Energy and Nutrient Intake Gaps and Socioeconomic Determinants of Ultra-Processed and Less-Processed Foods Consumed in Ethiopia: Evidence from National Food Consumption Survey
Abstract
1. Introduction
2. Materials and Methods
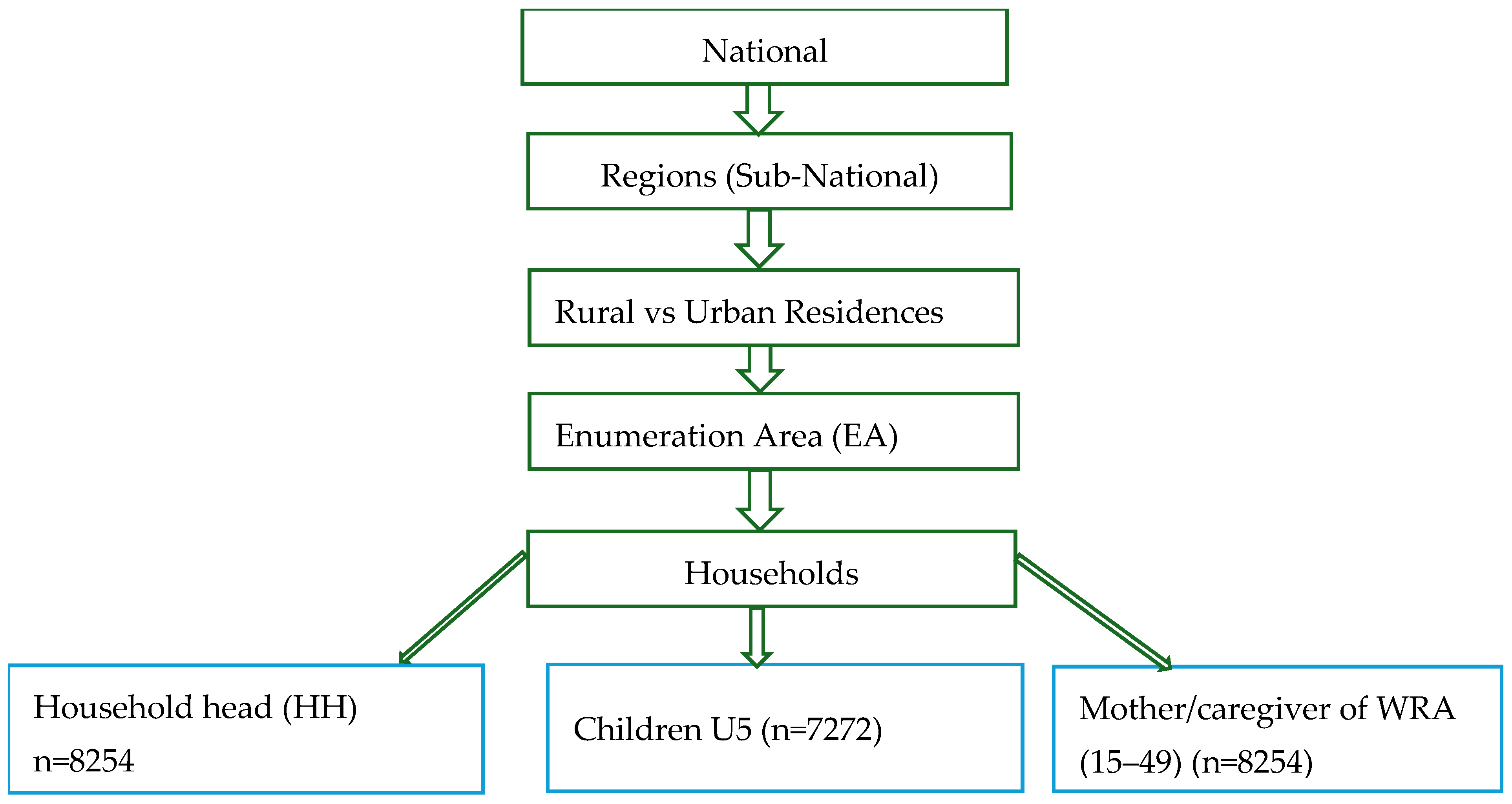
2.1. Survey Data Collection
2.2. Dietary Assessment and Food Classification
2.3. Anthropometric Measurement and Categorization
2.4. Household Socioeconomic Status (SES)
2.5. Statistical Analysis
β3xMother’s Education (vjk) + β4xResidence (vjk) + β5xmother’s Age (vjk) + uk)
β4xSocioeconomic Status (ijk) + β5xMothe’s Education (ijk) +
β6xMother’s Job (ijk) + uk + vjk)
3. Results
4. Discussion
5. Limitations and Strengths
6. Implications of the Study to UPF Intake
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marino, M.; Puppo, F.; Del Bo’, C.; Vinelli, V.; Riso, P.; Porrini, M.; Martini, D. A systematic review of worldwide consumption of ultra-processed foods: Findings and criticisms. Nutrients 2021, 13, 2778. [Google Scholar] [CrossRef]
- Gibney, M.J. Ultra-processed foods: Definitions and policy issues. Curr. Dev. Nutr. 2019, 3, nzy077. [Google Scholar] [CrossRef]
- Koiwai, K.; Takemi, Y.; Hayashi, F.; Ogata, H.; Matsumoto, S.; Ozawa, K.; MacHado, P.P.; Monteiro, C.A. Consumption of ultra-processed foods decreases the quality of the overall diet of middle-aged Japanese adults. Public Health Nutr. 2019, 22, 2999–3008. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Shim, S.Y.; Cha, H.J.; Kim, J.; Kim, H.C. Association between Ultra-processed Food Consumption and Dietary Intake and Diet Quality in Korean Adults. J. Acad. Nutr. Diet. 2022, 122, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Braesco, V.; Souchon, I.; Sauvant, P.; Haurogné, T.; Maillot, M.; Féart, C.; Darmon, N. Ultra-processed foods: How functional is the NOVA system? Eur. J. Clin. Nutr. 2022, 76, 1245–1253. [Google Scholar] [CrossRef]
- Popkin, B.M.; Barquera, S.; Corvalan, C.; Hofman, K.J.; Monteiro, C.; Ng, S.W.; Swart, E.C.; Taillie, L.S. Towards unified and impactful policies to reduce ultra-processed food consumption and promote healthier eating. Lancet Diabetes Endocrinol. 2021, 9, 462–470. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Reviews. Obes Rev. 2013, 14 (Suppl. S2), 21–28. [Google Scholar] [CrossRef]
- Da Costa Louzada, M.L.; Ricardo, C.Z.; Steele, E.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. The share of ultra-processed foods determines the overall nutritional quality of diets in Brazil. Public Health Nutr. 2018, 21, 94–102. [Google Scholar] [CrossRef]
- Petrus, R.R.; do Amaral Sobral, P.J.; Tadini, C.C.; Gonçalves, C.B. The NOVA classification system: A critical perspective in food science. Trends Food Sci. Technol. 2021, 116, 603–608. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Reardon, T.; Tschirley, D.; Liverpool-tasie, L.S.O.; Awokuse, T.; Fanzo, J.; Minten, B.; Vos, R.; Dolislager, M.; Sauer, C.; Dhar, R.; et al. The processed food revolution in African food systems and the double burden of malnutrition. Glob. Food Secur. 2021, 28, 100466. [Google Scholar] [CrossRef]
- Hussein, A.; Girma, M.; Samuel, A.; Habte, K.; Moges, T. Trends in Calorie Intake from Sugar-Sweetened Beverages and Sugar-Sweetened Snacks in Ethiopia (2010–2016); National Information Platforms for Nutrition: Lusaka, Zambia, 2021. [Google Scholar]
- GAIN. Food Systems Dashboard Ethiopia; GAIN: Geneva, Switzerland, 2024. [Google Scholar]
- GAIN. FSCI Indicators Breakdown; GAIN: Geneva, Switzerland, 2024; Available online: https://www.foodsystemsdashboard.org/countries/eth/fsci (accessed on 19 November 2024).
