Personalized Nutrition Biomarkers and Dietary Strategies for Atherosclerosis Risk Management: A Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Eligibility Criteria for Study Inclusion
2.3. Search Strategy and Identification of Eligible Studies
2.4. Data Extraction Process
2.5. Data Synthesis
2.6. Quality Appraisal and Risk of Bias Assessment of Studies
3. Results
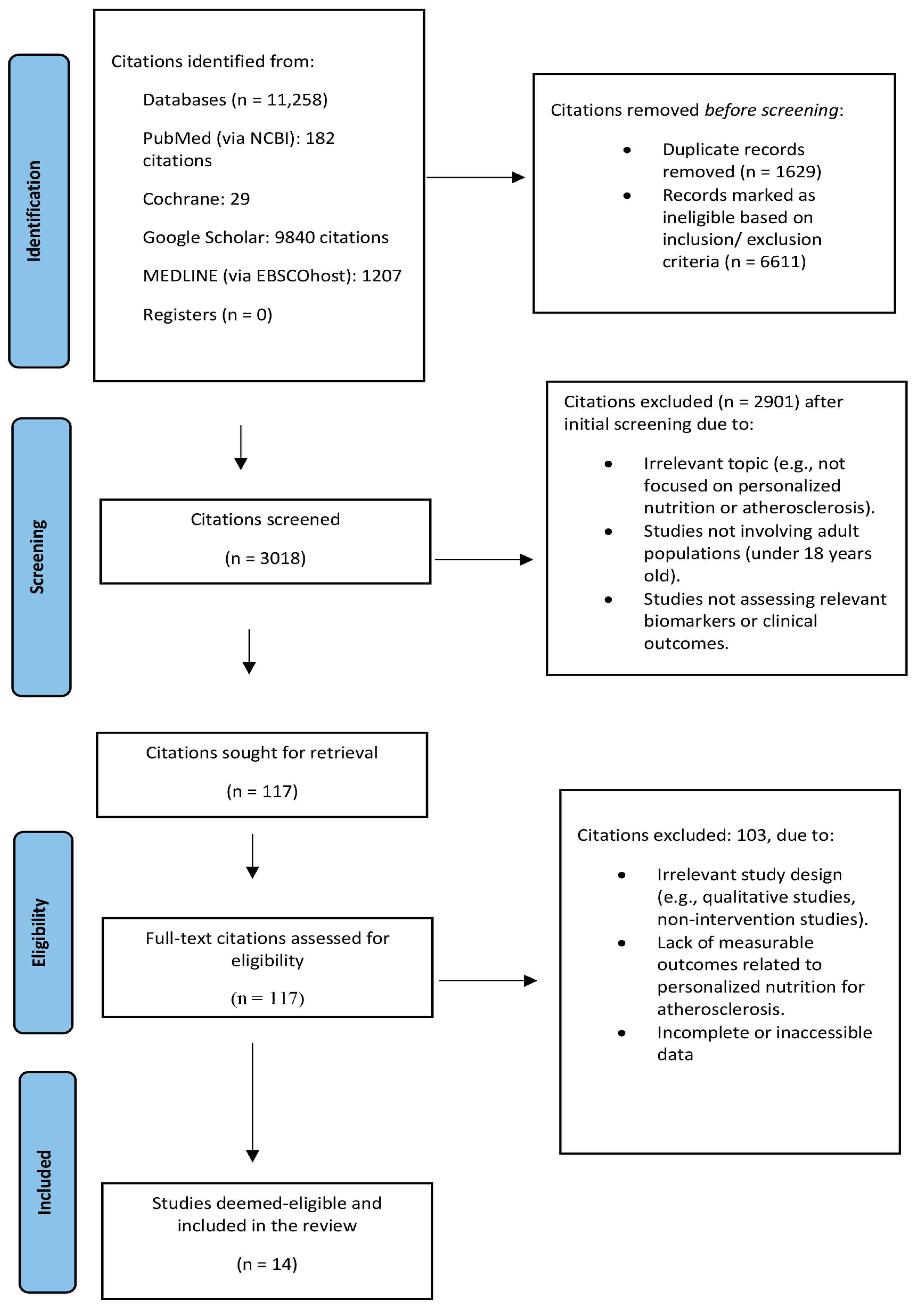
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Summary of Findings
3.4. Personalized Biomarkers in Atherosclerosis
3.4.1. Genetic Biomarkers
Lipoprotein (a)
MicroRNA and Non-Coding RNA
3.4.2. Microbiome-Associated Biomarkers
3.4.3. Metabolomic Biomarkers
3.5. Navigating Personalized Nutrition Approaches: Tailoring Strategies for Atherosclerosis Management
3.6. Personalized Nutrition in Clinical Settings: Opportunities and Challenges
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, M.M.; Rafi, U.; Imran, A.; Rehman, M.U.; Abbas, S.K. Risk Factors Associated with Cardiovascular Disorders: Risk Factors Associated with Cardiovascular Disorders. Pak. Biomed. J. 2024, 7, 3–10. [Google Scholar] [CrossRef]
- Ware, L.; Vermeulen, B.; Maposa, I.; Flood, D.; Brant, L.C.C.; Khandelwal, S.; Singh, K.; Soares, S.; Jessen, N.; Perman, G. Comparison of Cardiovascular Health Profiles Across Population Surveys From 5 High-to Low-Income Countries. CJC Open 2024, 6, 582–596. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Wang, Y.; Han, W.; Xu, W.; Liao, X.; Zhang, T.; Wang, G. Matrix Stiffness, Endothelial Dysfunction and Atherosclerosis. Mol. Biol. Rep. 2023, 50, 7027–7041. [Google Scholar] [CrossRef]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-Density Lipoprotein Revisited: Biological Functions and Clinical Relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Kamzolas, O.; Papazoglou, A.S.; Gemousakakis, E.; Moysidis, D.V.; Kyriakoulis, K.G.; Brilakis, E.S.; Milkas, A. Concomitant Coronary Artery Disease in Identical Twins: Case Report and Systematic Literature Review. J. Clin. Med. 2023, 12, 5742. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.; Nohara, A.; Sekiya, T.; Watanabe, A.; Takamura, M. Genetic Counseling and Genetic Testing for Familial Hypercholesterolemia. Genes 2024, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, L.; Chen, C.; Li, P.; Lu, B. The Gut Microbiota-Artery Axis: A Bridge between Dietary Lipids and Atherosclerosis? Prog. Lipid Res. 2023, 89, 101209. [Google Scholar] [CrossRef] [PubMed]
- Faghy, M.A.; Yates, J.; Hills, A.P.; Jayasinghe, S.; da Luz Goulart, C.; Arena, R.; Laddu, D.; Gururaj, R.; Veluswamy, S.K.; Dixit, S. Cardiovascular Disease Prevention and Management in the COVID-19 Era and beyond: An International Perspective. Prog. Cardiovasc. Dis. 2023, 76, 102–111. [Google Scholar] [CrossRef]
- Torres, N.; Guevara-Cruz, M.; Velázquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary Prevention of Cardiovascular Disease: A Review of Contemporary Guidance and Literature. JRSM Cardiovasc. Dis. 2017, 6, 2048004016687211. [Google Scholar] [CrossRef]
- Bush, C.L.; Blumberg, J.B.; El-Sohemy, A.; Minich, D.M.; Ordovás, J.M.; Reed, D.G.; Behm, V.A.Y. Toward the Definition of Personalized Nutrition: A Proposal by the American Nutrition Association. J. Am. Coll. Nutr. 2020, 39, 5–15. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Caffrey, A.; Christensen, L.; Mezgec, S.; Surendran, S.; Hjorth, M.F.; McNulty, H.; Pentieva, K.; Roager, H.M.; Seljak, B.K.; et al. Diets, nutrients, genes and the microbiome: Recent advances in personalised nutrition. Br. J. Nutr. 2021, 126, 1489–1497. [Google Scholar] [CrossRef]
- Chen, Y. Effect of chrono-nutrition–based dietary intervention on metabolic disease. Precis. Nutr. 2024, 3, e00076. [Google Scholar]
- Waffenschmidt, S.; Knelangen, M.; Sieben, W.; Bühn, S.; Pieper, D. Single Screening versus Conventional Double Screening for Study Selection in Systematic Reviews: A Methodological Systematic Review. BMC Med. Res. Methodol. 2019, 19, 132. [Google Scholar] [CrossRef]
