Leveraging Deep Learning to Enhance Malnutrition Detection via Nutrition Risk Screening 2002: Insights from a National Cohort
Abstract
1. Introduction
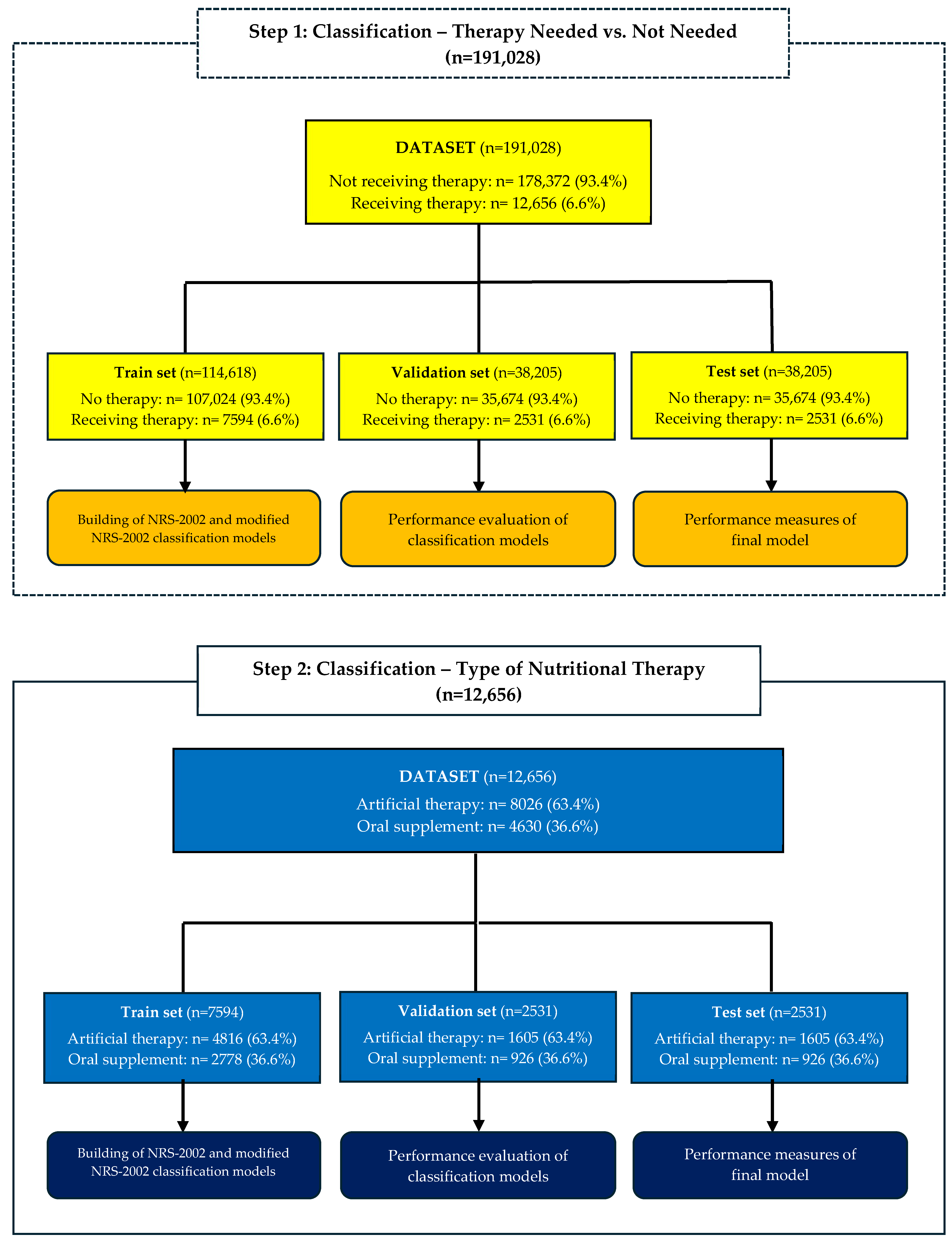
2. Methods
2.1. Data Collection
2.2. Statistical Analysis
