Maternal Adherence to Healthy Dietary Patterns During Pregnancy and Gestational Weight Gain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dietary Assessments
2.3. Gestational Weight Gain (GWG)
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
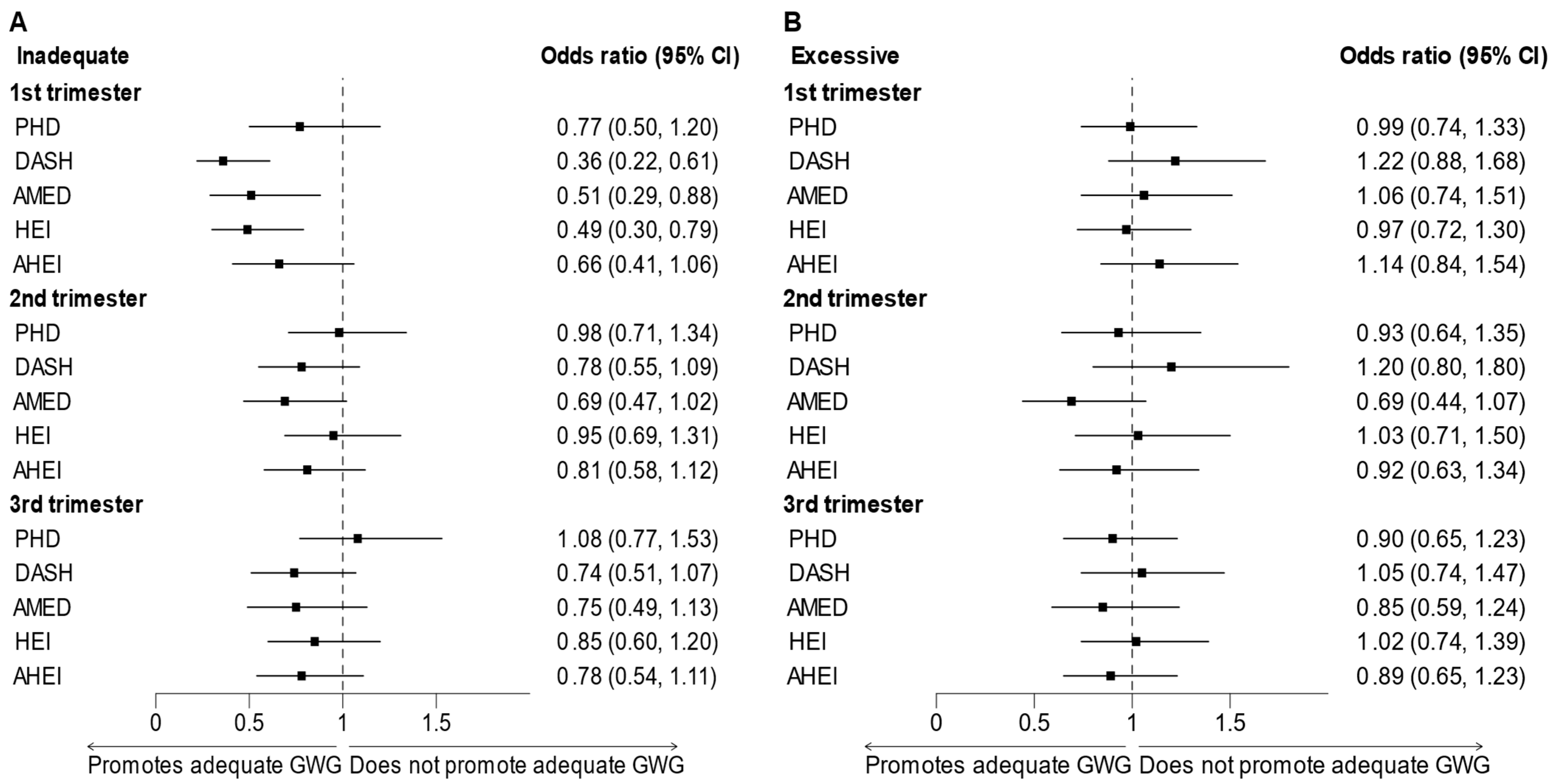
3.2. Adherence to Dietary Patterns and Gestational Weight Gain
4. Discussion
4.1. Comparison of Findings with Existing Studies
4.2. Potential Biological Mechanisms
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Attestation
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHEI | Alternate Healthy Eating Index-2010 |
| AHEI-P | Alternate Healthy Eating Index for Pregnancy |
| AMED | Alternative Mediterranean diet |
| ASA24 | Automated Self-Administered 24-Hour Dietary Assessment Tool |
| BMI | Body Mass Index |
| DAG | Directed Acyclic Graph |
| DASH | Dietary Approaches to Stop Hypertension |
| DGA | Dietary Guidelines for Americans |
| DGAC | Dietary Guidelines Advisory Committee |
| DHQ-II | Diet History Questionnaire-II |
| HEI | Healthy Eating Index-2010 |
| IOM | Institute of Medicine |
| FFQ | Food-Frequency Questionnaire |
| GWG | Gestational Weight Gain |
| MPED | My Pyramid Equivalent Database |
| NICHD | Eunice Kennedy Shriver National Institute of Child health and Human Development |
| PHD | Planetary Health Diet |
| US | United States |
| USDA | United States Department of Agriculture |
References
- Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Champion, M.L.; Harper, L.M. Gestational Weight Gain: Update on Outcomes and Interventions. Curr. Diabetes Rep. 2020, 20, 11. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Chen, L.; Klein, M.; Franke, B.; Chen, J.; Buitelaar, J. Maternal gestational weight gain and offspring’s neurodevelopmental outcomes: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 153, 105360. [Google Scholar] [CrossRef]
- Dalfra, M.G.; Burlina, S.; Lapolla, A. Weight gain during pregnancy: A narrative review on the recent evidences. Diabetes Res. Clin. Pract. 2022, 188, 109913. [Google Scholar] [CrossRef] [PubMed]
