Natural Products as Modulators of Iron Metabolism and Ferroptosis in Diabetes and Its Complications
Abstract
1. Introduction
2. Iron–Diabetes Pathophysiological Interplay
2.1. Dysregulated Iron Homeostasis in Diabetes
2.2. Iron’s Association with Different Types of Diabetes
2.2.1. Iron and Type 1 Diabetes
2.2.2. Iron and Type 2 Diabetes
2.2.3. Iron and Gestational Diabetes
2.3. Diabetes in Hereditary Iron Overload Disorders—Hereditary Hemochromatosis and Thalassemia
2.3.1. Hereditary Hemochromatosis-Associated Diabetes
2.3.2. Thalassemia-Associated Diabetes
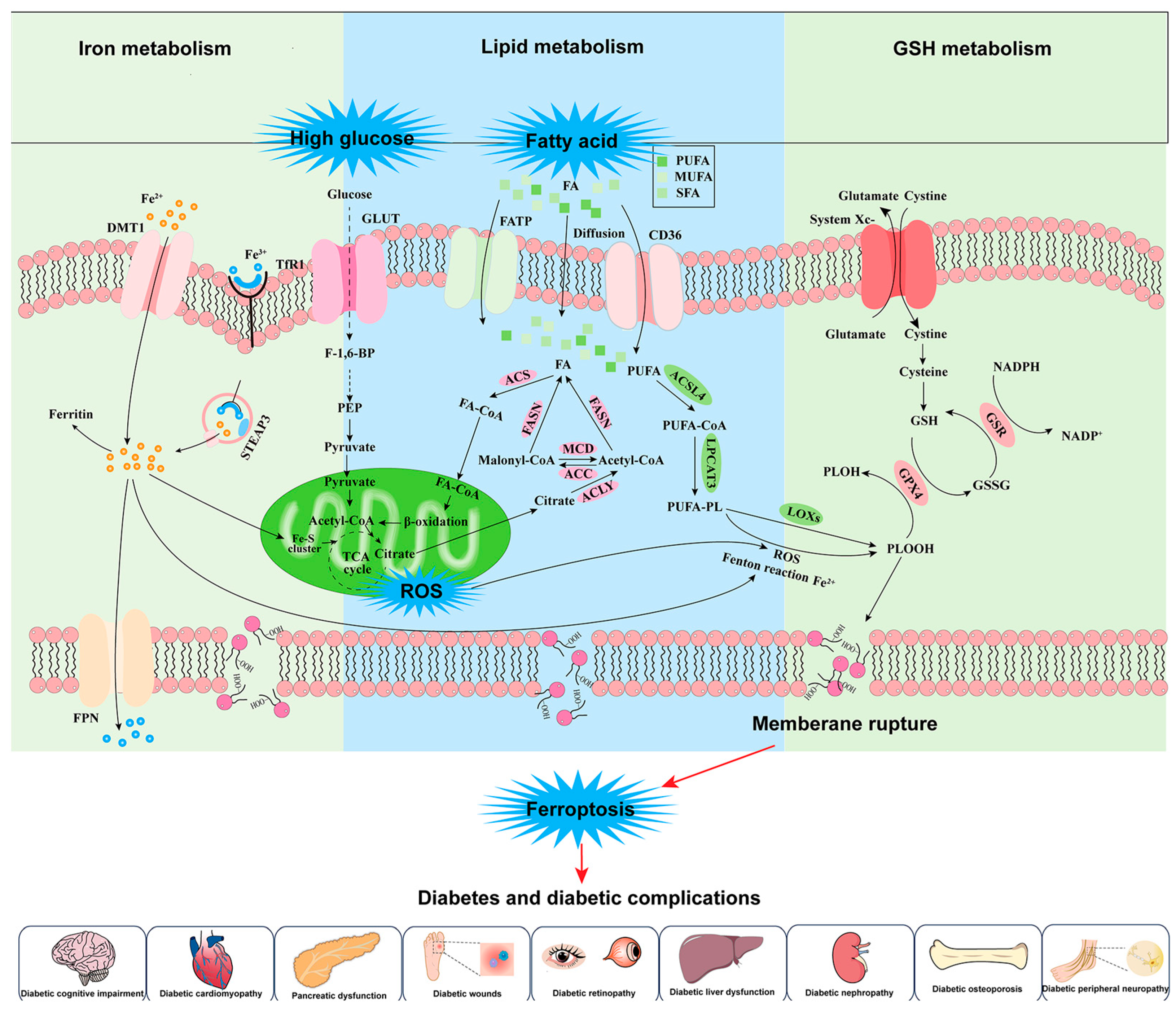
2.4. Iron-Mediated Oxidative Stress in Diabetes Pathogenesis
2.5. The Impact of Iron Homeostasis on Glycemic Control and Insulin Regulation
2.5.1. Impacts of Iron Level Variations on Glycemic Control
2.5.2. Iron Regulation in Pancreatic β Cell Function and Insulin Sensitivity
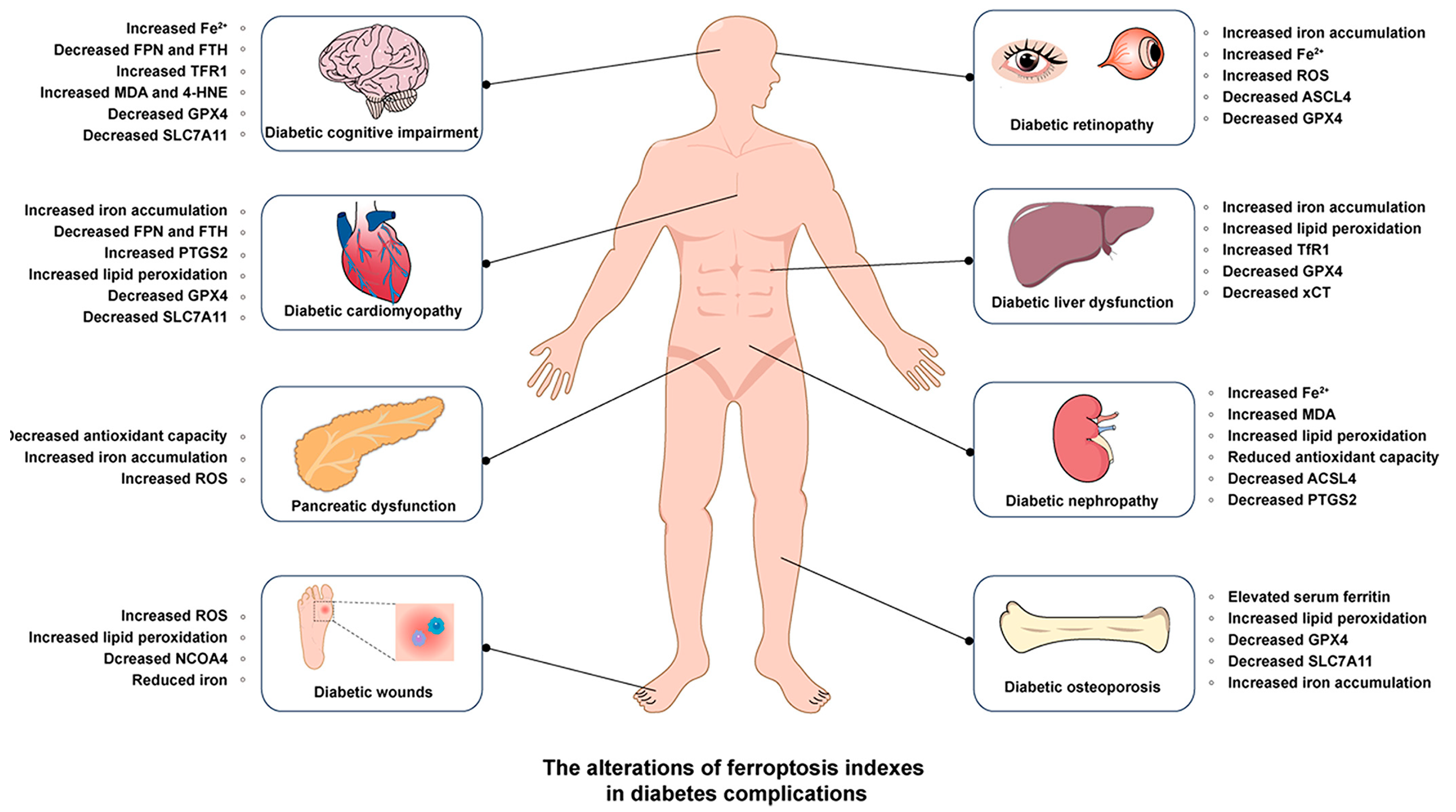
3. Dysregulated Iron Metabolism, Ferroptosis, and Diabetes
3.1. Diabetic Nephropathy (DN)
3.2. Diabetic Osteoporosis (DOP)
3.3. Diabetic Peripheral Neuropathy (DPN)
3.4. Diabetic Cognitive Impairment
3.5. Diabetic Cardiovascular Diseases
3.6. Diabetic Liver Dysfunction
3.7. Diabetic Retinopathy (DR)
3.8. Diabetic Wounds
4. Iron Metabolism and Ferroptosis-Targeted Therapeutics with Natural Products in Diabetes
4.1. Diabetic Nephropathy
4.1.1. Flavonoids
4.1.2. Terpenoids
4.1.3. Alkaloids
4.1.4. Lignans
4.1.5. Anthraquinones
| Compound | Diabetic Models | Changes of Ferroptotic Biological Events | Ref. | |||
|---|---|---|---|---|---|---|
| Animal Model | Cell Model | Iron Metabolism | Lipid Metabolism | GSH Metabolism | ||
| Flavones | SD rats (200–220 g) induced by HFD, unilateral nephrectomy and STZ (35 mg/kg) | Murine PTEC cell line NRK-52E under AGE | Decreased nonheme iron content Decreased expression of TfR1 | Decreased MDA and ROS | Increased expression of GPX4 | [120] |
| Vitexin | Male Sprague Dawley rats induced by HFD (3 weeks)/STZ (35 mg/kg) | HK-2 cells under 50 mM glucose | Decreased Fe2+ content | Decreased MDA | Increased content of GSH Increased expression of SCL7A11 and GPX4 | [121] |
| Glabridin | Male Sprague Dawley rats induced by HFD (3 weeks)/STZ (40 mg/kg, 10 days) | NRK-52E rat renal tubular epithelia cells under 30 mM glucose | Decreased iron content in kidney tissue Decreased expression of TfR1 | Decreased MDA | Increased content of superoxide dismutase (SOD) and GSH Increased expression of SLC7A11, solute carrier family 3 member 2 (SLC3A2), catalase (CAT), and GPX4 | [123] |
| Quercetin | / | HK-2 cells under 30 mM glucose | Decreased iron content Decreased TfR1 Increased FTH1 | Decreased MDA and 4-HNE | Increased content of GSH Increased expression of SCL7A11 and GPX4 | [124,125] |
| Tanshinone IIA | 10-week-old db/db mice | Mouse glomerular podocyte MPC5 cells under 30 mM glucose | Decreased Fe2+ content | Decreased ROS and MDA | Increased GSH | [126] |
| Ginkgolide B | C57BL/KsJ db/db mice | Mouse renal podocyte MPC5 under 25 mM glucose | Decreased expression of TfR1 Increased expression of FTH1 | / | Increased expression of GPX4 | [127] |
| Hederagenin | 6–8-week-old male C57BL/6J mice induced by STZ (50 mg/kg) | HK-2 cells under 25 mM glucose | Decreased lipid ROS and MDA | Increased expression of GPX4 | [128] | |
| Platycodin D | / | Human proximal renal tubule cell line HK-2 under 30 mM glucose | Decreased labile iron content Decreased expression of TfR1 Increased expression of FTH1 | Decreased lipid peroxide and MDA Decreased expression of ACSL4 | Increased content of GSH Increased expression of SCL7A11 | [129] |
| Leonurine | 6-week-old C57BL/6 mice induced by STZ (50 mg/kg, 5 days) and HFD (2 weeks) | Human umbilical vein endothelial cells (HUVECs) under 30 mM glucose | Increased expression of FTH1 and FTL Decreased iron content | Decreased MDA | Increased GPX4 and Nrf2 Increased GSH content | [130] |
| Berberine | 8-week-old male C57BL/6J mice induced by STZ (65 mg/kg) | HK-2 cells under 5.5 mM glucose | Decreased expression of FTH1 Decreased iron content | Decreased MDA | Increased expression of GPX4 | [131] |
| Schisandrin A | 5–6-week-old C57BL/6 mice induced by HFD (12 weeks)/STZ (30 mg/kg, 7 days) | Human renal glomerular endothelial cells under 20 mM glucose | Decreased iron content | Decreased MDA | Increased SOD, CAT, and GSH Increased expression of GPX4 | [132] |
| Umbelliferone | 10-week-old C57BLKS/J db/db male mice | Human proximal renal tubule cell line HK-2 under 30 mM glucose | / | Decreased expression of ACSL4 | Increased expression of GPX4 | [135] |
| Rhein | 6–8-week-old male C57BL/6J mice induced by STZ (50 mg/kg, 5 days) | Mouse glomerular podocyte MPC5 cells under 30 mM glucose | Decreased Fe2+ content Decreased expression of TfR | Decreased MDA | Increased expression of GPX4 and SLC7A11 Increased GSH and SOD content | [136] |
| Chicoric acid | C57BL/6 mice (5–6 weeks, 18–20 g) induced by HFD and STZ (30 mg/kg) | NRK-52E cells stimulated with 20 mmol/L d-glucose | Decreased iron concentration | / | Increased GSH activity and GPX4 expression | [137] |
| Rosa laevigata Michx. polysaccharide | C57BL/6 mice (20–22 g) induced by high-glucose and high-fat (HGHF) (8 weeks) STZ (30 mg/kg) | / | Decreased expression of transferrin and Steap3 | Decreased ROS and 4-HNE | Increased expression of GPx4 | [138] |
4.1.6. Polysaccharides
4.2. Diabetic Osteoporosis
| Compound | Diabetic Models | Changes of Ferroptotic Biological Events | Ref. | |||
|---|---|---|---|---|---|---|
| Animal Model | Cell Model | Iron Metabolism | Lipid Metabolism | GSH Metabolism | ||
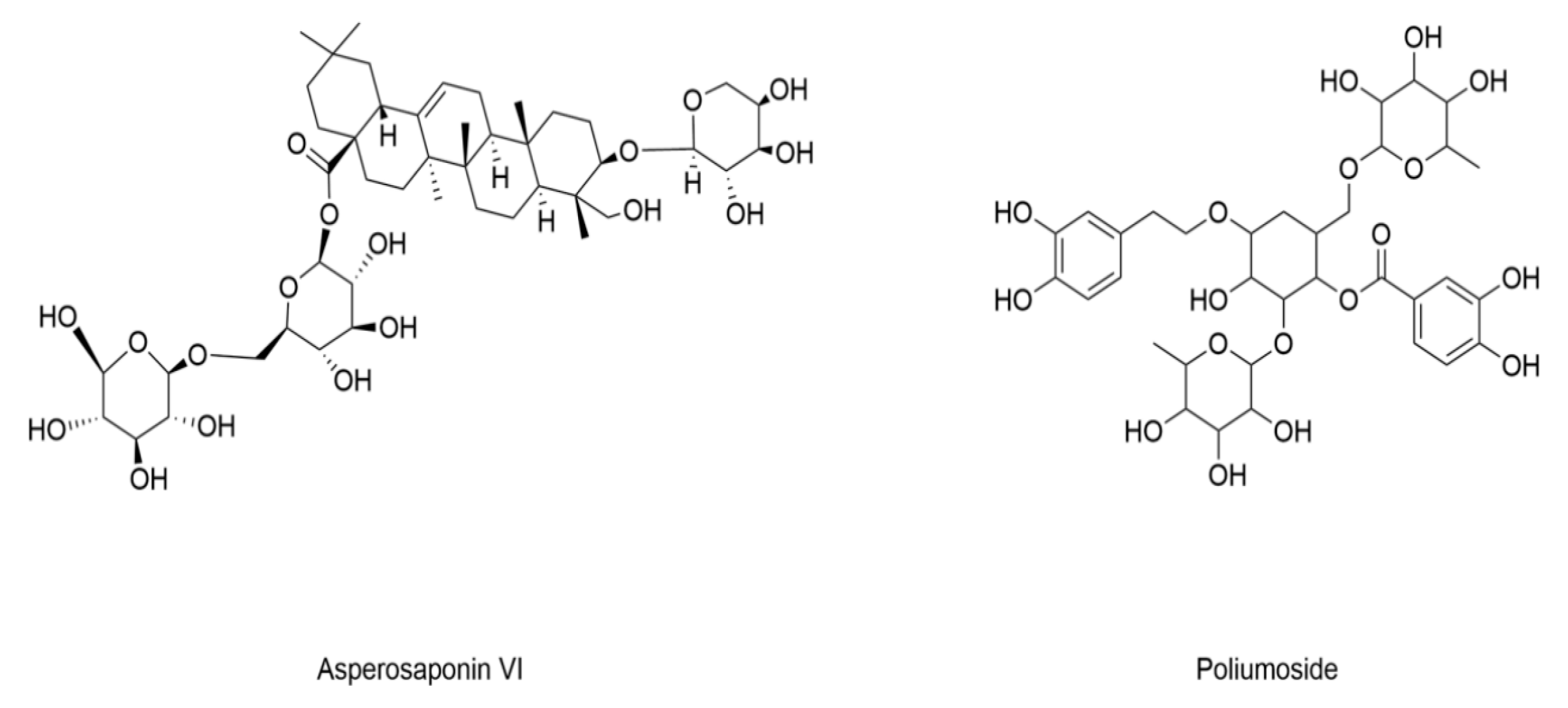
| Poliumoside | C57BL/6 mice (25 ± 2 g) induced by HGHF combined with STZ (minimal dosage) | Bone mesenchymal stem cells (BMSCs) cultured in HGHF conditions | / | Decreased lipid peroxidation | Increased GSH levels | [139] |
| Asperosaponin VI | C57BL/6J mice induced by HFD and STZ (25 mg/kg) | Primary osteoblasts treated with high glucose and palmitic acid (HGPA) | Increased iron accumulation | Decreased lipid peroxidation | Increased expression of GPX4 | [140] |
4.3. Diabetic Cardiomyopathy
4.4. Diabetic Pancreas Injury
4.5. Diabetic Recognitive Impairment
4.6. Diabetic Retinopathy
4.7. Diabetic Peripheral Neuropathy
4.8. Diabetic Atherosclerosis
| Compound | Diabetic Models | Changes of Ferroptotic Biological Events | Ref. | |||
|---|---|---|---|---|---|---|