- Askari, M.; Heshmati, J.; Shahinfar, H.; Tripathi, N.; Daneshzad, E. Ultra-processed food and the risk of overweight and obesity: A systematic review and meta-analysis of observational studies. Int. J. Obes. 2020, 44, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Hajihashemi, P.; Mohammadifard, N.; Najafi, F.; Farshidi, H.; Lotfizadeh, M.; Kazemi, T.; Karimi, S.; Shirani, S.; Solati, K.; et al. Association between ultra-processed foods consumption and micronutrient intake and diet quality in Iranian adults: A multicentric study. Public Health Nutr. 2023, 26, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-processed foods and health outcomes: A narrative review. Nutrients 1997 2020, 12, 1955. [Google Scholar] [CrossRef]
- Silva Meneguelli, T.; Viana Hinkelmann, J.; Hermsdorff, H.H.M.; Zulet, M.Á.; Martínez, J.A.; Bressan, J. Food consumption by degree of processing and cardiometabolic risk: A systematic review. Int. J. Food Sci. Nutr. 2020, 71, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Almeida, L.B.; Scagliusi, F.B.; Duran, A.C.; Jaime, P.C. Barriers to and facilitators of ultra-processed food consumption: Perceptions of Brazilian adults. Public Health Nutr. 2018, 21, 68–76. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Rossi, V.; Santero, S.; Bianchi, A.; Zuccotti, G. Ultra-Processed Food, Reward System and Childhood Obesity. Children 2023, 10, 804. [Google Scholar] [CrossRef]
- Colozza, D. A qualitative exploration of ultra-processed foods consumption and eating out behaviours in an Indonesian urban food environment. Nutr. Health 2024, 30, 613–623. [Google Scholar] [CrossRef]
- Tseng, M.; Grigsby, C.J.; Austin, A.; Amin, S.; Nazmi, A. Sensory-Related Industrial Additives in the US Packaged Food Supply. Front. Nutr. 2022, 8, 762814. [Google Scholar] [CrossRef]
- Martins, C.A.; Andrade, G.C.; de Oliveira, M.F.B.; Rauber, F.; de Castro, I.R.R.; Couto, M.T.; Levy, R.B. “Healthy”, “usual” and “convenience” cooking practices patterns: How do they influence children’s food consumption? Appetite 2021, 158, 105018. [Google Scholar] [CrossRef]
- Trübswasser, U.; Verstraeten, R.; Salm, L.; Holdsworth, M.; Baye, K.; Booth, A.; Feskens, E.J.M.; Gillespie, S.; Talsma, E.F. Factors influencing obesogenic behaviours of adolescent girls and women in low- and middle-income countries: A qualitative evidence synthesis. Obes. Rev. 2021, 22, e13163. [Google Scholar] [CrossRef] [PubMed]
- Awedew, A.F.; Berheto, T.M.; Dheresa, M.; Tadesse, S.; Hailmariam, A.; Tollera, G.; Mohammed, S.; Acham, Y.; Walker, A.; Worku, A.; et al. The Burden of Non-Communicable Diseases and Its Implications for Sustainable Development Goals Across Regions in Ethiopia. Ethiop. J. Health Dev. 2023, 37, S1–S2. [Google Scholar]
- CSA. Analytical Report on The 2013 National Labour Force Survey; Central Statistical Agency: Addis Ababa, Ethiopia, 2014; pp. 1–116. [Google Scholar]
- Gibson, R.S.; Ferguson, E.L. An interactive 24-hour recall for assessing the adequacy of iron and zinc intakes in developing countries. Heat Transf. Eng. 2008, 10, 60–62. [Google Scholar]
- Woldeyohannes, M.; Girma, M.; Petros, A.; Hussen, A.; Samuel, A.; Dinssa, D.A.; Challa, F.; Laillou, A.; Chitekwe, S.; Baye, K.; et al. Ethiopia National Food and Nutrition Survey to inform the Ethiopian National Food and Nutrition Strategy: A study protocol. BMJ Open 2023, 13, e067641. [Google Scholar] [CrossRef]
- Coates, J.; Rogers, B.L.; Blau, A.; Lauer, J.; Roba, A. Filling a dietary data gap? Validation of the adult male equivalent method of estimating individual nutrient intakes from household-level data in Ethiopia and Bangladesh. Food Policy 2017, 72, 27–42. [Google Scholar] [CrossRef]
- EHNRI. Food Composition Table for Use in Ethiopia Part III and IV; EHNRI: Addis Ababa, Ethiopia, 1997. [Google Scholar]
- Allen, L. Guidelines on Food Fortification with Micronutrients [Internet]; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2006; 341p, Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=19d204d5568d78c25c7f3feedeb53c1ae87b5ce2 (accessed on 22 June 2025).
- FAO/WHO. Human Vitamin and Mineral Requirements. In Report of a Joint FAO/WHO Expert Consultation Bangkok, Thailand; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Institute of Medicine. Dietary reference intakes for energy 2021, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. In Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academies: Washington, DC, USA, 2005; 1331p. [Google Scholar] [CrossRef]
- WHO. Feeding and Nutrition of Infants and Young Children: Guidelines for the WHO European Region, with Emphasis on the Former Soviet Countries [Internet]. 2003. Available online: https://iris.who.int/bitstream/handle/10665/272658/9789289013543-eng.pdf?sequence=3&isAllowed=y (accessed on 11 May 2025).
- WHO. Vitamin and Mineral Requirements in Human Nutrition [Internet]; World Health Organization: Geneva, Switzerland, 2004; 341p, Available online: https://iris.who.int/bitstream/handle/10665/42716/9241546123.pdf (accessed on 11 May 2025).
- WHO. Carbohydrate Intake for Adults and Children WHO Guideline. 2023. Available online: https://iris.who.int/bitstream/handle/10665/370420/9789240073593-eng.pdf (accessed on 24 May 2025).
- Moubarac, J.-C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef]
- WHO. Malnutrition in Women [Internet]. 2024. Available online: https://www.who.int/data/nutrition/nlis/info/malnutrition-in-women (accessed on 14 December 2024).
- Gebretsadik, G.G.; Adhanu, A.K.; Mulugeta, A. Magnitude and determinants of animal source food consumption among children aged 6–23 months in Ethiopia: Secondary analysis of the 2016 Ethiopian demographic and health survey. BMC Public Health 2022, 22, 453. [Google Scholar] [CrossRef]
- USAID. The DHS Program, Wealth- Index-Construction [Internet]. 2016. Available online: https://dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm (accessed on 27 December 2023).
- WHO. WHO Updates Guidelines on Fats and Carbohydrates [Internet]. 2023. Available online: https://www.who.int/news/item/17-07-2023-who-updates-guidelines-on-fats-and-carbohydrates (accessed on 19 May 2025).
- García-Blanco, L.; de la Víctor, O.; Santiago, S.; Pouso, A.; Martínez-González, M.Á.; Martín-Calvo, N. High consumption of ultra-processed foods is associated with increased risk of micronutrient inadequacy in children: The SENDO project. Eur. J. Pediatr. 2023, 182, 3537–3547. [Google Scholar] [CrossRef]
- Katidi, A.; Vlassopoulos, A.; Noutsos, S.; Kapsokefalou, M. Ultra-Processed Foods in the Mediterranean Diet according to the NOVA Classification System; A Food Level Analysis of Branded Foods in Greece. Foods 2023, 12, 1520. [Google Scholar] [CrossRef]
- Marrón-Ponce, J.A.; Sánchez-Pimienta, T.G.; Da Costa Louzada, M.L.; Batis, C. Energy contribution of NOVA food groups and sociodemographic determinants of ultra-processed food consumption in the Mexican population. Public Health Nutr. 2018, 21, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Martínez Steele, E.; Popkin, B.M.; Swinburn, B.; Monteiro, C.A.; Steele, E.M.; Popkin, B.M.; Swinburn, B.; Monteiro, C.A. The share of ultra-processed foods and the overall nutritional quality of diets in the US: Evidence from a nationally representative cross-sectional study. Popul. Health Metr. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Romero Ferreiro, C.; Lora Pablos, D.; Gómez de la Cámara, A. Two dimensions of nutritional value: Nutri-score and nova. Nutrients 2021, 13, 2783. [Google Scholar] [CrossRef]
- Seid, Y.M. Innovations in Data Dissemination at the Central Statistical Agency of Ethiopia. IASSIST Q. Fall 2009, 33, 26. [Google Scholar] [CrossRef]
- Melesse, M.B.; de Brauw, A.; Abate, G.T. Understanding Urban Consumers’ Food Choice Behavior in Ethiopia: Promoting Demand for Healthy Foods [Internet]. Addis Ababa, Ethiopia; 2019. (131). Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/49c72416-20d1-4807-8bdc-6a12abfced94/content (accessed on 14 May 2025).