- Hoelscher, D.M.; Tobias, D.; Deierlein, A.; Gardner, C.; Giovannucci, E.; Anderson, C.A.M.; Booth, S.; Fung, T.; Raynor, H.; Stanford, F.C.; et al. Dietary Patterns and Growth, Body Composition, and Risk of Obesity: A Systematic Review; Nutrition Evidence Systematic Review; U.S. Department of Agriculture, Food and Nutrition Service, Center for Nutrition Policy and Promotion: Beltsville, MD, USA, 2024. [Google Scholar] [CrossRef]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary patterns associated with obesity outcomes in adults: An umbrella review of systematic reviews. Public Health Nutr. 2021, 24, 6390–6414. [Google Scholar] [CrossRef] [PubMed]
- English, L.K.; Raghavan, R.; Obbagy, J.E.; Callahan, E.H.; Fultz, A.K.; Nevins, J.E.H.; Scinto-Madonich, S.; Reigh, N.A.; Stoody, E.E. Dietary Patterns and Health: Insights From NESR Systematic Reviews to Inform the Dietary Guidelines for Americans. J. Nutr. Educ. Behav. 2024, 56, 75–87. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Frank, S.M.; Jaacks, L.M.; Adair, L.S.; Avery, C.L.; Meyer, K.; Rose, D.; Taillie, L.S. Adherence to the Planetary Health Diet Index and correlation with nutrients of public health concern: An analysis of NHANES 2003–2018. Am. J. Clin. Nutr. 2024, 119, 384–392. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the Healthy Eating Index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2015, 115, 780–800.e785. [Google Scholar] [CrossRef]
- Grewal, J.; Grantz, K.L.; Zhang, C.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Cohort Profile: NICHD Fetal Growth Studies-Singletons and Twins. Int. J. Epidemiol. 2018, 47, 25–251. [Google Scholar] [CrossRef]
- Grantz, K.L.; Grewal, J.; Kim, S.; Grobman, W.A.; Newman, R.B.; Owen, J.; Sciscione, A.; Skupski, D.; Chien, E.K.; Wing, D.A.; et al. Unified standard for fetal growth: The Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies. Am. J. Obstet. Gynecol. 2022, 226, 576–587.e572. [Google Scholar] [CrossRef]
- Hinkle, S.N.; Zhang, C.; Grantz, K.L.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; D’Alton, M.E.; Skupski, D.; Nageotte, M.P.; et al. Nutrition during Pregnancy: Findings from the National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singleton Cohort. Curr. Dev. Nutr. 2021, 5, nzaa182. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.A.; Friday, J.E.; Moshfegh, A. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004 [Online] Food Surveys Research Group; Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture: Beltsville, MD, USA, 2008. Available online: http://www.ars.usda.gov/ba/bhnrc/fsrg (accessed on 13 August 2025).
