Dietary Iron Intake Impacts the Microbial Composition of the Murine Intestinal and Lung Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Iron Concentration Measurements
2.3. Microbiome Analysis
2.4. Data Handling
2.5. Statistical Analysis
3. Results
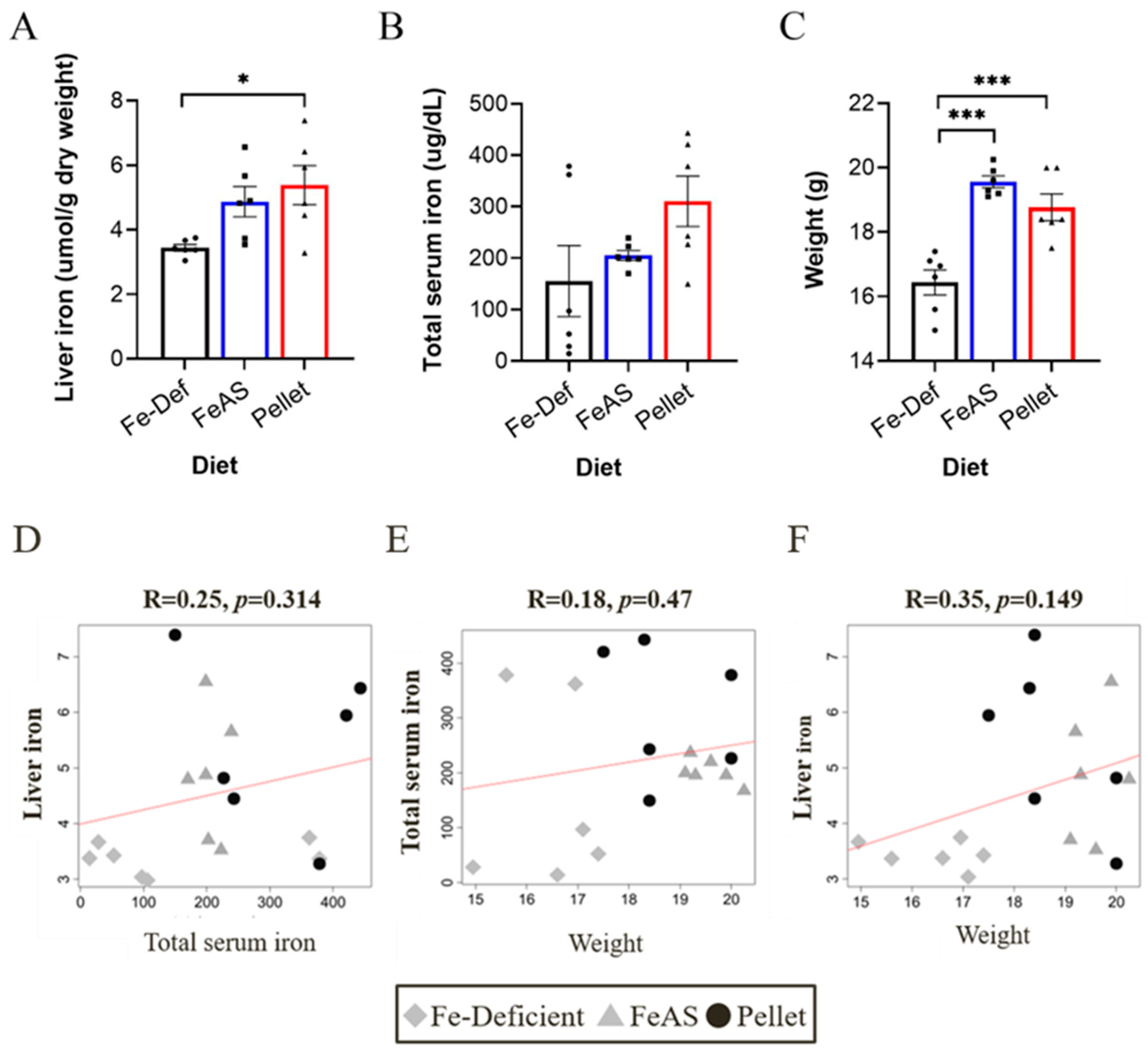
3.1. Iron Status and Weight
3.2. Iron Supplementation Has a Small Effect on the Microbiome of the Lung
3.3. Iron Supplementation Significantly Changes the Microbiome of the Duodenum
3.4. Iron Supplementation Significantly Changes the Microbiome of the Colon
3.5. Changes in Microbial Taxa Between Diets in the Lung, Duodenum and Colon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Cote d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Tompkins, G.R.; O’dell, N.L.; Bryson, I.T.; Pennington, C.B. The effects of dietary ferric iron and iron deprivation on the bacterial composition of the mouse intestine. Curr. Microbiol. 2001, 43, 38–42. [Google Scholar] [CrossRef]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutri. 2012, 142, 271–277. [Google Scholar] [CrossRef]
- Zakrzewski, M.A.-O.; Wilkins, S.A.-O.; Helman, S.A.-O.; Brilli, E.A.-O.; Tarantino, G.A.-O.; Anderson, G.A.-O.; Frazer, D.A.-O. Supplementation with Sucrosomial® iron leads to favourable changes in the intestinal microbiome when compared to ferrous sulfate in mice. Biometals 2022, 35, 27–38. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12, S150–S156. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Reid, D.W.; Lam, Q.T.; Schneider, H.; Walters, E.H. Airway iron and iron-regulatory cytokines in cystic fibrosis. Eur. Respir. J. 2004, 24, 286–291. [Google Scholar] [CrossRef]
- Ghio, A.J.; Roggli, V.L.; Soukup, J.M.; Richards, J.H.; Randell, S.H.; Muhlebach, M.S. Iron accumulates in the lavage and explanted lungs of cystic fibrosis patients. J. Cyst. Fibros. 2013, 12, 390–398. [Google Scholar] [CrossRef]
- Ali, M.A.-O.; Kim, R.Y.; Brown, A.C.; Donovan, C.; Vanka, K.S.; Mayall, J.R.; Liu, G.A.-O.; Pillar, A.L.; Jones-Freeman, B.; Xenaki, D.; et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J. Pathol. 2020, 251, 49–62. [Google Scholar] [CrossRef]
- Ali, M.K.; Kim, R.Y.; Brown, A.C.; Mayall, J.R.; Karim, R.; Pinkerton, J.W.; Liu, G.A.-O.; Martin, K.L.; Starkey, M.R.; Pillar, A.L.; et al. Crucial role for lung iron level and regulation in the pathogenesis and severity of asthma. Eur. Respir. J. 2020, 55, 1901340. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Braat, S.; Hasan, M.I.; Bennett, C.; Barrios, M.; Jones, N.; Abdul Azeez, I.; Wilcox, S.; Roy, P.K.; Bhuiyan, M.S.A.; et al. Effects of iron supplements and iron-containing micronutrient powders on the gut microbiome in Bangladeshi infants: A randomized controlled trial. Nat. Commun. 2024, 15, 8640. [Google Scholar] [CrossRef]
- Celis, A.I.; Relman, D.A.; Huang, K.C. The impact of iron and heme availability on the healthy human gut microbiome in vivo and in vitro. Cell Chem. Biol. 2023, 30, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Coe, G.L.; Pinkham, N.V.; Celis, A.I.; Johnson, C.; DuBois, J.L.; Walk, S.A.-O. Dynamic Gut Microbiome Changes in Response to Low-Iron Challenge. Appl. Environ. Microbiol. 2021, 87, e02307-20. [Google Scholar] [CrossRef]