- Godbharle, S.; Kesa, H.; Jeyakumar, A.; Shambharkar, P. Socio-demographic and economic factors associated with the consumption of processed foods in South Africa—Evidence from Demographic and Health Survey VII. Public Health 2024, 226, 190–198. [Google Scholar] [CrossRef]
- Wanjohi, M.N.; Asiki, G.; Wilunda, C.; Holdsworth, M.; Pradeilles, R.; Paulo, L.S.; Langat, N.; Amugsi, D.A.; Kimenju, S.; Kimani-Murage, E.W.; et al. Ultra-Processed Food Consumption Is Associated With Poor Diet Quality and Nutrient Intake Among Adolescents in Urban Slums, Kenya. Int. J. Public Health 2024, 69, 1607891. [Google Scholar] [CrossRef] [PubMed]
- D’avila, H.F.; Kirsten, V.R. Energy intake from ultra-processed foods among adolescents. Rev. Paul. De Pediatr. 2017, 35, 54–60. [Google Scholar] [CrossRef]
- Simões, B.D.S.; Cardoso, L.D.O.; Benseñor, I.J.M.; Schmidt, M.I.; Duncan, B.B.; Luft, V.C.; del Carmen Bisi Molina, M.; Barreto, S.M.; Levy, R.B.; Giatti, L. O consumo de alimentos ultraprocessados e nível socioeconômico: Uma análise transversal do estudo longitudinal de Saúde do Adulto, Brasil. Cad. Saude Publica 2018, 34, 1–13. [Google Scholar] [CrossRef]
- Zapata, M.E.; Cediel, G.; Arrieta, E.; Rovirosa, A.; Carmuega, E.; Monteiro, C.A. Ultra-processed foods consumption and diet quality among preschool children and women of reproductive age from Argentina. Public Health Nutr. 2023, 26, 2304–2313. [Google Scholar] [CrossRef]
- Khandpur, N.; Cediel, G.; Obando, D.A.; Jaime, P.C.; Parra, D.C. Sociodemographic factors associated with the consumption of ultra-processed foods in Colombia. Rev. Saude Publica 2020, 54, 19. [Google Scholar] [CrossRef] [PubMed]
- Mediratta, S.; Ghosh, S.; Mathur, P. Intake of ultra-processed food, dietary diversity and the risk of nutritional inadequacy among adults in India. Public Health Nutr. 2023, 26, 2849–2858. [Google Scholar] [CrossRef]
- Marchese, L.; Livingstone, K.M.; Woods, J.L.; Wingrove, K.; MacHado, P. Ultra-processed food consumption, socio-demographics and diet quality in Australian adults. Public Health Nutr. 2022, 25, 94–104. [Google Scholar] [CrossRef]
- Colombet, Z.; Schwaller, E.; Head, A.; Kypridemos, C.; Capewell, S.; O’Flaherty, M. OP12 Social inequalities in ultra-processed food intakes in the United Kingdom: A time trend analysis (2008–2018). In Proceedings of the SSM Annual Scientific Meeting; Commercial Determinants of Health, Glasgow, UK, 30 October–30 December 2024. [Google Scholar] [CrossRef]
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition quality of food purchases varies by household income: The SHoPPER study. BMC Public Health 2019, 19, 231. [Google Scholar] [CrossRef]
- Sauer, C.M.; Reardon, T.; Tschirley, D.; Liverpool-Tasie, S.; Awokuse, T.; Alphonce, R.; Ndyetabula, D.; Waized, B. Consumption of processed food & food away from home in big cities, small towns, and rural areas of Tanzania. Agric. Econ. 2021, 52, 749–770. [Google Scholar] [CrossRef]
- de Paula Costa, D.V.; Lopes, M.S.; de Mendonça, R.D.; Malta, D.C.; de Freitas, P.P.; Lopes, A.C.S. Food consumption differences in brazilian urban and rural areas: The national health survey. Cienc. E Saude Coletiva 2021, 26, 3805–3813. [Google Scholar] [CrossRef]
- de Xavier, I.C.V.M.; Hardman, C.M.; de Andrade, M.L.S.S.; de Barros, M.V.G. Frequência de consumo de frutas, hortaliças e refrigerantes: Estudo comparativo entre adolescentes residentes em área urbana e rural. Rev. Bras. Epidemiol. 2014, 17, 371–380. [Google Scholar]
- Fasil, N.; Berhane, H.; Beyan, S.; Foti, K.; Khandpur, N.; Steffen, L.; Zhao, D.; Marklund, M.; Appel, L.; Henry, M. Predictors of Frequency of Ultra-Processed Food Purchase Among Adult Consumers in Addis Ababa, Ethiopia. Curr. Dev. Nutr. 2024, 8, 102935. [Google Scholar] [CrossRef]
- Costa, C.D.S.; Sattamini, I.F.; Steele, E.M.; Louzada, M.L.D.C.; Claro, R.M.; Monteiro, C.A. Consumption of ultra-processed foods and its association with sociodemographic factors in the adult population of the 27 Brazilian state capitals. Rev. Saude Publica 2021, 55, 47. [Google Scholar]
- Silveira, V.N.C.; dos Santos, A.M.; França, A.K.T.C. Determinants of the Consumption of ultra-Processed Foods in the Brazilian Population. Br. J. Nutr. 2024, 132, 1104–1109. Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/abs/determinants-of-the-consumption-of-ultraprocessed-foods-in-the-brazilian-population/C0A95F8C5C65B7B679837043DF6039E5#access-block (accessed on 16 May 2025). [CrossRef]
- dos Santos Costa, C.; Steele, E.M.; de Faria, F.R.; Monteiro, C.A. Score of ultra-processed food consumption and its association with sociodemographic factors in the Brazilian National Health Survey. Cad. Saude Publica 2019, 38, e00119421. [Google Scholar] [CrossRef]
- Magalhães, V.; Severo, M.; Correia, D.; Torres, D.; Costa De Miranda, R.; Rauber, F.; Levy, R.; Rodrigues, S.; Lopes, C. Associated factors to the consumption of ultra-processed foods and its relation with dietary sources in Portugal. J. Nutr. Sci. 2021, 10, e89. [Google Scholar] [CrossRef]
- Bookari, K. A cross-sectional exploratory study of food literacy among Saudi parents of adolescent children aged 10 to 19 years. Front. Nutr. 2023, 9, 1083118. [Google Scholar] [CrossRef]
- Baye, K.; Hirvonen, K.; Dereje, M.; Remans, R. Energy and nutrient production in Ethiopia, 2011–2015: Implications to supporting healthy diets and food systems. PLoS ONE 2019, 14, e0213182. [Google Scholar] [CrossRef]