- Divison of Cancer Control and Population Science, National Cancer Institute. Diet History Questionnaire II. Available online: https://epi.grants.cancer.gov/dhq2/database/nutrient.html (accessed on 13 August 2025).
- Epidemiology and Genomics Research Program, National Cancer Institute. Diet*Calc Analysis Program, Version 1.4.3; 2005. Available online: https://epi.grants.cancer.gov/dhq/dietcalc/download.html (accessed on 13 August 2025).
- Zhang, C.; Hediger, M.L.; Albert, P.S.; Grewal, J.; Sciscione, A.; Grobman, W.A.; Wing, D.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; et al. Association of Maternal Obesity with Longitudinal Ultrasonographic Measures of Fetal Growth: Findings From the NICHD Fetal Growth Studies–Singletons. JAMA Pediatr. 2018, 172, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.J.; Ortega-Villa, A.M.; Grobman, W.; Hinkle, S.N.; Newman, R.B.; Hediger, M.; Grewal, J.; Wing, D.A.; Albert, P.S.; Grantz, K.L. Longitudinal changes in maternal anthropometry in relation to neonatal anthropometry. Public Health Nutr. 2019, 22, 797–804. [Google Scholar] [CrossRef]
- Wagner, K.A.; Chen, Z.; Hinkle, S.N.; Gleason, J.L.; Lee, W.; Grobman, W.A.; Owen, J.; Newman, R.B.; Skupski, D.W.; He, D.; et al. Relationship between gestational weight gain with fetal body composition and organ volumes in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Dimensional Study: A prospective pregnancy cohort. Am. J. Clin. Nutr. 2025, 121, 367–375. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2004, 36, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; di Bartolo, I.; Savasi, V.M.; Cetin, I. Micronutrient supplementation in pregnancy: Who, what and how much? Obstet. Med. 2019, 12, 5–13. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Tomova, G.D.; Arnold, K.F.; Gilthorpe, M.S.; Tennant, P.W.G. Adjustment for energy intake in nutritional research: A causal inference perspective. Am. J. Clin. Nutr. 2022, 115, 189–198. [Google Scholar] [CrossRef]
- Rothman, K.J. Curbing type I and type II errors. Eur. J. Epidemiol. 2010, 25, 223–224. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Dikgale, B.; Dlakavu, F.; Masenge, A.; De Gouveia, S.; Adam, S. Pregnant women’s dietary patterns and knowledge of gestational weight gain: A cross-sectional study. Int. J. Gynecol. Obstet. 2024, 166, 871–878. [Google Scholar] [CrossRef]
- Caut, C.; Leach, M.; Steel, A. Dietary guideline adherence during preconception and pregnancy: A systematic review. Matern. Child Nutr. 2020, 16, e12916. [Google Scholar] [CrossRef]
- Ferreira, L.B.; Lobo, C.V.; Miranda, A.; Carvalho, B.D.C.; Santos, L.C.D. Dietary Patterns during Pregnancy and Gestational Weight Gain: A Systematic Review. Rev. Bras. Ginecol. Obstet. 2022, 44, 540–547. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, X.; Yi, H.; Tang, S.; You, H. Determinants of excessive gestational weight gain: A systematic review and meta-analysis. Arch. Public Health 2022, 80, 129. [Google Scholar] [CrossRef]
- Montvignier Monnet, A.; Savoy, D.; Préaubert, L.; Hoffmann, P.; Bétry, C. In Underweight Women, Insufficient Gestational Weight Gain Is Associated with Adverse Obstetric Outcomes. Nutrients 2022, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013; Available online: https://www.taylorfrancis.com/books/mono/10.4324/9780203771587/statistical-power-analysis-behavioral-sciences-jacob-cohen (accessed on 13 August 2025).
