksen, H.R. Diurnal Cortisol Rhythm: Associated with Anxiety and Depression, or Just an Indication of Lack of Energy? Psychiatry Res. 2015, 228, 209–215. [Google Scholar] [CrossRef]
- Foster, R.G. Sleep, circadian rhythms and health. Interface Focus. 2020, 10, 20190098. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Jahdali, H. The consequences of sleep deprivation on cognitive performance. Neurosciences 2023, 28, 91–99. [Google Scholar] [CrossRef]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404. [Google Scholar] [CrossRef]
- Xiang, T.; Liao, J.; Cai, Y.; Fan, M.; Li, C.; Zhang, X.; Li, H.; Chen, Y.; Pan, J. Impairment of GABA inhibition in insomnia disorders: Evidence from the peripheral blood system. Front. Psychiatry 2023, 14, 1134434. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Cheng, H.; Li, N.; Zhang, B.; Dai, W.; Wu, X.; Zhang, D.; Feng, W.; Li, S.; et al. GABA and its receptors’ mechanisms in the treatment of insomnia. Heliyon 2024, 10, e40665. [Google Scholar] [CrossRef]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Singh, K.K.; Ghosh, S.; Bhola, A.; Verma, P.; Amist, A.D.; Sharma, H.; Sachdeva, P.; Sinha, J.K. Sleep and Immune System Crosstalk: Implications for Inflammatory Homeostasis and Disease Pathogenesis. Ann. Neurosci. 2024, 32, 196–206. [Google Scholar] [CrossRef]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and Circadian Regulation of Cortisol: A Short Review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef]
- Andreadi, A.; Andreadi, S.; Todaro, F.; Ippoliti, L.; Bellia, A.; Magrini, A.; Chrousos, G.P.; Lauro, D. Modified Cortisol Circadian Rhythm: The Hidden Toll of Night-Shift Work. Int. J. Mol. Sci. 2025, 26, 2090. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Meléndez-Fernández, O.H.; Liu, J.A.; Nelson, R.J. Circadian Rhythms Disrupted by Light at Night and Mistimed Food Intake Alter Hormonal Rhythms and Metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef]
- Arora, S.; Sahadevan, P.; Sundarakumar, J.S. Association of sleep quality with physical and psychological health indicators in overweight and obese rural Indians. Sleep Med. X 2024, 7, 100112. [Google Scholar] [CrossRef]
- Alhusseini, N.K.; Elaasser, B.; Alshaar, B.; Alyousof, S.; Boukhet, S.; Omair, A. Obesity, Quality of Sleep and Anxiety in Saudi Arabia. J. Contemp. Med. Sci. 2021, 7, 235–241. [Google Scholar] [CrossRef]
- Fusco, S.F.B.; Amancio, S.C.P.; Pancieri, A.P.; Alves, M.V.M.F.F.; Spiri, W.C.; Braga, E.M. Anxiety, sleep quality, and binge eating in overweight or obese adults. Rev. Esc. Enferm. USP 2020, 54, e03656. [Google Scholar] [CrossRef]
- Hussenoeder, F.S.; Conrad, I.; Engel, C.; Zachariae, S.; Zeynalova, S.; Glaesmer, H.; Hinz, A.; Witte, V.; Tönjes, A.; Löffler, M.; et al. Analyzing the link between anxiety and eating behavior as a potential pathway to eating-related health outcomes. Sci. Rep. 2021, 11, 14717. [Google Scholar] [CrossRef]
- Cifuentes, L.; Campos, A.; Silgado, M.L.R.; Kelpin, S.; Stutzman, J.; Hurtado, M.D.; Grothe, K.; Hensrud, D.D.; Clark, M.M.; Acosta, A. Association between anxiety and eating behaviors in patients with obesity. Obes. Pillars 2022, 3, 100021. [Google Scholar] [CrossRef] [PubMed]
- Witaszek, T.; Babicki, M.; Brytek-Matera, A.; Mastalerz-Migas, A.; Kujawa, K.; Kłoda, K. Maladaptive Eating Behaviours, Generalised Anxiety Disorder and Depression Severity: A Comparative Study between Adult Women with Overweight, Obesity, and Normal Body Mass Index Range. Nutrients 2023, 16, 80. [Google Scholar] [CrossRef]