- Valsesia, A.; Egli, L.; Bosco, N.; Magkos, F.; Kong, S.C.; Sun, L.; Goh, H.J.; Weiting, H.; Arigoni, F.; Leow, M.K.-S.; et al. Clinical- and Omics-Based Models of Subclinical Atherosclerosis in Healthy Chinese Adults: A Cross-Sectional Exploratory Study. Am. J. Clin. Nutr. 2021, 114, 1752–1762. [Google Scholar] [CrossRef]
- Gomez-Delgado, F.; Alcala-Diaz, J.F.; Leon-Acuña, A.; Lopez-Moreno, J.; Delgado-Lista, J.; Gomez-Marin, B.; Roncero-Ramos, I.; Yubero-Serrano, E.M.; Rangel-Zuñiga, O.A.; Vals-Delgado, C.; et al. Apolipoprotein E Genetic Variants Interact with Mediterranean Diet to Modulate Postprandial Hypertriglyceridemia in Coronary Heart Disease Patients: CORDIOPREV Study. Eur. J. Clin. Investig. 2019, 49, e13146. [Google Scholar] [CrossRef]
- Marcotte, B.V.; Guénard, F.; Lemieux, S.; Couture, P.; Rudkowska, I.; Calder, P.C.; Minihane, A.M.; Vohl, M.-C. Fine Mapping of Genome-Wide Association Study Signals to Identify Genetic Markers of the Plasma Triglyceride Response to an Omega-3 Fatty Acid Supplementation. Am. J. Clin. Nutr. 2019, 109, 176–185. [Google Scholar] [CrossRef]
- Perng, W.; Villamor, E.; Shroff, M.R.; Nettleton, J.A.; Pilsner, J.R.; Liu, Y.; Diez-Roux, A.V. Dietary Intake, Plasma Homocysteine, and Repetitive Element DNA Methylation in the Multi-Ethnic Study of Atherosclerosis (MESA). Nutr. Metab. Cardiovasc. Dis. 2014, 24, 614–622. [Google Scholar] [CrossRef]
- Meng, T.; Kubow, S.; Nielsen, D.E. Common Variants in the CD36 Gene Are Associated with Dietary Fat Intake, High-Fat Food Consumption and Serum Triglycerides in a Cohort of Quebec Adults. Int. J. Obes. 2021, 45, 1193–1202. [Google Scholar] [CrossRef]
- García-Calzón, S.; Martínez-González, M.A.; Razquin, C.; Corella, D.; Salas-Salvadó, J.; Martínez, J.A.; Zalba, G.; Marti, A. Pro12Ala Polymorphism of the PPARγ2 Gene Interacts with a Mediterranean Diet to Prevent Telomere Shortening in the PREDIMED-NAVARRA Randomized Trial. Circ. Cardiovasc. Genet. 2015, 8, 91–99. [Google Scholar] [CrossRef]
- Roessler, C.; Kuhlmann, K.; Hellwing, C.; Leimert, A.; Schumann, J. Impact of Polyunsaturated Fatty Acids on MiRNA Profiles of Monocytes/Macrophages and Endothelial Cells—A Pilot Study. Int. J. Mol. Sci. 2017, 18, 284. [Google Scholar] [CrossRef]
- Gomez-Delgado, F.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Lopez-Moreno, J.; Tinahones, F.J.; Ordovas, J.M.; Garaulet, M.; et al. Chronic Consumption of a Low-Fat Diet Improves Cardiometabolic Risk Factors According to the CLOCK Gene in Patients with Coronary Heart Disease. Mol. Nutr. Food Res. 2015, 59, 2556–2564. [Google Scholar] [CrossRef]
- Ulven, S.M.; Christensen, J.J.; Nygård, O.; Svardal, A.; Leder, L.; Ottestad, I.; Lysne, V.; Laupsa-Borge, J.; Ueland, P.M.; Midttun, Ø.; et al. Using Metabolic Profiling and Gene Expression Analyses to Explore Molecular Effects of Replacing Saturated Fat with Polyunsaturated Fat—A Randomized Controlled Dietary Intervention Study. Am. J. Clin. Nutr. 2019, 109, 1239–1250. [Google Scholar] [CrossRef]
- Rudkowska, I.; Pérusse, L.; Bellis, C.; Blangero, J.; Després, J.-P.; Bouchard, C.; Vohl, M.-C. Interaction between Common Genetic Variants and Total Fat Intake on Low-Density Lipoprotein Peak Particle Diameter: A Genome-Wide Association Study. Lifestyle Genom. 2015, 8, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, B.L.; Cormier, H.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.-C. Association between Polymorphisms in Phospholipase A 2 Genes and the Plasma Triglyceride Response to an N-3 PUFA Supplementation: A Clinical Trial. Lipids Health Dis. 2015, 14, 12. [Google Scholar] [CrossRef]