2.3. Classification Analysis
3. Results
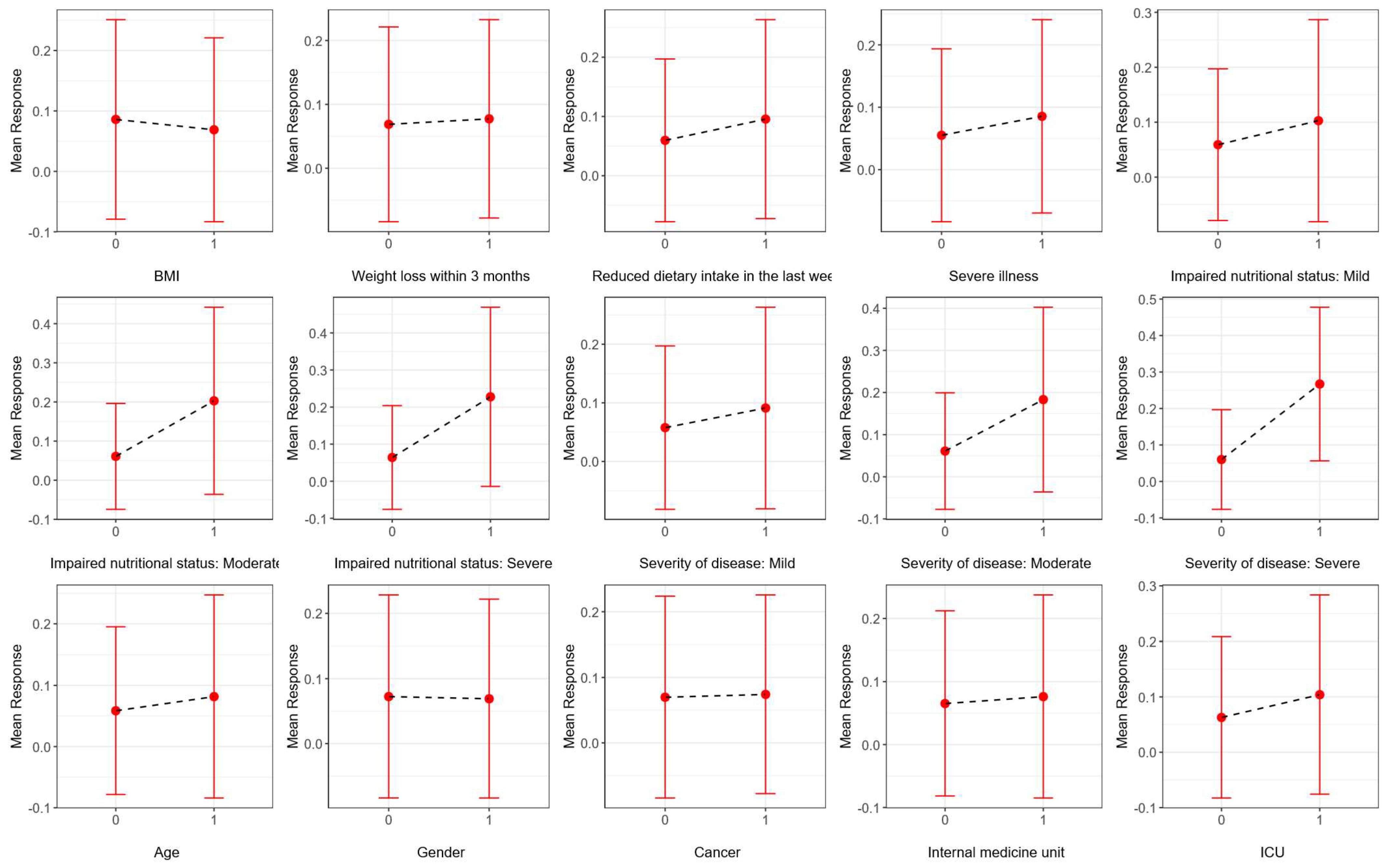
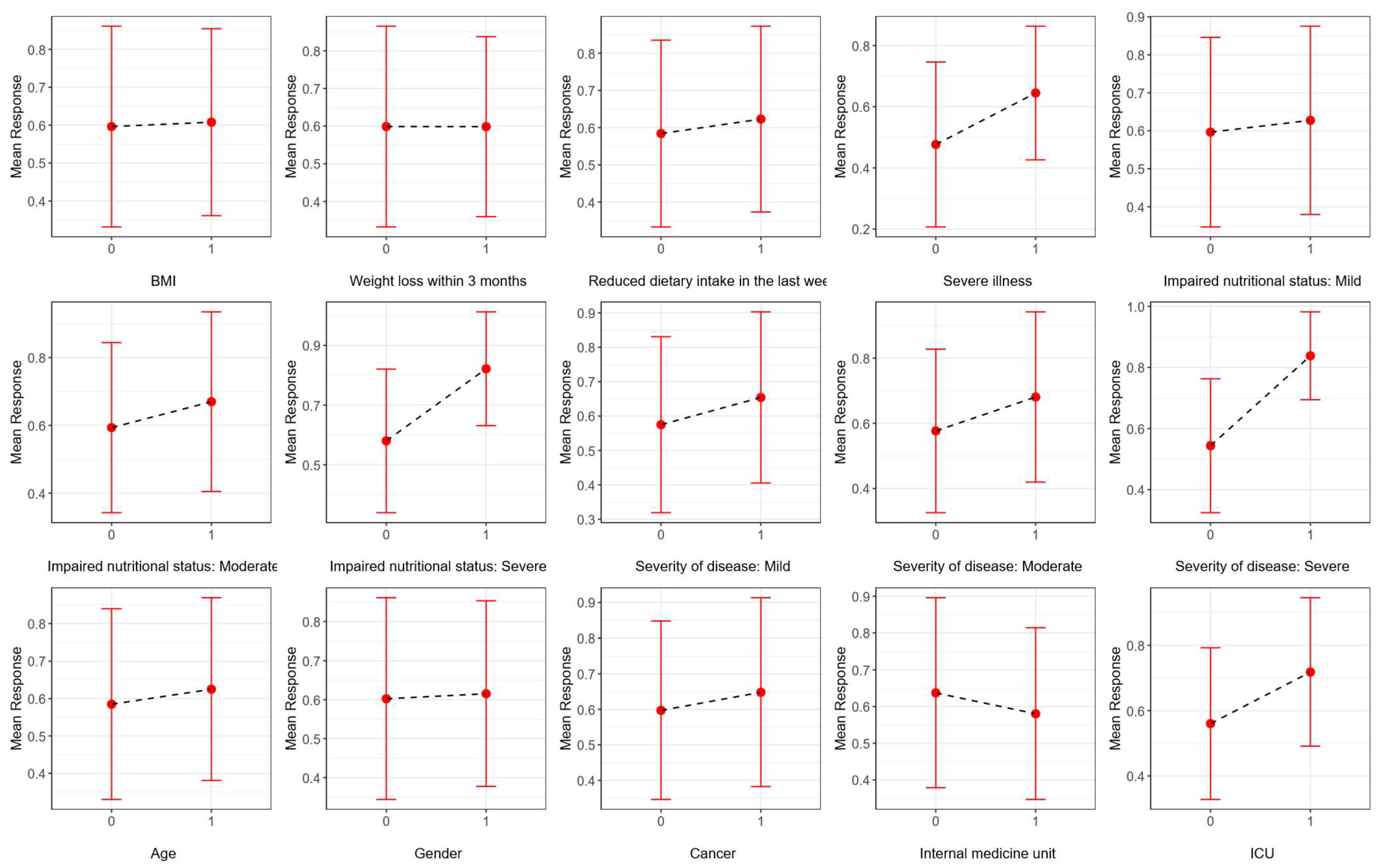
3.1. Descriptive Statistics and Univariate Analysis
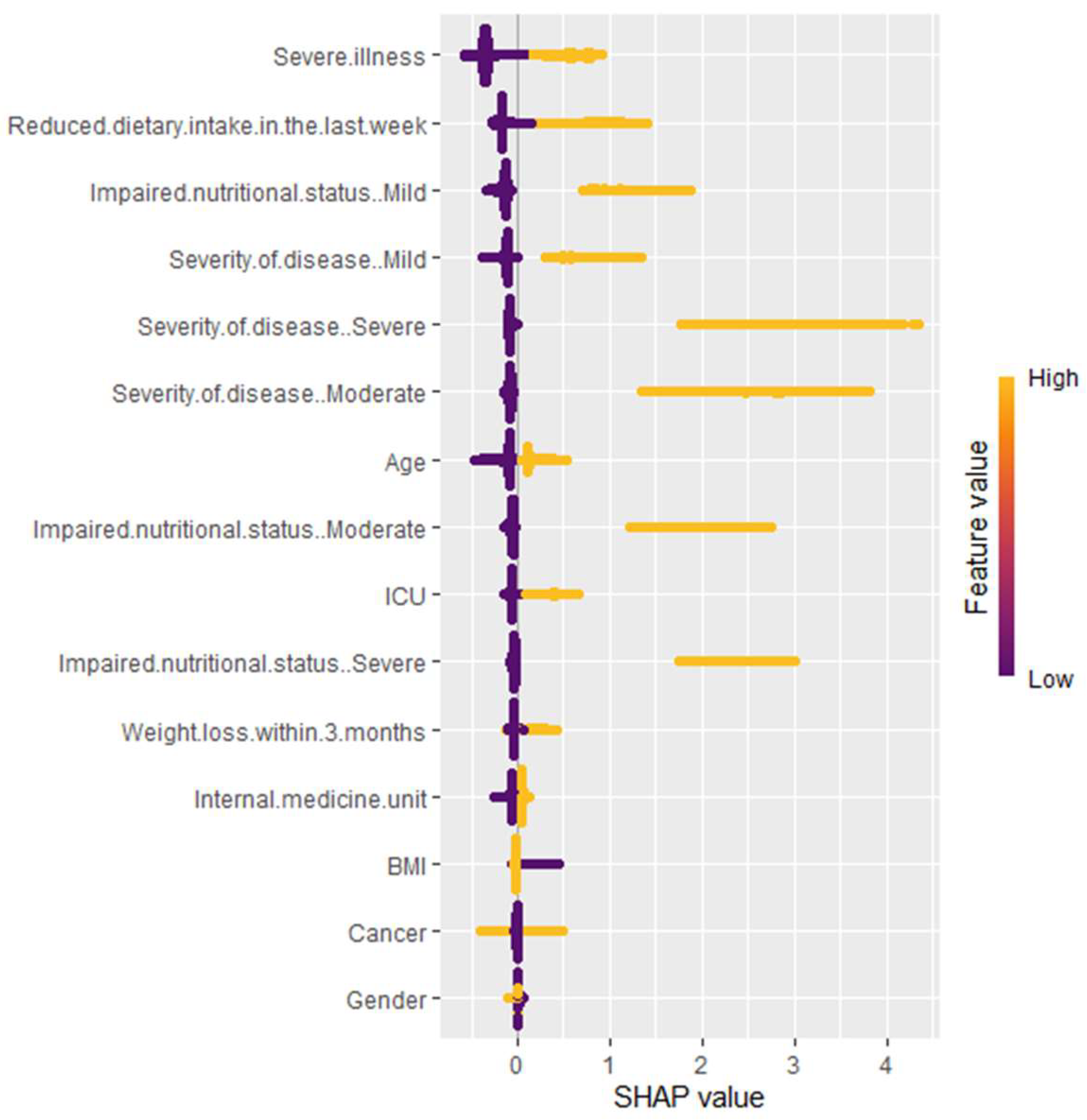