- Elms, L.; Hand, B.; Skubisz, M.; Best, K.P.; Grzeskowiak, L.E.; Rogers, G.B.; Green, T.J.; Taylor, S.L. The Effect of Iron Supplements on the Gut Microbiome of Females of Reproductive Age: A Randomized Controlled Trial. J. Nutr. 2024, 154, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Kortman, G.A.M.; Reijnders, D.; Swinkels, D.A.-O. Oral iron supplementation: Potential implications for the gut microbiome and metabolome in patients with CKD. Hemodial. Int. 2017, 21, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- La Carpia, F.; Wojczyk, B.S.; Annavajhala, M.K.; Rebbaa, A.; Culp-Hill, R.; D’Alessandro, A.; Freedberg, D.E.; Uhlemann, A.C.; Hod, E.A. Transfusional iron overload and intravenous iron infusions modify the mouse gut microbiota similarly to dietary iron. NPJ Biofilms Microbiomes 2019, 5, 26. [Google Scholar] [CrossRef]
- Liu, H.; Wu, W.; Luo, Y.A.-O. Oral and intravenous iron treatment alter the gut microbiome differentially in dialysis patients. Int. Urol. Nephrol. 2023, 55, 759–767. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef]
- Parmanand, B.A.; Kellingray, L.; Le Gall, G.; Basit, A.W.; Fairweather-Tait, S.; Narbad, A. A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J. Nutr. Biochem. 2019, 67, 20–27. [Google Scholar] [CrossRef]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency-A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef]
- Gifford, A.H.; Alexandru, D.M.; Li, Z.; Dorman, D.B.; Moulton, L.A.; Price, K.E.; Hampton, T.H.; Sogin, M.L.; Zuckerman, J.B.; Parker, H.W.; et al. Iron supplementation does not worsen respiratory health or alter the sputum microbiome in cystic fibrosis. J. Cyst. Fibros. 2014, 13, 311–318. [Google Scholar] [CrossRef]
- Noordine, M.L.; Seyoum, Y.; Bruneau, A.; Baye, K.; Lefebvre, T.; Cherbuy, C.A.-O.; Canonne-Hergaux, F.; Nicolas, G.A.-O.; Humblot, C.A.-O.; Thomas, M.A.-O. The microbiota and the host organism switch between cooperation and competition based on dietary iron levels. Gut Microbes 2024, 16, 2361660. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.; Munoz-Wolf, N.; Strydom, J.; Faherty, L.; Williams, N.C.; Kenny, S.; Donnelly, S.C.; Cloonan, S.M. Nutritional immunity: The impact of metals on lung immune cells and the airway microbiome during chronic respiratory disease. Respir. Res. 2021, 22, 133. [Google Scholar] [CrossRef]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Singer, B.H.; Newstead, M.W.; Falkowski, N.R.; Erb-Downward, J.R.; Standiford, T.J.; Huffnagle, G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016, 1, 16113. [Google Scholar] [CrossRef]
- Eladham, M.W.; Selvakumar, B.; Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Ibrahim, S.M.; Halwani, R. Unraveling the gut-Lung axis: Exploring complex mechanisms in disease interplay. Heliyon 2024, 10, e24032. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Wilkins, S.J.; Darshan, D.; Mirciov, C.S.G.; Dunn, L.A.; Anderson, G.J. Ferroportin Is Essential for Iron Absorption During Suckling, But Is Hyporesponsive to the Regulatory Hormone Hepcidin. Cell. Mol. Gastroenterol. Hepatol. 2016, 3, 410–421. [Google Scholar] [CrossRef]