- Worku, I.H.; Dereje, M.; Minten, B.; Hirvonen, K. Diet transformation in Africa: The case of Ethiopia. Agric. Econ. 2017, 48, 73–86. [Google Scholar] [CrossRef]
- Sheehy, T.; Carey, E.; Sharma, S.; Biadgilign, S. Trends in energy and nutrient supply in Ethiopia: A perspective from FAO food balance sheets. Nutr. J. 2019, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Moges, T.; Brouwer, I.D.; Delbiso, T.D.; Remans, R.; Baudron, F.; Belachew, T.; Groot, J.C.J. Spatial farming systems diversity and micronutrient intakes of rural children in Ethiopia. Matern. Child Nutr. 2022, 18, e13242. [Google Scholar] [CrossRef] [PubMed]
- Bosha, T.; Lambert, C.; Riedel, S.; Gola, U.; Melesse, A.; Biesalski, H.K. Validation of the CIMI-Ethiopia program and seasonal variation in maternal nutrient intake in enset (False banana) growing areas of Southern Ethiopia. Int. J. Environ. Res. Public Health 2019, 16, 2852. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, K.; Taffesse, A.S.; Worku Hassen, I. Seasonality and household diets in Ethiopia. Public Health Nutr. 2016, 19, 1723–1730. [Google Scholar] [CrossRef]
- Abegaz, G.A.; Hassen, I.W.; Minten, B. Consumption of Animal-Source Foods in Ethiopia: Patterns, Changes, and Determinants; The International Food Policy Research Institute: Washington, DC, USA, 2018; Volume 113. [Google Scholar]
- Zemene, M.A.; Kebede, N.; Anteneh, R.M.; Moges, N.; Tsega, S.S.; Dessie, A.M.; Belete, M.A.; Anley, D.T.; Alemayehu, E.; Chanie, E.S.; et al. Determinants of animal source food consumption among children aged 6–23 months in sub-Saharan Africa: Multilevel mixed effect model. Sci. Rep. 2024, 14, 26294. [Google Scholar] [CrossRef]
- Endawkie, A.; Gedefie, A.; Muche, A.; Mohammed, A.; Ayres, A.; Melak, D.; Abeje, E.T.; Bayou, F.D.; Belege Getaneh, F.; Asmare, L. Household- and community-level factors of zero vegetable or fruit consumption among children aged 6–23 months in East Africa. Front. Nutr. 2024, 11, 1363061. [Google Scholar] [CrossRef]
- Gelibo, T.; Amenu, K.; Taddele, T.; Taye, G.; Getnet, M.; Getachew, T.; Defar, A.; Teklie, H.; Bekele, A.; Shiferaw, F.; et al. Low fruit and vegetable intake and its associated factors in Ethiopia: A community based cross sectional NCD steps survey. Ethiop. J. Health Dev. 2017, 31, 355–361. [Google Scholar]
- Semagn, B.E.; Abubakari, A. Zero fruits/vegetables consumption and associated factors among Children aged 6–23 months in Ethiopia: Mixed effect logistic regression analysis. PLoS ONE 2023, 18, e0288732. [Google Scholar] [CrossRef]
- Baffa, L.D.; Angaw, D.A.; Abriham, Z.Y.; Gashaw, M.; Agimas, M.C.; Sisay, M.; Muhammad, E.A.; Mengistu, B.; Belew, A.K. Prevalence of iodine deficiency and associated factors among school-age children in Ethiopia: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Belachew, T.; Nida, H.; Getaneh, T.; Woldemariam, D.; Getinet, W. Calcium Deficiency and Causation of Rickets in Ethiopian Children. East Afr. Med. J. 2005, 82, 154–160. Available online: https://www.ajol.info/index.php/eamj/article/view/9273 (accessed on 24 May 2025).
- Belay, A.; Joy, E.J.M.; Chagumaira, C.; Zerfu, D.; Ander, E.L.; Young, S.D.; Bailey, E.H.; Lark, R.M.; Broadley, M.R.; Gashu, D. Selenium deficiency is widespread and spatially dependent in ethiopia. Nutrients 2020, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- EPHI. Ethiopian National Micronutrient Survey Report [Internet]. October 2016. Available online: https://www.exemplars.health/-/media/files/egh/resources/stunting/ethiopia/ethiopian-national-micronutrient-survey-report.pdf (accessed on 15 May 2025).
- Zerfu, T.; Girma, M.; Genye, T.; Muleta, A.; Tessema, M.; Samuel, A. Prevalence, Biomarkers, and Cut-Offs for Assessing Vitamin D Deficiency (VDD) in Ethiopia: A systematic Review and Meta-Analysis [Internet]. Addis Ababa; 2024. Available online: https://www.nipn.ephi.gov.et/sites/default/files/2025-04/VDD_2_pages_Taddese.pdf (accessed on 24 May 2025).
- Rahman, S.; Shaheen, N. Phytate-iron molar ratio and bioavailability of iron in Bangladesh. Trop. Med. Int. Health 2022, 27, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alba, V.; Lazarte, C.E.; Bergenståhl, B.; Granfeldt, Y. Phytate, iron, zinc, and calcium content of common Bolivian foods and their estimated mineral bioavailability. Food Sci. Nutr. 2019, 7, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Al Hasan, S.M.; Hassan, M.; Saha, S.; Islam, M.; Billah, M.; Islam, S. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: A cross-sectional study. BMC Nutr. 2016, 2, 24. [Google Scholar] [CrossRef]
- Abdulwaliyu, I.; Arekemase, S.O.; Adudu, J.A.; Batari, M.L.; Egbule, M.N.; Okoduwa, S.I.R. Investigation of the medicinal significance of phytic acid as an indispensable anti-nutrient in diseases. Clin. Nutr. Exp. 2019, 28, 42–61. [Google Scholar] [CrossRef]
| NOVA1 | NOVA2 | NOVA3 | NOVA4 | |
|---|---|---|---|---|
| Other names of NOVA foods | Unprocessed, minimally or less-processed foods | Processed culinary ingredients | Processed foods | Ultra-processed foods (UPF) |