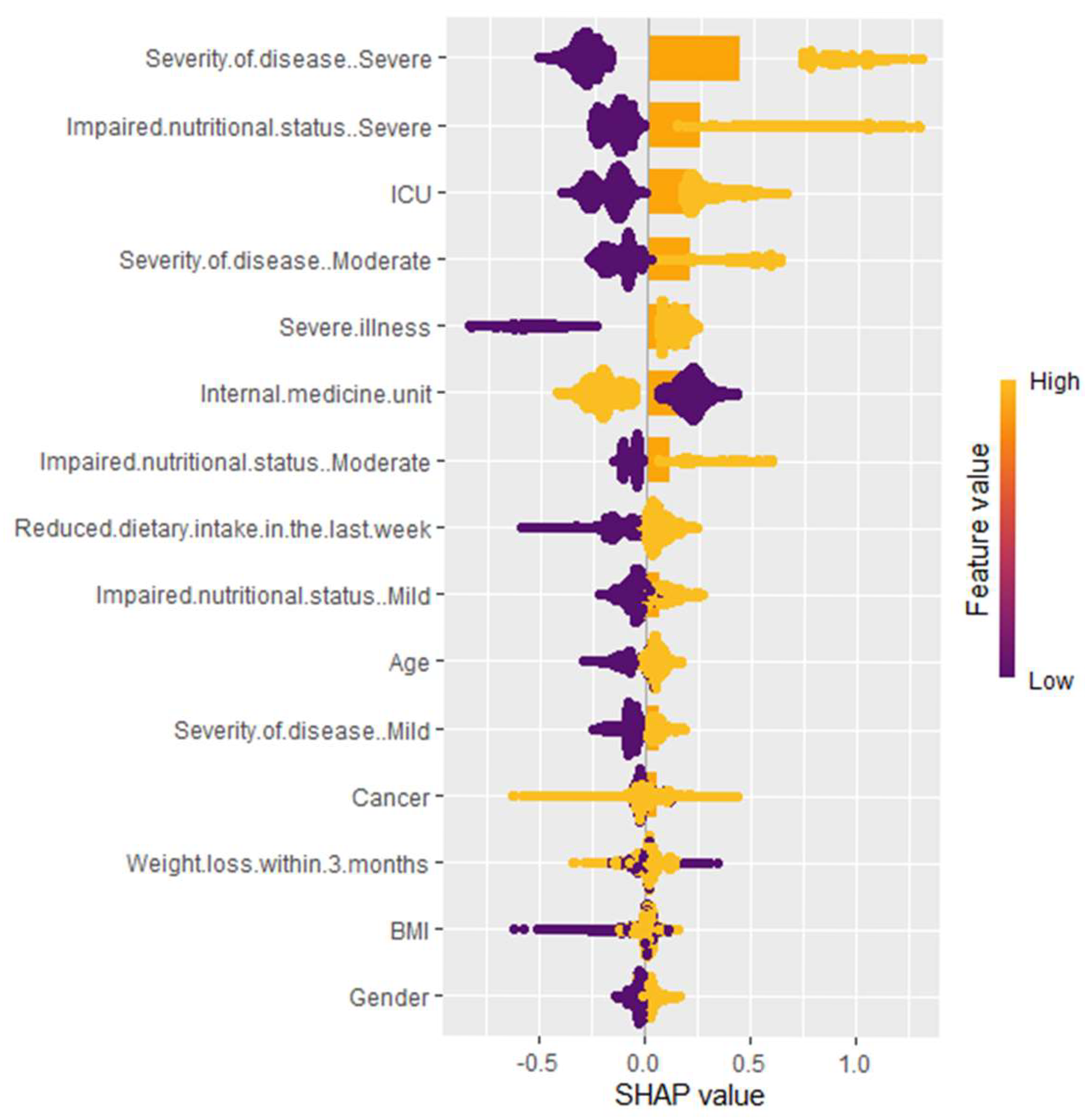
3.2. Variable Importance
- Severe illness and reduced dietary intake in the last week have a wide range of SHAP values (yellow and purple), showing that high and low values greatly impact the predictions of the model.
