Malnutrition and Nutrition Impact Symptoms in Kuwaiti Colorectal Cancer Patients: Validation of PG-SGA Short Form
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
2.2. Data Collection
2.3. Survey Tool and Data Components
2.4. Dietary Intake Method
2.5. Assessment of Nutritional Status
2.6. Dietary Assessment
2.7. Missing Data Handling
2.8. Statistical Analysis
3. Results
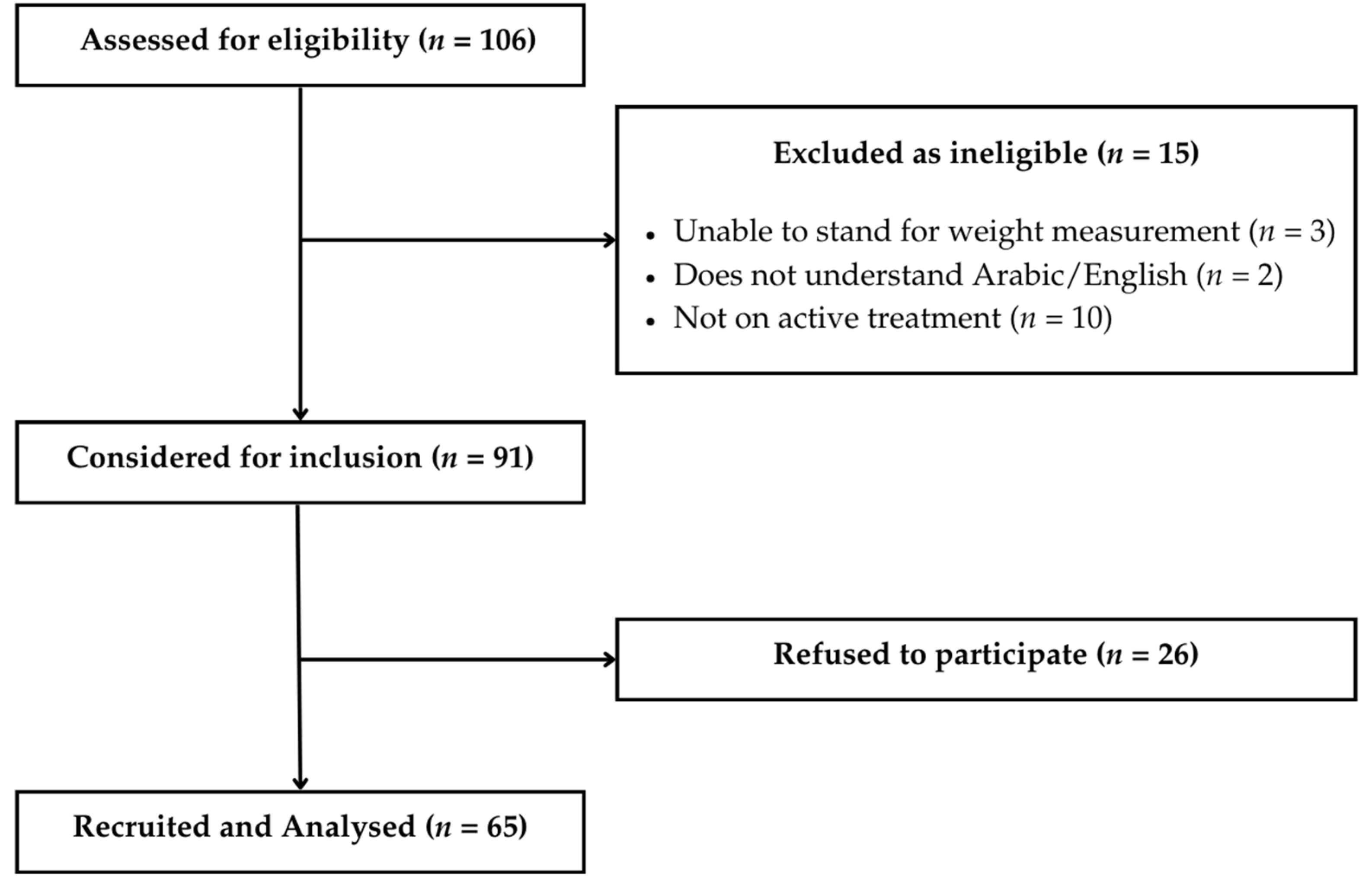
3.1. Recruitment and Sample Size Considerations
3.2. Sociodemographic Characteristics
3.3. Clinical Characteristics by Nutritional Status
3.4. Nutritional Status of CRC Patients
3.5. Anthropometrics and Malnutrition
3.6. Dietary Intake and Malnutrition
3.7. Biochemical Data
3.8. Referral to a Dietitian and Malnutrition Status
3.9. NIS According to Malnutrition Status
3.10. Predictors of Malnutrition
3.11. Agreement Between Screening and Assessment Tools
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| PG-SGA | Patient-Generated Subjective Global Assessment |
| PG-SGA SF | Patient-Generated Subjective Global Assessment Short Form |
| MST | Malnutrition Screening Tool |
| NIS | Nutrition Impact Symptoms |
| KCCC | Kuwait Cancer Control Center |
| BMI | Body Mass Index |
| NFPE | Nutrition-Focused Physical Examination |
| IBW | Ideal Body Weight |
| ABW | Adjusted Body Weight |
| RDA | Recommended Dietary Allowance |
| CI | Confidence Interval |
| OR | Odds Ratio |
| R | R Statistical Software |
| IQR | Interquartile Range |
| HIS | Hospital Information System |
| RBC | Red Blood Cells |
| EHR | Electronic Health Record |
| EER | Estimated Energy Requirements |
| MCH | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| MCV | Mean Corpuscular Volume |
| RDW | Red Cell Distribution Width |
| PT-Global | Platform for PG-SGA scoring tool |
References
- World Health Organization. Cancer. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 10 March 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kuwait Cancer Registry. Kuwait Cancer Registry Annual Report 2010–2019; Ministry of Health: 2023. Available online: https://www.moh.gov.kw/UserGuides/Cancer%20Registry%20Book%202019.pdf (accessed on 10 March 2025).
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in hospitalized old patients: Screening and diagnosis, clinical outcomes, and management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef]