- Mirciov, C.S.G.; Wilkins, S.J.; Dunn, L.A.; Anderson, G.J.; Frazer, D.M. Characterization of Putative Erythroid Regulators of Hepcidin in Mouse Models of Anemia. PLoS ONE 2017, 12, e0171054. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.E.; Sun, Y.; Wolcott, R.D.; Domingo, A.; Carroll, J.A. Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: Bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathog. Dis. 2008, 5, 459–472. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Srinivas, G.; Kuenzel, S.; Linnenbrink, M.; Alnahas, S.; Bruce, K.D.; Steinhoff, U.; Baines, J.F.; Schaible, U.E. Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS ONE 2014, 9, e113466. [Google Scholar] [CrossRef]
- Scheiermann, J.; Klinman, D.M. Three distinct pneumotypes characterize the microbiome of the lung in BALB/cJ mice. PLoS ONE 2017, 12, e0180561. [Google Scholar] [CrossRef]
- Barfod, K.K.; Roggenbuck, M.; Hansen, L.H.; Schjorring, S.; Larsen, S.T.; Sorensen, S.J.; Krogfelt, K.A. The murine lung microbiome in relation to the intestinal and vaginal bacterial communities. BMC Microbiol. 2013, 13, 303. [Google Scholar] [CrossRef]
- Wang, K.; Wu, W.; Jiang, X.; Xia, J.; Lv, L.; Li, S.; Zhuge, A.; Wu, Z.; Wang, Q.; Wang, S.; et al. Multi-Omics Analysis Reveals the Protection of Gasdermin D in Concanavalin A-Induced Autoimmune Hepatitis. Microbiol. Spectr. 2022, 10, e0171722. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, S.; Zhang, X.; Lin, Y.; Liu, J.; Cheng, W.; Zeng, Y. The role of the microbiota and metabolites in the treatment of pulmonary fibrosis with UC-MSCs: Integrating fecal metabolomics and 16S rDNA analysis. PLoS ONE 2025, 20, e0313989. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, X.; Guo, J.; Han, Y.; You, J.; Tian, Z.; Zheng, X.; Zheng, S.; Ling, Y.; Pei, L.; et al. Triangle correlations of lung microbiome, host physiology and gut microbiome in a rat model of idiopathic pulmonary fibrosis. Sci. Rep. 2024, 14, 28743. [Google Scholar] [CrossRef]
- Sverrild, A.; Kiilerich, P.; Brejnrod, A.; Pedersen, R.; Porsbjerg, C.; Bergqvist, A.; Erjefalt, J.S.; Kristiansen, K.; Backer, V. Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J. Allergy Clin. Immunol. 2017, 140, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Philippot, Q.; Deslée, G.; Adair-Kirk, T.L.; Woods, J.C.; Byers, D.; Conradi, S.; Dury, S.; Perotin, J.M.; Lebargy, F.; Cassan, C.; et al. Increased Iron Sequestration in Alveolar Macrophages in Chronic Obtructive Pulmonary Disease. PLoS ONE 2014, 9, e96285. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, S.M.; Mumby, S.; Adcock, I.M.; Choi, A.M.K.; Chung, K.F.; Quinlan, G.J. The “Iron”-y of Iron Overload and Iron Deficiency in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 1103–1112. [Google Scholar] [CrossRef]