| Definition of NOVA foods | Foods directly obtained from natural sources [9,10]. They are minimally processed or unprocessed foods; either consumed directly from the natural source or by removal of inedible/unwanted parts with or without the process of drying, powdering, squeezing, crushing, grinding, fractioning, steaming, poaching, boiling, roasting, pasteurization, chilling, freezing, vacuum packaging, non-alcoholic fermentation, etc., processes not adding salt, sugar, oils/fats. | Food ingredients that are not consumed alone but added as culinary ingredients. | For food products manufactured by industry, essentially, they essentially add salt, sugar, or other substances to processed foods to make them stable and more palatable. | Formulations or ingredients are typically created by a series of industrial techniques and processes. |
| Foods as an example | Fruit, seeds, leaves, stems, roots, tubers or animals (muscle, fat, offal, eggs, and milk); fungi, algae, and water after separation from nature [11]. | Oil, butter, lard, sugar, salt, spice, and herbs that used as culinary | Bottles of vegetables or legumes (pulses) preserved in brine and vinegar, fruits, syrups, meat products, and canned fish, smoked fish, freshly baked bread, and homemade cheeses with added salt [10]. | Sweets, fatty or salty packaged snacks, confectionaries and candies, packaged bread and buns, pastries, cookies, chips, biscuits, fish and poultry nuggets, carbonated and sugary drinks, margarines, other spreads, breakfast cereals, energy drinks, pizza, sausages, burgers, hot dogs, instant soups, baby formula, noodles, and desserts [10]. |
| Variable | Women (15–49 Years) | Children (6–45 Months) | ||||
|---|---|---|---|---|---|---|
| Level | Frequency | (%) | Level | Frequency | % | |
| Residence | Urban | 2142 | 28.1 | Urban | 1945 | 26.8 |
| Rural | 5492 | 71.9 | Rural | 5327 | 73.2 | |
| Age | Teen/young adult (15–21) | 1296 | 17.0 | 6–12 months | 1804 | 24.8 |
| Early adulthood (22–34) | 4982 | 65.3 | 13–24 months | 3102 | 42.66 | |
| Early–late middle age (35–45) | 1356 | 17.8 | 25–36 months | 2336 | 32.1 | |
| 37–45 months | 30 | 0.41 | ||||
| BMI | Thin (<18.5) | 2080 | 27.3 | Under wt | 337 | 4.6 |
| Normal (18.5–24.9) | 4824 | 63.4 | Normal | 5849 | 80.4 | |
| Over wt./obese (≥25) | 708 | 9.3 | Over wt/obese | 1086 | 14.9 | |
| Region | Tigray | 738 | 9.7 | Tigray | 724 | 10.0 |
| Afar | 550 | 7.2 | Afar | 563 | 7.7 | |
| Amhara | 979 | 12.8 | Amhara | 924 | 12.7 | |
| Oromia | 1027 | 13.5 | Oromia | 989 | 13.6 | |
| Somali | 646 | 8.5 | Somali | 642 | 8.8 | |
| Benishangul | 595 | 7.8 | Benishangul | 546 | 7.5 | |
| SNNPR | 897 | 11.8 | SNNPR | 947 | 13.0 | |
| Gambella | 490 | 6.4 | Gambella | 435 | 6.0 | |
| Harari | 475 | 6.2 | Harari | 419 | 5.8 | |
| Addis Ababa | 764 | 10.0 | Addis Ababa | 610 | 8.4 | |
| Dire Dawa | 473 | 6.2 | Dire Dawa | 473 | 6.5 | |
| Mother’s education | No formal education | 4,78 | 62.7 | Child sex | ||
| Primary school (G: 1–8) | 1820 | 23.8 | ||||
| High school (G: 9–12) | 792 | 10.4 | Male | 3888 | 53.5 | |
| Tertiary (G: >12) | 239 | 3.1 | Female | 3384 | 46.5 | |
| Mother’s Job | Employed | 2193 | 28.7 | |||
| Per time or irregularly paid | 1021 | 13.4 | ||||
| Housewife | 3447 | 45.2 | ||||
| Unpaid family worker | 973 | 12.8 | ||||
| Wealth Index (SES) | Poorest | 3024 | 39.6 | |||
| Middle | 1501 | 19.7 | ||||
| Richest | 3109 | 40.7 | ||||
| Frequency or Variety of Any Fruit Consumed p/24HDR | Frequency or Variety of Any Vegetables Consumed p/24HDR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | WRA | Children | WRA | ||||||||
| Diversity (n) and/or Frequency of Fruits Consumed | n | % | Diversity (n) and/or Frequency of Fruits Consumed | n | % | Diversity (n) and/or Frequency of Vegetables Consumed | n | % | Diversity (n) and/or Frequency of Vegetables Consumed | n | % |
| None consumed (0) | 6861 | 94.4 | None consumed (0) | 5430 | 65.79 | None consumed (0) | 2839 | 39.0 | None consumed (0) | 1989 | 24.1 |
| 1 | 300 | 4.1 | 1 | 1045 | 12.7 | 1 | 900 | 12.4 | 1 | 763 | 9.2 |
| 2 | 77 | 1.1 | 2 | 1039 | 12.6 | 2 | 1278 | 17.6 | 2 | 1587 | 19.2 |
| ≥3 | 34 | 0.5 | ≥3 | 740 | 9.0 | 3 | 800 | 11.0 | 3 | 1312 | 15.9 |
| 4 | 685 | 9.4 | 4 | 1055 | 12.8 | ||||||
| ≥5 | 770 | 10.6 | ≥5 | 1548 | 18.8 | ||||||
| NOVA Foods Consumed in WRA (n = 8254) | |||||
|---|---|---|---|---|---|
| Nutrient Types | NOVA1 | NOVA2 | NOVA3 | NOVA4 | NOVA Total |
| p50 (p25, p75, p95) | p50 (p25, p75, p95) | p50 (p25, p75, p95) | p50 (p25, p75, p95) | p50 (p25, p75, p95) | |
| Vit A (µg RAE) | 29.9 (4.1, 205.8, 1573.1) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0.0, 6.4) | 0 (0.0, 0.0, 0.0) | 32.7 (5.3, 207.0, 1576.6) |
| Thiamin, B1 (mg) | 1.2 (0.7, 1.8, 3.1) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0.0, 0.1) | 0 (0.0, 0.0, 0.0) | 1.2 (0.8, 1.8, 3.2) |
| Riboflavin, B2 (mg) | 0.9 (0.5, 1.4, 2.6) | 0.0 (0.0, 0.0, 0.01) | 0.0 (0.0, 0.0, 0.3) | 0 (0.0, 0.0, 0.0) | 0.9 (0.5, 1.4, 2.7) |
| Niacin, B3 (mg) | 7.8 (4.9, 12.1, 22.4) | 0.0 (0.0, 0.0, 0.1) | 0.0 (0.0, 0.0, 0.6) | 0 (0.0, 0.0, 0.0) | 8.0 (4.97, 12.4, 23.3) |
| Vit C (mg) | 21.7 (9.8, 46.8, 137.6) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0, 3.5) | 0 (0.0, 0.0, 0.0) | 22.8 (10.4, 48.1, 139.6) |
| Calcium (mg) | 332.4 (169.9, 598.0, 1473.5) | 1.2 (0.4, 2.5, 7.0) | 0.0 (0.0, 0.0, 60.2) | 0 (0.0, 0.0, 0.0) | 347.0 (180.9, 618.6, 1501.8) |
| Iron (mg) | 36.89 (21.42, 63.28, 144.97) | 0.02 (0.0, 0.04, 0.1) | 0.0 (0.0, 0.0, 4.5) | 0 (0.0, 0.0, 0.0) | 38.0 (22.2, 65.3, 147.8) |
| Zinc (mg) | 6.4 (3.5, 10.5, 20.5) | 0.0 (0.0, 0.0, 0.1) | 0.0 (0.0, 0.0, 0.4) | 0 (0.0, 0.0, 0.0) | 6.6 (3.7, 10.9, 21.3) |