- Ouellette, C.; Rudkowska, I.; Lemieux, S.; Lamarche, B.; Couture, P.; Vohl, M.-C. Gene-Diet Interactions with Polymorphisms of the MGLL Gene on Plasma Low-Density Lipoprotein Cholesterol and Size Following an Omega-3 Polyunsaturated Fatty Acid Supplementation: A Clinical Trial. Lipids Health Dis. 2014, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Vallée Marcotte, B.; Cormier, H.; Guénard, F.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.-C. Novel Genetic Loci Associated with the Plasma Triglyceride Response to an Omega-3 Fatty Acid Supplementation. Lifestyle Genom. 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mehramiz, M.; Ghasemi, F.; Esmaily, H.; Tayefi, M.; Hassanian, S.M.; Sadeghzade, M.; Sadabadi, F.; Moohebati, M.; Azarpazhooh, M.R.; Parizadeh, S.M.R.; et al. Interaction between a Variant of CDKN2A/B-Gene with Lifestyle Factors in Determining Dyslipidemia and Estimated Cardiovascular Risk: A Step toward Personalized Nutrition. Clin. Nutr. 2018, 37, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Long, H.A.; French, D.P.; Brooks, J.M. Optimising the Value of the Critical Appraisal Skills Programme (CASP) Tool for Quality Appraisal in Qualitative Evidence Synthesis. Res. Methods Med. Health Sci. 2020, 1, 31–42. [Google Scholar] [CrossRef]
- Nair, A.S.; Borkar, N.K. Various Biases in Systematic Review and Meta-Analysis and Their Assessment. Indian J. Anaesth. 2025, 69, 138–142. [Google Scholar] [CrossRef]
- Boffetta, P.; Winn, D.M.; Ioannidis, J.P.; Thomas, D.C.; Little, J.; Smith, G.D.; Cogliano, V.J.; Hecht, S.S.; Seminara, D.; Vineis, P. Recommendations and Proposed Guidelines for Assessing the Cumulative Evidence on Joint Effects of Genes and Environments on Cancer Occurrence in Humans. Int. J. Epidemiol. 2012, 41, 686–704. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.; Quispe, R.; Das, T.; Juraschek, S.P.; Martin, S.S.; Michos, E.D. The Use of Blood Biomarkers in Precision Medicine for the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Review. Expert. Rev. Precis. Med. Drug Dev. 2021, 6, 247–258. [Google Scholar] [CrossRef]
- Riley, T.M.; Sapp, P.A.; Kris-Etherton, P.M.; Petersen, K.S. Effects of saturated fatty acid consumption on lipoprotein (a): A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2024, 120, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Nayor, M.; Brown, K.J.; Vasan, R.S. The Molecular Basis of Predicting Atherosclerotic Cardiovascular Disease Risk. Circ. Res. 2021, 128, 287–303. [Google Scholar] [CrossRef]
- Gareev, I.; Kudriashov, V.; Sufianov, A.; Begliarzade, S.; Ilyasova, T.; Liang, Y.; Beylerli, O. The Role of Long Non-Coding RNA ANRIL in the Development of Atherosclerosis. Non-Coding RNA Res. 2022, 7, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Melander, O.; Martínez, J.A.; Toledo, E.; Carpéné, C.; Corella, D. Advances in Integrating Traditional and Omic Biomarkers When Analyzing the Effects of the Mediterranean Diet Intervention in Cardiovascular Prevention. Int. J. Mol. Sci. 2016, 17, 1469. [Google Scholar] [CrossRef]
- Solly, E.L.; Dimasi, C.G.; Bursill, C.A.; Psaltis, P.J.; Tan, J.T.M. MicroRNAs as Therapeutic Targets and Clinical Biomarkers in Atherosclerosis. J. Clin. Med. 2019, 8, 2199. [Google Scholar] [CrossRef]
- Kura, B.; Parikh, M.; Slezak, J.; Pierce, G.N. The Influence of Diet on MicroRNAs That Impact Cardiovascular Disease. Molecules 2019, 24, 1509. [Google Scholar] [CrossRef]
- Walsh, R.; Jurgens, S.J.; Erdmann, J.; Bezzina, C.R. Genome-Wide Association Studies of Cardiovascular Disease. Physiol. Rev. 2023, 103, 2039–2055. [Google Scholar] [CrossRef]
- Hedman, Å.K.; Mendelson, M.M.; Marioni, R.E.; Gustafsson, S.; Joehanes, R.; Irvin, M.R.; Zhi, D.; Sandling, J.K.; Yao, C.; Liu, C.; et al. Epigenetic patterns in blood associated with lipid traits predict incident coronary heart disease events and are enriched for results from genome-wide association studies. Circ. Cardiovasc. Genet. 2017, 10, e001487. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L. Lipoprotein (a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, W. Lipoprotein (a). 2023. Available online: https://www.heart.org/en/health-topics/cholesterol/genetic-conditions/lipoprotein-a (accessed on 30 March 2025).
