The Effect of Nutrition Impact Symptoms on Nutrition Status After Completion of Curative-Intent Treatment for Gastric, Oesophageal, and Pancreatic Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
2.4. Result Synthesis
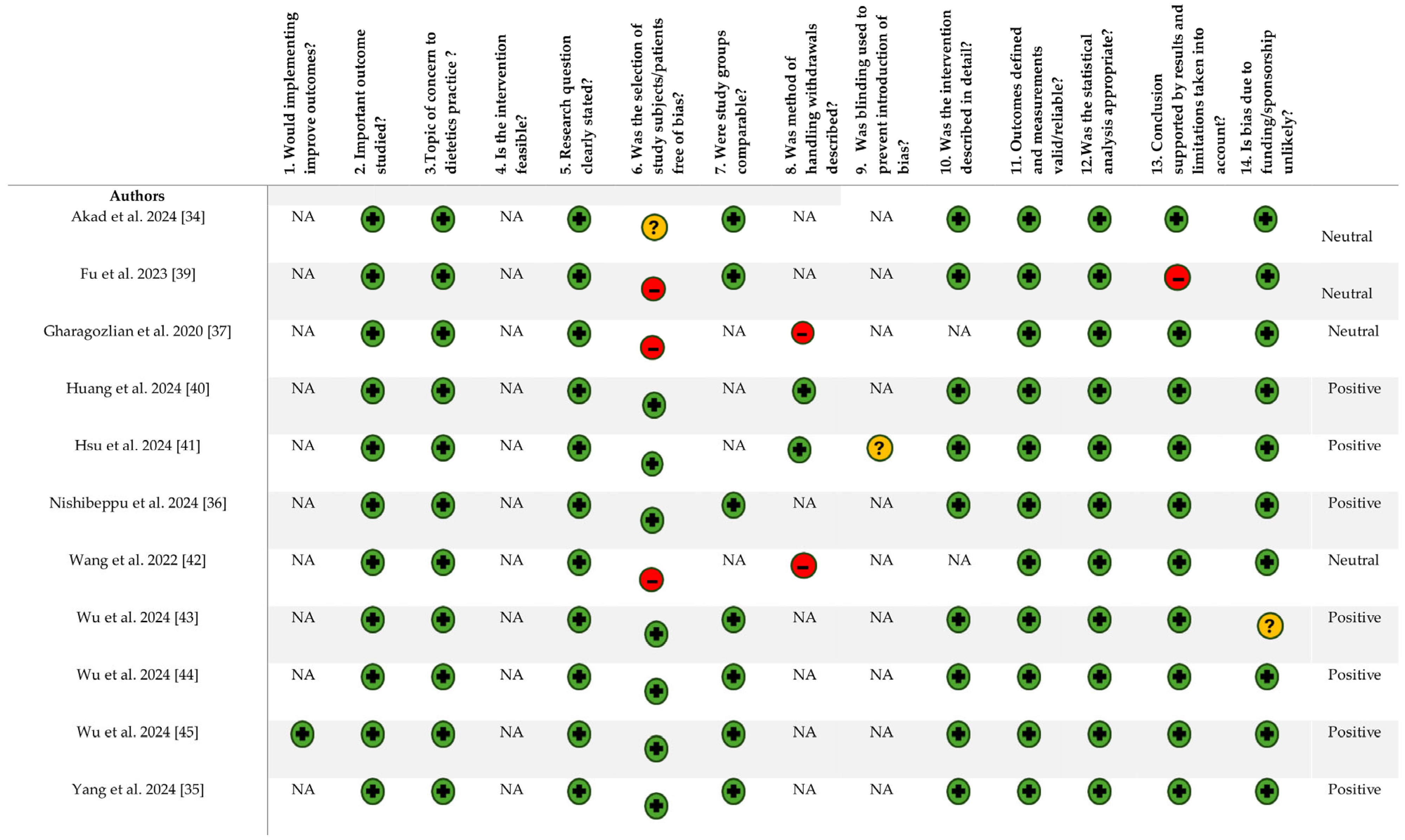
2.5. Quality Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Nutrition Impact Symptoms
3.5. Reflux
3.6. Abdominal Pain
3.7. Diarrhoea
3.8. Constipation
3.9. Dysphagia
3.10. Fatigue
3.11. Dumping Syndrome
3.12. Increased Flatus
3.13. Indigestion
3.14. Appetite
3.15. Other Nutrition Impact Symptoms
3.16. Nutrition Outcomes
3.17. Malnutrition Risk
3.18. Nutrition Status
3.19. Weight Change
3.20. Body Mass Index (BMI)
3.21. Muscle Mass
3.22. Muscle Strength
3.23. Fat Mass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UGI | Upper gastrointestinal tract |
| QoL | Quality of life |
| PEI | Pancreatic exocrine insufficiency |
| BMI | Body mass index |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| SWiM | Synthesis Without Meta-analysis |
| PGSAS-45 | Postgastrectomy Syndrome Assessment Scale-45 |
| GLIM | Global Leadership Initiative on Malnutrition |
| PGSAS-37 | Postgastrectomy Syndrome Assessment Scale-37 |
| GERD | Gastroesophageal reflux disease |
| FACT-Ga | Functional Assessment of Cancer Therapy-Gastric |
| GSRS | Gastrointestinal Symptom Rating Scale |
| SGA | Subjective Global Assessment |
| RG | Robotic gastrectomy |
| LG | Laparoscopic gastrectomy |
| PG-SGA | Patient-Generated Subjective Global Assessment |
| EORTC | European Organisation for Research and Treatment of Cancer |
| FOIS | Functional Oral Intake Scale |
| SOA | Side overlap anastomosis |
| DTA | Double-tract anastomosis |
| SNAQ | Simplified Nutritional Appetite Questionnaire |
| SF-36 | Short Form 36 Health Survey Questionnaire |
| CCRT | Concurrent chemoradiotherapy |
| NRS | Nutritional Risk Screening |
| MUST | Malnutrition Universal Screening tool |
| MNA | Mini Nutritional Assessment |
| PNI | Prognostic Nutritional Index |
| BIA | Bioelectrical impedance analysis |
| HGS | Hand grip strength |
| CT | Computed tomography |
| CGM | Continuous glucose monitoring |
| ORD | Observer-reported dysphagia |
| ASMI | Appendicular skeletal muscle index |
| BCM | Body cell mass |
| FFM | Fat free mass |
| LBM | Lean body mass |
| PARA | Phase angle right arm |
| PALA | Phase angle left arm |
| PATR | Phase angle trunk |
| PARL | Phase angle right leg |
| PALL | Phase angle left leg |
| BF | Body fat percentage |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| PMI | Psoas muscle index |
References
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 15 May 2025).
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.; Lam, F.; Bray, F.; Ervik, M.; Soerjomataram, I. ICBP SURVMARK-2 online tool: International Cancer Survival Benchmarking. Available online: https://gco.iarc.fr/survival/survmark/ (accessed on 15 May 2025).
