Associations Between Diet, Oral Health, and General Development in Romanian School-Age Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Study Design, and Ethical Considerations
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Future Directions of Research
- First, longitudinal studies are needed to establish temporal relationships between dietary patterns, oral health behaviors, body composition, and the development of dental caries. Such designs would allow for a more accurate evaluation of causality and behavioral change over time, particularly during critical periods of dentition transition.
- Second, future research should aim to improve the precision of dietary intake assessment through multi-method approaches, such as combining food frequency questionnaires with 24 h dietary recalls or objective biomarkers. This would reduce the impact of reporting bias and enhance the validity of nutritional exposure measurements.
- Third, larger and more diverse samples are recommended to increase the statistical power of subgroup analyses and to better capture variability in socioeconomic and parental factors. In particular, stratified recruitment strategies may help clarify the role of maternal education, body weight status, and access to dental care in caries risk.
- Lastly, intervention studies targeting modifiable risk factors (e.g., sugar intake, oral hygiene habits, dairy consumption) would be valuable in assessing the effectiveness of prevention strategies tailored to specific populations, especially in regions with high caries prevalence.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pujia, R.; Ferro, Y.; Maurotti, S.; Khoory, J.; Gazzaruso, C.; Pujia, A.; Montalcini, T.; Mazza, E. The Effects of COVID-19 on the Eating Habits of Children and Adolescents in Italy: A Pilot Survey Study. Nutrients 2021, 13, 2641. [Google Scholar] [CrossRef] [PubMed]
- Baban, R.S.; Kasar, K.A.; Al-Karawi, I.N. Fasting glucose to leptin ratio as a new diagnostic marker in patients with diabetes mellitus. Oman Med. J. 2010, 25, 269–275. [Google Scholar] [CrossRef]
- Wulandari, L.P. Overview of nutrition intake of rural and urban adolescents. MIKIA Mimb. Ilm. Kesehat. Ibu Dan Anak (Matern. Neonatal Health J.) 2021, 56–64. [Google Scholar] [CrossRef]
- Galler, J.R.; Koethe, J.R.; Yolken, R.H. Neurodevelopment: The Impact of Nutrition and Inflammation During Adolescence in Low-Resource Settings. Pediatrics 2017, 139 (Suppl. S1), S72–S84. [Google Scholar] [CrossRef]
- Isabirye, N.; Bukenya, J.N.; Nakafeero, M.; Ssekamatte, T.; Guwatudde, D.; Fawzi, W. Dietary diversity and associated factors among adolescents in eastern Uganda: A cross-sectional study. BMC Public Health 2020, 20, 534. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Taylor, G.W.; Manz, M.C.; Yoshihara, A.; Sato, M.; Muramatsu, K.; Watanabe, R.; Miyazaki, H. Oral health status: Relationship to nutrient and food intake among 80-year-old Japanese adults. Community Dent. Oral Epidemiol. 2014, 42, 441–450. [Google Scholar] [CrossRef]
- Furuya, J.; Suzuki, H.; Hidaka, R.; Nakagawa, K.; Yoshimi, K.; Nakane, A.; Yamaguchi, K.; Shimizu, Y.; Itsui, Y.; Saito, K.; et al. Factors Related to Oral Intake of Food by Hospitalized Patients with Malnutrition under the Care of a Nutrition Support Team. Int. J. Environ. Res. Public Health 2021, 18, 11725. [Google Scholar] [CrossRef]
- Aida, J. Oral Health and Nutrition: Epidemiology, Clinical, and Social Aspects. J. Nutr. Sci. Vitaminol. 2022, 68, S26–S27. [Google Scholar] [CrossRef]
- Salcudean, A.; Trusculescu, L.M.; Popovici, R.A.; Serb, N.; Pasca, C.; Bodo, C.R.; Craciun, R.E.; Olariu, I. Dental anxiety—A psychosocial cause affecting the quality of life—A systematic review. Rom. J. Oral Rehabil. 2024, 4, 471–483. [Google Scholar] [CrossRef]
- Sălcudean, A.; Cîmpian, D.-M.; Popovici, R.-A.; Forna, N.; Corodan-Comiati, D.-M.; Sasu, A.-B.; Cozma, M.-M.; Bodo, C.-R.; Enache, E.-C.; Păcurar, M.; et al. Dietary habits and their influence on the microbiome and mental health in adolescents. Nutrients 2025, 17, 1496. [Google Scholar] [CrossRef]
- Bodo, C.R.; Sălcudean, A.; Nireștean, A.; Lukacs, E.; Lică, M.M.; Muntean, D.L.; Anculia, R.C.; Popovici, R.A.; Neda Stepan, O.; Enătescu, V.R.; et al. Association between chronic misophonia-induced stress and gastrointestinal pathology in children—A hypothesis. Children 2024, 11, 699. [Google Scholar] [CrossRef]
- Sahab, L.; Newton, J.T.; Sabbah, W. The Nutritional Pathway Between Tooth Loss and Healthy Ageing: A Longitudinal Study of Older American Adults. Nutrients 2025, 17, 719. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tsang, Y.C.; Jiang, C.M.; Leung, K.C.M.; Lo, E.C.M.; Chu, C.H. Diet, Nutrition, and Oral Health in Older Adults: A Review of the Literature. Dent. J. 2023, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Badrasawi, M.M.H.; Hijjeh, N.H.; Amer, R.S.; Allan, R.M.; Altamimi, M. Nutrition Awareness and Oral Health among Dental Patients in Palestine: A Cross-Sectional Study. Int. J. Dent. 2020, 2020, 3472753. [Google Scholar] [CrossRef] [PubMed]
- Aslan Ceylan, J.; Aslan, Y.; Ozcelik, A.O. The effects of socioeconomic status, oral and dental health practices, and nutritional status on dental health in 12-year-old school children. Egypt. Pediatr. Assoc. Gaz. 2022, 70, 13. [Google Scholar] [CrossRef]
- Carvalho Silva, C.; Gavinha, S.; Vilela, S.; Rodrigues, R.; Manso, M.C.; Severo, M.; Lopes, C.; Melo, P. Dietary Patterns and Oral Health Behaviours Associated with Caries Development from 4 to 7 Years of Age. Life 2021, 11, 609. [Google Scholar] [CrossRef]
- Manohar, N.; Hayen, A.; Arora, A. Obesity and dental caries in early childhood: A systematic review protocol. JBI Evid. Synth. 2020, 18, 135–145. [Google Scholar] [CrossRef]
- Shivakumar, S.; Srivastava, A.C.; Shivakumar, G. Body Mass Index and Dental Caries: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2018, 11, 228–232. [Google Scholar] [CrossRef]