| Animal Model | Cell Model | Iron Metabolism | Lipid Metabolism | GSH Metabolism | ||
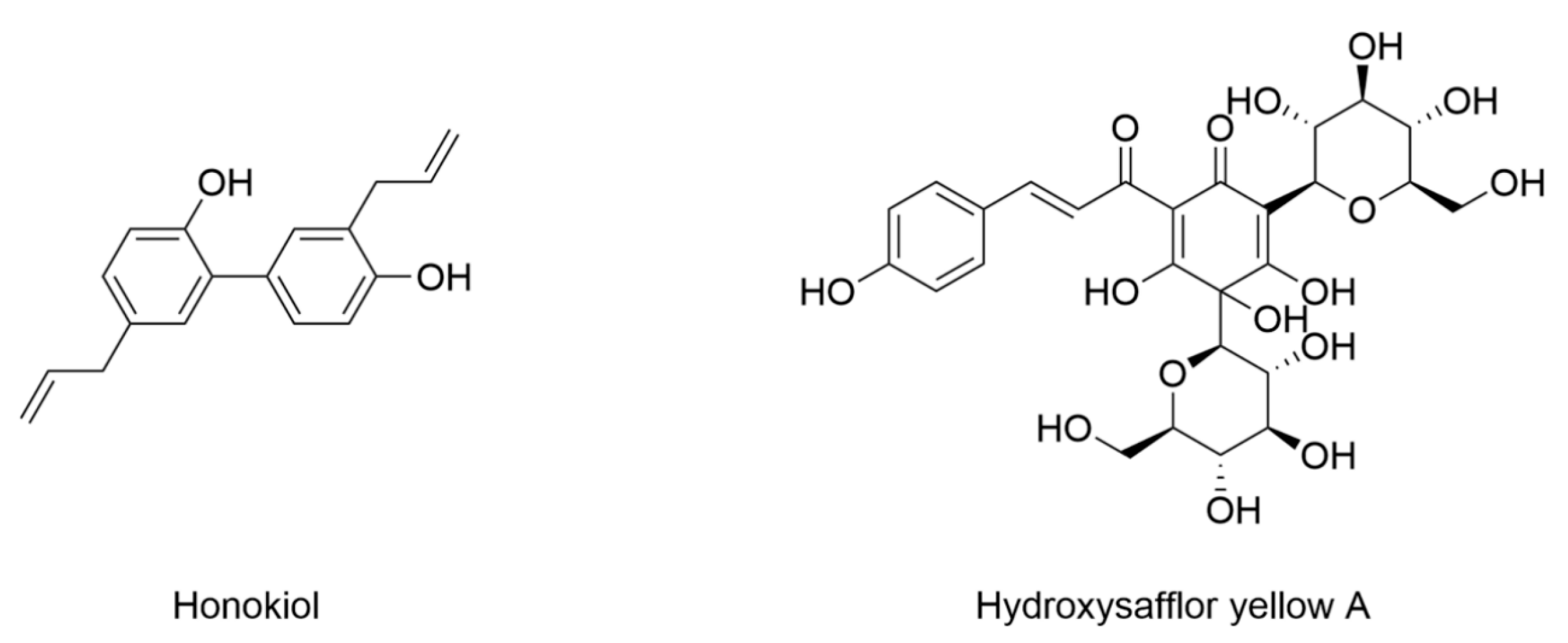
| Honokiol | 6–8-week-old male SD rats induced by STZ (60 mg/kg) | Rat Schwann cell line RSC96 under 150 mM glucose | Decreased Fe2+ Decreased expression of TfR1 Increased expression of FTH | Decreased MDA and ROS | Increased expression of GPX4, NRF2, and SLC7A11 Increased GSH | [162] |
| Hydroxysafflor yellow A | ApoE−/−-deficient C57BL/6 mice induced by HFD (4 weeks)/STZ (30 mg/kg, 3 days) | Human umbilical vein endothelial cells under 33.3 mM glucose | Decreased iron | Decreased MDA and ROS Decreased expression of ACSL4 | Increased expression of GPX4, GSH, and SLC7A11 | [165] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TCM | Traditional Chinese medicine |
| T2DM | Type 2 diabetes mellitus |
| IB-AC | Antioxidant capacity |
| CP | Ceruloplasmin |
| NTBI | Non-transferrin-bound iron |
| FPN | Ferroportin |
| SLC11A2 | Solute carrier family 11 member 2 |
| STZ | Streptozotocin |
| DMT1 | Divalent metal transporter 1 |
| FLVCR1 | Feline leukemia virus subgroup C receptor 1 |
| ZIP14 | Zrt/Irt-like protein 14 |
| TfRC | Transferrin receptor |
| HFE | Hemochromatosis |
| HMOX1 | Heme oxygenase 1 |
| TMPRS | Transmembrane protease, serine |
| SMAD7 | Decapentaplegic homolog 7 |
| ROS | Reactive oxygen species |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| GPX4 | Glutathione peroxidase 4 |
| TIBC | Total iron binding capacity |
| SLC40A1 | Solute carrier family 40 member 1 (ferroportin) |
| BMP6 | Bone morphogenetic protein 6 |
| AMPK | Adenosine monophosphate-activated protein kinase |
| GLUT4 | Glucose transporter type 4 |
| GSK3β | Glycogen synthase kinase 3β |
| Akt | Protein kinase B |
| Heph | Hephaestin |
| FAC | Ferric ammonium citrate |
| DFP | Deferiprone |
| IL-1β | Interleukin-1β |
| TNFα | Tumor necrosis factor α |
| TCA | Tricarboxylic acid |
| G6P | Glucose-6-phosphate |
| F-1,6-BP | Fructose-1,6-bisphosphate |
| PFK-1 | Phosphofructokinase-1 |
| PEP | Phosphoenolpyruvate |
| TfR1 | Transferrin receptor 1 |
| PDH | Pyruvate dehydrogenase complex |
| SFAs | Saturated fatty acids |
| MUFAs | Monounsaturated fatty acids |
| PUFAs | Polyunsaturated fatty acids |
| FA-CoA | Fatty acyl-CoA |
| CD36 | Cluster of Differentiation 36 |
| ACS | Acyl-CoA synthetase |
| ACLY | ATP-citrate lyase |
| Malonyl-CoA | Malonyl-coenzyme A |
| ACC | Acetyl-CoA carboxylase |
| PLOOHs | Phospholipid hydroperoxides |
| GSSG | Oxidized glutathione |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NADP+ | Nicotinamide adenine dinucleotide phosphate (oxidized form) |
| Kim-1 | Kidney injury molecule-1 |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| STEAP3 | Six-transmembrane epithelial antigen of prostate 3 |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| DN | Diabetic nephropathy |
| SD | Sprague Dawley |
| DOP | Diabetic osteoporosis |
| AGEs | Advanced glycation end products |
| FTL | Ferritin light chain |
| FTH1 | Ferritin heavy chain |
| HFD | High-fat diet |
| DCM | Diabetic cardiomyopathy |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| DPN | Diabetic peripheral neuropathy |
| SLC7A11 | Solute carrier family 7 member 11 |
| DR | Diabetic retinopathy |
| CA | Chicoric acid |
| PAQR3 | AdipoQ receptor family member 3 |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| AVI | Asperosaponin VI |
| SIRT1 | Silent information regulator 1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1α |
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Total Diabetes-Related Health Expenditure (USD Million). Available online: https://diabetesatlas.org/data-by-indicator/diabetes-related-health-expenditure/total-diabetes-related-health-expenditure-usd-million/ (accessed on 18 July 2025).
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Sun, Z.Z.; Tang, Y.T.; Xu, C.; Jiao, X.Y. Hepcidin expression and iron parameters change in Type 2 diabetic patients. Diabetes Res. Clin. Pract. 2011, 93, 43–48. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on haemochromatosis. J. Hepatol. 2022, 77, 479–502. [Google Scholar] [CrossRef]
- Huang, J.; Jones, D.; Luo, B.; Sanderson, M.; Soto, J.; Abel, E.D.; Cooksey, R.C.; McClain, D.A. Iron overload and diabetes risk: A shift from glucose to Fatty Acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 2011, 60, 80–87. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, P.; Xing, Z.; Yang, B.; Zheng, F.; Yu, Z.; Zhang, W.; Yang, X.; Luo, J.; Tang, T.; et al. Regulation of ferroptosis with natural compounds: Potential applications in the treatment of acquired brain injury. Phytochem. Rev. 2025, 1–36. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Van Campenhout, C.; Lagrou, A.R.; Moorkens, G.; De Block, C.; Manuel-y-Keenoy, B. Iron-binding antioxidant capacity is impaired in diabetes mellitus. Free Radic. Biol. Med. 2006, 40, 1749–1755. [Google Scholar] [CrossRef]
- Lee, D.-H.; Liu, D.Y.; Jacobs, D.R., Jr.; Shin, H.-R.; Song, K.; Lee, I.-K.; Kim, B.; Hider, R.C. Common Presence of Non–Transferrin-Bound Iron Among Patients With Type 2 Diabetes. Diabetes Care 2006, 29, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Rodríguez, A.; Becerril, S.; Valentí, V.; Salvador, J.; Frühbeck, G.; Fernández-Real, J.M. Increased small intestine expression of non-heme iron transporters in morbidly obese patients with newly diagnosed type 2 diabetes. Mol. Nutr. Food Res. 2017, 62, 1700301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bartnikas, T.; Chu, X.; Klein, J.; Yun, C.; Srinivasan, S.; He, P. Hyperglycemia promotes microvillus membrane expression of DMT1 in intestinal epithelial cells in a PKCalpha-dependent manner. FASEB J. 2019, 33, 3549–3561. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Upson, S.E.; Banyard, K.L.; Knight, R.; Mace, K.A.; Hardman, M.J. Reduced iron in diabetic wounds: An oxidative stress-dependent role for STEAP3 in extracellular matrix deposition and remodeling. J. Investig. Dermatol. 2019, 139, 2368–2377.e2367. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Rodríguez, A.; Ortega, F.; Becerril, S.; Sabater-Masdeu, M.; Latorre, J.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Increased adipose tissue heme levels and exportation are associated with altered systemic glucose metabolism. Sci. Rep. 2017, 7, 5305. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Fei, L.; Wang, X.; Lei, Y.; Yu, L.; Xu, W.; Chen, J.; Zhu, E.; Zhong, M.; Huang, M.; et al. ZIP14 is involved in iron deposition and triggers ferroptosis in diabetic nephropathy. Metallomics 2022, 14, mfac034. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Wang, F. Pleiotropic actions of iron balance in diabetes mellitus. Rev. Endocr. Metab. Disord. 2015, 16, 15–23. [Google Scholar] [CrossRef]