- Boyle, E.; Elliott, J.A. Novel nutrition strategies in gastric and esophageal cancer. Expert Rev. Gastroenterol. Hepatol. 2025, 19, 89–104. [Google Scholar] [CrossRef]
- Jacobson, R.; Gurd, E.N.; Pimiento, J.M. Long-term nutrition alterations after surgery for gastrointestinal cancers. Nutr. Clin. Pract. 2023, 38, 721–730. [Google Scholar] [CrossRef]
- Teixeira Farinha, H.; Bouriez, D.; Grimaud, T.; Rotariu, A.M.; Collet, D.; Mantziari, S.; Gronnier, C. Gastro-Intestinal Disorders and Micronutrient Deficiencies following Oncologic Esophagectomy and Gastrectomy. Cancers 2023, 15, 3554. [Google Scholar] [CrossRef]
- Gallanis, A.F.; Mannes, A.J.; Davis, J.L. Gastric Cancer Surgery. In Anesthesia for Oncological Surgery; Springer International Publishing: Cham, Switzerland, 2023; pp. 257–261. [Google Scholar]
- Bolger, J.C.; Donohoe, C.L.; Lowery, M.; Reynolds, J.V. Advances in the curative management of oesophageal cancer. Br. J. Cancer 2022, 126, 706–717. [Google Scholar] [CrossRef]
- Cobani, E.; Al Hallak, M.N.; Shields, A.F.; Maier, J.; Kelly, T.E.; Naidoo, N.; Tobon, M.; Kim, S.; Beal, E.W. Gastric Cancer Survivorship: Multidisciplinary Management, Best Practices and Opportunities. J. Gastrointest. Cancer 2024, 55, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Khorasanchi, A.; Nemani, S.; Pandey, S.; Del Fabbro, E. Managing Nutrition Impact Symptoms in Cancer Cachexia: A Case Series and Mini Review. Front. Nutr. 2022, 9, 831934. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, T.; Charkaoui, M.; Marref, I.; Lepage, C. Nutritional management after cancer related gastrectomy. Hépato Gastro Oncol. Dig. 2020, 27, 438–445. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Wilson, J.S.; Smith, R.C. Diagnosis and management of pancreatic exocrine insufficiency. Med. J. Aust. 2017, 207, 161–165. [Google Scholar] [CrossRef]
- Chaudhary, A.; Domínguez-Muñoz, J.E.; Layer, P.; Lerch, M.M. Pancreatic Exocrine Insufficiency as a Complication of Gastrointestinal Surgery and the Impact of Pancreatic Enzyme Replacement Therapy. Dig. Dis. 2020, 38, 53–68. [Google Scholar] [CrossRef]
- Surmelioglu, A.; Ozkardesler, E.; Tilki, M.; Yekrek, M. Exocrine pancreatic insufficiency in long-term follow-up after curative gastric resection with D2 lymphadenectomy: A cross-sectional study: Exocrine pancreatic insufficiency after curative gastric resection. Pancreatology 2021, 21, 975–982. [Google Scholar] [CrossRef]
- Shah, N.D.; Wall, E.; Macaire, G.; Diller, H. Nutrition Care for Patients with Upper GI Malignancies: Part 2—Gastric Cancer. Pract. Gastroenterol. 2023, 47, 20–37. [Google Scholar]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef]