| Energy (kcal) | 1349.8 (912.2, 1979.9, 3294.7) | 42.1 (0.0, 185.9, 627.9) | 0.0 (0.0, 0.0, 348.6) | 0.0 (0.0, 0.0, 263.4) | 1587.0 (1095.9, 2254.9, 3621.4) |
| Tot protein (kcal) | 145.9 (93.8, 217.7, 378.1) | 0.0 (0.0, 0.0, 0.7) | 0.0 (0.0, 0.0, 14.6) | 0.0 (0.0, 0.0, 0.0) | 150.7 (96.9, 223.9, 387.3) |
| Tot CHO (kcal) | 1069.7 (722.1, 1572.7, 2662.0) | 0.0 (0.0, 33.4, 255.2) | 0.0 (0.0, 0.0, 50.2) | 0.0 (0.0, 0.0, 0.0) | 1136.5 (778.2, 1648.2, 2715.6) |
| Total fat (kcal) | 106.5 (60.6, 186.3, 431.7) | 0.4 (0.0, 108.5, 503.5) | 0.0 (0.0, 0.0, 27.1) | 0.0 (0.0, 0.0, 254.8) | 211.9 (114.5, 380.3, 854.5) |
| Phy (mg) | 1135.1 (647.4, 1833.0, 3476.5) | 0.0 (0.0, 0.0, 0.0) | 0 (0.0, 0.0, 0.0) | 0 (0.0, 0.0, 0.0) | 1190.2 (683.4, 1930.0, 3862.4) |
| Phy:Fe | 2.3 (1.5, 4.2, 7.4) | 0.0 (0.0, 0.0, 0.0) | 0 (0.0, 0.0, 0.0) | 0 (0.0, 0.0, 0.0) | 3.1 (1.9, 6.1, 24.2) |
| Phy:Zn | 18.4 (11.8, 29.7, 76.4) | 0.0 (0.0, 0.0, 0.0) | 0 (0.0, 3.4, 34.6) | 0 (0.0, 28.7, 31.5) | 18.0 (11.4, 29.1, 72.9) |
| Phy:Ca | 0.2 (0.1, 0.3, 0.9) | 0.0 (0.0, 0.0, 0.0) | 0 (0.0, 0.0, 0.3) | 0 (0.0, 0.3, 0.5) | 0.4 (0.2, 0.5, 0.8) |
| PhyxCa/Zn | 142.8 (61.5, 338.2, 1405.6) | 0.0 (0.0, 0.0, 0.0) | 0 (0.0, 0.3, 38.9) | 0 (0.0, 4.3, 88.4) | 148.8 (93.0, 272.7, 380.2) |
| Added salt (g) | 6.2 (2.6, 12.6, 30.3) | ||||
| Added oil (g) | 6.7 (0.0, 26.4, 94.3) | ||||
| Added sugar (g) | 0.0 (0.0, 18.8, 116.1) | ||||
| NOVA Foods Consumed in Children (n = 7272) | |||||
|---|---|---|---|---|---|
| Nutrient Types | NOVA1 | NOVA2 | NOVA3 | NOVA4 | NOVA Total |
| p50 (p25, p75, p95) | p50 (p25, p75, p95) | p50 (p25, p75, p95) | p50 (p25, p75, p95) | p50 (p25, p75, p95) | |
| Vit A (µg RAE) | 20.8 (1.1, 120.9, 513.1) | 0.0 (0.0, 0.0, 6.4) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0.0, 0.0) | 24.4 (1.4, 129.0, 528.7) |
| Thiamin, B1 (mg) | 0.3 (0.2, 0.6, 1.2) | 0.0 (0.0, 0.0, 0.2) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0.0, 0.0) | 0.4 (0.2, 0.7, 1.3) |
| Riboflavin, B2 (mg) | 0.3 (0.1, 0.6, 1.3) | 0.0 (0.0, 0.0, 0.2) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0.0, 0.0) | 0.4 (0.2, 0.7, 1.5) |
| Niacin, B3 (mg) | 2.4 (1.1, 4.5, 9.4) | 0.0 (0.0, 0.0, 1.0) | 0.0 (0.0, 0.0, 0.1) | 0.0 (0.0, 0.0, 0.2) | 2.7 (1.3, 4.9, 10.5) |
| Vit C (mg) | 7.6 (2.6, 17.1, 56.8) | 0.0 (0.0, 0.0, 1.6) | 0.0 (0.0, 0.0, 0.1) | 0.0 (0.0, 0.0, 0.0) | 8.0 (2.8, 18.1, 59.6) |
| Calcium (mg) | 134.2 (50.7, 301.5, 776.3) | 0.4 (0.0, 1.9, 67.2) | 0.0 (0.0, 0.0, 10.0) | 0.0 (0.0, 0.0, 4.2) | 152.6 (59.9, 334.5, 849.7) |
| Iron (mg) | 9.0 (4.0, 17.6, 43.6) | 0.0 (0.0, 0.0, 3.8) | 0.0 (0.0, 0.0, 0.6) | 0.0 (0.0, 0.0, 0.2) | 9.9 (4.4, 19.2, 46.5) |
| Zinc (mg) | 1.8 (0.9, 3.4, 7.6) | 0.0 (0.0, 0.0, 0.9) | 0.0 (0.0, 0.0, 0.1) | 0.0 (0.0, 0.0, 0.1) | 2.0 (1.0, 3.7, 8.3) |
| Energy (kcal) | 435.3 (224.4, 742.8, 1395.5) | 23.2 (0.0, 112.5, 387.4) | 0.0 (0.0, 0.0, 40.7) | 0.0 (0.0, 0.0, 147.2) | 553.0 (298.0, 912.8, 1648.0) |
| Tot CHO (kcal) | 300.6 (136.9, 546.1, 1077.9) | 0.0 (0.0, 43.8, 224.0) | 0.0 (0.0, 0.0, 10.5) | 0.0 (0.0, 0.0, 40.7) | 364.7 (181.8, 619.9, 1164.1) |
| Tot protein (kcal) | 49.3 (24.5, 86.6, 169.0) | 0.0 (0.0, 0.0, 20.4) | 0.0 (0.0, 0.0, 2.8) | 0.0 (0.0, 0.0, 3.1) | 54.7 (27.8, 93.4, 179.0) |
| Total Fat (kcal) | 51.5 (20.2, 114.0, 298.3) | 0.0 (0.0, 38.8, 217.4) | 0.0 (0.0, 0.0, 3.9) | 0.0 (0.0, 0.0, 94.9) | 96.2 (40.7, 200.5, 474.1) |
| Phy (mg) | 283.6 (96.4, 584.0, 1322.9) | 0.0 (0.0, 0.0, 115.1) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0.0, 17.4) | 313.1 (116.4, 625.9, 1414.1) |
| Phy:Fe | 2.2 (1.4, 4.0, 7.9) | 0.0 (0.0, 0.0, 4.2) | 0.8 (0.0, 2.4, 13.5) | 14.1 (4.2, 14.1, 15.6) | 2.3 (1.4, 4.0, 7.6) |
| Phy:Zn | 15.3 (7.3, 25.8, 62.6) | 0.0 (0, 0.0, 0.0, 33.0) | 0.0 (0.0, 0.0, 0.0) | 0.0 (0.0, 0.0, 28.7) | 15.3 (7.6, 25.3,59.8) |
| Phy:Ca | 0.2 (0.1, 0.3, 0.8) | 0.0 (0.0, 0.0, 0.5) | 0.1 (0.0, 0.2, 1.0) | 0.3 (0.3, 0.3, 0.5) | 0.6 (0.3, 0.9, 1.4) |
| PhyxCa/Zn | 40.5 (11.8, 109.7, 417.7) | 0.0 (0.0, 0.0, 14.2) | 1.0 (0.0, 15.9, 274.0) | 3.2 (1.8, 8.9, 27.1) | 59.4 (32.1, 106.5, 171.9) |
| Added Salt (g) | 0.8 (0.0, 3.5, 57.9) | ||||
| Added Oil (g) | 0.0 (0.0, 5.1, 41.2) | ||||
| Added Sugar (g) | 0.0 (0.0, 2.3, 52.6) | ||||
| Subjects by Age Category | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children, 6–12 Months Old (n = 1804) | Children, 1–4 Years Old (n = 5468) | Women (15–18) Years Old (n = 388) | Women, 19+ Years Old (n = 7866) | |||||||||
| Nutrients and Energy | Intake, Median (IQR) | RNI | % NRI Achieved | Intake, Median (IQR) | RNI | % NRI Achieved | Intake, Median (IQR) | RNI | % NRI Achieved | Intake, Median (IQR) | RNI | % NRI Achieved |
| Energy (Kcal) | 282.4 (358.3) | 872.5 | 32.4 | 654.5 (621.1) | 1230 | 53.2 | 1523.8 (1110.4) | 2200 | 69.3 | 1586.2 (1158.4) | 2200 | 72.1 |
| CHO (g) | 38.2 (59.3) | 95 | 40.2 | 110.7 (110.1) | 130 | 84.7 | 271.9 (211.5) | 130 | 209.2 | 284.8 (217.7) | 130 | 219.1 |
| Protein (g) | 7.0 (10.5) | 11.0 | 63.6 | 16.0 (16.7) | 13.0 | 123.1 | 39.6 (32.3) | 46.0 | 86.1 | 37.6 (31.6) | 46.0 | 81.7 |
| Fat (kcal) | 72.6 (140) | 30%E | 85.7 | 104.0 (163.8) | 30%E | 53.0 | 276.8 (312) | 30%E | 60.6 | 208.9 (260.4) | 30%E | 43.9 |
| Fe (mg) | 4.0 (7.0) | 9.3 | 43.0 | 12.5 (15.7) | 5.8 | 215.5 | 44.3 (48.7) | 31.0 | 142.9 | 37.8 (42.8) | 29.4 | 128.6 |
| Ca (mg) | 118.3 (282.5) | 400 | 29.6 | 164.3 (278.8) | 500 | 32.9 | 361.4 (409.3) | 1000 | 36.1 | 346.5 (439.6) | 1000 | 34.7 |
| Zn (mg) | 1.0 (1.4) | 8.4 | 11.9 | 2.5 (3.0) | 8.3 | 30.1 | 7.5 (7.6) | 9.8 | 76.5 | 6.6 (7.2) | 9.8 | 67.4 |