- Aoun, C.; Nassar, L.; Soumi, S.; El Osta, N.; Papazian, T.; Rabbaa Khabbaz, L. The Cognitive, Behavioral, and Emotional Aspects of Eating Habits and Association With Impulsivity, Chronotype, Anxiety, and Depression: A Cross-Sectional Study. Front. Behav. Neurosci. 2019, 13, 204. [Google Scholar] [CrossRef]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The Association of Emotional Eating with Overweight/Obesity, Depression, Anxiety/Stress, and Dietary Patterns: A Review of the Current Clinical Evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef] [PubMed]
- Braden, A.; Musher-Eizenman, D.; Watford, T.; Emley, E. Eating when depressed, anxious, bored, or happy: Are emotional eating types associated with unique psychological and physical health correlates? Appetite 2018, 125, 410–417. [Google Scholar] [CrossRef]
- Schrempft, S.; Jiménez-Sánchez, C.; Baysson, H.; Zaballa, M.E.; Lamour, J.; Stringhini, S.; Guessous, I.; Nehme, M. Specchio study group. Pathways linking BMI trajectories and mental health in an adult population-based cohort: Role of emotional eating and body dissatisfaction. Int. J. Obes. 2025, 49, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.L.; Rowe, A.C.; Robinson, E.; Hardman, C.A. Explaining the relationship between attachment anxiety, eating behaviour and BMI. Appetite 2018, 127, 214–222. [Google Scholar] [CrossRef]
- Ha, O.R.; Lim, S.L. The role of emotion in eating behavior and decisions. Front. Psychol. 2023, 14, 1265074. [Google Scholar] [CrossRef]
- Arexis, M.; Feron, G.; Brindisi, M.C.; Billot, P.É.; Chambaron, S. A scoping review of emotion regulation and inhibition in emotional eating and binge-eating disorder: What about a continuum? J. Eat. Disord. 2023, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, N.K.O.D.; Costa, M.A.; Gosmann, N.P.; Dalle Molle, R.; Gonçalves, F.G.; Silva, A.C.; Rodrigues, Y.; Silveira, P.P.; Manfro, G.G. Emotional eating in women with generalized anxiety disorder. Trends Psychiatry Psychother. 2023, 45, e20210399. [Google Scholar] [CrossRef] [PubMed]
- Leehr, E.J.; Krohmer, K.; Schag, K.; Dresler, T.; Zipfel, S.; Giel, K.E. Emotion regulation model in binge eating disorder and obesity—A systematic review. Neurosci. Biobehav. Rev. 2015, 49, 125–134. [Google Scholar] [CrossRef]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology 2017, 42, 1375–1389. [Google Scholar] [CrossRef]
- Ghabashi, M.A. Increased self-regulation of eating behavior is associated with reduced generalized anxiety disorder in Saudi Arabia. Front. Psychol. 2024, 15, 1480812. [Google Scholar] [CrossRef] [PubMed]
- Bouillon-Minois, J.B.; Trousselard, M.; Thivel, D.; Gordon, B.A.; Schmidt, J.; Moustafa, F.; Oris, C.; Dutheil, F. Ghrelin as a Biomarker of Stress: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 784. [Google Scholar] [CrossRef]
- Kuckuck, S.; van der Valk, E.S.; Scheurink, A.J.W.; van der Voorn, B.; Iyer, A.M.; Visser, J.A.; Delhanty, P.J.D.; van den Berg, S.A.A.; van Rossum, E.F.C. Glucocorticoids, stress and eating: The mediating role of appetite-regulating hormones. Obes. Rev. 2023, 24, e13539. [Google Scholar] [CrossRef]
- Comeras, L.B.; Herzog, H.; Tasan, R.O. Neuropeptides at the crossroad of fear and hunger: A special focus on neuropeptide Y. Ann. N. Y. Acad. Sci. 2019, 1455, 59–80. [Google Scholar] [CrossRef]
- Krauss, S.; Dapp, L.C.; Orth, U. The Link Between Low Self-Esteem and Eating Disorders: A Meta-Analysis of Longitudinal Studies. Clin. Psychol. Sci. 2023, 11, 1141–1158. [Google Scholar] [CrossRef]