- Kostner, K.; Kostner, G.M. Lipoprotein (a): Metabolism, Pathophysiology, and Impact on Diabetes Mellitus. In Lipoproteins in Diabetes Mellitus; Springer: Berlin/Heidelberg, Germany, 2023; pp. 247–274. [Google Scholar]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F. Lipoprotein (a) in Atherosclerotic Cardiovascular Disease and Aortic Stenosis: A European Atherosclerosis Society Consensus Statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Berglund, L. Lipoprotein (a) and Effects of Diet: Time for Reassessment. Nutrients 2025, 17, 1714. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J.; Courville, A.B.; Boring, J.; Brychta, R.; Chen, K.Y.; Darcey, V.; Forde, C.G.; Gharib, A.M.; Gallagher, I.; et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat. Med. 2021, 27, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Fasolo, F.; Di Gregoli, K.; Maegdefessel, L.; Johnson, J.L. Non-Coding RNAs in Cardiovascular Cell Biology and Atherosclerosis. Cardiovasc. Res. 2019, 115, 1732–1756. [Google Scholar] [CrossRef]
- MacDonald-Ramos, K.; Martínez-Ibarra, A.; Monroy, A.; Miranda-Ríos, J.; Cerbón, M. Effect of Dietary Fatty Acids on MicroRNA Expression Related to Metabolic Disorders and Inflammation in Human and Animal Trials. Nutrients 2021, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.R.; Ferreira, V.V.; Pereira-da-Silva, T.; Ferreira, R.C. The Role of MiRNAs in the Diagnosis of Stable Atherosclerosis of Different Arterial Territories: A Critical Review. Front. Cardiovasc. Med. 2022, 9, 1040971. [Google Scholar] [CrossRef]
- Lin, C.-M.; Wang, B.-W.; Pan, C.-M.; Fang, W.-J.; Chua, S.-K.; Cheng, W.-P.; Shyu, K.-G. Chrysin Boosts KLF2 Expression through Suppression of Endothelial Cell-Derived Exosomal MicroRNA-92a in the Model of Atheroprotection. Eur. J. Nutr. 2021, 60, 4345–4355. [Google Scholar] [CrossRef]
- Reyes-Pérez, S.D.; González-Becerra, K.; Barrón-Cabrera, E.; Muñoz-Valle, J.F.; Armendáriz-Borunda, J.; Martínez-López, E. FADS1 Genetic Variant and Omega-3 Supplementation Are Associated with Changes in Fatty Acid Composition in Red Blood Cells of Subjects with Obesity. Nutrients 2024, 16, 3522. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, K.; Lian, P.; Hu, Y.; Shuai, Z.; Gao, S.; Ge, S.; Xu, T.; Xiao, Q.; Chen, Z. Pathological Bases and Clinical Application of Long Non-coding RNAs in Cardiovascular Diseases. Hypertension 2021, 78, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.H.; Luo, H.Q.; Chen, L.H.; Xu, M.; Li, G.Y.; Li, J.M. Impact of high fat diet on long non-coding RNAs and messenger RNAs expression in the aortas of ApoE(−/−) mice. Sci. Rep. 2016, 6, 34161. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Al Samarraie, A.; Pichette, M.; Rousseau, G. Role of the Gut Microbiome in the Development of Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 5420. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, Y.; Hu, G.; Zhang, Q.; Zheng, L. Gut Microbiome and Metabolites, the Future Direction of Diagnosis and Treatment of Atherosclerosis? Pharmacol. Res. 2023, 187, 106586. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Djekic, D.; Nicoll, R.; Novo, M.; Henein, M. Metabolomics in Atherosclerosis. IJC Metab. Endocr. 2015, 8, 26–30. [Google Scholar] [CrossRef][Green Version]
- Jung, S.; Song, S.-W.; Lee, S.; Kim, S.H.; Ann, S.; Cheon, E.J.; Yi, G.; Choi, E.-Y.; Lee, S.H.; Joo, H.-C.; et al. Metabolic Phenotyping of Human Atherosclerotic Plaques: Metabolic Alterations and Their Biological Relevance in Plaque-Containing Aorta. Atherosclerosis 2018, 269, 21–28. [Google Scholar] [CrossRef]
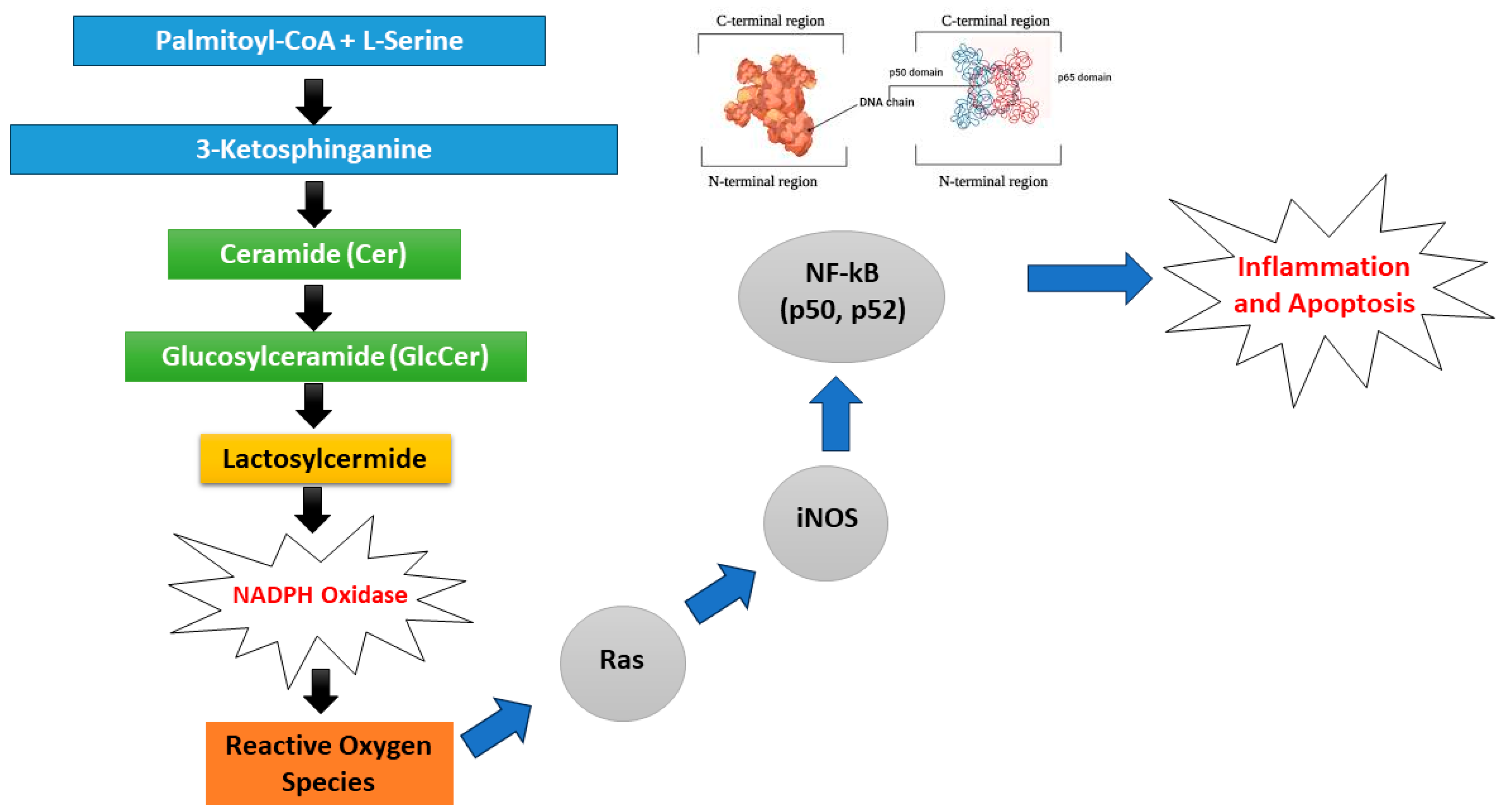
- Vorkas, P.A.; Shalhoub, J.; Isaac, G.; Want, E.J.; Nicholson, J.K.; Holmes, E. Metabolic phenotyping of atherosclerotic plaques reveals latent associations between free cholesterol and ceramide metabo-lism in atherogenesis. J. Proteome Res. 2015, 14, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Edsfeldt, A.; Dunér, P.; Ståhlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O.; et al. Sphingolipids Contribute to Human Atherosclerotic Plaque Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef]