- Mąkosza, K.M.; Muc-Wierzgoń, M.; Dzięgielewska-Gęsiak, S. Nutrition and Selected Lifestyle Elements as a Tertiary Prevention in Colorectal Cancer Patients. Nutrients 2024, 16, 3129. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Song, C.; Xu, H.; Song, C.; Wang, C.; Fu, Z.; Ba, Y.; Wu, J.; Xie, C.; Chen, G.; et al. The patient-generated subjective global assessment is a promising screening tool for cancer cachexia. BMJ Support. Palliat. Care 2022, 12, e39–e46. [Google Scholar] [CrossRef] [PubMed]
- Yule, M.S.; Brown, L.R.; Waller, R.; Wigmore, S.J. Cancer cachexia. BMJ 2024, 387, e080040. [Google Scholar] [CrossRef]
- De Moura, A.; Turpin, A.; Neuzillet, C. Nutritional supportive care in the course of patients with esophagogastric cancers. Bull. du Cancer 2023, 110, 540–551. [Google Scholar] [CrossRef]
- Seid, A.; Debebe, Z.; Ayelign, A.; Abeje, M.; Endris, B.S.; Assefa, M.; Jemal, A. Malnutrition Diagnosed by Patient-Generated Subjective Global Assessment and the Risk of All-Cause Mortality in Adults With Gastrointestinal Cancer: A Systematic Review and Meta-Analysis. J. Hum. Nutr. Diet. 2025, 38, e70012. [Google Scholar] [CrossRef]
- Deftereos, I.; Yeung, J.M.C.; Arslan, J.; Carter, V.M.; Isenring, E.; Kiss, N. On Behalf of The Nourish Point Prevalence Study Group Assessment of Nutritional Status and Nutrition Impact Symptoms in Patients Undergoing Resection for Upper Gastrointestinal Cancer: Results from the Multi-Centre NOURISH Point Prevalence Study. Nutrients 2021, 13, 3349. [Google Scholar] [CrossRef]
- Mekal, D.; Sobocki, J.; Badowska-Kozakiewicz, A.; Sygit, K.; Cipora, E.; Bandurska, E.; Czerw, A.; Deptala, A. Evaluation of Nutritional Status and the Impact of Nutritional Treatment in Patients with Pancreatic Cancer. Cancers 2023, 15, 3816. [Google Scholar] [CrossRef]
- Kiss, N.; Loeliger, J.; Findlay, M.; Isenring, E.; Baguley, B.J.; Boltong, A.; Butler, A.; Deftereos, I.; Eisenhuth, M.; Fraser, S.F.; et al. Clinical Oncology Society of Australia: Position statement on cancer-related malnutrition and sarcopenia. Nutr. Diet. 2020, 77, 416–425. [Google Scholar] [CrossRef]
- Haiducu, C.; Buzea, A.; Mirea, L.E.; Dan, G.A. The prevalence and the impact of sarcopenia in digestive cancers. A systematic review. Rom. J. Intern. Med. 2021, 59, 328–344. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Laviano, A.; Di Lazzaro Giraldi, G.; Koverech, A. Practical Management of Cancer Cachexia. Oncol. Ther. 2017, 5, 125–134. [Google Scholar] [CrossRef]
- Dunne, R.F.; Roeland, E.J. The Interplay Among Pancreatic Cancer, Cachexia, Body Composition, and Diabetes. Hematol. Oncol. Clin. 2022, 36, 897–910. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Clarivate. EndNote, Clarivate Analytics: Philadelphia, PA, USA, 2025.
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Covidence Systematic Review Software, Veritas Health Innovation: Melbourne, Australia, 2025. Available online: https://app.covidence.org/reviews/530647 (accessed on 11 February 2025).