- Impaired nutritional status (mild and moderate), severity of disease (mild, moderate, and severe), age, ICU, and weight loss within 3 months show a medium range of SHAP values. This means that their impact on the model’s prediction varies moderately with different values, but overall, they are not as strong in influence compared to other top variables.
- Cancer and gender display a narrow range of SHAP values, indicating that while they contribute to the predictions, their impact is relatively stable and less variable.
- Severity of disease (particularly severe cases), ICU admission, and impaired nutritional status are the most influential factors. Both high and low categories of these variables may greatly impact the model’s predictions.
- Severe illness and internal medicine unit admission also contribute substantially, highlighting the importance of hospitalization context.
- Reduced dietary intake in the last week indicates that larger reductions in intake are associated with higher model predictions, while smaller reductions result in lower predictions.
- Age, cancer status, and weight loss within 3 months, as well as moderate illness severity and mild to moderate impaired nutritional status, have a medium range of SHAP scores. Their impact on the model’s predictions is noticeable, although not as strong as the top variables.
- BMI and gender have little effect on model predictions.
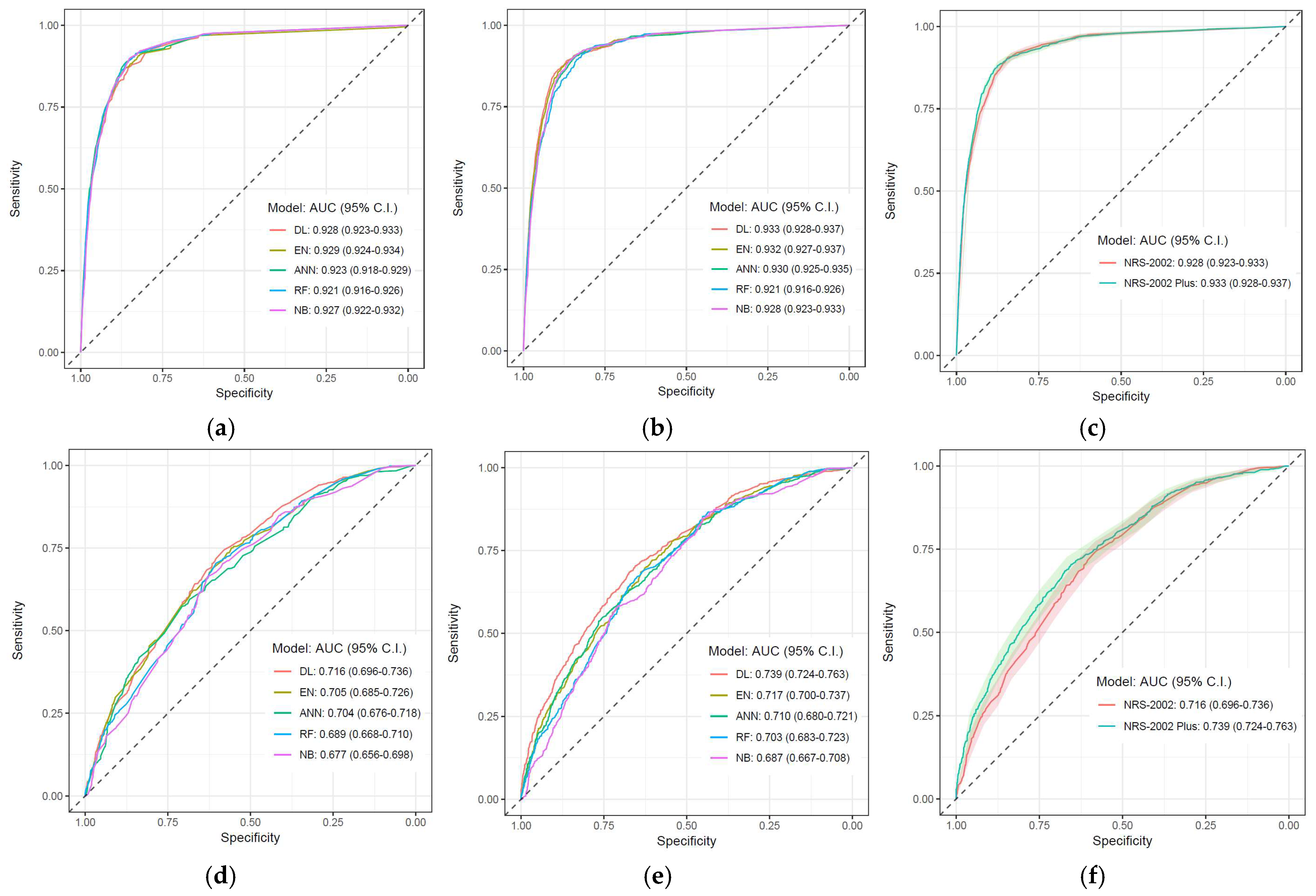
3.3. Classification Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Hersberger, L.; Bargetzi, L.; Bargetzi, A.; Tribolet, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: Secondary analysis of a prospective randomised trial. Clin. Nutr. 2020, 39, 2720–2729. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Krznaric, Z.; Vranesic Bender, D.; Blaz Kovac, M.; Cuerda, C.; van Ginkel-Res, A.; Hiesmayr, M.; Marinho, A.; Mendive, J.; Monteiro, I.; Pirlich, M.; et al. Clinical nutrition in primary care: ESPEN position paper. Clin. Nutr. 2024, 43, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Akmansu, M.; Gungor, L.; Halil, M.; Bicakli, D.H.; Koc, N.; Ozogul, Y.; Sungurtekin, H.; Abbasoglu, O.; Kepan. Optimal use of oral nutritional supplements (ONS) in medical nutrition therapy: ONS consensus report from KEPAN. Eur. J. Clin. Nutr. 2023, 77, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- de Man, F.; Barazonni, R.; Garel, P.; van Ginkel-Res, A.; Green, C.; Koltai, T.; Pichard, C.; Roller-Wirnsberger, R.; Sieber, C.; Smeets, M.; et al. Towards optimal nutritional care for all: A multi-disciplinary patient centred approach to a complex challenge. Clin. Nutr. 2020, 39, 1309–1314. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Trollebo, M.A.; Skeie, E.; Revheim, I.; Stangeland, H.; Erstein, M.H.; Gronning, M.K.; Tangvik, R.J.; Morken, M.H.; Nygard, O.; Eagan, T.M.L.; et al. Comparison of nutritional risk screening with NRS2002 and the GLIM diagnostic criteria for malnutrition in hospitalized patients. Sci. Rep. 2022, 12, 19743. [Google Scholar] [CrossRef]
- Arslan, S.; Dal, N.; Tari Selcuk, K.; Sahin, K.; Atan, R.M. Identifying malnutrition risk in hospitalized patients: An analysis of five tools in the light of GLIM criteria. Postgrad. Med. 2024, 136, 504–513. [Google Scholar] [CrossRef]
- Raslan, M.; Gonzalez, M.C.; Dias, M.C.; Nascimento, M.; Castro, M.; Marques, P.; Segatto, S.; Torrinhas, R.S.; Cecconello, I.; Waitzberg, D.L. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition 2010, 26, 721–726. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, MI, USA, 1988. [Google Scholar]
- R Core Team. RA Language and Environment for Statistical Computing; R Foundation for Statistical: Vienna, Austria, 2020. [Google Scholar]
- Salvatore, M. rcompanion: Functions to Support Extension Education Program Evaluation; R Foundation for Statistical Computing (via CRAN): Vienna, Austria, 2021; Volume 2. [Google Scholar]