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef]
- Stordal, K.; McArdle, H.J.; Hayes, H.; Tapia, G.; Viken, M.K.; Lund-Blix, N.A.; Haugen, M.; Joner, G.; Skrivarhaug, T.; Marild, K.; et al. Prenatal iron exposure and childhood type 1 diabetes. Sci. Rep. 2018, 8, 9067. [Google Scholar] [CrossRef]
- Wójciak, R.W.; Mojs, E.; Stanisławska-Kubiak, M. The Occurrence of Iron-Deficiency Anemia in Children with Type 1 Diabetes. J. Investig. Med. 2023, 62, 865–867. [Google Scholar] [CrossRef]
- Vreugdenhil, M.; Akkermans, M.D.; van Swelm, R.P.L.; Laarakkers, C.M.; Houdijk, E.; Bakker, B.; Clement-de Boers, A.; van der Kaay, D.C.M.; de Vries, M.C.; Woltering, M.C.; et al. Serum hepcidin concentrations in relation to iron status in children with type 1 diabetes. Pediatr. Hematol. Oncol. 2021, 38, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Orban, E.; Schwab, S.; Thorand, B.; Huth, C. Association of iron indices and type 2 diabetes: A meta-analysis of observational studies. Diabetes/Metab. Res. Rev. 2014, 30, 372–394. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Huang, Y.; Li, Y.; Qin, L.; Wei, Q.; Chen, X.; Yang, C.; Zhang, M. Association between systemic iron status and beta-cell function and insulin sensitivity in patients with newly diagnosed type 2 diabetes. Front. Endocrinol. 2023, 14, 1143919. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Kopf, S.; Schmidt, J.; Mudder, K.; da Silva, A.R.; Nawroth, P.; Muckenthaler, M.U. Uncoupled iron homeostasis in type 2 diabetes mellitus. J. Mol. Med. 2017, 95, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Shahinfar, H.; Jayedi, A.; Shab-Bidar, S. Dietary iron intake and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2022, 61, 2279–2296. [Google Scholar] [CrossRef]
- Pradeepa, R.; Shreya, L.; Anjana, R.M.; Jebarani, S.; Kamal Raj, N.; Kumar, M.S.; Jayaganesh, P.; Swami, O.C.; Mohan, V. Frequency of iron deficiency anemia in type 2 diabetes—Insights from tertiary diabetes care centres across India. Diabetes Metab. Syndr. 2022, 16, 102632. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Alam, F.; Moin, S.; Noor, N.; Arif, S.H. Role of ferritin and oxidative stress index in gestational diabetes mellitus. J. Diabetes Metab. Disord. 2021, 20, 1615–1619. [Google Scholar] [CrossRef]
- Zein, S.; Sitti, F.; Osman, M.; Arnaud, J.; Batandier, C.; Gauchez, A.-S.; Rachidi, S.; Couturier, K.; Hininger-Favier, I. Middle iron-enriched fructose diet on gestational diabetes risk and on oxidative stress in offspring rats. Biol. Trace Elem. Res. 2016, 175, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Zein, S.; Rachidi, S.; Hininger-Favier, I. Is oxidative stress induced by iron status associated with gestational diabetes mellitus? J. Trace Elem. Med. Biol. 2014, 28, 65–69. [Google Scholar] [CrossRef]
- Zaugg, J.; Melhem, H.; Huang, X.; Wegner, M.; Baumann, M.; Surbek, D.; Korner, M.; Albrecht, C. Gestational diabetes mellitus affects placental iron homeostasis: Mechanism and clinical implications. FASEB J. 2020, 34, 7311–7329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gao, H.; Zhou, W.; Cai, H.; Liao, L.; Wang, C. Circular RNA HIPK3 facilitates ferroptosis in gestational diabetes mellitus by regulating glutathione peroxidase 4 DNA methylation. J. Gene Med. 2023, 25, e3526. [Google Scholar] [CrossRef] [PubMed]
- Kataria, Y.; Wu, Y.; Horskjaer, P.H.; Mandrup-Poulsen, T.; Ellervik, C. Iron status and gestational diabetes-A meta-analysis. Nutrients 2018, 10, 621. [Google Scholar] [CrossRef]
- Adams, P.C.; Jeffrey, G.; Ryan, J. Haemochromatosis. Lancet 2023, 401, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Baschant, U.; Altamura, S.; Steele-Perkins, P.; Muckenthaler, M.U.; Spasić, M.V.; Hofbauer, L.C.; Steinbicker, A.U.; Rauner, M. Iron effects versus metabolic alterations in hereditary hemochromatosis driven bone loss. Trends Endocrinol. Metab. 2022, 33, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Y.; Zhang, L.; Peng, G.; Zhang, F.; Zhao, X. Iron overload due to SLC40A1 mutation of type 4 hereditary hemochromatosis. Med. Mol. Morphol. 2023, 56, 233–238. [Google Scholar] [CrossRef]
- Kawabata, H. The mechanisms of systemic iron homeostasis and etiology, diagnosis, and treatment of hereditary hemochromatosis. Int. J. Hematol. 2017, 107, 31–43. [Google Scholar] [CrossRef]
- Wu, H.X.; Liu, J.Y.; Yan, D.W.; Li, L.; Zhuang, X.H.; Li, H.Y.; Zhou, Z.G.; Zhou, H.D. Atypical juvenile hereditary hemochromatosis onset with positive pancreatic islet autoantibodies diabetes caused by novel mutations in HAMP and overall clinical management. Mol. Genet. Genom. Med. 2020, 8, e1522. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kowdley, K.V. Hereditary hemochromatosis and diabetes mellitus: Implications for clinical practice. Nat. Rev. Endocrinol. 2010, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]
- McClain, D.A.; Abraham, D.; Rogers, J.; Brady, R.; Gault, P.; Ajioka, R.; Kushner, J.P. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia 2006, 49, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Kattamis, A.; Kwiatkowski, J.L.; Aydinok, Y. Thalassaemia. Lancet 2022, 399, 2310–2324. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Z.; Jiang, Z.; Liu, Z.; Hou, L.; Cai, G.; Ou, H.; Huang, S.; Song, Q.; Fang, J.; et al. Indicators of glucose dysregulation and the relationship with iron overload in Chinese children with beta thalassemia major. Pediatr. Diabetes 2022, 23, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Aljwaid, H.; White, D.L.; Collard, K.J.; Moody, A.J.; Pinkney, J.H. Non-transferrin-bound iron is associated with biomarkers of oxidative stress, inflammation and endothelial dysfunction in type 2 diabetes. J. Diabetes Complicat. 2015, 29, 943–949. [Google Scholar] [CrossRef]
- Stechemesser, L.; Eder, S.K.; Wagner, A.; Patsch, W.; Feldman, A.; Strasser, M.; Auer, S.; Niederseer, D.; Huber-Schonauer, U.; Paulweber, B.; et al. Metabolomic profiling identifies potential pathways involved in the interaction of iron homeostasis with glucose metabolism. Mol. Metab. 2017, 6, 38–47. [Google Scholar] [CrossRef]