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Akad, F.; Filip, B.; Preda, C.; Zugun-Eloae, F.; Peiu, S.N.; Akad, N.; Crauciuc, D.V.; Vatavu, R.; Gavril, L.C.; Sufaru, R.F.; et al. Assessing Nutritional Status in Gastric Cancer Patients after Total versus Subtotal Gastrectomy: Cross-Sectional Study. Nutrients 2024, 16, 1485. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.; Meng, X.; Xu, X.; Wang, C.; Zhang, M.; Zhang, T. Complete laparoscopic and Da Vinci robot esophagogastric anastomosis double muscle flap plasty for radical resection of proximal gastric cancer. Front. Oncol. 2024, 14, 1395549. [Google Scholar] [CrossRef]
- Nishibeppu, K.; Kubota, T.; Yubakami, M.; Ohashi, T.; Kiuchi, J.; Shimizu, H.; Arita, T.; Yamamoto, Y.; Konishi, H.; Morimura, R.; et al. Impact of hypoglycemia after gastrectomy on Global Leader Initiative on Malnutrition-defined malnutrition: A retrospective study. Surg. Today 2024, 54, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Gharagozlian, S.; Mala, T.; Brekke, H.K.; Kolbjornsen, L.C.; Ullerud, A.A.; Johnson, E. Nutritional status, sarcopenia, gastrointestinal symptoms and quality of life after gastrectomy for cancer—A cross-sectional pilot study. Clin. Nutr. ESPEN 2020, 37, 195–201. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2016. [Google Scholar]
- Fu, J.; Li, Y.; Liu, X.; Jiao, X.; Qu, H.; Wang, Y.; Niu, Z. Effects of robotic and laparoscopic-assisted surgery on lymph node dissection and quality of life in the upper third of gastric cancer: A retrospective cohort study based on propensity score matching. Front. Surg. 2023, 9, 1057496. [Google Scholar] [CrossRef]
- Huang, S.C.; Yang, L.Y.; Chao, Y.K.; Chang, W.Y.; Tsao, Y.T.; Chou, C.Y.; Hsiao, C.C.; Chiu, C.H. Improved functional oral intake and exercise training attenuate decline in aerobic capacity following chemoradiotherapy in patients with esophageal cancer. J. Rehabil. Med. 2024, 56, 25906. [Google Scholar] [CrossRef]
- Hsu, L.F.; Lee, Y.H.; Yang, H.Y.; Chou, Y.J.; Tien, Y.W.; Liu, C.Y.; Shun, S.C. Changes in nutritional status and fatigue and their associations with quality of life in patients with pancreatic cancer after surgery: A 12-month longitudinal study. Appl. Nurs. Res. 2024, 80, 151858. [Google Scholar] [CrossRef]
- Wang, H.M.; Wang, T.J.; Huang, C.S.; Liang, S.Y.; Yu, C.H.; Lin, T.R.; Wu, K.F. Nutritional Status and Related Factors in Patients with Gastric Cancer after Gastrectomy: A Cross-Sectional Study. Nutrients 2022, 14, 2634. [Google Scholar] [CrossRef]
- Wu, C.Y.; Zhong, W.J.; Ye, K. Comparison of the efficacy, safety and postoperative quality of life between modified side overlap anastomosis and double-tract anastomosis after laparoscopic proximal gastrectomy. Updat. Surg. 2024, 76, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Huang, Q.Z.; Ye, K. Comparison of short-term clinical efficacy between modified Kamikawa anastomosis and double tract anastomosis after laparoscopic proximal gastrectomy. Front. Oncol. 2024, 14, 1414120. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, J.A.; Ye, K. Clinical efficacy of modified Kamikawa anastomosis in patients with laparoscopic proximal gastrectomy. World J. Gastrointest. Surg. 2024, 16, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Tanabe, K.; Yoshida, M.; Kawahira, H.; Inada, T.; Okabe, H.; Urushihara, T.; Kawashima, Y.; Fukushima, N.; Nakada, K. Postgastrectomy Syndrome Assessment Scale (PGSAS)-45 and changes in body weight are useful tools for evaluation of reconstruction methods following distal gastrectomy. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), 370–378. [Google Scholar] [CrossRef]