- Mayer, M. shapviz: SHAP Visualizations; R Package Version 0.9.0; CRAN (R Foundation for Statistical Computing): Vienna, Austria, 2023. [Google Scholar]
- Fryda, T.; LeDell, E.; Gill, N.; Aiello, S.; Fu, A.; Candel, A.; Click, C.; Kraljevic, T.; Nykodym, T.; Aboyoun, P.; et al. h2o: R Interface for the ‘H2O’ Scalable Machine Learning Platform. R Package Version 3.46.0.2. 2024. Available online: https://github.com/h2oai/h2o-3 (accessed on 3 March 2025).
- Wickham, H.; Wickham, H. Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Auguie, B.; Antonov, A. gridExtra: Miscellaneous Functions for “Grid” Graphics; R Foundation for Statistical Computing (via CRAN): Vienna, Austria, 2017; Volume 2. [Google Scholar]
- Thomas, D.M.; Kleinberg, S.; Brown, A.W.; Crow, M.; Bastian, N.D.; Reisweber, N.; Lasater, R.; Kendall, T.; Shafto, P.; Blaine, R.; et al. Machine learning modeling practices to support the principles of AI and ethics in nutrition research. Nutr. Diabetes 2022, 12, 48. [Google Scholar] [CrossRef]
- Raschka, S. Model evaluation, model selection, and algorithm selection in machine learning. arXiv 2018, arXiv:1811.12808. [Google Scholar]
- Li, Z.; Yoon, J.; Zhang, R.; Rajabipour, F.; Srubar, W.V., III; Dabo, I.; Radlińska, A. Machine learning in concrete science: Applications, challenges, and best practices. npj Comput. Mater. 2022, 8, 127. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Dag, O.; Kasikci, M.; Karabulut, E.; Alpar, R. Diverse classifiers ensemble based on GMDH-type neural network algorithm for binary classification. Commun. Stat.-Simul. Comput. 2022, 51, 2440–2456. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wan, Z.; Zhu, Y.; Zhang, L.; Zhang, L.; Wan, H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: A multi-center study. Nutrition 2021, 83, 111072. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc, E.W.G. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Barbosa, A.A.O.; Vicentini, A.P.; Langa, F.R. Comparison of NRS-2002 criteria with nutritional risk in hospitalized patients. Cien Saude Colet. 2019, 24, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Sanson, G.; Sadiraj, M.; Barbin, I.; Confezione, C.; De Matteis, D.; Boscutti, G.; Zaccari, M.; Zanetti, M. Prediction of early- and long-term mortality in adult patients acutely admitted to internal medicine: NRS-2002 and beyond. Clin. Nutr. 2020, 39, 1092–1100. [Google Scholar] [CrossRef]
- Zhang, K.; Gui, H.; Cong, J.; He, P. A modified nutrition risk screening 2002 predicts the risk of death among hospitalized patients with COVID-19. Clin. Nutr. ESPEN 2022, 52, 365–370. [Google Scholar] [CrossRef]
- Wunderle, C.; Suter, S.S.; Endner, N.; Haenggi, E.; Kaegi-Braun, N.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. Sex differences in clinical presentation, treatment response, and side effects of nutritional therapy among patients at nutritional risk: A secondary analysis of the randomized clinical trial EFFORT. Am. J. Clin. Nutr. 2024, 120, 1225–1232. [Google Scholar] [CrossRef]
- Wu, T.; Xu, H.; Li, W.; Zhou, F.; Guo, Z.; Wang, K.; Weng, M.; Zhou, C.; Liu, M.; Lin, Y.; et al. The potential of machine learning models to identify malnutrition diagnosed by GLIM combined with NRS-2002 in colorectal cancer patients without weight loss information. Clin. Nutr. 2024, 43, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, C.; Wang, Y.; Yu, Z.; Wang, S.; Yang, J.; Lu, S.; Zhou, C.; Wu, E.; Chen, J. Predicting malnutrition in gastric cancer patients using computed tomography(CT) deep learning features and clinical data. Clin. Nutr. 2024, 43, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Veraar, C.; Worf, I.; Tarantino, S.; Kiss, N.; Schuh, C.; Hiesmayr, M. More Nutritional Support on the Wards after a Previous Intensive Care Unit Stay: A nutritionDay Analysis in 136,667 Patients. Nutrients 2023, 15, 3545. [Google Scholar] [CrossRef]