- Reber, E.; Schönenberger, K.A.; Vasiloglou, M.F.; Stanga, Z. Nutritional Risk Screening in Cancer Patients: The First Step Toward Better Clinical Outcome. Front. Nutr. 2021, 8, 603936. [Google Scholar] [CrossRef]
- Opanga, S.A.; Ronoh, V.; Mutai, K.; Tenge, C.; Wanyoike, M.; Macharia, M. Nutritional status of cancer outpatients using scored patient-generated subjective global assessment: A Kenyan cross-sectional study. Nutrients 2017, 9, 1136. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Ravasco, P. Nutrition in cancer patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef]
- Thompson, K.L.; Elliott, L.; Fuchs—Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence--Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e47. [Google Scholar] [CrossRef] [PubMed]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Carriço, M.; Guerreiro, C.S.; Parreira, A. The validity of the Patient-Generated Subjective Global Assessment Short-Form© in cancer patients undergoing chemotherapy. Clin. Nutr. ESPEN 2021, 43, 296–301. [Google Scholar] [CrossRef]
- Thompson, F.E.; Subar, A.F. Dietary assessment methodology. In Nutrition in the Prevention and Treatment of Disease, 4th ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Delahanty, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 5–48. [Google Scholar] [CrossRef]
- Vasson, M.-P.; Talvas, J.; Perche, O.; Dillies, A.-F.; Bachmann, P.; Pezet, D.; Achim, A.-C.; Pommier, P.; Racadot, S.; Weber, A.; et al. Immunonutrition stimulates immune functions and antioxidant defense capacities of leukocytes in radiochemotherapy-treated head & neck and esophageal cancer patients: A double-blind randomized clinical trial. Clin. Nutr. 2014, 33, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, V.E.; Dietrich, A.M.; Estabrooks, P.A.; Savla, J.; Serrano, E.; Davy, B.M. Dietary biomarkers: Advances, limitations and future directions. Nutr. J. 2012, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Marques Vidal, P.; Camilo, M.E. Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- PT-Global. PG-SGA Tool (Web-Based Platform). Available online: https://pt-global.org/pt-global-app/ (accessed on 8 July 2025).