- Radwan, H.; Hashim, M.; Hasan, H.; Abbas, N.; Obaid, R.R.S.; Al Ghazal, H.; Naja, F. Adherence to the Mediterranean diet during pregnancy is associated with lower odds of excessive gestational weight gain and postpartum weight retention: Results of the Mother-Infant Study Cohort. Br. J. Nutr. 2022, 128, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: A US cohort. J. Am. Diet Assoc. 2009, 109, 1004–1011. [Google Scholar] [CrossRef]
- Berube, L.T.; Deierlein, A.L.; Woolf, K.; Messito, M.J.; Gross, R.S. Prenatal Dietary Patterns and Associations with Weight-Related Pregnancy Outcomes in Hispanic Women with Low Incomes. Child. Obes. 2024, 20, 198–207. [Google Scholar] [CrossRef]
- Cacau, L.T.; Benseñor, I.M.; Goulart, A.C.; Cardoso, L.O.; Lotufo, P.A.; Moreno, L.A.; Marchioni, D.M. Adherence to the Planetary Health Diet Index and Obesity Indicators in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutrients 2021, 13, 3691. [Google Scholar] [CrossRef]
- Forbes, L.E.; Graham, J.E.; Berglund, C.; Bell, R.C. Dietary Change during Pregnancy and Women’s Reasons for Change. Nutrients 2018, 10, 1032. [Google Scholar] [CrossRef]
- Jin, J. Behavioral Interventions for Healthy Weight Gain During Pregnancy. JAMA 2021, 325, 2126. [Google Scholar] [CrossRef]
- Chen, L.; Dai, J.; Yu, G.; Pang, W.W.; Rahman, M.L.; Liu, X.; Fiehn, O.; Guivarch, C.; Chen, Z.; Zhang, C. Metabolomic Biomarkers of Dietary Approaches to Stop Hypertension (DASH) Dietary Patterns in Pregnant Women. Nutrients 2024, 16, 492. [Google Scholar] [CrossRef]
- Donat-Vargas, C.; Gea, A.; Sayon-Orea, C.; Carlos, S.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Association between dietary intakes of PCBs and the risk of obesity: The SUN project. J. Epidemiol. Community Health 2014, 68, 834. [Google Scholar] [CrossRef]
- Khoury, N.; Martínez, M.Á.; Paz-Graniel, I.; Martínez-González, M.Á.; Corella, D.; Castañer, O.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; Vioque, J.; et al. Dietary intake of polychlorinated dibenzo-p-dioxins and furans, adiposity and obesity status. Environ. Res. 2023, 227, 115697. [Google Scholar] [CrossRef] [PubMed]
- Chetrit, L.; Frenoy, P.; Artaud, F.; Marques, C.; Ren, X.; Severi, G.; Mancini, F.R. Evidence of a positive association between dietary exposure to polychlorinated biphenyl (PCB) and weight gain among women in the E3N prospective cohort. Sci. Total Environ. 2024, 957, 177587. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Landry, D.; Little, J.; Minelli, C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med. Res. Methodol. 2017, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Cuparencu, C.; Bulmuş-Tüccar, T.; Stanstrup, J.; La Barbera, G.; Roager, H.M.; Dragsted, L.O. Towards nutrition with precision: Unlocking biomarkers as dietary assessment tools. Nat. Metab. 2024, 6, 1438–1453. [Google Scholar] [CrossRef] [PubMed]