- Sheean, P.M.; Peterson, S.J.; Chen, Y.; Liu, D.; Lateef, O.; Braunschweig, C.A. Utilizing multiple methods to classify malnutrition among elderly patients admitted to the medical and surgical intensive care units (ICU). Clin. Nutr. 2013, 32, 752–757. [Google Scholar] [CrossRef]
- Bond, A.; McCay, K.; Lal, S. Artificial intelligence & clinical nutrition: What the future might have in store. Clin. Nutr. ESPEN 2023, 57, 542–549. [Google Scholar] [CrossRef]
- Erickson, B.J. Basic Artificial Intelligence Techniques: Machine Learning and Deep Learning. Radiol. Clin. N. Am. 2021, 59, 933–940. [Google Scholar] [CrossRef]
| Frequency (n) | Percent (%) | |
|---|---|---|
| BMI | ||
| <20.50 | 15,389 | 8.1 |
| ≥20.50 | 175,639 | 91.9 |
| Total | 191,028 | 100.0 |
| Weight loss within 3 months | ||
| Present | 25,904 | 13.6 |
| Absent | 165,124 | 86.4 |
| Total | 191,028 | 100.0 |
| Reduced dietary intake in the last week | ||
| Present | 31,016 | 16.2 |
| Absent | 160,012 | 83.8 |
| Total | 191,028 | 100.0 |
| Severe illness | ||
| Present | 60,013 | 31.4 |
| Absent | 131,015 | 68.6 |
| Total | 191,028 | 100.0 |
| Impaired nutritional status | ||
| Absent | 158,508 | 82.9 |
| Mild | 22,502 | 11.8 |
| Moderate | 6094 | 3.2 |
| Severe | 3924 | 2.1 |
| Total | 191,028 | 100.0 |
| Severity of disease | ||
| Absent | 140,618 | 73.6 |
| Mild | 36,325 | 19.0 |
| Moderate | 7447 | 3.9 |
| Severe | 6638 | 3.5 |
| Total | 191,028 | 100.0 |
| Age | ||
| <70 | 116,226 | 60.8 |
| ≥70 | 74,802 | 39.2 |
| Total | 191,028 | 100.0 |
| Gender | ||
| Male | 104,219 | 54.6 |
| Female | 86,809 | 45.4 |
| Total | 191,028 | 100.0 |
| Cancer | ||
| Absent | 173,378 | 90.8 |
| Present | 17,650 | 9.2 |
| Total | 191,028 | 100.0 |
| Unit | ||
| Internal medicine | 95,364 | 49.9 |
| Surgery | 67,398 | 35.3 |
| Intensive care | 28,266 | 14.8 |
| Total | 191,028 | 100.0 |
| Not Receiving Nutritional Therapy (n = 178,372, 93.4%) | Receiving Nutritional Therapy (n = 12,656, 6.6%) | |||||
|---|---|---|---|---|---|---|
| Frequency (n) | Percent (%) | Frequency (n) | Percent (%) | p-Value | Effect Size | |
| BMI | ||||||
| <20.50 (n = 15,389, 8.1%) | 12,578 | 81.7 | 2811 | 18.3 | <0.001 | 0.139 |
| ≥20.50 (n = 175,639, 91.9%) | 165,794 | 94.4 | 9845 | 5.6 | ||
| Weight loss within 3 months | ||||||
| Present (n = 25,904, 13.6%) | 19,546 | 75.5 | 6358 | 24.5 | <0.001 | 0.285 |
| Absent (n = 165,124, 86.4%) | 158,826 | 96.2 | 6298 | 3.8 | ||
| Reduced dietary intake in the last week | ||||||
| Present (n = 31,016, 16.2%) | 23,154 | 74.7 | 7862 | 25.3 | <0.001 | 0.331 |
| Absent (n = 160,012, 83.8%) | 155,218 | 97.0 | 4794 | 3.0 | ||
| Severe illness | ||||||
| Present (n = 60,013, 31.4%) | 49,682 | 82.8 | 10,331 | 17.2 | <0.001 | 0.288 |
| Absent (n = 131,015, 68.6%) | 128,690 | 98.2 | 2325 | 1.8 | ||
| Impaired nutritional status | ||||||
| Absent (n = 158,508, 83%) | 155,352 | 98.0 | 3156 | 2.0 | <0.001 | 0.452 |
| Mild (n = 22,502, 11.8%) | 17,576 | 78.1 | 4926 | 21.9 | ||
| Moderate (n = 6094, 3.2%) | 3521 | 57.8 | 2573 | 42.2 | ||
| Severe (n = 3924, 2.1%) | 1923 | 49.0 | 2001 | 51.0 | ||
| Severity of disease | ||||||
| Absent (n = 140,618, 73.6%) | 138,861 | 98.8 | 1757 | 1.2 | <0.001 | 0.457 |
| Mild (n = 36,325, 19%) | 31,515 | 86.8 | 4810 | 13.2 | ||
| Moderate (n = 7447, 3.9%) | 4488 | 60.3 | 2959 | 39.7 | ||
| Severe (n = 6638, 3.5%) | 3508 | 52.8 | 3130 | 47.2 | ||
| Age | ||||||
| <70 (n = 116,226, 60.8%) | 111,810 | 96.2 | 4416 | 3.8 | <0.001 | 0.142 |
| ≥70 (n = 74,802, 39.2%) | 66,562 | 89.0 | 8240 | 11.0 | ||
| Gender | ||||||
| Male (n = 104,219, 54.6%) | 97,065 | 93.1 | 7154 | 6.9 | <0.001 | 0.011 |
| Female (n = 86,809, 45.4%) | 81,307 | 93.7 | 5502 | 6.3 | ||
| Cancer | ||||||
| Absent (n = 173,378, 90.8%) | 163,173 | 94.1 | 10,205 | 5.9 | <0.001 | 0.093 |
| Present (n = 17,650, 9.2%) | 15,199 | 86.1 | 2451 | 13.9 | ||
| Unit | ||||||
| Surgery (n = 67,398, 35.3%) | 66,146 | 98.1 | 1252 | 1.9 | <0.001 | 0.207 |