- Abbott, J.; Teleni, L.; McKavanagh, D.; Watson, J.; McCarthy, A.L.; Isenring, E. Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) is a valid screening tool in chemotherapy outpatients. Support. Care Cancer 2016, 24, 3883–3887. [Google Scholar] [CrossRef]
- De Groot, L.M.; Lee, G.; Ackerie, A.; van der Meij, B.S. Malnutrition screening and assessment in the cancer care ambulatory setting: Mortality predictability and validity of the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) and the GLIM criteria. Nutrients 2020, 12, 2287. [Google Scholar] [CrossRef]
- Mueller, C.; Compher, C.; Ellen, D.M.; American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. Clinical guidelines: Nutrition screening, assessment, and intervention in adults. J. Parenter. Enter. Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef]
- MDCalc. Ideal Body Weight (IBW) and Adjusted Body Weight (ABW) Calculators [Mobile Application]. Version 5.4.0. 2025. Available online: https://www.mdcalc.com (accessed on 10 May 2025).
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.E.; den Braver, N.R.; Berkhof, J.; Langius, J.A.E.; Verheul, H.M.W. Loss of muscle mass during chemotherapy is predictive of poor survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Muscaritoli, M.; The PreMiO Study Group; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef]
- Ho, C.Y.; Ibrahim, Z.; Abu Zaid, Z.; Mat Daud, Z.; Md Yusop, N.B. Clinical malnutrition predictive model among gynecologic cancer patients prior to elective operation: A cross-sectional study. Clin. Nutr. 2021, 40, 4373–4379. [Google Scholar] [CrossRef]
- Durán Poveda, M.; Suárez-de-la-Rica, A.; Minchot, E.C.; Ocón Bretón, J.; Sánchez Pernaute, A.; Rodríguez Caravaca, G.; PREMAS Study Group. The prevalence and impact of nutritional risk and malnutrition in gastrointestinal surgical oncology patients: A prospective, observational, multicenter, and exploratory study. Nutrients 2023, 15, 3283. [Google Scholar] [CrossRef]
- Yi, H.C.; Hong, B.Z. High prevalence of malnutrition and associated factors in newly diagnosed upper gastrointestinal cancer patients: A cross-sectional study. Asian Pac. J. Cancer Care 2024, 9, 267–275. [Google Scholar] [CrossRef]
- Alsaleh, K.; Almomen, F.A.; Altaweel, A.; Barasain, O.; Alqublan, A.; Binsalamah, A.; Almashham, A. Malnutrition in cancer patients receiving chemotherapy in a single oncology center. J. Nat. Sci. Med. 2021, 7, 147–152. [Google Scholar] [CrossRef]
- Shakhshir, M.H.; Salameh, H.T.; Amer, R.; Zyoud, S.H. An evaluation of nutritional impact symptoms and their association with reduced dietary intake in patients with solid tumors at tertiary care hospitals: A multicenter, cross-sectional study from Palestine. BMC Cancer 2024, 24, 239. [Google Scholar] [CrossRef]