- Fu, T.; Wang, J.; Xu, S.; Yu, J.; Sun, G. Media Internalized Pressure and Restrained Eating Behavior in College Students: The Multiple Mediating Effects of Body Esteem and Social Physique Anxiety. Front. Psychol. 2022, 13, 887124. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, P. The Role of Stress and Mental Health in Obesity. Obesities 2025, 5, 20. [Google Scholar] [CrossRef]
- Mhanna, A.; Martini, N.; Hmaydoosh, G.; Hamwi, G.; Jarjanazi, M.; Zaifah, G.; Kazzazo, R.; Haji Mohamad, A.; Alshehabi, Z. The correlation between gut microbiota and both neurotransmitters and mental disorders: A narrative review. Medicine 2024, 103, e37114. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, Y. The microbiota-gut-brain axis and central nervous system diseases: From mechanisms of pathogenesis to therapeutic strategies. Front. Microbiol. 2025, 16, 1583562. [Google Scholar] [CrossRef]
- Gill, H.; Gill, B.; El-Halabi, S.; Chen-Li, D.; Lipsitz, O.; Rosenblat, J.D.; Van Rheenen, T.E.; Rodrigues, N.B.; Mansur, R.B.; Majeed, A.; et al. Antidepressant Medications and Weight Change: A Narrative Review. Obesity 2020, 28, 2064–2072. [Google Scholar] [CrossRef]
- Gunturu, S. The Potential Role of GLP-1 Agonists in Psychiatric Disorders: A Paradigm Shift in Mental Health Treatment. Indian J. Psychol. Med. 2024, 46, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Kornelius, E.; Huang, J.Y.; Lo, S.C.; Huang, C.N.; Yang, Y.S. The risk of depression, anxiety, and suicidal behavior in patients with obesity on glucagon like peptide-1 receptor agonist therapy. Sci. Rep. 2024, 14, 24433. [Google Scholar] [CrossRef]
- De Giorgi, R.; Ghenciulescu, A.; Dziwisz, O.; Taquet, M.; Adler, A.I.; Koychev, I.; Upthegrove, R.; Solmi, M.; McCutcheon, R.; Pillinger, T.; et al. An analysis on the role of glucagon-like peptide-1 receptor agonists in cognitive and mental health disorders. Nat. Ment. Health 2025, 3, 354–373. [Google Scholar] [CrossRef]


| Mechanistic Pathways | Key Findings Linking GAD and Obesity | Representative References | Estimated Number of Studies (2015–2025) |
|---|---|---|---|
| HPA Axis Dysfunction | Chronic and early-life stress dysregulate the HPA axis (↑CRH/ACTH → sustained cortisol), impairing emotion regulation and heightening anxiety. Elevated cortisol increases appetite, promotes fat storage and metabolic dysregulation, raising obesity risk. Adverse childhood experiences further disrupt HPA function and foster obesogenic eating; emotional eating often serves as a coping mechanism, coupling HPA hyperactivity with weight gain and creating a self-reinforcing GAD and obesity cycle. | [40,44,46] | ~10 |
| Oxidative Stress | Oxidative stress and obesity-related low-grade inflammation (↑IL-6, TNF-α) disrupt neurotransmitter balance (↓serotonin, altered GABA), impair neural function, and promote anxiety. Shared mechanisms include chronic inflammation, mitochondrial dysfunction, NADPH oxidase activity, and reduced antioxidant defenses, leading to ROS overproduction, oxidative brain damage, appetite dysregulation, and a mutually reinforcing relationship of metabolic and neuropsychiatric disturbances. | [48,51,54,68,79,84] | ~35 |
| Insulin Resistance | IR is closely associated with visceral obesity, where chronic low-grade inflammation (↑IL-6, TNF-α) disrupts insulin signaling. Reduced insulin action in the CNS may affect the frontal lobe and hippocampus, leading to HPA axis hyperactivity, cortisol dysregulation, and serotonergic imbalance, which could increase susceptibility to GAD. Conversely, chronic anxiety stimulates HPA axis overactivity and persistent cortisol release, which impair insulin-mediated glucose uptake, promote lipogenesis, dyslipidemia, and visceral fat accumulation, and further amplify inflammation. | [88,91,93,97,99] | ~20 |