- Baig, M.N.; Kumar, P.; Kalimireddy, P.; Pradhan, P.K.; Bharateesh, J.V.; Issrani, R. Influence of Unhealthy Diet and Sedentary Behavior on the Oral Health-Related Quality of Life on Child and Adolescent Age Groups: An Original Research. J. Pharm. Bioallied Sci. 2025, 17 (Suppl. S2), S1796–S1798. [Google Scholar] [CrossRef]
- Montero, J.; Costa, J.; Bica, I.; Barrios, R. Caries and quality of life in Portuguese adolescents: Impact of diet and behavioural risk factors. J. Clin. Exp. Dent. 2018, 10, e218–e223. [Google Scholar] [CrossRef]
- Lendrawati, L.; Pintauli, S.; Rahardjo, A.; Bachtiar, A.; Maharani, D. Risk factors of dental caries: Consumption of sugary snacks among indonesian adolescents. Pesqui. Bras. Em Odontopediatria E Clín. Integr. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Hong, J.; Whelton, H.; Douglas, G.; Kang, J. Consumption frequency of added sugars and UK children’s dental caries. Community Dent. Oral. Epidemiol. 2018, 46, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Lee, Y.C.; Hsu, L.Y.; Chang, H.J.; Chi, L.Y. Association between sugary drinks consumption and dental caries incidence among Taiwanese schoolchildren with mixed dentition. Community Dent. Oral. Epidemiol. 2022, 50, 384–390. [Google Scholar] [CrossRef]
- Al-Zahrani, A.; Al-Qahtani, M.; Al-Barti, M.; Bakhurji, E.A. Dietary Determinants of Dental Caries Prevalence and Experience in Saudi Schoolchildren: Frequency versus Quantity. Sci. World J. 2022, 2022, 5447723. [Google Scholar] [CrossRef] [PubMed]
- Punitha, V.C.; Amudhan, A.; Sivaprakasam, P.; Rathanaprabu, V. Role of dietary habits and diet in caries occurrence and severity among urban adolescent school children. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. S1), S296–S300. [Google Scholar] [CrossRef]
- Echeverria, M.S.; Schuch, H.S.; Cenci, M.S.; Motta, J.V.S.; Bertoldi, A.D.; Hallal, P.C.; Demarco, F.F. Trajectories of Sugar Consumption and Dental Caries in Early Childhood. J. Dent. Res. 2022, 101, 724–730. [Google Scholar] [CrossRef]
- Xu, H.; Ma, X.; Wang, J.; Chen, X.; Zou, Q.; Ban, J. Exploring the state and influential factors of dental caries in preschool children aged 3-6 years in Xingtai City. BMC Oral Health 2024, 24, 951. [Google Scholar] [CrossRef] [PubMed]
- Prabakar, J.; Arumugham, I.M.; Sri Sakthi, D.; Kumar, R.P.; Leelavathi, L. Prevalence and Comparison of Dental Caries experience among 5 to 12 year old school children of Chandigarh using dft/ DMFT and SiC Index: A Cross-sectional study. J. Fam. Med. Prim. Care 2020, 9, 819–825. [Google Scholar] [CrossRef]
- Hiremath, A.; Murugaboopathy, V.; Ankola, A.V.; Hebbal, M.; Mohandoss, S.; Pastay, P. Prevalence of Dental Caries Among Primary School Children of India—A Cross-Sectional Study. J. Clin. Diagn. Res. 2016, 10, ZC47–ZC50. [Google Scholar] [CrossRef]
- Giri, M.; Pandit, S.; Oli, H.; Giri, S. Prevalence and associated factors of dental caries among basic school children in Kathmandu Metropolitan City, Nepal: A cross-sectional study. MedS Alliance J. Med. Med. Sci. 2021, 1, 89–94. [Google Scholar] [CrossRef]
- Seth, N.; Shivalingesh, K.K.; Anand, R.; Sharma, A.; Singh Thakar, S.; Khan, K. Caries prevalence and oral hygiene status among 7-12 years old school children from rural and urban areas of Gautam Budh Nagar, U.P. J. Adv. Oral Res. 2016, 7, 35–40. [Google Scholar] [CrossRef]
- Shakuntala, B.S.; Sirisha, P.; Kulkarni, M.P. Dental Caries Experience among School-going Children Based on Diet, Physical Activity, and Adiposity in South Bengaluru: A Questionnaire Study. J. Health Sci. Res. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Shaffer, J.R.; Polk, D.E.; Feingold, E.; Wang, X.; Cuenco, K.T.; Weeks, D.E.; DeSensi, R.S.; Weyant, R.J.; Crout, R.; McNeil, D.W.; et al. Demographic, socioeconomic, and behavioral factors affecting patterns of tooth decay in the permanent dentition: Principal components and factor analyses. Community Dent. Oral Epidemiol. 2013, 41, 364–373. [Google Scholar] [CrossRef]
- Krol, D.M.; Whelan, K. Section On Oral Health. Maintaining and Improving the Oral Health of Young Children. Pediatrics 2023, 151, e2022060417. [Google Scholar] [CrossRef]
- Bud, A.; Bud, E.; Eșian, D.; Pop, S.; Bechir, A.; Păcurar, M.; Curt-Mola, F.; Ţărmure, V. Interrelation between salivary pH, buffer capacity and dental caries in underweight, normal weight and overweight children. Rev. De Chim. 2017, 68, 1255–1258. [Google Scholar] [CrossRef]
- Balaji, K.; Kumar, P.; Pradeep, D. Analysis Of Factors Associated With Dental Caries In Children Aged 6-12 Years Visiting A Private Dental Hospital. J. Contemp. Issues Bus. Gov. 2021, 27, 3346–3354. [Google Scholar] [CrossRef]
- Rangeela, M.; Santhanam, A.; Rajendran, D. Dental Caries Among Children Of Age 6–12 Years Old Visiting A Dental Institution—A Retrospective Study. J. Contemp. Issues Bus. Gov. 2021, 27, 3363–3370. [Google Scholar]
- Kuremoto, K.; Okawa, R.; Matayoshi, S.; Kokomoto, K.; Nakano, K. Estimation of dental age based on the developmental stages of permanent teeth in Japanese children and adolescents. Sci. Rep. 2022, 12, 3345. [Google Scholar] [CrossRef]
- Balel, Y.; Sağtaş, K.; Bülbül, H.N. Development and evaluation of a deep learning-based system for dental age estimation using the Demirjian method on panoramic radiographs. BMC Oral Health 2025, 25, 1172. [Google Scholar] [CrossRef]
- Esan, T.A.; Yengopal, V.; Schepartz, L.A. The Demirjian versus the Willems method for dental age estimation in different populations: A meta-analysis of published studies. PLoS ONE 2017, 12, e0186682. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health Surveys: Basic Methods, 4th ed.; World Health Organization: Geneva, Switzerland, 1997. Available online: https://apps.who.int/iris/handle/10665/41905 (accessed on 1 July 2025).