- Kim, D.H. Nutritional issues in patients with cancer. Intest. Res. 2019, 17, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Cushen, S.J.; Orsso, C.E.; Ryan, A.M. Sarcopenia and cachexia in the era of obesity: Clinical and nutri-tional impact. Proc. Nutr. Soc. 2016, 75, 188–198. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Castillo, A.L.; Quesenberry, C.P.; Weltzien, E. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.M.; Loeliger, J.; Nolte, L.; Kelaart, A.; Kiss, N. Prevalence of malnutrition and impact on clinical outcomes in cancer services: A comparison of two time points. Clin. Nutr. 2019, 38, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Cereda, E.; Pinto, C.; Cotogni, P.; Farina, G.; Gavazzi, C.; Pedrazzoli, P. Awareness and consideration of malnutrition among oncologists: Insights from an exploratory survey. Nutrition 2016, 32, 1028–1032. [Google Scholar] [CrossRef]
- Lorton, C.M.; Griffin, O.; Higgins, K.; Roulston, F.; Stewart, G.; Gough, N.; Barnes, E.; Aktas, A.; Walsh, T.D. Late referral of cancer patients with malnutrition to dietitians: A prospective study of clinical practice. Support. Care Cancer 2020, 28, 2351–2360. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poirée, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Lewandowska, A.; Religioni, U.; Czerw, A.; Deptała, A.; Karakiewicz, B.; Partyka, O.; Pajewska, M.; Sygit, K.; Cipora, E.; Kmieć, K.; et al. Nutritional Treatment of Patients with Colorectal Cancer. Int. J. Environ. Res. Public Health 2022, 19, 6881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Bayyari, N.; Hailat, M.; Baylin, A. Gender-specific malnutrition and muscle depletion in gastric and colorectal cancer: Role of dietary intake in a Jordanian cohort. Nutrients 2024, 16, 4000. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A.; Quatrara, B.; Parkhurst, M.L.; Malone, A.M.; Fanjiang, G.; Ziegler, T.R. Critical role of nutrition in improving quality of care: An interdisciplinary call to action to address adult hospital malnutrition. J. Acad. Nutr. Diet. 2013, 113, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
| N (%) | Malnourished | Well Nourished | p-Value | ||
|---|---|---|---|---|---|
| Gender | Female | 28 (43.1) | 18 (64.3) | 10 (35.7) | 0.890 |
| Male | 37 (56.9) | 22 (59.5) | 15 (40.5) | ||
| Marital status | Married | 56 (86.2) | 32 (57.1) | 24 (42.9) | 0.122 |
| Divorced/Widowed | 8 (12.3) | 7 (87.5) | 1 (12.5) | ||
| Single | 1 (1.5) | 0 (0) | 1 (100) | ||
| Age (years) | 18–39 | 3 (4.6) | 3 (100) | 0 (0) | 0.079 |
| 40–59 | 34 (52.3) | 20 (58.8) | 14 (41.2) | ||
| 60+ | 28 (43.1) | 16(47.8) | 12(52.2) | ||
| Governorate | Hawalli | 17 (26.2) | 7 (41.2) | 10 (58.8) | 0.146 |
| Farwaniya | 14 (21.5) | 8 (57.1) | 6 (42.9) | ||
| Capital | 14 (21.5) | 11 (78.6) | 3 (21.4) | ||
| Al-Ahmadi | 11 (16.9) | 8 (72.7) | 3 (27.3) | ||
| Jahra | 5 (7.7) | 4 (80) | 1 (20) | ||
| Mubarak Al-Kabeer | 4 (6.2) | 1 (25) | 3 (75) | ||
| Employment | Unemployed | 15 (23.1) | 11 (73.3) | 4 (26.7) | 0.415 |
| Employed | 27 (41.5) | 17 (63) | 10 (37) | ||
| Retired | 23 (35.4) | 12 (52.2) | 11 (47.8) | ||
| Monthly income (KWD) | <300 | 6 (9.3) | 3 (50) | 3 (50) | 0.437 |
| 301–1000 | 24 (36.9) | 17 (57.1) | 7 (42.9) | ||
| >1000 | 35 (53.8) | 20 (57.1) | 15 (42.9) | ||
| Nationality | Kuwaiti | 32 (49.2) | 19 (59.4) | 13 (40.6) | 1.000 |
| Non-Kuwaiti | 33 (50.8) | 20 (60.6) | 13 (39.4) | ||