| Internal medicine (n = 95,364, 49.9%) | 88,982 | 93.3 | 6382 | 6.7 | ||
| Intensive care (n = 28,266, 14.8%) | 23,244 | 82.2 | 5022 | 17.8 | ||
| Enteral/Combined/Parenteral Nutritional Therapy (n = 8026, 63.4%) | Oral Supplement (n = 4630, 36.6%) | |||||
|---|---|---|---|---|---|---|
| Frequency (n) | Percent (%) | Frequency (n) | Percent (%) | p-Value | Effect Size | |
| BMI | ||||||
| <20.50 (n = 2811, 22.2%) | 1660 | 59.1% | 1151 | 40.9% | <0.001 | 0.048 |
| ≥20.50 (n = 9845, 77.8%) | 6366 | 64.7% | 3479 | 35.3% | ||
| Weight loss within 3 months | ||||||
| Present (n = 6358, 50.2%) | 3917 | 61.6% | 2441 | 38.4% | <0.001 | 0.038 |
| Absent (n = 6298, 49.8%) | 4109 | 65.2% | 2189 | 34.8% | ||
| Reduced dietary intake in the last week | ||||||
| Present (n = 7862, 62.1%) | 5003 | 63.6% | 2859 | 36.4% | 0.513 | 0.006 |
| Absent (n = 4794, 37.9%) | 3023 | 63.1% | 1771 | 36.9% | ||
| Severe illness | ||||||
| Present (n = 10,331, 81.6%) | 7088 | 68.6% | 3243 | 31.4% | <0.001 | 0.227 |
| Absent (n = 2325, 18.4%) | 938 | 40.3% | 1387 | 59.7% | ||
| Impaired nutritional status | ||||||
| Absent (n = 3156, 24.9%) | 1883 | 59.7% | 1273 | 40.3% | <0.001 | 0.125 |
| Mild (n = 4926, 38.9%) | 3045 | 61.8% | 1881 | 38.2% | ||
| Moderate (n = 2573, 20.3%) | 1553 | 60.4% | 1020 | 39.6% | ||
| Severe (n = 2001, 15.8%) | 1545 | 77.2% | 456 | 22.8% | ||
| Severity of disease | ||||||
| Absent (n = 1757, 13.9%) | 752 | 42.8% | 1005 | 57.2% | <0.001 | 0.305 |
| Mild (n = 4810, 38%) | 2593 | 53.9% | 2217 | 46.1% | ||
| Moderate (n = 2959, 23.4%) | 2015 | 68.1% | 944 | 31.9% | ||
| Severe (n = 3130, 24.7%) | 2666 | 85.2% | 464 | 14.8% | ||
| Age | ||||||
| <70 (n = 4416, 34.9%) | 2657 | 60.2% | 1759 | 39.8% | <0.001 | 0.049 |
| ≥70 (n = 8240, 65.1%) | 5369 | 65.2% | 2871 | 34.8% | ||
| Gender | ||||||
| Male (n = 7154, 56.5%) | 4444 | 62.1% | 2710 | 37.9% | 0.001 | 0.031 |
| Female (n = 5502, 43.5%) | 3582 | 65.1% | 1920 | 34.9% | ||
| Cancer | ||||||
| Absent (n = 10,205, 80.6%) | 6640 | 65.1% | 3565 | 34.9% | <0.001 | 0.07 |
| Present (n = 2451, 19.4%) | 1386 | 56.5% | 1065 | 43.5% | ||
| Unit | ||||||
| Surgery (n = 1252, 9.9%) | 723 | 57.7% | 529 | 42.3% | <0.001 | 0.253 |
| Internal medicine (n = 6382, 50.4%) | 3368 | 52.8% | 3014 | 47.2% | ||
| Intensive care (n = 5022, 39.7%) | 3935 | 78.4% | 1087 | 21.6% | ||
| NRS-2002 | Modified NRS-2002 |
|---|---|
| BMI ≥ 20.5 | BMI ≥ 20.5 |
| Weight loss within 3 months | Weight loss within 3 months |
| Reduced dietary intake in the last week | Reduced dietary intake in the last week |
| Severe illness | Severe illness |
| Impaired nutritional status (absent, mild, moderate, severe) | Impaired nutritional status (absent, mild, moderate, severe) |
| Severity of disease (absent, mild, moderate, severe) | Severity of disease (absent, mild, moderate, severe) |
| Age ≥ 70 | Age ≥ 70 |
| Gender | |
| Cancer | |
| Unit (surgical unit, internal medicine unit, ICU) |
| Method | RF | ANN | DL | EN | NB | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable Set | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 |
| AUC (95% C.I.) | 0.921 (0.916–0.926) | 0.921 (0.916–0.926) | 0.923 (0.918–0.929) | 0.930 (0.925–0.935) | 0.928 (0.923–0.933) | 0.933 (0.928–0.937) | 0.929 (0.924–0.934) | 0.932 (0.927–0.937) | 0.927 (0.922–0.932) | 0.928 (0.923–0.933)) |
| Accuracy | 0.939 | 0.937 | 0.925 | 0.931 | 0.935 | 0.926 | 0.920 | 0.926 | 0.930 | 0.932 |
| Balanced Accuracy | 0.579 | 0.547 | 0.797 | 0.801 | 0.782 | 0.828 | 0.826 | 0.826 | 0.792 | 0.777 |
| MCC | 0.308 | 0.241 | 0.504 | 0.527 | 0.522 | 0.539 | 0.520 | 0.536 | 0.513 | 0.505 |
| Sensitivity | 0.165 | 0.098 | 0.650 | 0.650 | 0.606 | 0.716 | 0.718 | 0.710 | 0.634 | 0.599 |
| Specificity | 0.994 | 0.997 | 0.944 | 0.951 | 0.959 | 0.941 | 0.934 | 0.941 | 0.951 | 0.955 |
| PPV | 0.652 | 0.671 | 0.452 | 0.487 | 0.510 | 0.463 | 0.435 | 0.461 | 0.477 | 0.489 |
| NPV | 0.944 | 0.940 | 0.974 | 0.975 | 0.972 | 0.979 | 0.979 | 0.979 | 0.973 | 0.971 |