- Lundell, L.R.; Dent, J.; Bennett, J.R.; Blum, A.L.; Armstrong, D.; Galmiche, J.P.; Johnson, F.; Hongo, M.; Richter, J.E.; Spechler, S.J.; et al. Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999, 45, 172–180. [Google Scholar] [CrossRef]
- Nakada, K.; Ikeda, M.; Takahashi, M.; Kinami, S.; Yoshida, M.; Uenosono, Y.; Kawashima, Y.; Oshio, A.; Suzukamo, Y.; Terashima, M.; et al. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: Newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer 2015, 18, 147–158. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Neto, R.M.L.; Herbella, F.A.M.; Schlottmann, F.; Patti, M.G. Does DeMeester score still define GERD? Dis. Esophagus. 2018, 32, doy118. [Google Scholar] [CrossRef]
- Eremenco, S.L.; Cashy, J.; Webster, K.; Ohashi, Y.; Locker, G.Y.; Pelletier, G.; Cella, D. FACT-Gastric: A new international measure of QOL in gastric cancer. J. Clin. Oncol. 2004, 22, 8123. [Google Scholar] [CrossRef]
- Revicki, D.A.; Wood, M.; Wiklund, I.; Crawley, J. Reliability and validity of the gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease. Qual. Life Res. 1997, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Duerksen, D.R.; Laporte, M.; Jeejeebhoy, K. Evaluation of Nutrition Status Using the Subjective Global Assessment: Malnutrition, Cachexia, and Sarcopenia. Nutr. Clin. Pract. 2021, 36, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Ottery, F. Patient-Generated Subjective Global Assessment. In The Clinical Guide to Oncology Nutrition; McCallum, P.D., Polisena, C.G., Eds.; The American Dietetic Association: Chicago, IL, USA, 2000; pp. 11–23. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Blazeby, J.M.; Conroy, T.; Bottomley, A.; Vickery, C.; Arraras, J.; Sezer, O.; Moore, J.; Koller, M.; Turhal, N.S.; Stuart, R.; et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur. J. Cancer 2004, 40, 2260–2268. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.C.; Groher, M.E. Initial Psychometric Assessment of a Functional Oral Intake Scale for Dysphagia in Stroke Patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Lau, S.; Pek, K.; Chew, J.; Lim, J.P.; Ismail, N.H.; Ding, Y.Y.; Cesari, M.; Lim, W.S. The Simplified Nutritional Appetite Questionnaire (SNAQ) as a Screening Tool for Risk of Malnutrition: Optimal Cutoff, Factor Structure, and Validation in Healthy Community-Dwelling Older Adults. Nutrients 2020, 12, 2885. [Google Scholar] [CrossRef] [PubMed]
- Hann, D.M.; Jacobsen, P.B.; Azzarello, L.M.; Martin, S.C.; Curran, S.L.; Fields, K.K.; Greenberg, H.; Lyman, G. Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Qual. Life Res. 1998, 7, 301–310. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Gandek, B. SF-36 Health Survey: Manual & Interpretation Guide. Health Inst. 1993, 6, 1–22. [Google Scholar]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.L.E.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Stratton, R.J.; King, C.L.; Stroud, M.A.; Jackson, A.A.; Elia, M. ‘Malnutrition Universal Screening Tool’ predicts mortality and length of hospital stay in acutely ill elderly. Br. J. Nutr. 2006, 95, 325–330. [Google Scholar] [CrossRef]
- Guigoz, Y. The mini nutritional assessment (MNA) review of the literature-what does it tell us? J. Nutr. Health Aging 2006, 10, 466. [Google Scholar] [PubMed]
- Demirelli, B.; Akgül, B.N.; Özlem, E.; Akif, Ö.M.; Serap, K.; Eda, T.; Süleyman, K.; Rahib, H.; Özkan, A.; Akın, T.T.; et al. Modified Glasgow Prognostic Score, Prognostic Nutritional Index and ECOG Performance Score Predicts Survival Better than Sarcopenia, Cachexia and Some Inflammatory Indices in Metastatic Gastric Cancer. Nutr. Cancer 2021, 73, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis-Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Ziosoft. Ziostation, Ziosoft Japan: Tokyo, Janpan, 2023.