- Willett, W. Nutritional Epidemiology. In Monographs in Epidemiology and Biostatistics, 3rd ed.; Oxford Academic: Oxford, UK, 2012. [Google Scholar]
- WHO. Growth Reference Data for 5–19 Years. 2007. Available online: https://www.who.int/growthref/who2007_bmi_for_age/en/ (accessed on 1 July 2025).
- WHO. Oral Health Surveys: Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013. Available online: https://iris.who.int/bitstream/handle/10665/97035/9789241548649_eng.pdf (accessed on 22 August 2025).
- Koivusilta, L.; Honkala, S.; Honkala, E.; Rimpela, A. Toothbrushing as part of the adolescent lifestyle predicts education level. J. Dent. Res. 2003, 82, 361–366. [Google Scholar] [CrossRef]
- Kopycka-Kedzierawski, D.T.; Billings, R.J.; Feng, C.; Ragusa, P.G.; Flint, K.; Watson, G.E.; Wong, C.L.; Manning, S.; Gill, S.R.; O’Connor, T.G. A Prospective Longitudinal Study of Early Childhood Caries Onset in Initially Caries-Free Children. JDR Clin. Trans. Res. 2023, 8, 394–401. [Google Scholar] [CrossRef]
- Ibrahim, S.; Nishimura, M.; Matsumura, S.; Rodis, O.M.M.; Nishida, A.; Yamanaka, K.; Shimono, T. A longitudinal study of early childhood caries risk, dental caries, and lifestyle. Pediatr. Dent. J. 2009, 19, 174–180. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Antoniadou, M.; Amargianitakis, M.; Gortzi, O.; Androutsos, O.; Varzakas, T. Nutritional Factors Associated with Dental Caries across the Lifespan: A Review. Appl. Sci. 2023, 13, 13254. [Google Scholar] [CrossRef]
- Bashirian, S.; Shirahmadi, S.; Seyedzadeh-Sabounchi, S.; Soltanian, A.R.; Karimi-Shahanjarini, A.; Vahdatinia, F. Association of caries experience and dental plaque with sociodemographic characteristics in elementary school-aged children: A cross-sectional study. BMC Oral Health 2018, 18, 7. [Google Scholar] [CrossRef]
- Coelho, M. ICDAS and DMFT/dmft. sensitivity and specificity, the importance of the index used: A systematic review. J. Dent. Public Health 2020, 11, 176–187. [Google Scholar] [CrossRef]
- Arslan, Z.B. Evaluation of the Relationship Between Oral Health and Body Mass Index. Eurasian J. Med. 2023, 55, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, C.; Chen, H. Global, regional, and national caries of permanent teeth incidence, prevalence, and disability-adjusted life years, 1990-2021: Analysis for the global burden of disease study. BMC Oral Health 2025, 25, 715. [Google Scholar] [CrossRef]
- Köksal, E.; Tekçiçek, M.; Yalcın, S.S.; Tuğrul, B.; Yalçın, S.; Pekcan, G. Association between anthropometric measurements and dental caries in Turkish school children. Cent. Eur. J. Public Health 2011, 19, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, E.; Şimşek, M.; Gündoğar, Z.; Aktan, A.M. Oral health survey of children referring to faculty of dentistry in Gaziantep. Gaziantep Med. J. 2015, 21, 118–124. [Google Scholar] [CrossRef]
- Güler, Ç.; Eltas, A.; Güneş, D.; Görgen, V.; Ersöz, M. Evaluation of oral dental health status of the children aged among 7–14 years in Malatya. İnönü Üniversitesi Sağlık Bilim. Derg. 2012, 2, 19–24. [Google Scholar]
- Huew, R.; Elsheibani, S.; Buzaribah, K.; Mansur, E. Deciduous and permanent dental caries status among primary schoolchildren of libya: A cross-sectional study. Int. J. Sci. Res. Arch. 2023, 8, 804–811. [Google Scholar] [CrossRef]
- Aqeeli, A.; Alsharif, A.T.; Kruger, E.; Tennant, M.; Bakeer, H. Caries prevalence and severity in association with sociodemographic characteristics of 9-to-12-year-old school children in Al-Madinah, Saudi Arabia. Saudi Dent. J. 2021, 33, 897–903. [Google Scholar] [CrossRef]
- Singh, A.; Peres, M.A.; Watt, R.G. The Relationship between Income and Oral Health: A Critical Review. J. Dent. Res. 2019, 98, 853–860. [Google Scholar] [CrossRef]
- Van Chuyen, N.; Van Du, V.; Van Ba, N.; Long, D.D.; Son, H.A. The prevalence of dental caries and associated factors among secondary school children in rural highland Vietnam. BMC Oral Health 2021, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Brandt, K.; Wienke, A.; Schaller, H.G. Regional Disparities in Caries Experience and Associating Factors of Ghanaian Children Aged 3 to 13 Years in Urban Accra and Rural Kpando. Int. J. Environ. Res. Public Health 2022, 19, 5771. [Google Scholar] [CrossRef]