| Characteristic 1,3 | PHD | DASH | AMED | HEI | AHEI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | |
| N | 510 | 510 | 443 | 443 | 340 | 322 | 510 | 510 | 510 | 510 |
| Age (years) | ||||||||||
| Mean (SD) | 26.3 (5.5) | 29.6 (5.2) 2 | 25.9 (5.5) | 30.0 (4.9) 2 | 26.5 (5.7) | 30.0 (5.3) 2 | 25.8 (5.4) | 30.1 (4.9) 2 | 25.8 (5.4) | 30.5 (4.7) 2 |
| Currently paid jobs | ||||||||||
| None | 164 (32%) | 142 (28%) | 153 (35%) | 123 (28%) | 115 (34%) | 91 (28%) | 162 (32%) | 151 (30%) | 153 (30%) | 154 (30%) |
| At least one paid job | 346 (68%) | 368 (72%) | 290 (65%) | 320 (72%) | 225 (66%) | 231 (72%) | 348 (68%) | 359 (70%) | 357 (70%) | 356 (70%) |
| Diet-based vegetarianism (FFQ) | ||||||||||
| Full vegetarian (lacto-ovo-, pesco-, and semi-vegetarians) | 63 (12%) | 150 (30%) 2 | 86 (19%) | 96 (22%) | 104 (31%) | 47 (15%) 2 | 116 (23%) | 110 (22%) | 121 (24%) | 102 (20%) |
| Non-vegetarian | 447 (88%) | 356 (70%) | 357 (81%) | 345 (78%) | 236 (69%) | 274 (85%) | 392 (77%) | 398 (78%) | 387 (76%) | 407 (80%) |
| Education | ||||||||||
| Education less than high school-level | 73 (14%) | 29 (6%) 2 | 66 (15%) | 34 (8%) 2 | 48 (14%) | 27 (8%) 2 | 66 (13%) | 46 (9%) 2 | 60 (12%) | 44 (9%) 2 |
| High school diploma or equivalent | 142 (28%) | 73 (15%) | 129 (29%) | 62 (14%) | 92 (27%) | 41 (13%) | 142 (28%) | 70 (14%) | 144 (28%) | 64 (13%) |
| Some college/associate degree | 179 (35%) | 144 (28%) | 157 (35%) | 110 (25%) | 120 (35%) | 83 (26%) | 190 (37%) | 133 (26%) | 195 (38%) | 117 (23%) |
| Undergraduate/postgraduate degree | 116 (23%) | 264 (52%) | 91 (21%) | 237 (53%) | 80 (24%) | 170 (53%) | 112 (22%) | 261 (51%) | 111 (22%) | 285 (56%) |
| Health insurance | ||||||||||
| Private/managed care | 248 (49%) | 375 (74%) 2 | 210 (47%) | 313 (71%) 2 | 184 (54%) | 232 (72%) 2 | 242 (47%) | 357 (70%) 2 | 250 (49%) | 377 (74%) 2 |
| Other/Medicaid/Self-paid | 262 (51%) | 135 (26%) | 233 (53%) | 130 (29%) | 156 (46%) | 90 (28%) | 268 (53%) | 153 (30%) | 260 (51%) | 133 (26%) |
| Marital status | ||||||||||
| Married or living with a partner | 315 (62%) | 414 (81%) 2 | 259 (59%) | 373 (84%) 2 | 225 (66%) | 257 (80%) 2 | 302 (59%) | 430 (84%) 2 | 299 (59%) | 443 (87%) 2 |
| Not married | 194 (38%) | 96 (19%) | 183 (41%) | 70 (16%) | 114 (34%) | 65 (20%) | 207 (41%) | 80 (16%) | 210 (41%) | 67 (13%) |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 65 (13%) | 160 (31%) 2 | 52 (12%) | 154 (35%) 2 | 72 (21%) | 81 (25%) 2 | 73 (14%) | 135 (26%) 2 | 88 (17%) | 137 (27%) 2 |