- Maniam, P.A.-O.; Essilfie, A.T.; Kalimutho, M.; Ling, D.; Frazer, D.M.; Phipps, S.; Anderson, G.J.; Reid, D.W. Increased susceptibility of cystic fibrosis airway epithelial cells to ferroptosis. Biol. Res. 2021, 54, 38. [Google Scholar] [CrossRef]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T.; et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019, 10, 3145. [Google Scholar] [CrossRef]
- Reid, D.W.; Carroll, V.; May, C.; Champion, A.; Kirov, S.M. Increased airway iron as a potential factor in the persistence of Pseudomonas aeruginosa infection in cystic fibrosis. Eur. Respir. J. 2007, 30, 286. [Google Scholar] [CrossRef]
- Smith, D.J.; Lamont, I.L.; Anderson, G.J.; Reid, D.W. Targeting iron uptake to control Pseudomonas aeruginosa infections in cystic fibrosis. Eur. Respir. J. 2013, 42, 1723. [Google Scholar] [CrossRef]
- Ghio, A.J.; Hilborn, E.D.; Stonehuerner, J.G.; Dailey, L.A.; Carter, J.D.; Richards, J.H.; Crissman, K.M.; Foronjy, R.F.; Uyeminami, D.L.; Pinkerton, K.E. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am. J. Respir. Crit. Care Med. 2008, 178, 1130–1138. [Google Scholar] [CrossRef]
- Hoo, Z.H.; Wildman, M.J. Intravenous iron among cystic fibrosis patients. J. Cyst. Fibros. 2012, 11, 560–562. [Google Scholar] [CrossRef]
- Norkina, O.; Burnett, T.G.; De Lisle, R.C. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect. Immun. 2004, 72, 6040–6049. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.G.; Fouhy, F.; Harrison, M.J.; Rea, M.C.; Cotter, P.D.; O’Sullivan, O.; Stanton, C.; Hill, C.; Shanahan, F.; Plant, B.J.; et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017, 17, 58. [Google Scholar] [PubMed]
- Wrigley-Carr, H.E.; van Dorst, J.M.; Ooi, C.Y. Intestinal dysbiosis and inflammation in cystic fibrosis impacts gut and multi-organ axes. Med. Microecol. 2022, 13, 100057. [Google Scholar] [CrossRef]
- Hogan, D.A.; Willger, S.D.; Dolben, E.L.; Hampton, T.H.; Stanton, B.A.; Morrison, H.G.; Sogin, M.L.; Czum, J.; Ashare, A. Analysis of Lung Microbiota in Bronchoalveolar Lavage, Protected Brush and Sputum Samples from Subjects with Mild-To-Moderate Cystic Fibrosis Lung Disease. PLoS ONE 2016, 11, e0149998. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, Z.; Zhang, X.; Huang, P.; Xie, J.; Jiang, Q.; Zhang, Q.; Chung, K.F. Altered gut microbiome compositions are associated with the severity of asthma. J. Thorac. Dis. 2021, 13, 4322–4338. [Google Scholar] [CrossRef]
- McCauley, K.; Durack, J.; Valladares, R.; Fadrosh, D.W.; Lin, D.L.; Calatroni, A.; LeBeau, P.K.; Tran, H.T.; Fujimura, K.E.; LaMere, B.; et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J. Allergy Clin. Immunol. 2019, 144, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Nelson, C.E.; Brodie, E.L.; Desantis, T.Z.; Baek, M.S.; Liu, J.; Woyke, T.; Allgaier, M.; Bristow, J.; Wiener-Kronish, J.P.; et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011, 127, 372–381.e3. [Google Scholar] [CrossRef]