- Ballo, L.; Arheiam, A.; Marhazlinda, J. Determinants of caries experience and the impact on the OHRQOL of 6-year-old Libyan children: A cross-sectional survey. BMC Oral Health 2021, 21, 320. [Google Scholar] [CrossRef]
- Sanjulián, L.; Fernández-Rico, S.; González-Rodríguez, N.; Cepeda, A.; Miranda, J.M.; Fente, C.; Lamas, A.; Regal, P. The Role of Dairy in Human Nutrition: Myths and Realities. Nutrients 2025, 17, 646. [Google Scholar] [CrossRef]
- Shkembi, B.; Huppertz, T. Impact of dairy products and plant-based alternatives on dental health: Food matrix effects. Nutrients 2023, 15, 1469. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, F.; Bourgeois, D.; Orilisi, G.; Orsini, G.; Carrouel, F. Non-Cariogenic Effect of Milk and Dairy Products on Oral Health in Children and Adolescents: A Scoping Review. Children 2024, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Grohe, B.; Mittler, S. Advanced non-fluoride approaches to dental enamel remineralization: The next level in enamel repair management. Biomater. Biosyst. 2021, 4, 100029. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Yin, I.X.; Yu, O.Y.; Chan, A.K.Y.; Chu, C.H. Preventing Dental Caries with Calcium-Based Materials: A Concise Review. Inorganics 2024, 12, 253. [Google Scholar] [CrossRef]
- Grenov, B.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Role of Milk and Dairy Products in Growth of the Child. Nestle Nutr. Inst. Workshop Ser. 2020, 93, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Esin, K.; Ballı-Akgöl, B.; Sözlü, S.; Kocaadam-Bozkurt, B. Association between dental caries and adherence to the Mediterranean diet, dietary intake, and body mass index in children. BMC Oral Health 2024, 24, 297. [Google Scholar] [CrossRef]
- Bassa, S.; Workie, S.B.; Kassa, Y.; Tegbaru, D.W. Prevalence of dental caries and relation with nutritional status among school-age children in resource-limited setting of southern Ethiopia. BMC Oral Health 2023, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Ma, T.; Liang, Q.; Sun, J.; Wu, X.; Song, Y.; Nie, H.; Huang, J.; Mu, G. Fermented Dairy Products as Precision Modulators of Gut Microbiota and Host Health: Mechanistic Insights, Clinical Evidence, and Future Directions. Foods 2025, 14, 1946. [Google Scholar] [CrossRef] [PubMed]
- Lempert, S.M.; Christensen, L.B.; Froberg, K.; Raymond, K.; Heitmann, B.L. Association between dairy intake and caries among children and adolescents. results from the Danish EYHS follow-up study. Caries Res. 2015, 49, 251–258. [Google Scholar] [CrossRef]
- Raj, A.; Kashyap, S.; Kundra, K.; Kandari, S.; Rela, R.; Naz, F. Correlation between bmi, caries prevalence, and sugar-containing beverage intake in 6–10 year old children. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. S1), S991–S994. [Google Scholar] [CrossRef]
- Petti, S.; Simonetti, R.; SimonettiD’Arca, A. The effect of milk and sucrose consumption on caries in 6-to-11-year-old Italian schoolchildren. Eur. J. Epidemiol. 1997, 13, 659–664. [Google Scholar] [CrossRef]
- Monteagudo, C.; Téllez, F.; Heras-González, L.; Ibañez-Peinado, D.; Mariscal-Arcas, M.; Olea-Serrano, F. School dietary habits and incidence of dental caries. Nutr. Hosp. 2015, 32, 383–388. [Google Scholar]
- Hooley, M.; Skouteris, H.; Boganin, C.; Satur, J.; Kilpatrick, N. Body mass index and dental caries in children and adolescents: A systematic review of literature published 2004 to 2011. Syst. Rev. 2012, 1, 57. [Google Scholar] [CrossRef]
- Sharma, S.; Hegde, A.M. Relationship between body mass index, caries experience and dietary preferences in children. J. Clin. Pediatr. Dent. 2009, 34, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Alm, A.; Fåhraeus, C.; Wendt, L.K.; Koch, G.; Andersson-Gäre, B.; Birkhed, D. Body adiposity status in teenagers and snacking habits in early childhood in relation to approximal caries at 15 years of age. Int. J. Paediatr. Dent. 2008, 18, 189–196. [Google Scholar] [CrossRef]
- Paisi, M.; Kay, E.; Bennett, C.; Kaimi, I.; Witton, R.; Nelder, R.; Lapthorne, D. Body mass index and dental caries in young people: A systematic review. BMC Pediatr. 2019, 19, 122. [Google Scholar] [CrossRef]