| Gut–Brain Axis and Microbiota Dysbiosis | Gut dysbiosis (↓diversity, altered Firmicutes/Bacteroidetes ratio, ↓SCFA-producing bacteria) is observed in both obesity and GAD. In obesity, it enhances energy harvest, fat storage, bile acid disturbances, and systemic inflammation via LPS translocation. In GAD, dysbiosis involves reduced beneficial taxa (e.g., Faecalibacterium, Lachnospira) and overgrowth of pathogenic bacteria (e.g., Escherichia/Shigella, Fusobacterium), impairing neurotransmitter pathways (serotonin, dopamine, GABA), increasing intestinal permeability, and reducing BDNF levels. These changes activate immune responses and HPA axis dysregulation, creating a bidirectional gut–brain feedback loop that exacerbates both metabolic and anxiety symptoms. | [112,115,116,117,118,119,126,127] | ~25 |
| Sleep Disturbance | Chronic insufficient or poor-quality sleep (<6 h/day or fragmented sleep) is associated with both obesity and GAD. In obesity, short sleep promotes hormonal imbalances (↓leptin, ↑ghrelin), HPA axis overactivation with elevated evening cortisol, systemic inflammation (↑IL-6, CRP, TNF-α), and increased intake of energy-dense foods. In GAD, sleep disturbances stem from chronic hyperarousal, glutamatergic overactivity, reduced GABAergic transmission, amygdala hyperactivity, and impaired mPFC regulation. Sleep deprivation further heightens emotional reactivity, neuroinflammation, and anxiety symptoms, contributing to metabolic and psychological dysregulation. | [132,135,137,139,150,166] | ~35 |
| Maladaptive eating Behaviors | GAD is strongly associated with maladaptive eating behaviors, especially EE and UE, driven by emotion regulation deficits, altered reward processing, hormonal/neuropeptide imbalances (e.g., leptin, ghrelin, NPY, CRH), and heightened impulsivity. These behaviors temporarily relieve stress via dopamine pathways but reduce CR and impair appetite regulation, promoting weight gain and reinforcing anxiety long-term. Stable traits like neuroticism may underlie both anxiety and disinhibited eating, suggesting that interventions improving emotion regulation and self-control (e.g., mindfulness) can benefit both weight and anxiety outcomes. | [169,170,171,173,179] | ~25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dymek, A.; Zielińska, M.; Englert-Bator, A.; Dereń, K.; Łuszczki, E. Generalized Anxiety Disorder and Obesity: Overlapping Neuroendocrine, Metabolic, and Behavioral Pathways. Nutrients 2025, 17, 2835. https://doi.org/10.3390/nu17172835
Dymek A, Zielińska M, Englert-Bator A, Dereń K, Łuszczki E. Generalized Anxiety Disorder and Obesity: Overlapping Neuroendocrine, Metabolic, and Behavioral Pathways. Nutrients. 2025; 17(17):2835. https://doi.org/10.3390/nu17172835
Chicago/Turabian StyleDymek, Agnieszka, Magdalena Zielińska, Anna Englert-Bator, Katarzyna Dereń, and Edyta Łuszczki. 2025. "Generalized Anxiety Disorder and Obesity: Overlapping Neuroendocrine, Metabolic, and Behavioral Pathways" Nutrients 17, no. 17: 2835. https://doi.org/10.3390/nu17172835
APA StyleDymek, A., Zielińska, M., Englert-Bator, A., Dereń, K., & Łuszczki, E. (2025). Generalized Anxiety Disorder and Obesity: Overlapping Neuroendocrine, Metabolic, and Behavioral Pathways. Nutrients, 17(17), 2835. https://doi.org/10.3390/nu17172835