| Non-Hispanic Black | 241 (47%) | 80 (16%) | 221 (50%) | 66 (15%) | 134 (39%) | 74 (23%) | 257 (50%) | 84 (16%) | 253 (50%) | 73 (14%) |
| Hispanic | 144 (28%) | 138 (27%) | 102 (23%) | 135 (30%) | 96 (28%) | 83 (26%) | 119 (23%) | 165 (32%) | 125 (25%) | 140 (27%) |
| Asian/Pacific Islander | 60 (12%) | 132 (26%) | 68 (15%) | 88 (20%) | 38 (11%) | 84 (26%) | 61 (12%) | 126 (25%) | 44 (9%) | 160 (31%) |
| Parity (number of births) | ||||||||||
| 0 | 218 (43%) | 251 (49%) 2 | 200 (45%) | 211 (48%) | 144 (42%) | 157 (49%) | 245 (48%) | 239 (47%) | 238 (47%) | 232 (45%) |
| 1 | 178 (35%) | 183 (36%) | 150 (34%) | 159 (36%) | 130 (38%) | 114 (35%) | 165 (32%) | 180 (35%) | 174 (34%) | 189 (37%) |
| 2 or more | 114 (22%) | 76 (15%) | 93 (21%) | 73 (16%) | 66 (19%) | 51 (16%) | 100 (20%) | 91 (18%) | 98 (19%) | 89 (17%) |
| Pre-pregnancy BMI (kg/m2) | ||||||||||
| Mean (SD) | 25.9 (5.3) | 24.4 (4.6) 2 | 25.8 (5.4) | 24.6 (4.4)2 | 26.2 (5.2) | 24.0 (4.1) 2 | 25.8 (5.3) | 24.7 (4.6) 2 | 26.0 (5.3) | 24.4 (4.4) 2 |
| 19 to <25.0 (normal) | 272 (53%) | 337 (66%) 2 | 233 (53%) | 286 (65%) 2 | 167 (49%) | 222 (69%) 2 | 271 (53%) | 325 (64%) 2 | 258 (51%) | 338 (66%) 2 |
| ≥25.0 to <30.0 (overweight) | 142 (28%) | 112 (22%) | 122 (28%) | 109 (25%) | 97 (29%) | 76 (24%) | 142 (28%) | 127 (25%) | 151 (30%) | 116 (23%) |
| ≥30.0 (obese) | 96 (19%) | 61 (12%) | 88 (20%) | 48 (11%) | 76 (22%) | 24 (7%) | 97 (19%) | 58 (11%) | 101 (20%) | 56 (11%) |
| Self-defined vegetarianism (FFQ) | ||||||||||
| Non-vegetarian | 485 (97%) | 451 (91%) 2 | 423 (98%) | 379 (88%) 2 | 327 (98%) | 283 (89%) 2 | 484 (97%) | 452 (90%) 2 | 481 (97%) | 454 (90%) 2 |
| Vegetarian | 16 (3%) | 45 (9%) | 9 (2%) | 54 (12%) | 5 (2%) | 35 (11%) | 16 (3%) | 48 (10%) | 17 (3%) | 50 (10%) |
| Total physical activity (MET-min/week) | ||||||||||
| Median (p25, p75) | 312 (209, 418) | 274 (212, 378) 2 | 295 (204, 388) | 292 (225, 394) | 295 (201, 416) | 294 (225, 400) | 304 (205, 417) | 279 (214, 384) | 306 (206, 407) | 275 (214, 378) |
| Energy (kcal/day), mean (SD) | 2595 (1224) | 1846 (730) 2 | 2182 (1122) | 2271 (919) 2 | 1661 (804) | 2554 (989) 2 | 2361 (1185) | 2006 (837) 2 | 2169 (1158) | 2121 (864) |
| Macronutrients, mean (SD) | ||||||||||
| Carbohydrates (g/day), | 342.6 (190.9) | 253.2 (112.4) 2 | 291.9 (183.4) | 312.1 (134.1) 2 | 224 (132) | 338 (142) 2 | 323.3 (190.7) | 268.7 (123.9) 2 | 303.1 (190.0) | 275.1 (125.1) 2 |
| Protein (g/day), | 101.3 (48.1) | 70.6 (30.1) 2 | 78.1 (38.8) | 93.5 (41.6) 2 | 59.9 (26.6) | 107.0 (45.0) 2 | 82.9 (43.5) | 84.3 (38.3) | 75.3 (40.1) | 90.7 (39.7) 2 |