- Alkarimi, H.A.; Watt, R.G.; Pikhart, H.; Sheiham, A.; Taskos, G. Dental caries and growth in school-age children. Pediatrics 2014, 133, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.; Ristic-Medic, D.; Vucic, V.; Mitrovic, G.; NkolicIvosevic, J.; Peric, T.; Karadzic, I. Association between being overweight and oral health in Serbian schoolchildren. Int. J. Paediatr. Dent. 2015, 25, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Herlo, L.F.; Sălcudean, A.; Sîrli, R.; Iurciuc, S.; Herlo, A.; Nelson-Twakor, A.; Alexandrescu, L.; Dumache, R. Gut microbiota signatures in colorectal cancer as a potential diagnostic biomarker in the future: A systematic review. Int. J. Mol. Sci. 2024, 25, 7937. [Google Scholar] [CrossRef]
- Sălcudean, A.; Lică, M.M. The role of systemic family psychotherapy in glycemic control for children with type 1 diabetes. Children 2024, 11, 104. [Google Scholar] [CrossRef]
- Tilincă, M.C.; Antal, C.; Balint, A.; Sălcudean, A.; Varga, A. The newest therapeutically approach of “diabesity” using GLP-1 RA molecules: Impact of the oral formulation. Farmacia 2023, 71, 1–10. [Google Scholar] [CrossRef]
- Yen, C.E.; Lin, Y.Y.; Hu, S.W. Anthropometric status, diet, and dental caries among schoolchildren. Int. J. Environ. Res. Public Health 2021, 18, 7027. [Google Scholar] [CrossRef]
- Manohar, N.; Hayen, A.; Scott, J.A.; Do, L.G.; Bhole, S.; Arora, A. Impact of Dietary Trajectories on Obesity and Dental Caries in Preschool Children: Findings from the Healthy Smiles Healthy Kids Study. Nutrients 2021, 13, 2240. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.J. Acceptability of delivery of dietary advice in the dentistry setting to address obesity in pre-school children: A case study of the Common Risk Factor Approach. Public Health Nutr. 2015, 18, 1801–1806. [Google Scholar] [CrossRef]
- Deng, Q.; Wong, H.M.; Peng, S. Alterations in salivary profile in individuals with dental caries and/or obesity: A systematic review and meta-analysis. J. Dent. 2024, 151, 105451. [Google Scholar] [CrossRef]
- Verma, A.; Priyank, H.P.R.; Kumari, M.; Sayed Abdul, N.; Shivakumar, S. A Systematic Review and Meta-Analysis on Oral Health Disparities Among the Indigenous Paediatric Population. Cureus. 2023, 15, e41673. [Google Scholar] [CrossRef] [PubMed]
- Hubert, H.; Guinhouya, C.B.; Allard, L.; Durocher, A. Comparison of the diagnostic quality of body mass index, waist circumference and waist-toheight ratio in screening skinfold-determined obesity among children. J. Sci. Med. Sport 2021, 12, 449–451. [Google Scholar] [CrossRef]
- Peng, S.M.; Wong, H.M.; King, N.M.; McGrath, C. Is dental caries experience associated with adiposity status in preschool children? Int. J. Pediatr. Dent. 2014, 24, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.M.; Wong, H.M.; King, N.M.; Mcgrath, C. Association between dental caries and adiposity status (general, central, and peripheral adiposity) in 12-year-old children. Caries Res. 2014, 48, 32–38. [Google Scholar] [CrossRef]
- Rafitha, H.; Machmud, P.B.; Djuwita, R.; Gayatri, D.; Ayub, F.A. Overweight and Obesity Status with Dental Caries among Children Aged 7–12 Years Old in Badung District, Bali 2018. Kesmas 2019, 14, 65–69. [Google Scholar] [CrossRef]
- Alswat, K.; Mohamed, W.S.; Wahab, M.A.; Aboelil, A.A. The Association Between Body Mass Index and Dental Caries: Cross-Sectional Study. J. Clin. Med. Res. 2016, 8, 147–152. [Google Scholar] [CrossRef]
- Nagy-Pénzes, G.; Vincze, F.; Sándor, J.; Bíró, É. Does Better Health-Related Knowledge Predict Favorable Health Behavior in Adolescents? Int. J. Environ. Res. Public Health 2020, 17, 1680. [Google Scholar] [CrossRef]
- Salcudean, A.; Popovici, R.A.; Pitic, D.E.; Sarbu, D.; Boroghina, A.; Jomaa, M.; Salehi, M.A.; Kher, A.A.M.; Lica, M.M.; Bodo, C.R.; et al. Unraveling the complex interplay between neuroinflammation and depression: A comprehensive review. Int. J. Mol. Sci. 2025, 26, 1645. [Google Scholar] [CrossRef]
- Salcudean, A.; Osz, B.E.; Bodo, C.R.; Muntean, D.L. Serum serotonin level can be used as a predictive marker for depression in patients with type 2 diabetes mellitus. True or false? Farmacia 2024, 72, 1283–1289. [Google Scholar] [CrossRef]
- Chen, L.; Hong, J.; Xiong, D.; Zhang, L.; Li, Y.; Huang, S.; Hua, F. Are parents’ education levels associated with either their oral health knowledge or their children’s oral health behaviors? A survey of 8446 families in Wuhan. BMC Oral Health 2020, 20, 203. [Google Scholar] [CrossRef]