| Vit A (µg RAE) | 31.7 (107.9) | 400 | 7.9 | 23.2 (140.1) | 400 | 5.8 | 46.1 (213.0) | 600 | 7.7 | 31.8 (201.2) | 500 | 6.4 |
| Thiamin (mg) | 0.2 (0.2) | 0.3 | 66.7 | 0.5 (0.5) | 0.5 | 100 | 1.2 (1.0) | 1.1 | 109.1 | 1.2 (1.0) | 1.1 | 109.1 |
| Riboflavin (mg) | 0.2 (0.4) | 0.4 | 50 | 0.4 (0.5) | 0.5 | 80 | 0.9 (0.9) | 1.0 | 90 | 0.9 (0.9) | 1.1 | 90 |
| Niacin (mg NE) | 1.3 (2.1) | 4.0 | 32.5 | 3.3 (3.8) | 6.0 | 55 | 8.0 (7.5) | 16.0 | 50 | 8.0 (7.4) | 14.0 | 57.1 |
| Vit C (mg) | 4.4 (10.0) | 30 | 14.7 | 9.7 (17.5) | 30 | 32.3 | 28.1 (40.6) | 40 | 70.3 | 22.3 (37.4) | 45 | 49.6 |
| Regions | Children | WRA | ||||||
|---|---|---|---|---|---|---|---|---|
| UPF (kcal) | 95% CI | Total Energy (kcal) | % UPF kcal | UPF (kcal) | 95% CI | Total Energy (kcal) | % UPF kcal | |
| Tigray (n = 812) | 22.8 | 17.5, 28.1 | 749.8 | 3.0 | 32.0 | 26.0, 38.0 | 2066.9 | 1.6 |
| Afar (n = 617) | 12.2 | 7.6, 16.8 | 713.8 | 1.7 | 14.3 | 7.4, 21.1 | 2210.9 | 0.7 |
| Amhara (n = 1077) | 21.1 | 16.3, 26.0 | 664.6 | 3.2 | 33.8 | 27.8, 39.9 | 2085.5 | 1.6 |
| Oromia (n = 1093) | 28.8 | 22.9, 34.6 | 715.2 | 4.0 | 33.0 | 24.6, 41.3 | 1860.3 | 1.8 |
| Somali (n = 687) | 23.9 | 17.0, 30.8 | 601.8 | 4.0 | 17.2 | 10.5, 24.0 | 1338.0 | 1.3 |
| Benishangul (n = 629) | 18.0 | 12.2, 23.8 | 633.9 | 2.8 | 22.2 | 17.3, 27.1 | 1812.3 | 1.2 |
| SNNPR (n = 1049) | 9.8 | 7.3, 12.2 | 543.7 | 1.8 | 20.6 | 15.5, 25.8 | 1610.7 | 1.3 |
| Gambela (n = 511) | 12.7 | 6.4, 19.1 | 569.6 | 2.2 | 1.5 | 0.2, 2.9 | 1557.1 | 0.1 |
| Harari (n = 488) | 50.5 | 37.3, 63.7 | 775.8 | 6.5 | 53.9 | 40.3, 67.5 | 1799.4 | 3.0 |
| Addis Ababa (n = 802) | 63.4 | 54.2, 72.6 | 750.6 | 8.5 | 186.3 | 166.3, 206.3 | 1554.0 | 12.0 |
| Dire Dawa (n = 489) | 35.5 | 26.6, 44.5 | 687.9 | 5.2 | 32.5 | 21.5, 43.5 | 1441.1 | 2.3 |
| National | 24.8 | 22.9, 26.8 | 669.6 | 3.7 | 42.0 | 22.9, 26.8 | 1780.4 | 2.4 |
| Predictor Variables | n | % | Daily Kcal Intake from UPF in Children | |||
|---|---|---|---|---|---|---|
| Exp (β) [95% CI] | SE | p-Value | Q-Value | |||
| Residence | ||||||
| Urban | 1945 | 26.75 | 2.55 [1.67, 3.87] | 0.54 | 0.000 * | 0.000 |
| Rural | 5327 | 73.25 | 1 | |||
| Child age | ||||||
| 6–12 months | 1804 | 24.81 | 1 | |||
| 13–24 months | 3102 | 42.66 | 1.27 [1.14, 1.41] | 0.07 | 0.000 * | 0.000 |
| 25–36 months | 2336 | 32.12 | 1.58 [1.41, 1.77] | 0.09 | 0.000 * | 0.000 |
| 37–45 months | 30 | 0.41 | 6.75 [3.36,13.55] | 5.37 | 0.000* | 0.000 |
| BMI z-score | ||||||
| Under Wt | 337 | 4.63 | 1 | |||
| Normal | 5849 | 80.43 | 1.13 [0.98, 1.31] | 0.08 | 0.10 | 0.10 |
| Over Wt | 1086 | 14.93 | 1.11 [0.86, 1.44] | 0.15 | 0.405 | 0.405 |
| SES | ||||||
| Poorest | 3187 | 43.83 | 1 | |||
| Middle | 1438 | 19.77 | 1.17 [1.03, 1.32] | 0.07 | 0.016 | 0.01 |
| Richest | 2647 | 36.40 | 1.23 [1.05, 1.44] | 0.10 | 0.010 | 0.008 |
| Mother’s Education | ||||||
| No formal education | 4675 | 64.29 | 1 | |||
| Primary school (G: 1–8) | 1683 | 23.14 | 1.51 [1.35, 1.70] | 0.09 | 0.000 * | 0.000 |
| High school (G: 9–12) | 717 | 9.86 | 1.77 [1.50, 2.10] | 0.15 | 0.000 * | 0.000 |
| Tertiary (G: >12) | 197 | 2.78 | 1.47 [1.12, 1.93] | 0.20 | 0.005 * | 0.006 |
| Mother job | ||||||
| Employed | 1946 | 26.76 | 1 | |||
| Per-time/irregularly paid | 1082 | 14.88 | 0.99 [0.86, 1.15] | 0.07 | 0.918 | |
| Housewife | 3730 | 51.29 | 0.85 [0.76, 0.96] | 0.05 | 0.007 * | 0.0071 |
| Other (family supporter) | 514 | 7.07 | 1.01 [0.84, 1.22] | 0.10 | 0.894 | |
| _cons | 4.85 [3.37, 6.98] | 0.90 | 0.000 | |||
| /logs | 0.42 [0.40, 0.43] | 0.01 | ||||
| Random effect | ||||||
| Region var(_cons) | 0.14 [0.04, 0.51] | 0.09 | ||||
| Region > cluster var(_cons) | 1.59 [1.34, 1.88] | 0.14 | ||||
| Predictor Variables | n | % | Children’s Daily Kcal Intake from Less-Processed (NOVA1) Foods | |||
|---|---|---|---|---|---|---|
| Exp (β) [95% CI] | S.E | p-Value | Q-Value | |||
| Residence | 0.05 | 0.617 | ||||
| Urban | 1945 | 26.75 | 1.02 [0.94, 1.12] | |||
| Rural | 5327 | 73.25 | 1 | |||
| Child age | ||||||
| 6–12 months | 1804 | 24.81 | 1 | |||
| 13–24 months | 3102 | 42.66 | 1.78 [1.70, 1.86] | 0.04 | 0.000 * | 0.000 |
| 25–36 months | 2336 | 32.12 | 2.43 [2.32, 2.55] | 0.06 | 0.000 * | 0.000 |
| 37–45 months | 30 | 0.41 | 2.16 [1.64, 2.85] | 0.30 | 0.000 * | 0.000 |
| BMI Z-score | ||||||
| Under Wt | 337 | 4.63 | 1.07 [1.00, 1.14] | 0.05 | 0.035 | 0.01 |
| Normal | 5849 | 80.43 | 1.15 [1.03, 1.29] | 0.07 | 0.012 | 0.008 |
| Over Wt | 1086 | 14.93 | 1 | |||
| SES | ||||||
| Poorest | 3187 | 43.83 | 1.23 [1.16, 1.31] | 0.04 | 0.000 * | 0.000 |
| Middle | 1438 | 19.77 | 1.14 [1.07, 1.21] | 0.04 | 0.000 * | 0.000 |
| Richest | 2647 | 36.40 | 1 | |||
| Mother’s Education | ||||||
| No formal education | 4675 | 64.29 | 1 | |||
| Primary school (G: 1–8) | 1683 | 23.14 | 1.00 [0.96, 1.05] | 0.02 | 0.997 | |
| High school (G: 9–12) | 717 | 9.86 | 1.16 [1.08, 1.25] | 0.04 | 0.000 * | 0.000 |
| Tertiary (>12) | 197 | 2.71 | 1.29 [1.15, 1.45] | 0.08 | 0.000 * | 0.000 |
| Mother job | ||||||
| Employed (formal) | 1946 | 26.76 | 1 | |||
| Per-time or irregularly paid | 1082 | 14.88 | 1.01 [0.96, 1.08] | 0.03 | 0.626 | |
| Housewife | 3730 | 51.29 | 0.99 [0.94, 1.03] | 0.02 | 0.539 | |
| Others (unpaid family supporter) | 514 | 7.07 | 1.36 [1.26, 1.47] | 0.05 | 0.000 * | 0.000 |
| _cons | 238.38 (221.60, 79.42) | 14.69 | 0.000 | |||
| /logs | −0.31 (−0.33, −0.29) | 0.01 | ||||