- Li, J.; Jia, L.; Ma, W.; Feng, Y.; Yu, H.; Du, H. Dietary iron modulates hepatic glucose homeostasis via regulating gluconeogenesis. J. Nutr. Biochem. 2022, 109, 109104. [Google Scholar] [CrossRef]
- Huang, J.; Simcox, J.; Mitchell, T.C.; Jones, D.; Cox, J.; Luo, B.; Cooksey, R.C.; Boros, L.G.; McClain, D.A. Iron regulates glucose homeostasis in liver and muscle via AMP-activated protein kinase in mice. FASEB J. 2013, 27, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Feng, Y.; Jia, L.; Li, S.; Li, J.; Wang, Z.; Chen, X.; Du, H. Dietary iron modulates glucose and lipid homeostasis in diabetic mice. Biol. Trace Elem. Res. 2019, 189, 194–200. [Google Scholar] [CrossRef] [PubMed]
- James, J.V.; Varghese, J.; McKie, A.T.; Vaulont, S.; Jacob, M. Enhanced insulin signaling and its downstream effects in iron-overloaded primary hepatocytes from hepcidin knock-out mice. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2020, 1867, 118621. [Google Scholar] [CrossRef]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Soliman, N. Iron deficiency anemia and glucose metabolism. Acta Biomed. 2017, 88, 112–118. [Google Scholar] [CrossRef]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, J.S.; Lee, H.J.; Kim, W.H.; Park, S.I.; Song, J. Effect of excess iron on oxidative stress and gluconeogenesis through hepcidin during mitochondrial dysfunction. J. Nutr. Biochem. 2015, 26, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Largajolli, A.; Bertoldo, A.; Marcovina, S.; Nelson, J.E.; Yeh, M.M.; Kowdley, K.V.; Kahn, S.E. Serum ferritin is associated with non-alcoholic fatty liver disease and decreased Β-cell function in non-diabetic men and women. J. Diabetes Its Complicat. 2014, 28, 177–184. [Google Scholar] [CrossRef]
- Kulaksiz, H.; Fein, E.; Redecker, P.; Stremmel, W.; Adler, G.; Cetin, Y. Pancreatic beta-cells express hepcidin, an iron-uptake regulatory peptide. J. Endocrinol. 2008, 197, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Backe, M.B.; Moen, I.W.; Ellervik, C.; Hansen, J.B.; Mandrup-Poulsen, T. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu. Rev. Nutr. 2016, 36, 241–273. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Anderson, C.P.; Neschen, S.; Zumbrennen-Bullough, K.B.; Romney, S.J.; Kahle-Stephan, M.; Rathkolb, B.; Gailus-Durner, V.; Fuchs, H.; Wolf, E.; et al. Irp2 regulates insulin production through iron-mediated Cdkal1-catalyzed tRNA modification. Nat. Commun. 2020, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zheng, J.; Liu, G.; Xu, E.; Wang, J.; Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J.; Chen, H. Ceruloplasmin and hephaestin jointly protect the exocrine pancreas against oxidative damage by facilitating iron efflux. Redox Biol. 2018, 17, 432–439. [Google Scholar] [CrossRef]
- Fuqua, B.K.; Lu, Y.; Frazer, D.M.; Darshan, D.; Wilkins, S.J.; Dunn, L.; Loguinov, A.V.; Kogan, S.C.; Matak, P.; Chen, H.; et al. Severe iron metabolism defects in mice with double knockout of the multicopper ferroxidases hephaestin and ceruloplasmin. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Pauk, M.; Kufner, V.; Rumenovic, V.; Dumic-Cule, I.; Farkas, V.; Milosevic, M.; Bordukalo-Niksic, T.; Vukicevic, S. Iron overload in aging Bmp6−/− mice induces exocrine pancreatic injury and fibrosis due to acinar cell loss. Int. J. Mol. Med. 2021, 47, 60. [Google Scholar] [CrossRef]
- Deng, L.; Mo, M.Q.; Zhong, J.; Li, Z.; Li, G.; Liang, Y. Iron overload induces islet beta cell ferroptosis by activating ASK1/P-P38/CHOP signaling pathway. PeerJ 2023, 11, e15206. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, S.; Karunakaran, U.; Moon, J.-S.; Won, K.-C. Ferroptosis signaling in pancreatic β-Cells: Novel insights & therapeutic targeting. Int. J. Mol. Sci. 2022, 23, 13679. [Google Scholar] [CrossRef]
- Blesia, V.; Patel, V.B.; Al-Obaidi, H.; Renshaw, D.; Zariwala, M.G. Excessive iron induces oxidative stress promoting cellular perturbations and insulin secretory dysfunction in MIN6 beta cells. Cells 2021, 10, 1141. [Google Scholar] [CrossRef]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Jouihan, H.A.; Cobine, P.A.; Cooksey, R.C.; Hoagland, E.A.; Boudina, S.; Abel, E.D.; Winge, D.R.; McClain, D.A. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol. Med. 2008, 14, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Karmi, O.; Sohn, Y.S.; Marjault, H.B.; Israeli, T.; Leibowitz, G.; Ioannidis, K.; Nahmias, Y.; Mittler, R.; Cabantchik, I.Z.; Nechushtai, R. A combined drug treatment that reduces mitochondrial iron and reactive oxygen levels recovers insulin secretion in NAF-1-deficient pancreatic cells. Antioxidants 2021, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, L.G.; Aikin, R.; Tonnesen, M.F.; Paraskevas, S.; Blaabjerg, L.; Storling, J.; Rosenberg, L.; Billestrup, N.; Maysinger, D.; Mandrup-Poulsen, T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes 2009, 58, 1807–1815. [Google Scholar] [CrossRef]
- Hansen, J.B.; Tonnesen, M.F.; Madsen, A.N.; Hagedorn, P.H.; Friberg, J.; Grunnet, L.G.; Heller, R.S.; Nielsen, A.O.; Storling, J.; Baeyens, L.; et al. Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic beta cell fate in response to cytokines. Cell Metab. 2012, 16, 449–461. [Google Scholar] [CrossRef]
- Kang, T.; Huang, H.; Mandrup-Poulsen, T.; Larsen, M.R. Divalent metal transporter 1 knock-down modulates IL-1β mediated pancreatic beta-cell pro-apoptotic signaling pathways through the autophagic machinery. Int. J. Mol. Sci. 2021, 22, 8013. [Google Scholar] [CrossRef]