- van der Tas, J.T.; Kragt, L.; Elfrink, M.E.C.; Bertens, L.C.M.; Jaddoe, V.W.V.; Moll, H.A.; Ongkosuwito, E.M.; Wolvius, E.B. Social inequalities and dental caries in six-year-old children from the Netherlands. J. Dent. 2017, 62, 18–24. [Google Scholar] [CrossRef]
- Marshall, T.A.; Eichenberger-Gilmore, J.M.; Broffitt, B.A.; Warren, J.J.; Levy, S.M. Dental caries and childhood obesity: Roles of diet and socioeconomic status. Community Dent. Oral Epidemiol. 2007, 35, 449–458. [Google Scholar] [CrossRef]
- Kalinovic, R.; Pascariu, A.; Vlad, G.; Nitusca, D.; Salcudean, A.; Sirbu, I.O.; Marian, C.; Enatescu, V.R. Involvement of the expression of G protein-coupled receptors in schizophrenia. Pharmaceuticals 2024, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Salcudean, A.; Strete, G.; Enatescu, V.R.; Tilinca, M.C.; Sasu, A.B.; Ceana, D.E.; Kiss, M.; Dumache, R.; Ősz, B.E.; Teodorescu, D.R.; et al. Pilot study to evaluate adherence to treatment using the MARS scale on a sample of Romanian patients with cardiovascular diseases. Farmacia 2024, 72, 66–77. [Google Scholar] [CrossRef]
- Salcudean, A.; Osz, B.E.; Nan, A.G.; Sasu, A.B.; Cozma, M.M.; Tilinca, M.C.; Bocsak-Pop, A.R.; Strete, E.G. The efficacy of antidepressive treatment on COVID-19 pandemic-related depressive symptoms—A prospective, single-center, questionnaire-based study. Farmacia 2023, 71, 631–637. [Google Scholar] [CrossRef]
- Rachita, A.I.C.; Strete, G.E.; Salcudean, A.; Ghiga, D.V.; Radulescu, F.; Calinescu, M.; Nan, A.G.; Sasu, A.B.; Suciu, L.M.; Marginean, C. Prevalence and risk factors of depression and anxiety among women in the last trimester of pregnancy: A cross-sectional study. Medicina 2023, 59, 1009. [Google Scholar] [CrossRef] [PubMed]
- Folayan, M.O.; Kolawole, K.A.; Oyedele, T.; Chukwumah, N.M.; Onyejaka, N.; Agbaje, H.; Oziegbe, E.O.; Oshomoji, O.V. Association between knowledge of caries preventive practices, preventive oral health habits of parents and children and caries experience in children resident in suburban Nigeria. BMC Oral Health 2014, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, R.; Sava-Rosianu, R.; Jumanca, D.; Balean, O.; Damian, L.R.; Fratila, A.D.; Maricutoiu, L.; Hajdu, A.I.; Focht, R.; Dumitrache, M.A.; et al. The Impact of Parental Education on Schoolchildren’s Oral Health-A Multicenter Cross-Sectional Study in Romania. Int. J. Environ. Res. Public Health 2022, 19, 11102. [Google Scholar] [CrossRef]
- Perpelea, A.-C.; Sfeatcu, R.; Tănase, M.; Meleșcanu Imre, M.; Ripszky Totan, A.; Cernega, A.; Funieru, C.; Pițuru, S.-M. A STEPwise Approach for Oral Hygiene Behavior of Schoolchildren in Romania. Healthcare 2024, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Sfeatcu, R.; Cărămidă, M.; Sava-Rosianu, R.; Matichescu, M.L.; Galuscan, A.; Dumitrache, M.A. Carious status and socio-behavioral risk factors among 12 year-old children in South-Central region in Romania. BMC Oral Health. 2023, 23, 644. [Google Scholar] [CrossRef]
- Bramantoro, T.; Santoso, C.M.A.; Hariyani, N.; Setyowati, D.; Zulfiana, A.A.; Nor, N.A.M.; Nagy, A.; Pratamawari, D.N.P.; Irmalia, W.R. Effectiveness of the school-based oral health promotion programmes from preschool to high school: A systematic review. PLoS ONE 2021, 16, e0256007. [Google Scholar] [CrossRef]
- Cuculescu, M.; Slusanschi, O.; Boscaiu, V.R.; Luis, H.P.S.; Fernandes Ribeiro Graça, S.M.; Ramos Esteves Gonçalves Dos Santos Albuquerque, T.M.B.; Abreu Assunção, V.; Galuscan, A.; Podariu, A.C.; Malmqvist, S.; et al. Self-reported oral health-related habits, attitudes and knowledge in adults from Portugal, Romania and Sweden-A comparative study. Int. J. Dent. Hyg. 2019, 17, 359–368. [Google Scholar] [CrossRef]
- Ondine Lucaciu, P.; Mester, A.; Constantin, I.; Orban, N.; Cosma, L.; Candrea, S.; Sava-Rosianu, R.; Mesaros, A.S. A WHO Pathfinder Survey of Dental Caries in 6 and 12-Year Old Transylvanian Children and the Possible Correlation with Their Family Background, Oral-Health Behavior, and the Intake of Sweets. Int. J. Environ. Res. Public Health 2020, 17, 4180. [Google Scholar] [CrossRef]
- Roșioară, A.-I.; Năsui, B.A.; Ciuciuc, N.; Sîrbu, D.M.; Curșeu, D.; Pop, A.L.; Popescu, C.A.; Popa, M. Status of Healthy Choices, Attitudes and Health Education of Children and Young People in Romania—A Literature Review. Medicina 2024, 60, 725. [Google Scholar] [CrossRef]