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.-D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Crowder, S.L.; Douglas, K.G.; Yanina Pepino, M.; Sarma, K.P.; Arthur, A.E. Nutrition impact symptoms and associated outcomes in post-chemoradiotherapy head and neck cancer survivors: A systematic review. J. Cancer Surviv. 2018, 12, 479–494. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Laviano, A.; Gillis, C.; Sung, A.D.; Gardner, M.; Yalcin, S.; Dixon, S.; Newman, S.M.; Bastasch, M.D.; Sauer, A.C.; et al. Examining guidelines and new evidence in oncology nutrition: A position paper on gaps and opportunities in multimodal approaches to improve patient care. Support. Care Cancer 2022, 30, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Vingrys, K.; Atkins, L.; Pape, E.; Shaw, A.; Drury, A. Illuminating the nutrition-related policy-practice gaps in colorectal cancer survivorship. Support. Care Cancer 2024, 32, 131, s00520–s024. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, R.A.; Clayton, M.F.; Collins, K.K.; Gold, H.T.; Laiyemo, A.O.; Truesdale, K.P.; Ritzwoller, D.P. The Pathways to Prevention program: Nutrition as prevention for improved cancer outcomes. J. Natl. Cancer Inst. 2023, 115, 886–895. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Isenring, E.A.; Capra, S.; Bauer, J.D. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br. J. Cancer 2004, 91, 447–452. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Trujillo, E.B.; Claghorn, K.; Dixon, S.W.; Hill, E.B.; Braun, A.; Lipinski, E.; Platek, M.E.; Vergo, M.T.; Spees, C. Inadequate Nutrition Coverage in Outpatient Cancer Centers: Results of a National Survey. J. Oncol. 2019, 2019, 7462940. [Google Scholar] [CrossRef]
- Hunter, J.; Smith, C.; Delaney, G.P.; Templeman, K.; Grant, S.; Ussher, J.M. Coverage of cancer services in Australia and providers’ views on service gaps: Findings from a national cross-sectional survey. BMC Cancer 2019, 19, 570. [Google Scholar] [CrossRef]
- Davis, A.M.; Vogelzang, J.L.; Affenito, S.G. The Shortage of Registered Dietitians or Nutritionists with a Terminal Degree: A Call to Action for the Profession. J. Acad. Nutr. Diet. 2023, 123, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Middleton, C. Dietitian-Nutritionists around the World-Their Education and Their Work; The International Confederation of Dietetic Associations (ICDA): Toronto, ON, Canada, 2021. [Google Scholar]
- Huggins, C.E.; Hanna, L.; Furness, K.; Silvers, M.A.; Savva, J.; Frawley, H.; Croagh, D.; Cashin, P.; Low, L.; Bauer, J.; et al. Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial. Nutrients 2022, 14, 3234. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L.; Wieben, A.; Stevens, J.; Tostrud, L.; Montgomery, K. Guideline-Recommended Symptom Management Strategies That Cross Over Two or More Cancer Symptoms. Oncol. Nurs. Forum. 2020, 47, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, F.; Hallemeier, C.L.; Lerut, T.; Fu, J. Oesophageal cancer. Lancet 2024, 404, 1991–2005. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.T.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Baguley, B.J.; Edbrooke, L.; Denehy, L.; Prado, C.M.; Kiss, N. A rapid review of nutrition and exercise approaches to managing unintentional weight loss, muscle loss, and malnutrition in cancer. Oncol. 2024, oyae261. [Google Scholar] [CrossRef] [PubMed]

| Author (Country) | Study Design | Cancer Type | Stage | Treatment | Sample Size | Age | Sex (% Female Total Sample) | Data Collection Timepoints | Intervention Provided | Outcomes of Interest |
|---|---|---|---|---|---|---|---|---|---|---|