| F1 Score | 0.264 | 0.172 | 0.534 | 0.557 | 0.554 | 0.562 | 0.542 | 0.560 | 0.544 | 0.538 |
| Method | RF | ANN | DL | EN | NB | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable Set | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 | NRS-2002 | Mod NRS-2002 |
| AUC (95% C.I.) | 0.689 (0.668–0.710) | 0.703 (0.683–0.723) | 0.704 (0.676–0.718) | 0.710 (0.680–0.721) | 0.716 (0.696–0.736) | 0.739 (0.724–0.763) | 0.705 (0.685–0.726) | 0.717 (0.70–0.737) | 0.677 (0.656–0.698) | 0.687 (0.667–0.708) |
| Accuracy | 0.595 | 0.594 | 0.605 | 0.612 | 0.585 | 0.665 | 0.628 | 0.629 | 0.573 | 0.589 |
| Balanced accuracy | 0.638 | 0.651 | 0.646 | 0.645 | 0.645 | 0.687 | 0.654 | 0.659 | 0.631 | 0.646 |
| MCC | 0.275 | 0.311 | 0.289 | 0.283 | 0.301 | 0.360 | 0.299 | 0.309 | 0.271 | 0.301 |
| Sensitivity | 0.479 | 0.437 | 0.493 | 0.522 | 0.422 | 0.606 | 0.558 | 0.546 | 0.414 | 0.431 |
| Specificity | 0.798 | 0.865 | 0.799 | 0.768 | 0.868 | 0.768 | 0.751 | 0.772 | 0.848 | 0.862 |
| PPV | 0.804 | 0.849 | 0.810 | 0.796 | 0.847 | 0.819 | 0.795 | 0.806 | 0.825 | 0.844 |
| NPV | 0.469 | 0.470 | 0.476 | 0.481 | 0.464 | 0.529 | 0.495 | 0.495 | 0.455 | 0.466 |
| F1 Score | 0.600 | 0.577 | 0.613 | 0.631 | 0.563 | 0.696 | 0.655 | 0.651 | 0.552 | 0.571 |
| Step 1 | Step 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable Set | NRS-2002 | Modified NRS-2002 | Increase/Decrease Absolute Effect (%) | Increase/Decrease Relative Effect (%) | NRS-2002 | Modified NRS-2002 | Increase/Decrease Absolute Effect (%) | Increase/Decrease Relative Effect (%) |
| AUC | 0.928 | 0.933 | 0.006 | 0.60% | 0.716 | 0.739 | 0.023 | 3.27% |
| Accuracy | 0.935 | 0.926 | −0.009 | −0.98% | 0.585 | 0.665 | 0.080 | 13.64% |
| Balanced Accuracy | 0.782 | 0.828 | 0.046 | 5.89% | 0.464 | 0.529 | 0.065 | 13.96% |
| MCC | 0.522 | 0.539 | 0.017 | 3.29% | 0.645 | 0.687 | 0.042 | 6.46% |
| Sensitivity | 0.606 | 0.716 | 0.110 | 18.13% | 0.301 | 0.360 | 0.060 | 19.91% |
| Specificity | 0.959 | 0.941 | −0.018 | −1.84% | 0.422 | 0.606 | 0.184 | 43.57% |
| PPV | 0.510 | 0.463 | −0.047 | −9.27% | 0.868 | 0.768 | −0.100 | −11.57% |
| NPV | 0.972 | 0.979 | 0.007 | 0.76% | 0.847 | 0.819 | −0.028 | −3.36% |
| F1 Score | 0.554 | 0.562 | 0.008 | 1.50% | 0.563 | 0.696 | 0.133 | 23.62% |
| Step 1 | Step 2 | |
|---|---|---|
| AUC (95%) | 0.933 (0.926–0.936) | 0.741 (0.722–0.761) |
| Accuracy | 0.923 | 0.651 |
| Balanced accuracy | 0.828 | 0.681 |
| MCC | 0.530 | 0.352 |
| Sensitivity | 0.937 | 0.569 |
| Specificity | 0.719 | 0.794 |
| PPV | 0.979 | 0.827 |
| NPV | 0.449 | 0.515 |
| F1 Score | 0.958 | 0.674 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalçın, N.; Kaşıkcı, M.; Kelleci-Çakır, B.; Demirkan, K.; Allegaert, K.; Halil, M.; Doğanay, M.; Abbasoğlu, O. Leveraging Deep Learning to Enhance Malnutrition Detection via Nutrition Risk Screening 2002: Insights from a National Cohort. Nutrients 2025, 17, 2716. https://doi.org/10.3390/nu17162716
Yalçın N, Kaşıkcı M, Kelleci-Çakır B, Demirkan K, Allegaert K, Halil M, Doğanay M, Abbasoğlu O. Leveraging Deep Learning to Enhance Malnutrition Detection via Nutrition Risk Screening 2002: Insights from a National Cohort. Nutrients. 2025; 17(16):2716. https://doi.org/10.3390/nu17162716
Chicago/Turabian StyleYalçın, Nadir, Merve Kaşıkcı, Burcu Kelleci-Çakır, Kutay Demirkan, Karel Allegaert, Meltem Halil, Mutlu Doğanay, and Osman Abbasoğlu. 2025. "Leveraging Deep Learning to Enhance Malnutrition Detection via Nutrition Risk Screening 2002: Insights from a National Cohort" Nutrients 17, no. 16: 2716. https://doi.org/10.3390/nu17162716
APA StyleYalçın, N., Kaşıkcı, M., Kelleci-Çakır, B., Demirkan, K., Allegaert, K., Halil, M., Doğanay, M., & Abbasoğlu, O. (2025). Leveraging Deep Learning to Enhance Malnutrition Detection via Nutrition Risk Screening 2002: Insights from a National Cohort. Nutrients, 17(16), 2716. https://doi.org/10.3390/nu17162716