- Bowerman, K.L.; Rehman, S.F.; Vaughan, A.; Lachner, N.; Budden, K.F.; Kim, R.Y.; Wood, D.L.A.; Gellatly, S.L.; Shukla, S.D.; Wood, L.G.; et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 2020, 11, 5886. [Google Scholar] [CrossRef]
- Einarsson, G.G.; Comer, D.M.; McIlreavey, L.; Parkhill, J.; Ennis, M.; Tunney, M.M.; Elborn, J.S. Community dynamics and the lower airway microbiota in stable chronic obstructive pulmonary disease, smokers and healthy non-smokers. Thorax 2016, 71, 795–803. [Google Scholar] [CrossRef]
- Garcia-Nuñez, M.; Millares, L.; Pomares, X.; Ferrari, R.; Pérez-Brocal, V.; Gallego, M.; Espasa, M.; Moya, A.; Monsó, E. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J. Clin. Microbiol. 2014, 52, 4217–4223. [Google Scholar] [CrossRef]
- Millares, L.; Pascual, S.; Montón, C.; García-Núñez, M.; Lalmolda, C.; Faner, R.; Casadevall, C.; Setó, L.; Capilla, S.; Moreno, A.; et al. Relationship between the respiratory microbiome and the severity of airflow limitation, history of exacerbations and circulating eosinophils in COPD patients. BMC Pulm. Med. 2019, 19, 112. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Ellermann, M.; Arthur, J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic. Biol. Med. 2017, 105, 68–78. [Google Scholar] [CrossRef]
- Johansson, M.E.; Jakobsson, H.E.; Holmén-Larsson, J.; Schütte, A.; Ermund, A.; Rodríguez-Piñeiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Gruss, A.; Borezée-Durant, E.; Lechardeur, D. Environmental heme utilization by heme-auxotrophic bacteria. Adv. Microb. Physiol. 2012, 61, 69–124. [Google Scholar] [PubMed]
- Arboleya, S.; Stanton, C.; Ryan, C.A.; Dempsey, E.; Ross, P.R. Bosom Buddies: The Symbiotic Relationship Between Infants and Bifidobacterium longum ssp. longum and ssp. infantis. Genetic and Probiotic Features. Annu. Rev. Food Sci. Technol. 2016, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 15, 925. [Google Scholar] [CrossRef]
- Tang, J.; Wei, Y.; Pi, C.; Zheng, W.; Zuo, Y.; Shi, P.; Chen, J.; Xiong, L.; Chen, T.; Liu, H.; et al. The therapeutic value of bifidobacteria in cardiovascular disease. Npj Biofilms Microbiomes 2023, 9, 82. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J.-z. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; López, P.; Donado, C.D.; Cuervo, A.; González, S.; Suárez, A.; Turroni, F.; Gueimonde, M.; Ventura, M.; et al. Allergic Patients with Long-Term Asthma Display Low Levels of Bifidobacterium adolescentis. PLoS ONE 2016, 11, e0147809. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. Methodological issues in the study of intestinal microbiota in irritable bowel syndrome. World J. Gastroenterol. WJG 2014, 20, 8821. [Google Scholar]
- Duytschaever, G.; Huys, G.; Bekaert, M.; Boulanger, L.; De Boeck, K.; Vandamme, P. Dysbiosis of bifidobacteria and Clostridium cluster XIVa in the cystic fibrosis fecal microbiota. J. Cyst. Fibros. 2013, 12, 206–215. [Google Scholar] [CrossRef]