- Rattanaporn, P.; Tongsima, S.; Mandrup-Poulsen, T.; Svasti, S.; Tanyong, D. Combination of ferric ammonium citrate with cytokines involved in apoptosis and insulin secretion of human pancreatic beta cells related to diabetes in thalassemia. PeerJ 2020, 8, e9298. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nature reviews. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Mechanisms of iron hepatotoxicity. J. Hepatol. 2016, 65, 226–227. [Google Scholar] [CrossRef]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Xing, G.; Meng, L.; Cao, S.; Liu, S.; Wu, J.; Li, Q.; Huang, W.; Zhang, L. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022, 23, e52280. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Fang, X.; Zhang, Y.; Wei, J.; Zhang, Y.; Tian, J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: Mechanisms and therapeutic opportunities. Cell Death Dis. 2023, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bi, R.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Cui, X.; Yang, H.; Gao, X.; Zhang, D. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur. J. Pharmacol. 2020, 888, 173574. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Y.; Yang, H.Z.; Wang, Y.J.; Chen, Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci. Rep. 2021, 41, BSR20202924. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, L.; Zhang, J.; Liu, X.; Wu, Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic. Biol. Med. 2021, 162, 435–449. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.W.; Joo, J.; Han, S.H.; Shin, H.; Nam, B.Y.; Park, J.; Yoo, T.H.; Kim, G.; Lee, P.; et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021, 12, 160. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Gao, Z.; Li, H. Iron increases diabetes-induced kidney injury and oxidative stress in rats. Biol. Trace Elem. Res. 2014, 160, 368–375. [Google Scholar] [CrossRef]
- Pena-Montes, D.J.; Huerta-Cervantes, M.; Rios-Silva, M.; Trujillo, X.; Cortes-Rojo, C.; Huerta, M.; Saavedra-Molina, A. Effects of dietary iron restriction on kidney mitochondria function and oxidative stress in streptozotocin-diabetic rats. Mitochondrion 2020, 54, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yan, Y.; Zuo, D.; Zhuo, Z.; Sun, T.; Lin, H.; Han, Z.; Zhao, Z.; Yu, H. Iron metabolism and ferroptosis in diabetic bone loss: From mechanism to therapy. Front. Nutr. 2023, 10, 1178573. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Jie, J.; Yao, J.; Li, W.; Cheng, Y.; Lu, W. Advanced glycation end products promote osteoporosis by inducing ferroptosis in osteoblasts. Mol. Med. Rep. 2022, 25, 140. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, Y.; Gao, Y.; Xu, Z.; Zhao, D.; Yang, M. ATF3 regulates osteogenic function by mediating osteoblast ferroptosis in type 2 diabetic osteoporosis. Dis. Markers 2022, 2022, 9872243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Meng, H.Z.; Yang, M.W. Regulation of DMT1 on Bone Microstructure in Type 2 Diabetes. Int. J. Med. Sci. 2015, 12, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, H.; Sun, J.; Zheng, T.; Zhao, P.; Li, H.; Yang, M. Mitochondrial ferritin deficiency promotes osteoblastic ferroptosis via mitophagy in type 2 diabetic osteoporosis. Biol. Trace Elem. Res. 2022, 200, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shen, X.; Ke, Y.; Lan, C.; Chen, X.; Liang, B.; Zhang, Y.; Yan, S. Activation of osteoblast ferroptosis via the METTL3/ASK1-p38 signaling pathway in high glucose and high fat (HGHF)-induced diabetic bone loss. FASEB J. 2022, 36, e22147. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Zhang, W.; Li, H.; Zhao, W.; Sun, J.; Yang, M. Melatonin suppresses ferroptosis induced by high glucose via activation of the Nrf2/HO-1 signaling pathway in type 2 diabetic osteoporosis. Oxidative Med. Cell. Longev. 2020, 2020, 9067610. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Y.; Wang, M.; Yuan, K.; Wang, Q.; Mu, P.; Du, J.; Yu, Z.; Yang, S.; Huang, K.; et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Kosacka, J.; Estrela-Lopis, I.; Woidt, K.; Serke, H.; Paeschke, S.; Stockinger, M.; Kloting, N.; Bluher, M.; Dorn, M.; et al. The role of nerve inflammation and exogenous iron load in experimental peripheral diabetic neuropathy (PDN). Metabolism 2016, 65, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, J.; Woidt, K.; Toyka, K.V.; Paeschke, S.; Kloting, N.; Bechmann, I.; Bluher, M.; Thiery, J.; Ossmann, S.; Baum, P.; et al. The role of dietary non-heme iron load and peripheral nerve inflammation in the development of peripheral neuropathy (PN) in obese non-diabetic leptin-deficient ob/ob mice. Neurol. Res. 2019, 41, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Paeschke, S.; Baum, P.; Toyka, K.V.; Bluher, M.; Koj, S.; Kloting, N.; Bechmann, I.; Thiery, J.; Kosacka, J.; Nowicki, M. The role of iron and nerve inflammation in diabetes mellitus type 2-induced peripheral neuropathy. Neuroscience 2019, 406, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, Y.; Oh, T.J.; Choi, S.H.; Jang, H.C. Association between Iron Intake and Diabetic Peripheral Neuropathy in Type 2 Diabetes: Significance of Iron Intake and the Ratio between Iron Intake and Polyunsaturated Fatty Acids Intake. Nutrients 2020, 12, 3365. [Google Scholar] [CrossRef]
- Tian, M.; Zhi, J.Y.; Pan, F.; Chen, Y.Z.; Wang, A.Z.; Jia, H.Y.; Huang, R.; Zhong, W.H. Bioinformatics analysis identifies potential ferroptosis key genes in the pathogenesis of diabetic peripheral neuropathy. Front. Endocrinol. 2023, 14, 1048856. [Google Scholar] [CrossRef]
- Wu, K.Y.; Deng, F.; Mao, X.Y.; Zhou, D.; Shen, W.G. Ferroptosis involves in Schwann cell death in diabetic peripheral neuropathy. Open Med. 2023, 18, 20230809. [Google Scholar] [CrossRef]
- Hao, L.; Mi, J.; Song, L.; Guo, Y.; Li, Y.; Yin, Y.; Zhang, C. SLC40A1 mediates ferroptosis and cognitive dysfunction in type 1 diabetes. Neuroscience 2021, 463, 216–226. [Google Scholar] [CrossRef]
- Guo, T.; Yu, Y.; Yan, W.; Zhang, M.; Yi, X.; Liu, N.; Cui, X.; Wei, X.; Sun, Y.; Wang, Z.; et al. Erythropoietin ameliorates cognitive dysfunction in mice with type 2 diabetes mellitus via inhibiting iron overload and ferroptosis. Exp. Neurol. 2023, 365, 114414. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Luo, X.; Yan, J.; Zhang, J.; Sun, R.; Luo, A.; Li, S. Activated AMPK mitigates diabetes-related cognitive dysfunction by inhibiting hippocampal ferroptosis. Biochem. Pharmacol. 2023, 207, 115374. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Luo, E.; Li, H.; Qin, Y.; Qiao, Y.; Yan, G.; Yao, Y.; Li, L.; Hou, J.; Tang, C.; Wang, D. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J Diabetes 2021, 12, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm. Sin. B 2022, 12, 708–722. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, J.; Wu, G.; Hu, Z.; Ying, P.; Bao, Z.; Ding, Z.; Tan, X. 6-Gingerol alleviates ferroptosis and inflammation of diabetic cardiomyopathy via the Nrf2/HO-1 pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3027514. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Wu, W.; Qi, L.; Tan, W.; Nagarkatti, P.; Nagarkatti, M.; Wang, X.; Cui, T. Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes 2020, 69, 2720–2734. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 141–149. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.S.; An, T.H.; Park, H.J.; Kim, W.K.; Bae, K.H.; Oh, K.J. Metabolic Spectrum of Liver Failure in Type 2 Diabetes and Obesity: From NAFLD to NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4495. [Google Scholar] [CrossRef] [PubMed]