| Random effect | ||||||
| Region var(_cons) | 0.02 (0.007, 0.05) | 0.009 | ||||
| Region > cluster var(_cons) | 0.04 (0.03, 0.05) | 0.005 | ||||
| Variables | n | (%) | Kcal Intake from UPF in WRA | |||
|---|---|---|---|---|---|---|
| Exp (β) [95% CI] | SE | p-Value | Q-Value | |||
| Residence | 0.87 | 0.000 * | 0.000 | |||
| Urban | 2142 | 28.06 | 3.77 [2.40, 5.92] | |||
| Rural | 5492 | 71.94 | 1 | |||
| Mother’s age (years) | ||||||
| Teen/young adult (15–21) | 1296 | 16.98 | 1.03 [0.91, 1.18] | 0.07 | 0.626 | |
| Early adulthood (22–34) | 4982 | 65.26 | 1.12 [1.01, 1.23] | 0.06 | 0.029 | 0.007 |
| Middle age (35–45) | 1356 | 17.76 | 1 | |||
| Mother BMI | ||||||
| Thin (BMI < 18.5) | 2080 | 27.33 | 1 | |||
| Normal (BMI 18.5–24.9) | 4824 | 63.37 | 1.05 [0.96, 1.1.15] | 0.05 | 2.73 | 2.73 |
| OverWt/obese (BMI ≥ 25) | 708 | 9.30 | 1.23 [1.06, 1.44] | 0.10 | 0.008 | 0.006 |
| SES | ||||||
| Poorest | 3024 | 39.61 | 1 | |||
| Middle | 1501 | 19.66 | 0.98 [0.88, 1.10] | 0.06 | 0.770 | 0.770 |
| Richest | 3109 | 40.73 | 1.39 [1.20, 1.63] | 0.11 | 0.000 * | 0.000 |
| Mother Education | ||||||
| No formal Education | 4783 | 62.65 | 1 | |||
| Primary (G: 1–8) | 1820 | 23.84 | 1.38 [1.24, 1.53] | 0.07 | 0.0008 * | 0.005 |
| High school (G: 9–12) | 792 | 10.37 | 1.66 [1.42, 1.94] | 0.13 | 0.000 * | 0.000 |
| Tertiary (G: >12) | 239 | 3.13 | 1.41 [1.12, 1.78] | 0.16 | 0.003 * | 0.005 |
| Mother job | ||||||
| Employed | 2193 | 28.73 | 1 | |||
| Per-time or irregularly paid | 1021 | 13.37 | 0.94 [0.82, 1.08] | 0.07 | 0.380 | 0.380 |
| Housewife | 3447 | 45.15 | 0.97 [0.88, 1.08] | 0.05 | 0.610 | 0.610 |
| Unpaid family worker | 973 | 12.75 | 1.01 [0.87, 1.18] | 0.08 | 0.898 | 0.898 |
| _cons | 4.24 [2.71, 6.65] | 0.97 | 0.000 | |||
| /logs | 0.37 [0.35, 0.38] | 0.01 | ||||
| Random effect | ||||||
| Region var(cons) | 0.50 [0.20, 1.29] | 0.24 | ||||
| Region > cluster var(cons) | 2.03 [1.72, 2.39] | 0.17 | ||||
| Variables | n | % | Kcal Intake from Less-Processed (NOVA1) Foods in WRA | |||
|---|---|---|---|---|---|---|
| Exp (β) [95% CI] | SE | p-Value | Q-Value | |||
| Residence | 0.03 | 0.000 * | 0.000 | |||
| Urban | 2142 | 28.06 | 0.80 [0.74, 0.86] | |||
| Rural | 5492 | 71.9 | 1 | |||
| Mother Age | ||||||
| Teen/young adult (15–21) | 1296 | 16.98 | 1.06 [1.02, 1.11] | 0.02 | 0.003 * | 0.004 |
| Early adulthood (22–34) | 4982 | 65.26 | 1.03 [1.00, 1.07] | 0.02 | 0.034 | 0.005 |
| Middle age (35–45) | 1356 | 17.76 | 1 | |||
| BMI Mother | ||||||
| Thin (BMI < 18.5) | 2080 | 27.33 | 1 | |||
| Normal (BMI 18.5–24.9) | 4824 | 63.37 | 1.01 [0.98, 1.04] | 0.01 | 0.563 | 0.563 |
| Overwt/obese (BMI ≥ 25) | 708 | 9.30 | 1.02 [0.97, 1.07] | 0.03 | 0.534 | 0.534 |
| SES | ||||||
| Poorest | 3024 | 39.61 | 1 | |||
| Middle | 1501 | 19.66 | 1.02 [0.98, 1.05] | 0.02 | 0.371 | 0.371 |
| Richest | 3109 | 40.73 | 1.02 [0.98, 1.06] | 0.02 | 0.390 | 0.390 |
| Mother Education | ||||||
| No formal education | 4783 | 62.65 | 1 | |||
| Elementary school (G: 1–8) | 1820 | 23.84 | 1.00 [0.97, 1.04] | 0.02 | 0.792 | 0.792 |
| High school (G: 9–12) | 792 | 10.37 | 1.03 [0.98, 1.08] | 0.03 | 0.236 | 0.236 |
| Tertiary (>12) | 239 | 3.13 | 1.06 [0.99, 1.14] | 0.04 | 0.104 | 0.104 |
| Mother job | ||||||
| Employed | 2193 | 28.73 | 1 | |||
| Per-time or irregularly paid | 1021 | 13.37 | 1.02 [0.98, 1.07] | 0.02 | 0.297 | 0.297 |
| Housewife | 3447 | 45.15 | 0.96 [0.93, 0.99] | 0.02 | 0.016 | 0.005 |
| Unpaid family worker | 973 | 12.75 | 0.98 [0.94, 1.03] | 0.02 | 0.460 | 0.460 |
| _cons | 1417.6 [1283.0, 1566.3] | 72.18 | 0.000 | |||
| /logs | −0.69 [−0.71, −0.68] | 0.01 | ||||
| Random effect | ||||||
| Region var(_cons) | 0.02 [0.01, 0.05] | 0.01 | ||||
| Region > cluster var(_cons) | 0.03 [0.03, 0.04] | 0.00 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcha, K.H.; Vandevijvere, S.; van Onselen, A.; Siwela, M.; Tessema, M.; Mkolo, N.M.; Moges, T.; Feskens, E.J.M.; Tesfaw, D.; Brouwer, I.D. Energy and Nutrient Intake Gaps and Socioeconomic Determinants of Ultra-Processed and Less-Processed Foods Consumed in Ethiopia: Evidence from National Food Consumption Survey. Nutrients 2025, 17, 2818. https://doi.org/10.3390/nu17172818
Balcha KH, Vandevijvere S, van Onselen A, Siwela M, Tessema M, Mkolo NM, Moges T, Feskens EJM, Tesfaw D, Brouwer ID. Energy and Nutrient Intake Gaps and Socioeconomic Determinants of Ultra-Processed and Less-Processed Foods Consumed in Ethiopia: Evidence from National Food Consumption Survey. Nutrients. 2025; 17(17):2818. https://doi.org/10.3390/nu17172818
Chicago/Turabian StyleBalcha, Kifle Habte, Stefanie Vandevijvere, Annette van Onselen, Muthulisi Siwela, Masresha Tessema, Nqobile Monate Mkolo, Tibebu Moges, Edith J. M. Feskens, Dejen Tesfaw, and Inge D. Brouwer. 2025. "Energy and Nutrient Intake Gaps and Socioeconomic Determinants of Ultra-Processed and Less-Processed Foods Consumed in Ethiopia: Evidence from National Food Consumption Survey" Nutrients 17, no. 17: 2818. https://doi.org/10.3390/nu17172818
APA StyleBalcha, K. H., Vandevijvere, S., van Onselen, A., Siwela, M., Tessema, M., Mkolo, N. M., Moges, T., Feskens, E. J. M., Tesfaw, D., & Brouwer, I. D. (2025). Energy and Nutrient Intake Gaps and Socioeconomic Determinants of Ultra-Processed and Less-Processed Foods Consumed in Ethiopia: Evidence from National Food Consumption Survey. Nutrients, 17(17), 2818. https://doi.org/10.3390/nu17172818