- Gengatharan, J.M.; Handzlik, M.K.; Chih, Z.Y.; Ruchhoeft, M.L.; Secrest, P.; Ashley, E.L.; Green, C.R.; Wallace, M.; Gordts, P.L.; Metallo, C.M. Altered sphingolipid biosynthetic flux and lipoprotein trafficking contribute to trans-fat-induced atherosclerosis. Cell Metab. 2025, 37, 274–290. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle Factors and High-Risk Atherosclerosis: Pathways and Mechanisms beyond Traditional Risk Factors. Eur. J. Prev. Cardiol. 2019, 27, 394–406. [Google Scholar] [CrossRef]
- Riccardi, G.; Giosuè, A.; Calabrese, I.; Vaccaro, O. Dietary Recommendations for Prevention of Atherosclerosis. Cardiovasc. Res. 2022, 118, 1188–1204. [Google Scholar] [CrossRef]
- Fanaroff, A.C.; Califf, R.M.; Windecker, S.; Smith, S.C.; Lopes, R.D. Levels of Evidence Supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA 2019, 321, 1069–1080. [Google Scholar] [CrossRef]
- Samuel, P.O.; Edo, G.I.; Emakpor, O.L.; Oloni, G.O.; Ezekiel, G.O.; Essaghah, A.E.A.; Agoh, E.; Agbo, J.J. Lifestyle Modifications for Preventing and Managing Cardiovascular Diseases. Sport. Sci. Health 2024, 20, 23–36. [Google Scholar] [CrossRef]
- Mullins, V.A.; Bresette, W.; Johnstone, L.; Hallmark, B.; Chilton, F.H. Genomics in Personalized Nutrition: Can You “Eat for Your Genes”? Nutrients 2020, 12, 3118. [Google Scholar] [CrossRef] [PubMed]
- Chatelan, A.; Bochud, M.; Frohlich, K.L. Precision Nutrition: Hype or Hope for Public Health Interventions to Reduce Obesity? Int. J. Epidemiol. 2019, 48, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Ordovás, J. A Multifaceted Approach to Precision Nutrition: The Genome, Epigenome, and Microbiome in the Prevention and Therapy of Cardiovascular Diseases. In Precision Nutrition; Elsevier: Amsterdam, The Netherlands, 2024; pp. 181–200. [Google Scholar]
- Döring, Y.; Noels, H.; Weber, C. The Use of High-Throughput Technologies to Investigate Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 182–195. [Google Scholar] [CrossRef]
- Vesnina, A.; Prosekov, A.; Atuchin, V.; Minina, V.; Ponasenko, A. Tackling Atherosclerosis via Selected Nutrition. Int. J. Mol. Sci. 2022, 23, 8233. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic Signatures Underlying Inflammation: An Interplay of Nutrition, Physical Activity, Metabolic Diseases, and Environmental Factors for Personalized Nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Mandaviya, P.R.; Stolk, L.; Heil, S.G. Homocysteine and DNA Methylation: A Review of Animal and Human Literature. Mol. Genet. Metab. 2014, 113, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Aavik, E.; Babu, M.; Ylä-Herttuala, S. DNA Methylation Processes in Atherosclerotic Plaque. Atherosclerosis 2019, 281, 168–179. [Google Scholar] [CrossRef]
- Boughanem, H.; Bandera-Merchán, B.; Hernández-Alonso, P.; Moreno-Morales, N.; Tinahones, F.J.; Lozano, J.; Morcillo, S.; Macias-Gonzalez, M. Association between the APOA2 Rs3813627 Single Nucleotide Polymorphism and HDL and APOA1 Levels through BMI. Biomedicines 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.-C.; Vohl, M.-C. Precision Nutrition for Cardiovascular Disease Prevention. Lifestyle Genom. 2023, 16, 73–82. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Mattingley, G.; Sorli, J.V.; Ordovas, J.M. Utilizing Nutritional Genomics to Tailor Diets for the Prevention of Cardiovascular Disease: A Guide for Upcoming Studies and Implementations. Expert. Rev. Mol. Diagn. 2017, 17, 495–513. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Davidson, M.H.; Hirsh, B.J.; Kathiresan, S.; Gaudet, D. Genetics and Causality of Triglyceride-Rich Lipoproteins in Atherosclerotic Cardiovascular Disease. J. Am. Coll. Cardiol. 2014, 64, 2525–2540. [Google Scholar] [CrossRef]
- Acharjee, S.; Chauhan, S.; Pal, R.; Tomar, R.S. Mechanisms of DNA Methylation and Histone Modifications. Prog. Mol. Biol. Transl. Sci. 2023, 197, 51–92. [Google Scholar]
- Hussain, Y.; Abdullah; Khan, F.; Alsharif, K.F.; Alzahrani, K.J.; Saso, L.; Khan, H. Regulatory Effects of Curcumin on Platelets: An Update and Future Directions. Biomedicines 2022, 10, 3180. [Google Scholar] [CrossRef]
- Marcum, J.A. Nutrigenetics/Nutrigenomics, Personalized Nutrition, and Precision Healthcare. Curr. Nutr. Rep. 2020, 9, 338–345. [Google Scholar] [CrossRef]