| Total fat (g/day), | 95.6 (49.0) | 66.6 (30.5) 2 | 81.5 (44.0) | 79.0 (35.4) 2 | 61.4 (33.0) | 92.6 (40.8) 2 | 85.7 (46.9) | 72.0 (32.4) 2 | 76.7 (44.5) | 78.9 (34.2) |
| Monounsaturated fat (g/day), | 36.0 (18.9) | 26.6 (13.3) 2 | 30.9 (16.8) | 31.0 (14.5) 2 | 22.6 (12.2) | 37.1 (16.7) 2 | 32.1 (17.8) | 29.0 (14.3) 2 | 28.8 (16.9) | 31.5 (14.6) 2 |
| Polyunsaturated fat (g/day), | 19.8 (10.9) | 14.7 (8.0) 2 | 17.2 (10.2) | 17.0 (7.7) | 12.5 (7.8) | 20.4 (8.8) 2 | 17.3 (10.2) | 16.0 (7.5) 2 | 15.5 (9.8) | 17.6 (7.9) 2 |
| Saturated fat (g/day), | 32.3 (17.2) | 19.9 (9.1) 2 | 27.3 (15.1) | 24.6 (12.5) 2 | 21.6 (12.0) | 27.7 (13.7) 2 | 29.7 (16.9) | 21.3 (9.9) 2 | 26.5 (15.7) | 23.4 (11.1) 2 |
| Dietary fiber (g/day), | 22.1 (13.9) | 22.9 (11.0) | 16.2 (9.0) | 29.2 (13.2) 2 | 13.1 (6.8) | 31.5 (13.6) 2 | 18.1 (10.8) | 25.9 (13.3) 2 | 16.3 (9.6) | 26.8 (13.1) 2 |
| Micronutrients, mean (SD) | ||||||||||
| Iron (mg/day) | 19.6 (10.0) | 16.8 (7.7) 2 | 15.7 (8.1) | 20.9 (9.3) 2 | 12.2 (5.3) | 23.4 (9.6) 2 | 17.6 (9.3) | 18.3 (8.7) | 16.1 (8.7) | 19.3 (9.2) 2 |
| Calcium (mg/day) | 1123.1 (677.9) | 876.2 (407.1) 2 | 801.0 (450.0) | 1255.8 (666.6) 2 | 732.1 (401.2) | 1217.3 (552.7) 2 | 940.4 (591.7) | 1049.5 (564.9) 2 | 888.7 (540.6) | 1066.6 (565.6) 2 |
| Total folate (dietary equivalent) (mcg/day) | 678.4 (364.7) | 595.7 (277.7) 2 | 543.3 (294.9) | 739.2 (346.2) 2 | 430.5 (207.4) | 812.2 (351.2) 2 | 612.8 (334.3) | 640.2 (314.8) | 558.3 (310.0) | 678.1 (333.6) 2 |
| Vitamin A (mcg of retinol equivalent/day) | 1516.6 (1114.8) | 1539.1 (998.6) | 1055.2 (737.3) | 2039.7 (1168.1) 2 | 893.0 (663.2) | 2227.8 (1226.4) 2 | 1163.9 (858.9) | 1863.2 (1185.1) 2 | 1062.2 (720.3) | 1953.0 (1267.2) 2 |
| Vitamin D (calciferol) (mcg/day) | 7.2 (4.8) | 4.5 (2.9) 2 | 4.9 (3.5) | 7.1 (5.0) 2 | 4.0 (3.0) | 7.5 (4.3) 2 | 5.1 (4.1) | 6.3 (4.1) 2 | 4.8 (3.8) | 6.6 (4.2) 2 |
| Gestational weight gain (kg) | ||||||||||
| Total GWG, median (p25, p75) | 11.7 (7.7, 15.6) | 12.6 (9.4, 16.0) 2 | 11.0 (6.8, 15.3) | 12.8 (9.7, 16.6) 2 | 11.5 (7.3, 16.1) | 12.9 (9.2, 16.7) 2 | 11.6 (7.7, 15.6) | 12.5 (9.5, 16.2) 2 | 11.5 (7.8, 16.1) | 12.8 (9.2, 16.3) 2 |
| Women with adequate GWG | ||||||||||
| Total | 158 (32.6%) | 171 (35.3%) | 128 (26.4%) | 156 (32.2%) 2 | 95 (19.6%) | 103 (21.2%) | 144 (29.7%) | 178 (36.7%) | 142 (29.3%) | 171 (35.3%) |
| 1st trimester | 166 (31.0%) | 185 (34.6%) 2 | 147 (27.5%) | 159 (29.7%) 2 | 107 (20.0%) | 117 (21.9%) 2 | 165 (30.8%) | 194 (36.3%) 2 | 174 (32.5%) | 193 (36.1%) 2 |
| 2nd trimester | 134 (30.1%) | 169 (38.0%) 2 | 111 (24.9%) | 152 (34.2%) 2 | 76 (17.1%) | 108 (24.3%) 2 | 136 (30.6%) | 169 (38.0%) | 123 (27.6%) | 168 (37.8%) 2 |