- Werner, T.; Wagner, S.J.; Martinez, I.; Walter, J.; Chang, J.S.; Clavel, T.; Kisling, S.; Schuemann, K.; Haller, D. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut 2011, 60, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, D.; Sun, Z.; Chen, X. Effects of intestinal Desulfovibrio bacteria on host health and its potential regulatory strategies: A review. Microbiol. Res. 2024, 284, 127725. [Google Scholar] [CrossRef]
- Ding, H.; Yu, X.; Chen, L.; Han, J.; Zhao, Y.; Feng, J. Tolerable upper intake level of iron damages the intestine and alters the intestinal flora in weaned piglets. Metallomics 2020, 12, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Xue, J.; Stevens, M.; Thomas, L.; White, J.R.; Dominguez Rieg, J.A. Intravenous ferric carboxymaltose and ferric derisomaltose alter the intestinal microbiome in female iron-deficient anemic mice. Biosci. Rep. 2023, 43, BSR20231217. [Google Scholar] [CrossRef]
- van den Bogert, B.; Erkus, O.; Boekhorst, J.; de Goffau, M.; Smid, E.J.; Zoetendal, E.G.; Kleerebezem, M. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol. Ecol. 2013, 85, 376–388. [Google Scholar] [CrossRef]
- Council, S.E. Prevotella melaninogenica, an Oral Anaerobic Bacterium, Prevalent in Cystic Fibrosis Chronic Lung Infection; University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2013. [Google Scholar]
- Althani, A.A.; Marei, H.E.; Hamdi, W.S.; Nasrallah, G.K.; El Zowalaty, M.E.; Al Khodor, S.; Al-Asmakh, M.; Abdel-Aziz, H.; Cenciarelli, C. Human Microbiome and its Association With Health and Diseases. J. Cell Physiol. 2016, 231, 1688–1694. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef]
- Comini, L.; Pasini, E.; Porta, R.; Olivares, A.; Testa, C.; Scalvini, S.; Vitacca, M. Dysbiosis and leaky gut in hyper-inflated COPD patients: Have smoking and exercise training any role? Respir. Med. Res. 2023, 83, 100995. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Xia, Y.; Sun, J. Salmonella infection upregulates the leaky protein claudin-2 in intestinal epithelial cells. PLoS ONE 2013, 8, e58606. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essilfie, A.-T.; Smith, A.; Watts, R.; Maniam, P.; Lamont, I.L.; Frazer, D.M.; Anderson, G.J.; Reid, D.W. Dietary Iron Intake Impacts the Microbial Composition of the Murine Intestinal and Lung Microbiome. Nutrients 2025, 17, 2696. https://doi.org/10.3390/nu17162696
Essilfie A-T, Smith A, Watts R, Maniam P, Lamont IL, Frazer DM, Anderson GJ, Reid DW. Dietary Iron Intake Impacts the Microbial Composition of the Murine Intestinal and Lung Microbiome. Nutrients. 2025; 17(16):2696. https://doi.org/10.3390/nu17162696
Chicago/Turabian StyleEssilfie, Ama-Tawiah, Alison Smith, Rebecca Watts, Pramila Maniam, Iain L. Lamont, David M. Frazer, Gregory J. Anderson, and David W. Reid. 2025. "Dietary Iron Intake Impacts the Microbial Composition of the Murine Intestinal and Lung Microbiome" Nutrients 17, no. 16: 2696. https://doi.org/10.3390/nu17162696
APA StyleEssilfie, A.-T., Smith, A., Watts, R., Maniam, P., Lamont, I. L., Frazer, D. M., Anderson, G. J., & Reid, D. W. (2025). Dietary Iron Intake Impacts the Microbial Composition of the Murine Intestinal and Lung Microbiome. Nutrients, 17(16), 2696. https://doi.org/10.3390/nu17162696