- Louca, P.; Menni, C.; Padmanabhan, S. Genomic Determinants of Hypertension with a Focus on Metabolomics and the Gut Microbiome. Am. J. Hypertens. 2020, 33, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, F.; Vickers, M.H.; Mithen, R.F. Epigenetics, MicroRNA and Metabolic Syndrome: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 5047. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.B. Understanding Human Disease in the Post-Genomic Era. In Essential Concepts in Molecular Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–111. [Google Scholar]
- Ceriani, F.; Montalvan, M.; Quintero, B.; Suárez, R.; Bautista-Valarezo, E.; Frias-Toral, E. Ethics of the Clinical Practice of Nutrigenetics and Nutrigenomics. Clin. Nutr. Open Sci. 2023, 49, 58–66. [Google Scholar] [CrossRef]
- Brennan, L.; de Roos, B. Nutrigenomics: Lessons Learned and Future Perspectives. Am. J. Clin. Nutr. 2021, 113, 503–516. [Google Scholar] [CrossRef]


| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Adults (≥18 years) with diagnosed atherosclerosis or at high risk of CVD. | Children, adolescents (<18 years), or unrelated populations (e.g., cancer patients, non-CVD conditions). |
| Intervention | Personalized nutrition interventions considering individual omics profiling (genetics, microbiome, metabolomics, epigenetics). | Generalized or non-personalized dietary advice. |
| Comparison | Standard care diets or control groups. | Studies without a comparison group. |
| Outcomes | Measured effects on atherosclerosis-related clinical outcomes, biomarkers, or metabolic profiles. | Studies that do not measure or report relevant clinical, metabolic, or biomarker outcomes. |
| Study Design | Primary studies only: randomized controlled trials (RCTs); cohort studies; case–control, cross-sectional studies with analytical focus. | Narrative reviews, systematic reviews, meta-analyses, opinion pieces, and conference abstracts without full papers. |
| Language | Studies published in English. | Non-English publications without available translations. |
| Publication Date | Studies published within the last 10 years (to capture contemporary personalized nutrition advancements). | Studies older than 10 years. |
| Study | Country | Sample/Population | Study Design | Sample Size | Intervention | Duration | Outcome Measured | Gene/Genetic Variant Tested | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| [17] | China | Healthy subjects with and without subclinical atherosclerosis (SA) | Cross-sectional exploratory study | 100 Chinese subjects (46 female, 54 male) | Mixed-meal test (low-fat (energy% < 30%) frozen meal) | 2 weeks | 164 blood biomarkers | Omics model | SA could not be accurately predicted using models, and it depends only on fasting biomarkers or baseline clinical characteristics. Conversely, an omics model based on the timing and quantity of postprandial biomarkers showed excellent performance [ROC AUC: 91%; 95% CI: 77–100]. |
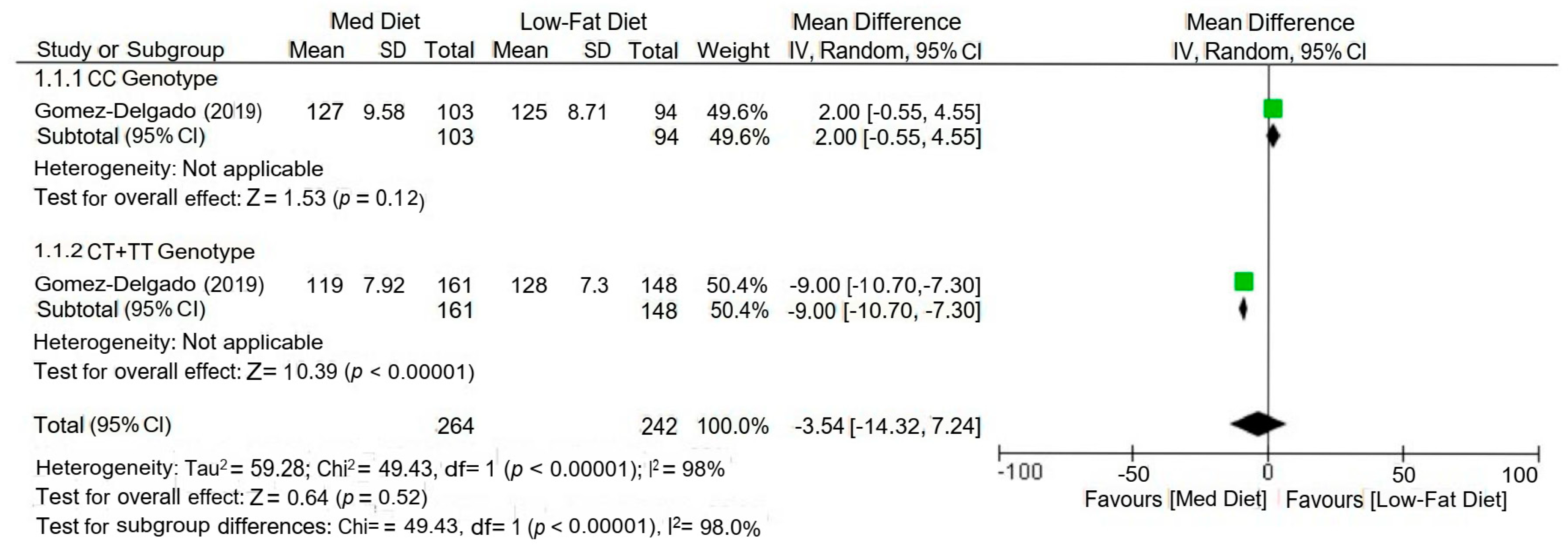
| [18] | Spain | CHD Patients | CORDIOPREV study (RCT) | 506 (male = 433, female = 73) | Mediterranean diet and Low-fat diet | 3 years | Postprandial TG and TRLs | APOE rs439401, rs440446, rs7412 | Using a gene–diet approach, the study analysed the interaction between the APOE rs439401 SNP and the MedDiet. Compared to CC patients, those in the MedDiet group who were carriers of the T-allele displayed a more significant decrease in postprandial triglycerides (TG: p = 0.03), as well as large triacylglycerol-rich lipoproteins (TRLs) TG (large TRLs TG; p = 0.01. Both the TG area under the curve (AUC-TG; P-interaction = 0.03) and the AUC-large TRLs TG (P-interaction = 0.02) showed consistent patterns that were significantly lower in T-allele carriers compared with levels in CC subjects. |
| [19] | Quebec, Canada | Caucasian subjects | Interventional | 208 | Omega-3 supplementation | 2 years, 3 months | Plasma lipids | 16 SNPs in IQCJ, 34 in NXPH1, 8 in PHF17, and 9 in MYB | The genotype risk score (GRS) accounted for 49.73 percent of the variation in TG response (p < 0.0001) in a general linear model that adjusted for age, sex, and body mass index. |