| Level of education | High school or lower | 37 (56.9) | 25 (67.6) | 12 (32.4) | 0.281 |
| Diploma | 8 (12.3) | 3 (37.5) | 5 (62.5) | ||
| Bachelor’s or higher | 20 (30.8) | 12 (60.0) | 8 (40.0) |
| N (%) | Malnourished | Well Nourished | p-Value | ||
|---|---|---|---|---|---|
| Number of comorbidities | 0 | 27 (42) | 14 (51.9) | 13 (48.1) | 0.50 |
| 1 | 19 (29) | 12 (63.2) | 7 (36.8) | ||
| ≥2 | 19 (29) | 13 (68.4) | 6 (31.6) | ||
| Current smoker | Yes | 11 (16.9) | 8 (72.7) | 3 (27.3) | 0.543 |
| No | 54 (83.1) | 31 (57.4) | 23 (42.6) | ||
| Stage of cancer | Early locally advanced | 2 (3.1) | 1 (50) | 1 (50.0) | 0.929 |
| Late locally advanced | 11 (16.9) | 7 (63.6) | 4 (36.4) | ||
| Metastasized | 52 (80.0) | 31 (59.6) | 21 (40.4) | ||
| Treatment type | Chemotherapy | 45 (69.2) | 28 (62.2) | 17 (37.8) | 0.209 |
| Target therapy | 4 (6.2) | 4 (100) | 0 (0) | ||
| Immunotherapy | 1 (1.5) | 1 (100) | 0 (0) | ||
| Combination | 15 (23.1) | 7 (46.7) | 8 (53.3) | ||
| Stoma | Yes | 23 (35.4) | 18 (78.3%) | 5 (21.7%) | 0.050 |
| No | 42 (64.6) | 21 (50.0%) | 21 (50.0%) | ||
| BMI category | Underweight | 2 (3.1) | 2 (100) | 0 (0) | 0.0565 |
| Normal | 25 (38.5) | 19 (76) | 6 (24) | ||
| Overweight | 18 (27.7) | 7 (38.9) | 11 (61.1) | ||
| Obese | 20 (30.8) | 11 (55) | 9 (45) |
| PG-SGA SF | PG-SGA | ||||
|---|---|---|---|---|---|
| Variable | Total Sample 65 (100) | At Risk 37 (56.9) | Not at Risk 28 (43.1) | Malnourished 39 (60) * | Well Nourished 26 (40) |
| NIS | |||||
| No appetite | 33 (51) | 27 (73) | 6 (21) | 27 (73) | 6 (21) |
| Nausea | 14 (22) | 14 (38) | 0 (0) | 7 (17.9) | 0 (0.0) |
| Dry mouth | 20 (31) | 19 (51) | 1 (4) | 19 (48.7) | 1 (3.8) |
| PG-SGA Total Score | 9.5 (6.0–15.0) | — | — | 11.0 (8.5–13.0) | 3.0 (0.25–4.75) |
| PG-SGA SF Total Score | 7 (4–13) | 11 (10–15) | 3.5 (1–5) | — | — |
| MST | Total Sample 65 (100) | At Risk 42 (64.6) | Not at Risk 23 (35.4) |
|---|---|---|---|
| Weight loss | |||
| No | 24 (37) | 7 (19) | 17 (61) |
| Unsure | 14 (22) | 11 (30) | 3 (11) |
| 1–5 kg | 9 (14) | 6 (16) | 3 (11) |
| 6–10 kg | 6 (9) | 5 (14) | 1 (4) |
| 11–15 kg | 3 (5) | 2 (5) | 1 (4) |
| >15 kg | 8 (12) | 6 (16) | 3 (11) |
| Poor appetite | 42 (65) | 31 (84) | 11 (39) |
| MST total score | 2 (0–3) | 3 (2–3) | 0 (0–2.25) |
| Variable | Malnourished Median (IQR) | Well Nourished Median (IQR) | p-Value | Effect Size r |
|---|---|---|---|---|
| % weight loss in 1 month | 1.25 (0.00–2.87) | 0.00 (0.00–0.00) | 0.001 | 0.42 |
| % weight loss in 6 months | 3.33 (0.00–13.77) | 0.00 (0.00–1.78) | 0.003 | 0.39 |
| Mid-arm | 27.35 (24.60–31.02) | 31.20 (28.06–35.01) | 0.01 | 0.31 |
| Mid-calf | 34.40 (32.10–37.62) | 37.72 (34.74–40.05) | 0.04 | 0.26 |
| CBW (kg) | 66.30 (58.00–81.30) | 78.40 (67.95–91.05) | 0.049 | 0.24 |
| Weight (last month) | 71.00 (57.50–82.00) | 77.00 (65.00–87.50) | 0.11 | 0.20 |
| BMI (kg/m2) | 24.50 (21.55–31.65) | 28.70 (24.35–32.60) | 0.15 | 0.18 |
| Weight (3 months) | 70.00 (57.00–81.00) | 76.00 (67.25–85.25) | 0.22 | 0.15 |
| Weight (6 months) | 74.00 (60.50–82.00) | 74.00 (67.25–84.75) | 0.55 | 0.07 |