- Funieru, C.; Twetman, S.; Funieru, E.; Dumitrache, A.M.; Sfeatcu, R.I.; Baicus, C. Caries experience in schoolchildren in Bucharest, Romania: The PAROGIM study. J. Public Health Dent. 2014, 74, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yekaninejad, M.S.; Eshraghian, M.R.; Nourijelyani, K.; Mohammad, K.; Foroushani, A.R.; Zayeri, F.; Pakpour, A.H.; Moscowchi, A.; Tarashi, M. Effect of a school-based oral health-education program on Iranian children: Results from a group randomized trial. Eur. J. Oral Sci. 2012, 120, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Cernega, A.; Mincă, D.G.; Furtunescu, F.L.; Radu, C.P.; Pârvu, S.; Pițuru, S.M. The Predictability of the Dental Practitioner in a Volatile Healthcare System: A 25-Year Study of Dental Care Policies in Romania (1999–2023). Healthcare 2025, 13, 249. [Google Scholar] [CrossRef]
| Characteristics | Bistriţa-Năsăud County, n (%) (n = 550) | Mureş County, n (%) (n = 550) |
|---|---|---|
| Sex | ||
| Male | 278 (50.5) | 276 (50.2) |
| Female | 272 (49.5) | 274 (49.8) |
| Age | ||
| 6 years | 275 (50) | 275 (50) |
| 10 years | 275 (50) | 275 (50) |
| Mother’s educational level | ||
| Primary level | 221 (40.2) | 98 (17.9) |
| Secondary level | 179 (32.5) | 168 (30.5) |
| Higher level | 150 (27.3) | 284 (51.6) |
| Father’s educational level | ||
| Primary level | 234 (42.5) | 87 (15.8) |
| Secondary level | 195 (35.5) | 202 (36.7) |
| Higher level | 121 (22.0) | 261 (47.5) |
| Mother’s employment status | ||
| Employed | 358 (65.1) | 387 (70.4) |
| Unemployed | 192 (34.9) | 163 (29.6) |
| Father’s employment status | ||
| Employed | 408 (74.2) | 417 (75.8) |
| Unemployed | 142 (25.8) | 133 (24.2) |
| Oral Health Behaviors | Children from Bistriţa-Năsăud County (n = 550) | Children from Mureş County (n = 550) | Total of School-Age Children (n = 1100) | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Tooth brushing frequency | |||||||
| Once/day | 262 | 47.6 | 220 | 40.0 | 477 | 43.4 | 0.177 a |
| Twice/day | 131 | 23.8 | 194 | 35.2 | 340 | 30.9 | |
| Irregular | 157 | 28.6 | 136 | 24.7 | 283 | 25.7 | |
| Using fluoride toothpaste | |||||||
| Yes | 387 | 70.4 | 408 | 74.2 | 795 | 72.3 | 0.608 a |
| No | 105 | 19.1 | 105 | 19.1 | 210 | 19.1 | |
| I do not know | 58 | 10.5 | 37 | 6.7 | 95 | 8.6 | |
| Frequency of dentist visits | |||||||
| Once/year | 37 | 6.7 | 5 | 0.9 | 42 | 3.8 | 0.005 b* |
| Twice/year | 27 | 4.9 | 84 | 15.3 | 110 | 10.0 | |
| When is a problem | 486 | 88.4 | 461 | 83.8 | 948 | 86.2 | |
| Daily Consumption | Bistriţa-Năsăud Children (n = 275) | Mureş Children (n = 275) | Daily Consumption | Bistriţa-Năsăud Children (n = 275) | Mureş Children (n = 275) |
|---|---|---|---|---|---|
| DMFT Mean ± SD | DMFT Mean ± SD | dmft Mean ± SD | dmft Mean ± SD | ||
| Milk | Milk | ||||
| Yes (n = 236) | 1.86 ± 1.7 | 2.66 ± 2.4 | Yes (n = 210) | 6.20 ± 3.4 | 16.47 ± 12.4 |
| No (n = 314) | 2.20 ± 2.0 | 3.49 ± 3.4 | No (n = 259) | 6.53 ± 3.4 | 17.20 ± 12.4 |
| p | 0.273 | 0.087 | p | 0.567 | 0.888 |
| Yogurt | Yogurt | ||||
| Yes (n = 160) | 2.03 ± 1.9 | 2.48 ± 2.7 | Yes (n = 137) | 6.32 ± 3.7 | 14.79 ± 12.2 |
| No (n = 390) | 2.24 ± 1.9 | 3.23 ± 3.8 | No (n = 332) | 6.40 ± 3.2 | 16.16 ± 11.4 |
| p | 0.285 | 0.740 | p | 0.897 | 0.055 |
| Cheese | Cheese | ||||
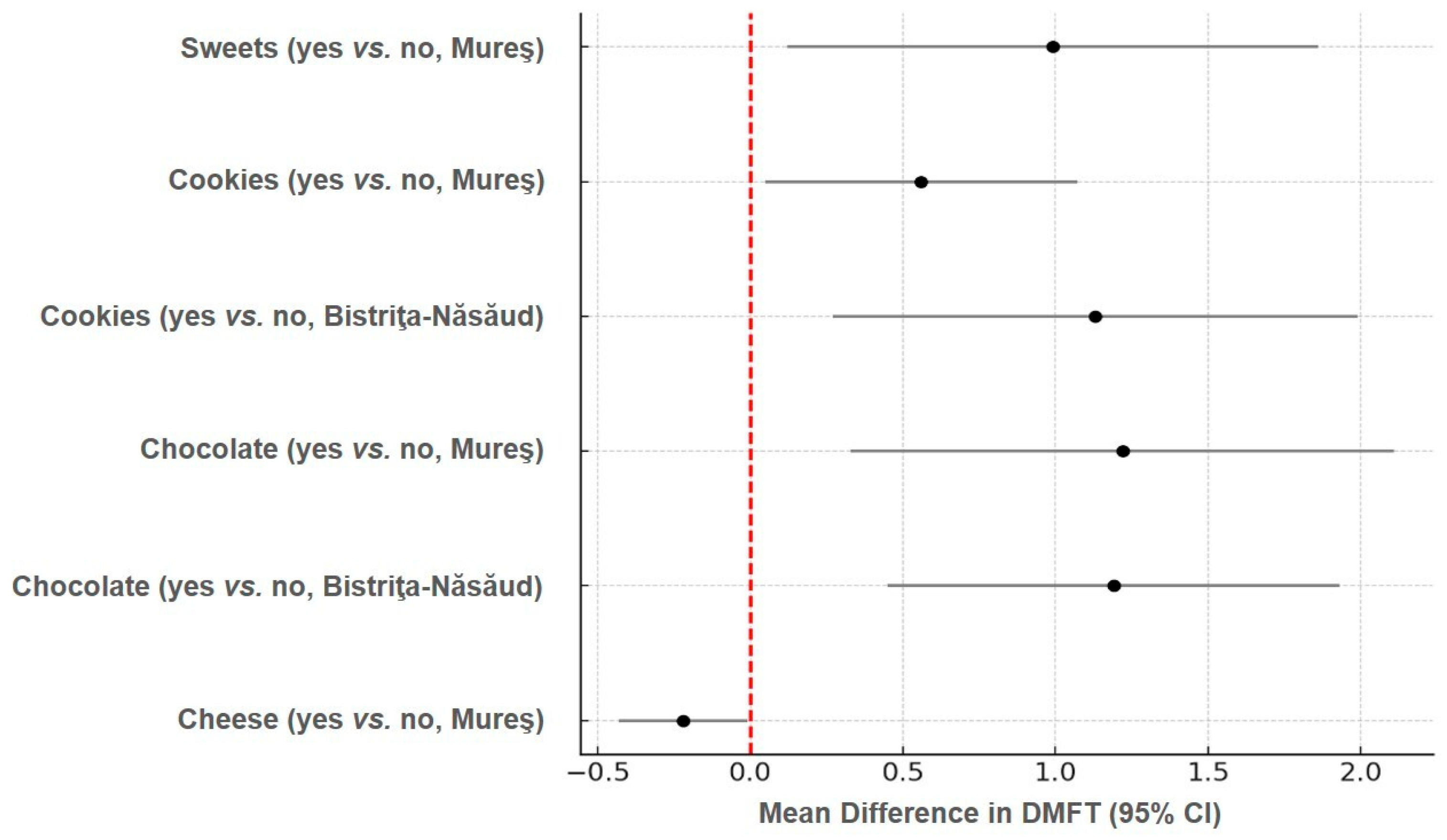
| Yes (n = 257) | 1.95 ± 1.8 | 2.99 ± 2.7 | Yes (n = 223) | 6.30 ± 3.4 | 16.91 ± 12.3 |
| No (n = 293) | 2.17 ± 1.9 | 5.02 ± 4.9 | No (n = 246) | 6.36 ± 3.4 | 17.77 ± 12.5 |
| p | 0.413 | 0.036 * | p | 0.908 | 0.474 |
| Other dairy products | Other dairy products | ||||
| Yes (n = 84) | 1.83 ± 1.6 | 2.61 ± 2.7 | Yes (n = 76) | 5.80 ± 3.4 | 14.29 ± 12.7 |
| No (n = 466) | 2.11 ± 1.9 | 3.23 ± 3.7 | No (n = 393) | 6.43 ± 3.5 | 17.91 ± 12.2 |
| p | 0.440 | 0.309 | 0.370 | 0.786 |
| Daily Consumption | Bistriţa-Năsăud Children (n = 275) | Mureş Children (n = 275) | Daily Consumption | Bistriţa-Năsăud Children (n = 275) | Mureş Children (n = 275) |
|---|---|---|---|---|---|
| DMFT Mean ± SD | DMFT Mean ± SD | dmft Mean ± SD | dmft Mean ± SD | ||
| Sugar | Sugar | ||||
| Yes (n = 162) | 2.29 ± 1.9 | 3.43 ± 2.4 | Yes (n = 136) | 6.35 ± 3.4 | 18.16 ± 13.6 |
| No (n = 388) | 1.98 ± 1.9 | 3.0 ± 1.6 | No (n = 333) | 6.28 ± 3.4 | 16.97 ± 11.8 |