| [20] | New York and Los Angeles, USA | White, Caucasian Black, African-American Hispanic | Cross-sectional | 987 (male: 469, female: 518) | - | 2 years | Plasma homocysteine, micronutrients | Long interspersed nucleotide 1 (LINE-1) and Alu | The LINE-1 methylation was 0.05 (0.01, 0.13), %5 mC higher for every 3 mmol/L increase in homocysteine. Furthermore, a positive correlation (p trend = 0.03) was found between BMI and LINE-1 methylation. The LINE-1 was 0.35 (0.03, 0.67), %5 mC higher in participants with a 40 kg/m2 BMI than in those with a normal BMI. A variation of 0.10 (0.02, 0.19), %5 mC in Alu methylation was also noted for every 10 cm of height. |
| [21] | Quebec, Canada | Middle-aged adults at higher risk of developing chronic diseases | Cross-sectional | CARTaGENE biobank (12,065) | - | 6 years | Lipid profile and intakes (%kcal/day) of total, saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acids | CD36 gene | Habitual fat consumption is linked to CD36 variations, which might explain later relationships with biomarkers associated with chronic diseases. Higher consumption of SFA was linked to rs1054516 and rs3173798 (both p < 0.05), and rs1054516 was also linked to higher levels of serum triglycerides (p = 0.0065). |
| [22] | Spain | Patients without a history of cardiovascular disease but at high cardiovascular risk | Mediterranean diet for primary prevention of cardiovascular diseases (Prevención con DietaMediterránea) randomized trial | 521 | Mediterranean Diet | 5 years | Telomere length | PPARγ2 locus, rs1801282, Ala allele | After five years of follow-up, the pro12Ala polymorphism is linked to TL homeostasis in persons at high cardiovascular risk. Furthermore, among Ala carriers, a stronger defence against telomere shortening is provided by a higher level of adherence to the MeDiet pattern. |
| [23] | Germany | Monocyte/macrophage cell line RAW264.7 and the endothelial cell line TIME | In vitro experiment | - | Docosahexaenoic acid (DHA; n-3-PUFA) or arachidonic acid (AA; n-6-PUFA) | - | miRNAs | miRNAs | PUFAs affect miRNA expression in both cell types under investigation, regardless of the presence of an inflammatory stimulant. Moreover, it was shown that cellular PUFA enrichment had an impact on certain miRNAs previously connected to vascular inflammation. |
| [24] | Spain | CHD Patients | CORDIOPREV study (RCT) | 897 | Low-fat (LF) diet and Mediterranean diet (MedDiet) | 12 months | hs-CRP, HDL/ApoA1 | CLOCK SNPs (rs1801260, rs3749474, rs4580704) | The LF diet and the rs4580704 SNP interact to improve the inflammation and dyslipidaemia associated with CHD. Compared to minor G allele carriers (G/G + C/G), major allele carriers C/C showed a higher drop in high-sensitivity C-reactive protein (p < 0.001) and a substantial rise in HDL/apolipoprotein A1 ratio (p = 0.029). |
| [25] | Oslo, Norway | Adults | RCT | 99 | PUFAs | 8 weeks | Lipoprotein subclasses, bile acids, proprotein convertase subtilisin/kexin type 9, acetate, and acetoacetate | mRNA levels of LXRA and LDLR, UCP2, and PPARD | Subclasses of lipoproteins, myristoyl- and palmitoyl-carnitine, and kynurenine decreased when PUFAs were substituted for SFAs. On the other hand, the intervention raised the levels of acetoacetate, bile acids, proprotein convertase subtilisin/kexin type 9, and acetate. The intervention also changed a few amino acids. After substituting SFAs with PUFAs, peripheral blood mononuclear cells showed a drop in the mRNA levels of UCP2 and PPARD and an increase in the mRNA levels of LXRA and LDLR, along with many genes implicated in inflammation and liver X receptor alpha target genes. |
| [26] | Quebec, Canada | Adults from Quebec Family Study (QFS)—observational | Quebec Family Study (QFS)—observational | 541 | - | - | Total fat intake; LDL-PPD | SNPs from a genome-wide association study (GWAS) | There is an interaction between dietary fat consumption and various SNPs in terms of variation in the LDL-PPD. |
| [27] | Quebec, Canada | Adults (18–50 years) | Interventional study | 208 | 3 g/day of n-3 PUFA | 6 weeks | TG levels | 5 SNPs in PLA2G2A, 6 in PLA2G2C, 8 in PLA2G2D, 6 in PLA2G2F, 22 in PLA2G4A, 5 in PLA2G6, and 9 in PLA2G7 were genotyped | These results suggest that SNPs in PLA2 genes could influence plasma TG levels when supplemented with n-3 PUFA. |