- Stancic, A.; Velickovic, K.; Markelic, M.; Grigorov, I.; Saksida, T.; Savic, N.; Vucetic, M.; Martinovic, V.; Ivanovic, A.; Otasevic, V. Involvement of ferroptosis in diabetes-induced liver pathology. Int. J. Mol. Sci. 2022, 23, 9309. [Google Scholar] [CrossRef]
- Silva, M.; de Brito Magalhaes, C.L.; de Paula Oliveira, R.; Silva, M.E.; Pedrosa, M.L. Differential expression of iron metabolism proteins in diabetic and diabetic iron-supplemented rat liver. J. Biochem. Mol. Toxicol. 2012, 26, 123–129. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Yang, J.; Wang, J.; Wu, Y.; Zhu, R.; Liu, Q.; Xie, P. lncRNA ZFAS1 positively facilitates endothelial ferroptosis via miR-7-5p/ACSL4 axis in diabetic retinopathy. Oxidative Med. Cell. Longev. 2022, 2022, 9004738. [Google Scholar] [CrossRef]
- Liu, C.; Sun, W.; Zhu, T.; Shi, S.; Zhang, J.; Wang, J.; Gao, F.; Ou, Q.; Jin, C.; Li, J.; et al. Glia maturation factor-beta induces ferroptosis by impairing chaperone-mediated autophagic degradation of ACSL4 in early diabetic retinopathy. Redox Biol. 2022, 52, 102292. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Bai, Z.; Zhang, L.; Zhang, F. Ferrostatin-1 alleviates tissue and cell damage in diabetic retinopathy by improving the antioxidant capacity of the Xc(-)-GPX4 system. Cell Death Discov. 2022, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liao, H.; Pang, M.; Pan, L.; Guan, Y.; Huang, X.; Hei, Z.; Luo, C.; Ge, M. Inhibition of the NADPH oxidase pathway reduces ferroptosis during septic renal injury in diabetic mice. Oxidative Med. Cell. Longev. 2022, 2022, 1193734. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Wu, Z.; Wu, Z.; Fang, H. Diabetic ferroptosis plays an important role in triggering on inflammation in diabetic wound. Am. J. Physiol. Metab. 2021, 321, E509–E520. [Google Scholar] [CrossRef]
- Wei, X.; Liu, M.; Zheng, Z.; Yu, S.; Huang, L.; Ma, J.; Gao, Y.; Peng, Y.; Chen, L.; Tan, R.; et al. Defective NCOA4-dependent ferroptosis in senescent fibroblasts retards diabetic wound healing. Cell Death Discov. 2023, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Z.; Cai, Y.; Li, Z.; Zhou, Q.; Chen, Q. Ferroptosis: Mechanisms and role in diabetes mellitus and its complications. Ageing Res. Rev. 2024, 94, 102201. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yin, X.D.; Zhang, F.; Zhu, Y.Z.; Li, Z.L. The regulatory effects of Traditional Chinese Medicine on ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 4578381. [Google Scholar] [CrossRef]
- Wang, M.Z.; Cai, Y.F.; Fang, Q.J.; Liu, Y.L.; Wang, J.; Chen, J.X.; Fu, Y.; Wan, B.Y.; Tu, Y.; Wu, W.; et al. Inhibition of ferroptosis of renal tubular cells with total flavones of Abelmoschus manihot alleviates diabetic tubulopathy. Anat Rec. 2023, 306, 3199–3213. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Wang, H.; Chen, Y. Vitexin ameliorated diabetic nephropathy via suppressing GPX4-mediated ferroptosis. Eur. J. Pharmacol. 2023, 951, 175787. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Zhong, B.; Liao, Q.; Wang, X.; Xie, Y.; He, X. Review on the Diverse Biological Effects of Glabridin. Drug Des. Dev. Ther. 2023, 17, 15–37. [Google Scholar] [CrossRef]
- Tan, H.; Chen, J.; Li, Y.; Li, Y.; Zhong, Y.; Li, G.; Liu, L.; Li, Y. Glabridin, a bioactive component of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol. Med. 2022, 28, 58. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, Y.; Qiao, Y.; Zheng, Y.; Yu, X.; Liu, F.; Wang, H.; Zheng, B.; Pan, S.; Ren, K.; et al. Quercetin ameliorates diabetic kidney injury by inhibiting ferroptosis via activating Nrf2/HO-1 signaling pathway. Am. J. Chin. Med. 2023, 51, 997–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Chang, L.; Ren, Y.; Sui, M.; Fu, Y.; Zhang, L.; Hao, L. Quercetin improves diabetic kidney disease by inhibiting ferroptosis and regulating the Nrf2 in streptozotocin-induced diabetic rats. Ren. Fail. 2024, 46, 2327495. [Google Scholar] [CrossRef]
- Zhu, S.; Kang, Z.; Zhang, F. Tanshinone IIA suppresses ferroptosis to attenuate renal podocyte injury in diabetic nephropathy through the embryonic lethal abnormal visual-like protein 1 and acyl-coenzyme A synthetase long-chain family member 4 signaling pathway. J. Diabetes Investig. 2024, 15, 1003–1016. [Google Scholar] [CrossRef]
- Chen, J.; Ou, Z.; Gao, T.; Yang, Y.; Shu, A.; Xu, H.; Chen, Y.; Lv, Z. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed. Pharmacother. 2022, 156, 113953. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Tan, R.; Xu, L.; Wang, H.; Li, J.; Su, H.; Zhong, X.; Liu, P.; Wang, L. Hederagenin improves renal fibrosis in diabetic nephropathy by regulating Smad3/NOX4/SLC7A11 signaling-mediated tubular cell ferroptosis. Int. Immunopharmacol. 2024, 135, 112303. [Google Scholar] [CrossRef]
- Huang, J.; Chen, G.; Wang, J.; Liu, S.; Su, J. Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4). Bioengineered 2022, 13, 6627–6637. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Zhang, Y.; Yin, K.; Chen, X.; Zhu, X. Leonurine Ameliorates Diabetic Nephropathy through GPX4-Mediated Ferroptosis of Endothelial Cells. Front Biosci. 2024, 29, 270. [Google Scholar] [CrossRef]
- Cai, S.; Zhu, H.; Chen, L.; Yu, C.; Su, L.; Chen, K.; Li, Y. Berberine Inhibits KLF4 Promoter Methylation and Ferroptosis to Ameliorate Diabetic Nephropathy in Mice. Chem. Res. Toxicol. 2024, 37, 1728–1737. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Sui, B.; Xu, M.; Pu, Z.; Qiu, T.; Zhang, Z. Schisandrin A from Schisandra chinensis attenuates ferroptosis and NLRP3 inflammasome-mediated pyroptosis in diabetic nephropathy through mitochondrial damage by AdipoR1 ubiquitination. Oxidative Med. Cell. Longev. 2022, 2022, 5411462. [Google Scholar] [CrossRef]