| Akad et al., 2024 [34] | Cross-sectional study | Gastric | I–III | Surgery; total and subtotal gastrectomy | 51 | 66.18 ± 12.93 | 33.3 | ‘Post-surgery’ (not described) | Standard nutrition supplements from the hospital | Nutrition status Performance status |
| Fu et al., 2023 [39] | Retrospective cohort study | Gastric | I–IV | Surgery; two surgical techniques: RG and LG for total gastrectomy | 409 | RG: 63.34 ± 7.91 LG: 64.08 ± 8.52 | 29 | Preoperative 3 months post-surgery 6 months post-surgery 1 year post-surgery | - | Nutrition status |
| Gharagozlian et al., 2020 [37] | Cross-sectional study | Gastric | I–III | Surgery; total or subtotal gastrectomy | 21 | 60 ± 12.6 | 48 | 28.7 (8.3) months post-surgery | 57% consulted a dietician before surgery 86% received dietetic follow-up in the hospital postoperatively | Nutrition status Symptom severity |
| Huang et al., 2024 [40] | Non-randomised controlled trial | Oesophageal | II–IV | Chemoradiotherapy | 67 | 57 ± 8 | 3 | Baseline 6–8 weeks post-completion of chemoradiotherapy | Exercise programme | Physical fitness Nutrition status Hand grip strength Body composition Functional oral intake |
| Hsu et al., 2024 [41] | Prospective cohort study | Pancreatic | I–IV | Surgery; distal pancreatectomy and splenectomy, total pancreatectomy, or bypass operation | 89 | 59.87 ± 11.70 | 49.4 | Before surgery 3 months post-surgery 6 months post-surgery 12 months post-surgery | - | Nutrition status Fatigue |
| Nishibeppu et al., 2024 [36] | Retrospective cohort study | Gastric | I–III | Surgery; distal or total gastrectomy | 69 | Data group by GLIM criteria: Normal/moderate malnutrition: 65.4 ± 10.0 Severe malnutrition: 66.4 ± 13.3 | 52 | Baseline 1 month post-surgery 1 year post-surgery | CGM monitoring | Glucose fluctuations Nutrition status |
| Wang et al., 2022 [42] | Cross-sectional study | Gastric | I–III | Surgery; total or subtotal gastrectomy | 101 | 66.5 ± 14.0, range: 25–89 | 47 | Mean: 10.9 ± 7.6 months post-surgery | - | Nutrition status |
| Wu et al., 2024 [43] | Retrospective cohort study | Gastric | I–II | Surgical techniques: SOA vs. DTA after laparoscopic proximal gastrectomy | 43 | SOA: 65.5 (60.8, 71.8) DTA: 70 (66, 76) median (range) | 16 | Baseline 3 months post-surgery 6 months post-surgery 12 months post-surgery | - | Nutrition status |
| Wu et al., 2024 [44] | Retrospective cohort study | Upper gastric and esophagogastric junction | I–II | Surgery; two surgical techniques; modified Kamikawa anastomosis and DTA after laparoscopic proximal gastrectomy | 42 | Range: 40–83 | 19 | Baseline 6 months post-surgery 12 months post-surgery | - | Postoperative nutrition status Gastroesophageal reflux |
| Wu et al., 2024 [45] | Case series | Esophagogastric and gastric | I–II | Surgery; modified Kamikawa anastomosis for laparoscopic proximal gastrectomy | 26 | 68.846 ± 1.352 | 15 | Baseline 6 months post-surgery 12 months post-surgery | - | Symptoms of reflux Nutrition status |
| Yang et al., 2024 [35] | Retrospective cohort study | Upper gastric | I–II | Surgery; laparoscopic surgery vs. Da Vinci robotic surgery for proximal subtotal gastrectomy | 35 | Median = 63 Range: 53–72 | 17 | 1–42 months post-surgery Median follow-up 24 months | - | Nutrition status |
| Author | Symptom Tool Used | Symptoms Reported | Baseline | Follow-Up Timepoint 1 | Follow-Up Timepoint 2 | Follow-Up Timepoint 3 |
|---|---|---|---|---|---|---|
| Akad et al., 2024 [34] | Postoperative: | |||||
| ORD 0 = no dysphagia 1 = symptomatic; able to eat regular diet 2 = symptomatic; altered eating/drinking | Dysphagia | Both groups: score 0: 34 (66.7%), score 1: 8 (15.7%), and score 2: 9 (17.6%) | ||||
| SNAQ (lower rating indicates better appetite and reduced risk of malnutrition) | Appetite | Both groups: low: 1 (2%), moderate: 2 (3.9%), and high: 48 (94.1%) | ||||