| 3rd trimester | 186 (30.9%) | 222 (36.9%) | 158 (26.3%) | 191 (31.8%) 2 | 113 (18.8%) | 139 (23.1%) | 186 (30.9%) | 223 (37.1%) | 172 (28.6%) | 228 (37.9%) 2 |
| Diet Index | Odds Ratios (95% Confidence Intervals) for Tertile 3 (High) vs. Tertile 1 (Low) 1 | |
|---|---|---|
| Inadequate vs. Adequate GWG | Excessive vs. Adequate GWG | |
| Full cohort (N = 1530) | ||
| PHD | 1.11 (0.80, 1.55) | 1.06 (0.76, 1.47) |
| DASH | 0.69 (0.48, 0.99) 2 | 1.17 (0.82, 1.67) |
| AMED | 0.87 (0.58, 1.31) | 1.10 (0.74, 1.62) |
| HEI | 0.78 (0.56, 1.10) | 0.95 (0.68, 1.32) |
| AHEI | 0.81 (0.57, 1.15) | 1.09 (0.78, 1.52) |
| Normal weight (N = 881) | ||
| PHD | 1.17 (0.78, 1.76) | 1.13 (0.70, 1.84) |
| DASH | 0.83 (0.53, 1.30) | 1.20 (0.70, 2.05) |
| AMED | 0.87 (0.53, 1.43) | 1.14 (0.65, 1.99) |
| HEI | 0.81 (0.53, 1.24) | 1.00 (0.61, 1.62) |
| AHEI | 0.82 (0.53, 1.27) | 1.14 (0.69, 1.87) |
| Overweight (N = 403) | ||
| PHD | 1.13 (0.48, 2.69) | 1.52 (0.83, 2.81) |
| DASH | 0.23 (0.08, 0.63) 2 | 1.01 (0.53, 1.91) |
| AMED | 0.91 (0.30, 2.70) | 1.67 (0.78, 3.56) |
| HEI | 0.45 (0.18, 1.10) | 0.93 (0.51, 1.72) |
| AHEI | 0.61 (0.24, 1.55) | 1.25 (0.67, 2.30) |
| Obese (N = 246) | ||
| PHD | 0.69 (0.25, 1.91) | 0.85 (0.35, 2.04) |
| DASH | 0.68 (0.20, 2.25) | 2.82 (1.02, 7.82) 2 |
| AMED | 0.68 (0.16, 2.94) | 1.33 (0.38, 4.66) |
| HEI | 0.94 (0.34, 2.60) | 1.25 (0.52, 3.01) |
| AHEI | 0.57 (0.20, 1.61) | 1.11 (0.46, 2.67) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.-X.; Wadhawan, S.; DeVilbiss, E.A.; Clayton, P.K.; Wagner, K.A.; Gleason, J.L.; Chen, Z.; Zhang, C.; Grantz, K.L.; Grewal, J. Maternal Adherence to Healthy Dietary Patterns During Pregnancy and Gestational Weight Gain. Nutrients 2025, 17, 2707. https://doi.org/10.3390/nu17162707
Lim S-X, Wadhawan S, DeVilbiss EA, Clayton PK, Wagner KA, Gleason JL, Chen Z, Zhang C, Grantz KL, Grewal J. Maternal Adherence to Healthy Dietary Patterns During Pregnancy and Gestational Weight Gain. Nutrients. 2025; 17(16):2707. https://doi.org/10.3390/nu17162707
Chicago/Turabian StyleLim, Shan-Xuan, Siona Wadhawan, Elizabeth A. DeVilbiss, Priscilla K. Clayton, Kathryn A. Wagner, Jessica L. Gleason, Zhen Chen, Cuilin Zhang, Katherine L. Grantz, and Jagteshwar Grewal. 2025. "Maternal Adherence to Healthy Dietary Patterns During Pregnancy and Gestational Weight Gain" Nutrients 17, no. 16: 2707. https://doi.org/10.3390/nu17162707
APA StyleLim, S.-X., Wadhawan, S., DeVilbiss, E. A., Clayton, P. K., Wagner, K. A., Gleason, J. L., Chen, Z., Zhang, C., Grantz, K. L., & Grewal, J. (2025). Maternal Adherence to Healthy Dietary Patterns During Pregnancy and Gestational Weight Gain. Nutrients, 17(16), 2707. https://doi.org/10.3390/nu17162707