| Nutrient Intake | Malnourished Median (IQR) | Well Nourished Median (IQR) | p-Value |
|---|---|---|---|
| Energy intake (kcal) | 1384.00 (1067.00–1762.50) | 1731.00 (1287.50–2296.75) | 0.03 |
| Protein (g) | 60.00 (43.00–78.50) | 101.50 (76.25–128.00) | 0.0001 |
| Protein intake (g/kg) | 0.82 (0.62–1.14) | 1.27 (0.96–1.57) | 0.004 |
| Met EER, n (%) | 16 (41.0) | 12 (46.2) | 0.88 |
| Seen by a Dietitian |
Malnourished,
n (%) |
Well Nourished,
n (%) | p-Value |
|---|---|---|---|
| Yes | 19 (70.4) | 8 (29.6) | 0.24 |
| No | 20 (52.6) | 18 (47.4) |
| Nutrition Impact Symptom | Malnourished n = 39 | Well Nourished n = 26 | p-Value |
|---|---|---|---|
| No appetite | 27 (69.2) | 6 (23.1) | 0.001 |
| Dry mouth | 19 (48.7) | 1 (3.8) | 0.0004 |
| Diarrhea | 16 (41.0) | 3 (11.5) | 0.02 |
| Nausea | 14 (35.9) | 0 (0.0) | 0.002 |
| Early Satiety | 13 (33.3) | 1 (3.8) | 0.01 |
| Constipation | 13 (33.3) | 5 (19.2) | 0.34 |
| Fatigue | 11 (28.2) | 2 (7.7) | 0.09 |
| Taste changes | 11 (28.2) | 2 (7.7) | 0.09 |
| Mouth sores | 9 (23.1) | 0 (0.0) | 0.02 |
| Pain | 7 (17.9) | 0 (0.0) | 0.06 |
| Vomiting | 7 (17.9) | 0 (0.0) | 0.06 |
| Problems swallowing | 5 (12.8) | 0 (0.0) | 0.15 |
| Hyperosmia | 5 (12.8) | 0 (0.0) | 0.15 |
| Variable | Odds Ratio (OR) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Dry mouth (yes vs. no) | 17.65 | 2.02–154.19 | 0.009 |
| Mid-arm muscle circumference (low vs. normal) | 5.21 | 1.25–21.78 | 0.023 |
| BMI (continuous) | 0.94 | 0.84–1.04 | 0.19 |
| Presence of stoma (yes vs. no) | 1.78 | 0.51–6.12 | 0.34 |
| Cancer stage (metastatic vs. early) | 3.1 | 0.85–11.30 | 0.08 |
| Age (per year) | 1.01 | 0.97–1.06 | 0.64 |
| Sex (female vs. male) | 0.88 | 0.30–2.55 | 0.79 |
| Metric | PG-SGA SF vs. PG-SGA | MST vs. PG-SGA |
|---|---|---|
| True Positives (TFs) | 34.0 | 31.0 |
| False Positives (FPs) | 3.0 | 11.0 |
| True Negatives (TNs) | 23.0 | 15.0 |
| False Negatives (FNs) | 5.0 | 8.0 |
| Sensitivity * | 87.2% [73.3–94.4] | 79.5% [64.5–89.2] |
| Specificity * | 88.5% [71.0–96.0] | 57.7% [38.9–74.5] |
| PPV * | 91.9% [78.7– 97.2] | 57.7% [38.9–74.5] |
| NPV * | 82.1% [64.4–92.1] | 65.2% [44.9–81.2] |
| Cohen’s Kappa (κ) * | 0.75 [0.58–0.91] | 0.38 [0.14–0.61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obaid, R.; Alkazemi, D. Malnutrition and Nutrition Impact Symptoms in Kuwaiti Colorectal Cancer Patients: Validation of PG-SGA Short Form. Nutrients 2025, 17, 2770. https://doi.org/10.3390/nu17172770
Obaid R, Alkazemi D. Malnutrition and Nutrition Impact Symptoms in Kuwaiti Colorectal Cancer Patients: Validation of PG-SGA Short Form. Nutrients. 2025; 17(17):2770. https://doi.org/10.3390/nu17172770
Chicago/Turabian StyleObaid, Raghad, and Dalal Alkazemi. 2025. "Malnutrition and Nutrition Impact Symptoms in Kuwaiti Colorectal Cancer Patients: Validation of PG-SGA Short Form" Nutrients 17, no. 17: 2770. https://doi.org/10.3390/nu17172770
APA StyleObaid, R., & Alkazemi, D. (2025). Malnutrition and Nutrition Impact Symptoms in Kuwaiti Colorectal Cancer Patients: Validation of PG-SGA Short Form. Nutrients, 17(17), 2770. https://doi.org/10.3390/nu17172770