- Naowaboot, J.; Somparn, N.; Saentaweesuk, S.; Pannangpetch, P. Umbelliferone improves an impaired glucose and lipid metabolism in high-fat diet/streptozotocin-induced type 2 diabetic rats. Phytother. Res. 2015, 29, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.A.M.; Khowailed, A.A.; Elattar, S.; Mahmoud, A.M. Umbelliferone ameliorates oxidative stress and testicular injury, improves steroidogenesis and upregulates peroxisome proliferator-activated receptor gamma in type 2 diabetic rats. J. Pharm. Pharmacol. 2022, 74, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Chen, C. Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem. Toxicol. 2022, 163, 112892. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Han, X.; Hu, W.; Xiong, D. Rhein inhibited ferroptosis and EMT to attenuate diabetic nephropathy by regulating the Rac1/NOX1/β-catenin Aaxis. Front. Biosci. 2023, 28, 100. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhou, J.; Qiu, T.; Xie, H.; Pu, Z. Chicoric acid advanced PAQR3 ubiquitination to ameliorate ferroptosis in diabetes nephropathy through the relieving of the interaction between PAQR3 and P110alpha pathway. Clin. Exp. Hypertens. 2024, 46, 2326021. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, W.; Wang, L.; Zhang, H.; Wang, Y.; Pan, B.; Li, H.; Ma, Z.; Xu, K.; Cui, H.; et al. Rosa laevigata Michx. polysaccharide ameliorates diabetic nephropathy in mice through inhibiting ferroptosis and PI3K/AKT pathway-mediated apoptosis and modulating tryptophan metabolism. J. Diabetes Res. 2023, 2023, 9164883. [Google Scholar] [CrossRef]
- Xu, C.Y.; Xu, C.; Xu, Y.N.; Du, S.Q.; Dai, Z.H.; Jin, S.Q.; Zheng, G.; Xie, C.L.; Fang, W.L. Poliumoside protects against type 2 diabetes-related osteoporosis by suppressing ferroptosis via activation of the Nrf2/GPX4 pathway. Phytomedicine 2024, 125, 155342. [Google Scholar] [CrossRef]
- Wei, F.; Ruan, B.; Dong, J.; Yang, B.; Zhang, G.; Kelvin Yeung, W.K.; Wang, H.; Cao, W.; Wang, Y. Asperosaponin VI inhibition of DNMT alleviates GPX4 suppression-mediated osteoblast ferroptosis and diabetic osteoporosis. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Mthembu, S.X.; Mazibuko-Mbeje, S.E.; Moetlediwa, M.T.; Muvhulawa, N.; Silvestri, S.; Orlando, P.; Nkambule, B.B.; Muller, C.J.; Ndwandwe, D.; Basson, A.K.; et al. Sulforaphane: A nutraceutical against diabetes-related complications. Pharmacol. Res. 2023, 196, 106918. [Google Scholar] [CrossRef]
- Hao, W.; Li, N.; Mi, C.; Wang, Q.; Yu, Y. Salidroside attenuates cardiac dysfunction in a rat model of diabetes. Diabet. Med. 2021, 39, e14683. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Q.; Hao, D.D.; Miao, H.X.; Wan, S.; Zhou, C.H.; Wang, S.Y.; Chen, S.Y.; Shang, J.; Feng, T.H. Gut microbiota profiling revealed the regulating effects of salidroside on iron metabolism in diabetic mice. Front. Endocrinol. 2022, 13, 1014577. [Google Scholar] [CrossRef]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Yin, M.; Li, Y.; Chen, C.; Zhang, J.; Sun, K.; Kong, X.; Chen, Z.; Qian, J. Isorhapontigenin Attenuates Cardiac Microvascular Injury in Diabetes via the Inhibition of Mitochondria-Associated Ferroptosis Through PRDX2-MFN2-ACSL4 Pathways. Diabetes 2023, 72, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Babiker, A.Y.; Almogbel, M.A.; Khan, A.A.; Husain Rahmani, A. 6-Gingerol, a bioactive compound of Ginger attenuates renal damage in streptozotocin-induced diabetic rats by regulating the oxidative stress and inflammation. Pharmaceutics 2021, 13, 317. [Google Scholar] [CrossRef]
- Wei, Z.; Shaohuan, Q.; Pinfang, K.; Chao, S.; Gasparotto Junior, A. Curcumin attenuates ferroptosis-induced myocardial injury in diabetic cardiomyopathy through the Nrf2 pathway. Cardiovasc. Ther. 2022, 2022, 3159717. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.K.; Mohandas, S.; Kunka Mohanram, R. Role of ferroptosis inhibitors in the management of diabetes. Biofactors 2023, 49, 270–296. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Chen, H.; Wei, S.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress- related PERK pathway in MIN6 cells. Toxicology 2022, 465, 153048. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.F.; Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin alleviates ferroptosis of pancreatic beta cells in type 2 diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef]
- Zhou, Y. The protective effects of cryptochlorogenic acid on beta-cells function in diabetes in vivo and vitro via inhibition of ferroptosis. Diabetes Metab. Syndr. Obes. 2020, 13, 1921–1931. [Google Scholar] [CrossRef]
- Wu, F.; Shang, C.; Jin, T.; Shi, L.; Ajiboye, B.O. Hispidin inhibits ferroptosis induced by high glucose via the miR-15b-5p/GLS2 axis in pancreatic beta cells. Evid.-Based Complement. Altern. Med. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
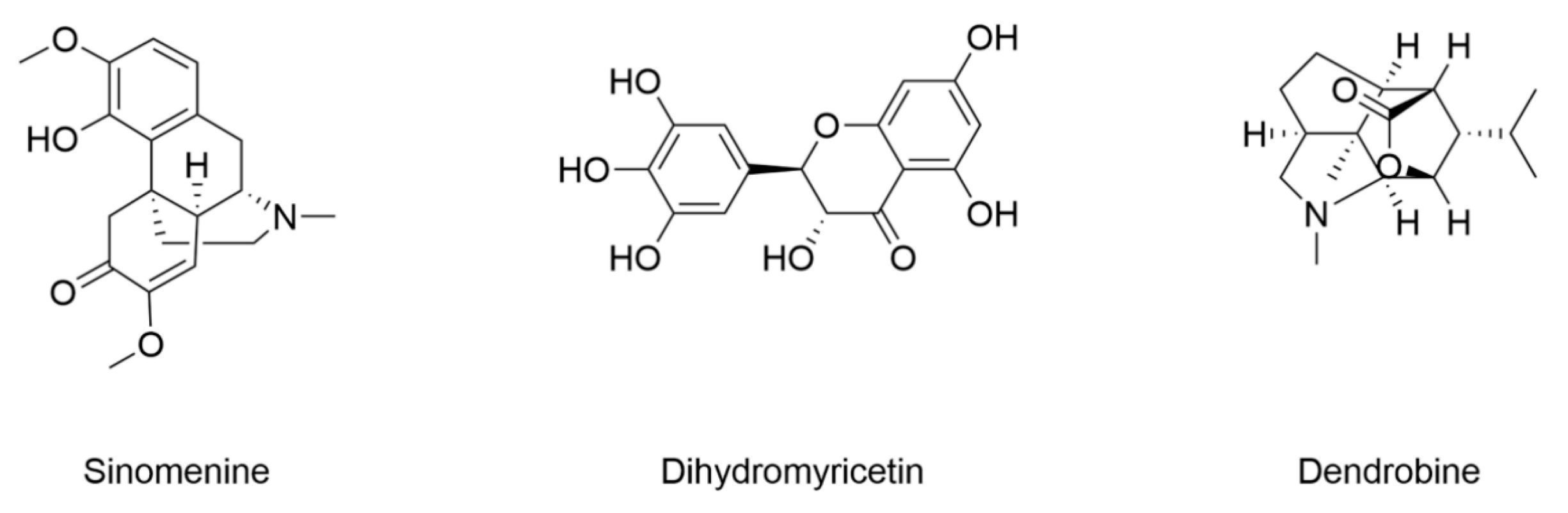
- Chen, J.; Guo, P.; Han, M.; Chen, K.; Qin, J.; Yang, F. Cognitive protection of sinomenine in type 2 diabetes mellitus through regulating the EGF/Nrf2/HO-1 signaling, the microbiota-gut-brain axis, and hippocampal neuron ferroptosis. Phytother. Res. 2023, 37, 3323–3341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, S.; Li, Q.; Song, Z.; He, J.; Yang, S.; Yan, C.; Ling, H. Dihydromyricetin alleviates hippocampal ferroptosis in type 2 diabetic cognitive impairment rats via inhibiting the JNK-inflammatory factor pathway. Neurosci. Lett. 2023, 812, 137404. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Lin, L.; Cheng, Y.; Zhao, Y.; Zhang, Y.; Pan, X. Dendrobine rescues cognitive dysfunction in diabetic encephalopathy by inhibiting ferroptosis via activating Nrf2/GPX4 axis. Phytomedicine 2023, 119, 154993. [Google Scholar] [CrossRef] [PubMed]
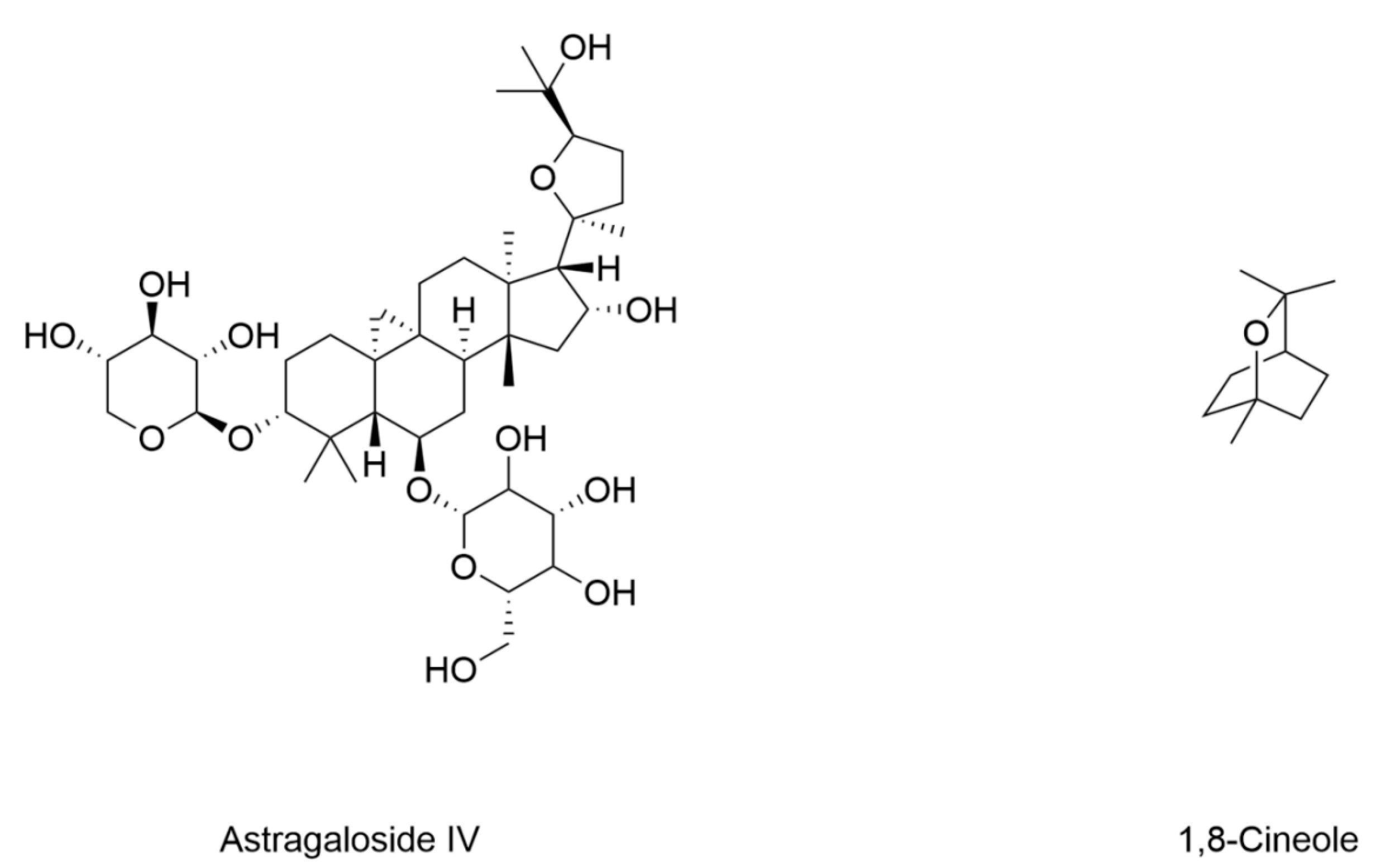
- Song, Q.; Zhao, Y.; Yang, Y.; Han, X.; Duan, J. Astragaloside IV protects against retinal iron overload toxicity through iron regulation and the inhibition of MAPKs and NF-κB activation. Toxicol. Appl. Pharmacol. 2021, 410, 115361. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Huang, D.; Shi, S.; Pei, C.; Wu, Y.; Shen, Z.; Wang, F.; Wang, Z. Astragaloside IV regulates the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to inhibit PM2.5-mediated lung injury in mice. Int. Immunopharmacol. 2022, 112, 109186. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Xu, J.; Liang, Q.; Hong, L.; Zhang, L.; Garcia-Rivas, G. Astragaloside IV inhibits bleomycin-induced ferroptosis in human umbilical vein endothelial cells by mediating LPC. Oxidative Med. Cell. Longev. 2021, 2021, 6241242. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.; Zhang, D.; Han, W. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered 2022, 13, 8240–8254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gan, S.; Fu, L.; Xu, Y.; Wang, S.; Zhang, G.; Pan, D.; Tao, L.; Shen, X. 1,8-Cineole ameliorates diabetic retinopathy by inhibiting retinal pigment epithelium ferroptosis via PPAR-γ/TXNIP pathways. Biomed. Pharmacother. 2023, 164, 114978. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, W.; Ye, C.; Hu, T.; Yu, Q.; Meng, M.; Sun, L.; Liang, J.; Chen, Y. Honokiol attenuates high glucose-induced peripheral neuropathy via inhibiting ferroptosis and activating AMPK/SIRT1/PGC-1α pathway in Schwann cells. Phytother. Res. 2023, 37, 5787–5802. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Peng, Y.; Li, J.; Wang, L.; Zhu, X.; Wang, N.; Yang, D.; Zhou, X.; Chang, D. Hydroxysafflor Yellow A alleviates acute myocardial ischemia/reperfusion injury in mice by inhibiting ferroptosis via the activation of the HIF-1α/SLC7A11/GPX4 signaling pathway. Nutrients 2023, 15, 3411. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, J.; Zhu, H.; Deng, S.; Gu, M.; Qu, S. Hydroxysafflor yellow A attenuates high glucose-induced human umbilical vein endothelial cell dysfunction. Hum. Exp. Toxicol. 2019, 38, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Li, C.; Zhang, Q.; Zheng, G.; Fan, W.; Pan, Z.; Shi, S. Hydroxysafflor yellow A inhibits endothelial cell ferroptosis in diabetic atherosclerosis mice by regulating miR-429/SLC7A11. Pharm. Biol. 2023, 61, 404–415. [Google Scholar] [CrossRef] [PubMed]


| Compound | Diabetic Models | Changes of Ferroptotic Biological Events | Ref. | |||
|---|---|---|---|---|---|---|
| Animal Model | Cell Model | Iron Metabolism | Lipid Metabolism | GSH Metabolism | ||
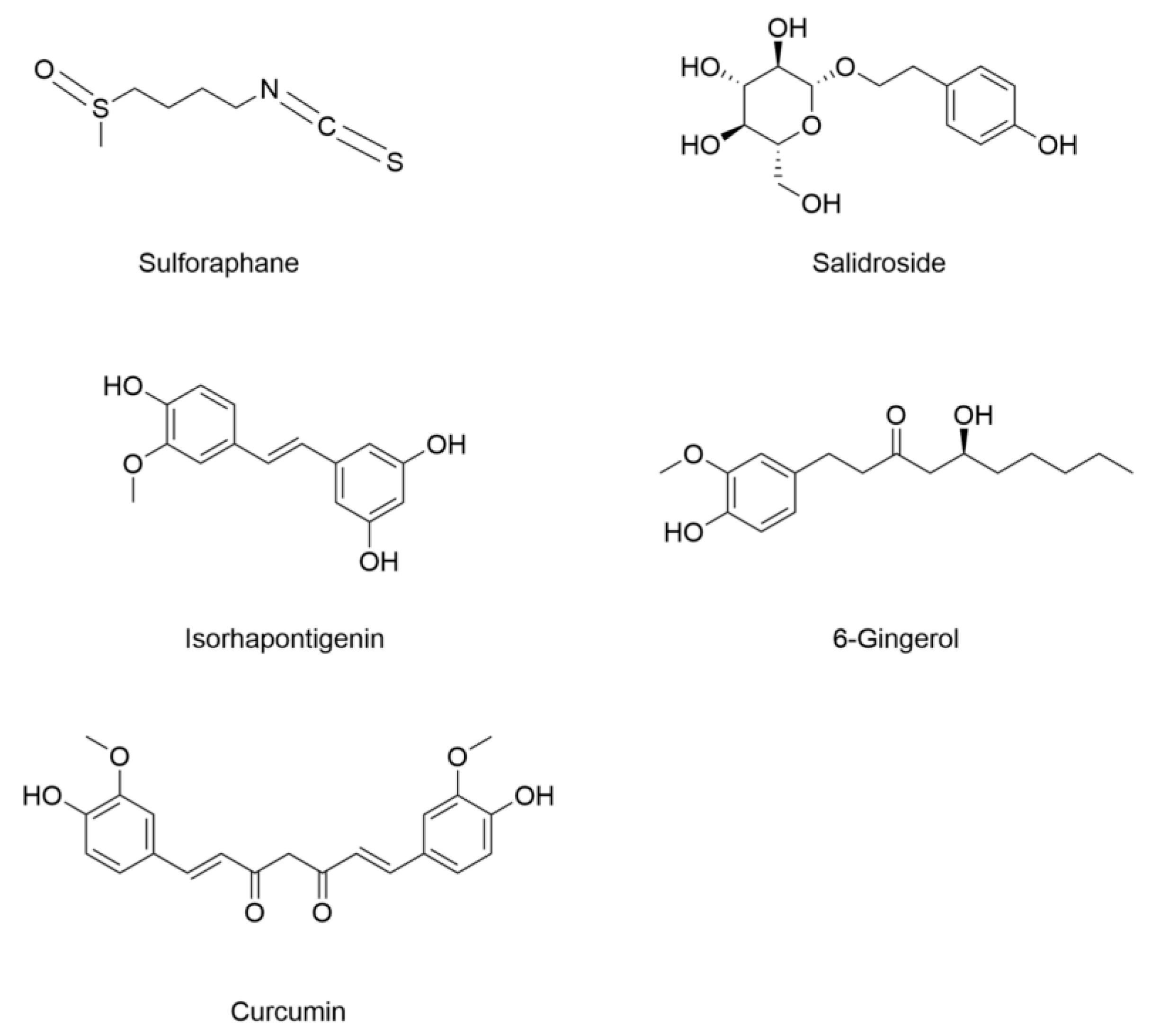
| Sulforaphane | 8-week-old male C57BL/6 mice induced by HFD (3 months)/STZ (100 mg/kg) | Primary cardiac cells from FVB pups | Increased expression of ferritin Decreased labile iron content | Decreased MDA | Decreased expression of prostaglandin-endoperoxide synthase (Ptsg2) Increased GSH content and GSH/GSSG ratio Increased expression of SLC7A11 | [105] |
| Salidroside | 8-week-old male BKS-Leprem2cd479/GPT (db/db) mice | / | Decreased serum transferrin and iron content | / | Increased expression of GPX4 | [143] |
| Isorhapontigenin | C57BLKS/J db/db mice induced by long-term diabetes (24 weeks) | Primary cardiac microvascular endothelial cells (CMECs) treated with high glucose (HG) and free fatty acids (FFAs) | Decreased ferrous iron content | Decreased lipid peroxidation | Increased GSH content and GPX4 expression | [145] |
| 6-Gingerol | 4–5-week-old male C57BL/6 mice induced by HFD (4 weeks)/STZ (50 mg/kg, 5 days) | H9c2 rat cardiomyoblast cells under 33 Mm glucose | Decreased iron content | Decreased MDA | Increased expression of GPX4 and SOD | [106] |
| Curcumin | 2-month-old male New Zealand rabbits induced by STZ (80 mg/kg) | Rat H9C2 cardiomyocytes under 30 mM glucose | / | / | Increased expression of GPX4 and HO-1 | [147] |
| Compound | Diabetic Models | Changes of Ferroptotic Biological Events | Ref. | |||
|---|---|---|---|---|---|---|
| Animal Model | Cell Model | Iron Metabolism | Lipid Metabolism | GSH Metabolism | ||
| Resveratrol | / | Min6 cells under 25 mM acrolein | / | Decreased MDA | Increased expression of GPX4 Decreased ACSL4 | [149] |
| Quercetin | 7–8-week-old C57BL/6 mice induced by HFD (4 months)/STZ (50 mg/kg, 5 days) | INS-1 cells under 11.1 Mm glucose | Decreased iron deposition and expression of FTL | Decreased MDA | Increased GSH content Increased expression of SOD and GPX4 Decreased expression of xCT | [151] |
| Cryptochlorogenic acid | SD rats (250–270 g) induced by STZ (50 mg/kg) | INS-1 cells under 50 mM glucose | Decreased expression of TfR1 Decreased iron | Activated Nrf2 Decreased MDA | Increased expression of GPX4 Increased content of GSH Decreased content of GSSG | [152] |
| Hispidin | / | Min6 cells under 10 mM glucose | Decreased Fe2+ | Decreased ROS and MDA | Increased content of GSH | [153] |
| Compound | Diabetic Models | Changes of Ferroptotic Biological Events | Ref. | |||
|---|---|---|---|---|---|---|
| Animal Model | Cell Model | Iron Metabolism | Lipid Metabolism | GSH Metabolism | ||
| Sinomenine | 8-week-old male SD rats induced by HGHF (4 weeks)/STZ (25 mg/kg) | Mouse hippocampal neuron cell line HT22 under 30 mM glucose | Decreased Fe2+ | Decreased MDA and ROS | / | [154] |
| Dihydromyricetin | 6–8-week-old male SD rats induced by HFD (4 weeks)/STZ (30 mg/kg) | / | Decreased Fe2+ | Decreased MDA and ROS Decreased expression of ACSL4 | Increased expression of GPX4 Increased GSH | [155] |
| Dendrobine | 12-week-old male db/db mice | Mouse hippocampal neuron cell line HT22 under 400 μg/mL AGEs | Decreased iron content Decreased expression of TfR1 Increased expression of FPN and FTH | Decreased MDA | Increased expression of GPX4, Nrf2, HO-1, and NQO1 Increased SOD and GSH | [156] |
| Compound | Diabetic Models | Changes of Ferroptotic Biological Events | Ref. | |||
|---|---|---|---|---|---|---|
| Animal Model | Cell Model | Iron Metabolism | Lipid Metabolism | GSH Metabolism | ||
| Astragaloside IV | / | Human retinal pigment epithelium ARPE-19 cells under 25 mM glucose | / | Decreased lipid peroxidation and ROS Activated Nrf2 | Increased expression of GPX4 and GCLC | [160] |
| 1,8-Cineole | 8-week-old male C57BL/6 mice induced by HFD (8 weeks) STZ (55 mg/kg) | ARPE-19 cells under 25 mM glucose | Decreased Fe2+ | Decreased MDA | Increased GSH Increased expression of GPX4 and FSP-1 | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Li, C.; Dong, X.; Wang, B.; Qin, J.; Lv, H. Natural Products as Modulators of Iron Metabolism and Ferroptosis in Diabetes and Its Complications. Nutrients 2025, 17, 2714. https://doi.org/10.3390/nu17162714
Xie Y, Li C, Dong X, Wang B, Qin J, Lv H. Natural Products as Modulators of Iron Metabolism and Ferroptosis in Diabetes and Its Complications. Nutrients. 2025; 17(16):2714. https://doi.org/10.3390/nu17162714
Chicago/Turabian StyleXie, Yuanfen, Chunqin Li, Xige Dong, Beilei Wang, Jiaxin Qin, and Huanhuan Lv. 2025. "Natural Products as Modulators of Iron Metabolism and Ferroptosis in Diabetes and Its Complications" Nutrients 17, no. 16: 2714. https://doi.org/10.3390/nu17162714
APA StyleXie, Y., Li, C., Dong, X., Wang, B., Qin, J., & Lv, H. (2025). Natural Products as Modulators of Iron Metabolism and Ferroptosis in Diabetes and Its Complications. Nutrients, 17(16), 2714. https://doi.org/10.3390/nu17162714