| p | 0.288 | 0.432 | p | 0.897 | 0.566 |
| Chocolate | Chocolate | ||||
| Yes (n = 55) | 3.14 ± 1.8 | 5.43 ± 4.6 | Yes (n = 50) | 5.31 ± 2.9 | 14.99 ± 10.6 |
| No (n = 495) | 1.95 ± 1.9 | 2.88 ± 2.3 | No (n = 419) | 6.45 ± 3.5 | 17.60 ± 12.5 |
| p | 0.011 * | 0.007 * | p | 0.173 | 0.336 |
| Sweets | Sweets | ||||
| Yes (n = 13) | 3.65 ± 2.3 | 7.65 ± 7.3 | Yes (n = 13) | 5.25 ± 4.4 | 17.05 ± 16.5 |
| No (n = 537) | 2.0 ± 1.9 | 3.02 ± 2.4 | No (n = 456) | 6.36 ± 3.4 | 17.33 ± 12.3 |
| p | 0.066 | 0.009 * | p | 0.477 | 0.976 |
| Cookies | Cookies | ||||
| Yes (n = 31) | 3.13 ± 2.1 | 4.38 ± 3.7 | Yes (n = 24) | 8.49 ± 2.9 | 24.71 ± 8.2 |
| No (n = 519) | 2.0 ± 1.9 | 3.06 ± 2.9 | No (n = 445) | 6.22 ± 3.4 | 16.93 ± 10.4 |
| p | 0.009 * | 0.215 | 0.056 | 0.028 * |
| Age Group | County | Total Number of Children | Children with dmft/DMFT > 0 | Caries Prevalence (%) |
|---|---|---|---|---|
| 6 years | Bistriţa-Năsăud | 275 | 224 | 81.5% |
| 6 years | Mureş | 275 | 215 | 78.2% |
| 10 years | Bistriţa-Năsăud | 275 | 183 | 66.5% |
| 10 years | Mureş | 275 | 167 | 60.7% |
| Dental Health Indicators | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group of Bistriţa-Năsăud Children (n = 275) | Group of Mureş Children (n = 275) | Group of Bistriţa-Năsăud Children (n = 275) | Group of Mureş Children (n = 275) | |||||
| Body Composition Measurement | DMFT | DMFT | dmft | dmft | ||||
| r | p | r | p | r | p | r | p | |
| Height [cm] | 0.471 | 0.005 ** | 0.387 | 0.005 ** | –0.407 | 0.005 ** | –0.192 | 0.017 * |
| Weight [kg] | 0.414 | 0.005 ** | 0.320 | 0.005 ** | 0.350 | 0.005 ** | –0.154 | 0.048 * |
| Waist [cm] | 0.247 | 0.006 ** | 0.194 | 0.014 * | –0.217 | 0.018 * | –0.122 | 0.180 |
| The ratio of waist to hip | 0.123 | 0.264 | 0.190 | 0.043 * | –0.036 | 0.729 | –0.022 | 0.826 |
| Body fat [%] | 0.094 | 0.124 | 0.039 | 0.332 | –0.090 | 0.325 | –0.109 | 0.158 |
| BMI [kg/m2] | 0.228 | 0.007 ** | 0.161 | 0.045 * | –0.187 | 0.049 * | –0.130 | 0.322 |
| Independent Variable | Crude Odds Ratio (COR) | p | Adjusted Odds Ratio (AOR) | p |
|---|---|---|---|---|
| Mother’s education level | ||||
| Primary level | 3.45 (1.29, 9.39) | 0.017 * | 2.69 (0.82, 9.06) | 0.013 * |
| Secondary level | 1.81 (0.97, 3.61) | 0.036 * | 1.29 (0.51, 3.38) | 0.078 |
| Higher level | 1 | |||
| Father’s education level | ||||
| Primary level | 2.45 (0.51, 3.38) | 0.022 * | 1.42 (0.61, 3.37) | 0.063 |
| Secondary level | 1.55 (0.65, 3.78) | 0.073 | 1.22 (0.50, 3.10) | 0.176 |
| Higher level | 1 | |||
| Frequency of daily brushing | ||||
| Once/day | 1 | |||
| Twice/day | 0.92 (0.48, 1.80) | 0.498 | ||
| Irregular | 1.32 (0.68, 2.65) | 0.704 | ||
| Frequency of daily sugar intake | ||||
| Daily | 1.66 (0.81, 3.46) | 0.059 | 1.72 (0.85, 3.53) | 0.032 * |
| Occasional | 1 | |||
| Body fat ratio | 1.13 (1.08, 1.26) | 0.009 * | 1.17 (1.09, 1.27) | 0.008 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seni, A.-G.; Sălcudean, A.; Popovici, R.-A.; Cîmpian, D.-M.; Olariu, T.; Olariu, I.; Păcurar, M.; Kiș, A.M.; Bădoiu, S.-C.; Jinga, V.; et al. Associations Between Diet, Oral Health, and General Development in Romanian School-Age Children. Nutrients 2025, 17, 2832. https://doi.org/10.3390/nu17172832
Seni A-G, Sălcudean A, Popovici R-A, Cîmpian D-M, Olariu T, Olariu I, Păcurar M, Kiș AM, Bădoiu S-C, Jinga V, et al. Associations Between Diet, Oral Health, and General Development in Romanian School-Age Children. Nutrients. 2025; 17(17):2832. https://doi.org/10.3390/nu17172832
Chicago/Turabian StyleSeni, Ana-Gabriela, Andreea Sălcudean, Ramona-Amina Popovici, Dora-Mihaela Cîmpian, Teodora Olariu, Iustin Olariu, Mariana Păcurar, Andreea Mihaela Kiș, Silviu-Constantin Bădoiu, Viorel Jinga, and et al. 2025. "Associations Between Diet, Oral Health, and General Development in Romanian School-Age Children" Nutrients 17, no. 17: 2832. https://doi.org/10.3390/nu17172832
APA StyleSeni, A.-G., Sălcudean, A., Popovici, R.-A., Cîmpian, D.-M., Olariu, T., Olariu, I., Păcurar, M., Kiș, A. M., Bădoiu, S.-C., Jinga, V., Blidaru, A., Dumitrescu, S.-I., Anculia, R.-C., Forna, N., Todor, L., & Tarcea, M. (2025). Associations Between Diet, Oral Health, and General Development in Romanian School-Age Children. Nutrients, 17(17), 2832. https://doi.org/10.3390/nu17172832