| [28] | Quebec, Canada | Adults (18–50 years) | Interventional study | 210 | 5 g/d of a fish oil supplement | 6 weeks | LDL-C, particle size | 18 SNPs of the MGLL gene | After supplementation with n-3 PUFA, plasma LDL-C levels and particle size may be modified by polymorphisms in the MGLL gene. |
| [29] | Quebec, Canada | Adults (18–50 years) | Interventional study | 208 | 5 g/day of fish oil | 6-week | Plasma TG | SNPs: IQCJ, NXPH1, PHF17 and MYB genes | Using fine-mapping at GWAS-associated loci, SNPs partially explaining the significant interindividual heterogeneity in plasma TG levels induced by an n-3 FA supplementation were identified. |
| [30] | Iran | Adults | Mashhad Stroke and Heart Atherosclerotic Disorders (MASHAD) cohort study | 1165 | - | 7 years | CVD risk, lipids | CDKN2A/B-rs10811661 locus | A strong correlation was observed between cardiovascular risk variables and dyslipidaemia, as well as the CDKN2A-rs10811661 polymorphism. |
| Biomarker Categories | Biological Markers | Mechanism/Aspects of Personalized Nutrition | Outcome | References |
|---|---|---|---|---|
| Genetic Markers | Lipoprotein (a) | The LPA gene, in particular, plays a major role in determining Lp(a) levels, but other treatments have also been proven to affect them. | Lp(a) significantly increases the risk of ASCVD that remains after statin treatment in individuals. | [34] |
| Mutations in PCSK9 (proprotein convertase subtilisin/kexin type 9) Protein | Lowers the amounts of LDLR expressed in peripheral tissues or the liver, which indirectly obstructs the absorption of LDL by hepatocytes and other tissues. | Interference with molecular pathways during the onset and development of atherosclerotic plaque | [35,36] | |
| Antisense noncoding RNA in the INK4 locus (ANRIL) | Regulate the division and death of cells | Change the arterial plaque size and the apoptotic debris removal process | [36] | |
| CDKN2A/2B Rs10811661 (C/T) polymorphism | A TT genotype has been linked to a higher risk of CVD, insulin resistance, and hypercholesterolemia. These effects were more noticeable in the subgroup with low physical activity levels and high dietary energy intake. | Genetic variation increases the risk of cardiovascular disease and dyslipidemia. | [30] | |
| ANRIL | Modify chromatin to control the growth of vascular smooth muscle cells (VSMCs) in plaques. Additionally, alter transcriptional levels to impact macrophage proliferation and death. | Atherosclerotic plaque growth is tightly linked to the proliferation and death of related cells. | [37] | |
| Circulating miRNAs | A PUFA-enriched normocaloric diet is linked to modifications in the circulating profile of miRNA. A high-fat diet demonstrated how TGRL uses miRNA to sway the endothelium pro-inflammatory response. | Decreased miR-21, miR-30, miR-126 and miR-221-3p; and increased miR-21, miR-92a and miR-99a with the progression and degradation of atherosclerosis phenotypes | [38,39,40] | |
| miR-24 miR-122 miR-185 miR-223 miR-486 Cholesterol homeostasis and reverse cholesterol transport | ||||
| miR-155 miR-378a Plaque rupture in atherosclerosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayyaz, K.; Din, M.S.u.; Bashir, H.; Ahmad, F.; Barrow, C.J.; Khalid, N. Personalized Nutrition Biomarkers and Dietary Strategies for Atherosclerosis Risk Management: A Systematic Review. Nutrients 2025, 17, 2804. https://doi.org/10.3390/nu17172804
Fayyaz K, Din MSu, Bashir H, Ahmad F, Barrow CJ, Khalid N. Personalized Nutrition Biomarkers and Dietary Strategies for Atherosclerosis Risk Management: A Systematic Review. Nutrients. 2025; 17(17):2804. https://doi.org/10.3390/nu17172804
Chicago/Turabian StyleFayyaz, Khadijah, Muhammad Saeed ud Din, Husnain Bashir, Firdos Ahmad, Colin J. Barrow, and Nauman Khalid. 2025. "Personalized Nutrition Biomarkers and Dietary Strategies for Atherosclerosis Risk Management: A Systematic Review" Nutrients 17, no. 17: 2804. https://doi.org/10.3390/nu17172804
APA StyleFayyaz, K., Din, M. S. u., Bashir, H., Ahmad, F., Barrow, C. J., & Khalid, N. (2025). Personalized Nutrition Biomarkers and Dietary Strategies for Atherosclerosis Risk Management: A Systematic Review. Nutrients, 17(17), 2804. https://doi.org/10.3390/nu17172804