| Fu et al., 2023 [39] | Quality of life questionnaire-stomach 22, qlq-sto22 + EORTC QLQ-30 (higher scores indicate worse condition) | Preoperative (RG vs. LG): | 3 months post-surgery (RG vs. LG): | 6 months post-surgery (RG vs. LG): | 12 months post-surgery (RG vs. LG): | |
| Dysphagia a | 10.3 ± 6.3 vs. 10.9 ± 5.7 | 27.8 ± 12.3 vs. 30.9 ± 10.8 | 16.4 ± 6.7 vs. 18.4 ± 9.7 | 12.3 ± 3.1 vs. 13.1 ± 2.9 | ||
| Sour regurgitation a | 9.2 ± 7.1 vs. 8.9 ± 6.5 | 10.3 ± 6.3 vs. 12.4 ± 7.1 | 8.2 ± 4.3 vs. 8.6 ± 3.7 | 8.1 ± 3.3 vs. 8.3 ± 3.7 | ||
| Belching a | 5.1 ± 4.2 vs. 5.4 ± 3.9 | 11.2 ± 6.1 vs. 14.1 ± 8.7 ↑ | 7.1 ± 3.9 vs. 9.4 ± 4.5 ↑ | 6.2 ± 2.2 vs. 6.7 ± 2.4 | ||
| Abdominal pain a | 8.1 ± 4.9 vs. 8.3 ± 4.1 | 15.7 ± 6.8 vs. 19.1 ± 7.3 ↑ | 12.3 ± 4.9 vs. 14.4 ± 5.7 ↑ | 9.3 ± 3.6 vs. 10.6 ± 3.3 ↑ | ||
| Diarrhoea a | 6.9 ± 5.1 vs. 7.1 ± 5.2 | 13.4 ± 7.1 vs. 16.6 ± 8.7 ↑ | 12.9 ± 5.3 vs. 14.1 ± 4.9 | 9.9 ± 4.7 vs. 10.1 ± 4.6 | ||
| Fatigue a | 7.1 ± 4.7 vs. 7.3 ± 4.4 | 18.7 ± 10.3 vs. 21.2 ± 9.8 | 11.3 ± 5.2 vs. 13.7 ± 6.5 ↑ | 8.4 ± 3.9 vs. 9.2 ± 3.1 | ||
| Correlation between nutrition status (using PG-SGA scoring) and symptoms (using QLQ-STO22 and QLQ-C30) (PG-SGA has a continuous score from 0 to 16, where higher scores indicate higher malnutrition risk) | Dysphagia b | 0–3 = 14.4 ± 6.1 4–8 = 15.2 ± 7.3 ≥ 9 = 17.8 ± 9.2 ↑ | ||||
| Sour regurgitation b | 0–3 = 7.2 ± 4.1 4–8 = 6.9 ± 3.4 ≥9 = 7.7 ± 5.4 ↑ | |||||
| Belching b | 0–3 = 8.4 ± 5.3 4–8 = 8.2 ± 4.6 ≥ 9 = 8.9 ± 3.8 ↑ | |||||
| Abdominal pain b | 0–3 = 16.3 ± 8.2 4–8 =19.4 ± 7.7 ≥9 = 22.6 ± 6.3 ↑ | |||||
| Diarrhoea b | 0–3 = 7.2 ± 5.2 4–8 = 9.3 ± 4.9 ≥ 9 = 12.2 ± 6.4 ↑ | |||||
| Fatigue b | 0–3 =21.1 ± 9.3 4–8 =22.5 ± 13.2 ≥9 = 22.9 ± 12.6 ↑ | |||||
| Gharagozlian et al., 2020 [37] | GSRS syndrome (7-point Likert scale, where higher scores indicate worse conditions) | Post-surgery: 28.7 (8.3) months | ||||
| Abdominal pain a | Well nourished = 2.0 (0.88) Malnourished = 2.9 (0.72) ↑ | |||||
| Diarrhoea a | Well nourished = 2.3 (1.5) Malnourished = 2.6 (1.3) | |||||
| Constipation a | Well nourished = 1.8 (0.84) Malnourished = 2.9 (1.4) | |||||
| Indigestion a | Well nourished = 2.9 (1.0) Malnourished = 3.5 (0.43) | |||||
| Reflux a | Well nourished = 1.5 (0.97) Malnourished = 2.3 (1.4) | |||||
| SF-36 scale (score out of 100, where higher scores indicate better conditions) | Bodily pain a | Well nourished = 79.2 (22.0) Malnourished = 47.6 (13.7) ↑ | ||||
| Huang et al., 2024 [40] | FOIS b (7-point Likert scale, 0 = NBM and 7 = oral intake with no restrictions) | Dysphagia b | Pre-CCRT: | Post-CCRT (6–8 weeks): | ||
| 5.5 ± 1.7 | 5.7 ± 1.6 | |||||
| Hsu et al., 2024 [41] | Fatigue Symptom Inventory (higher scores indicate higher level of fatigue, ranging from 0 to 127 points) | Fatigue b | Before surgery: | 3 months: | 6 months: | 12 months: |
| 18.57 ± 22.50 | 21.91 ± 23.88 | 16.31 ± 21.26 | 16.42 ± 20.81 | |||
| Nishibeppu, et al., 2024 [36] | PGSAS-37 (7-point Likert scale, where higher scores indicate worse conditions) | 1 month (normal/moderate malnutrition vs. severe): | 1 year (normal/moderate malnutrition vs. severe): | |||
| Oesophageal reflux a | 2.0 ± 0.9 vs. 1.99 ± 0.9 | 1.7 ± 1.0 vs. 1.7 ± 0.6 | ||||
| Abdominal pain a | 2.1 ± 0.8 vs. 2.2 ± 0.9 | 1.5 ± 0.7 vs. 1.5 ± 0.7 | ||||
| Indigestion a | 2.2 ± 0.8 vs. 2.0 ± 0.7 | 2.2 ± 0.9 vs. 2.2 ± 1.0 | ||||
| Diarrhoea a | 1.7 ± 0.7 vs. 2.1 ± 1.1 | 1.8 ± 0.7 vs. 2.6 ± 1.1 ↑ | ||||
| Constipation a | 2.2 ± 0.9 vs. 2.5 ± 1.1 | 2.2 ± 0.9 vs. 2.1 ± 1.0 | ||||
| Dumping a | 1.8 ± 1.0 vs. 2.0 ± 1.2 | 1.5 ± 0.9 vs. 2.1 ± 1.2 ↑ | ||||
| Wang et al., 2022 [42] | Gastric Cancer Subscale of the FACT-Ga. (4-point Likert scale—0, not at all; 4, very much) | Post-surgery: | ||||
| Being bothered by gas (flatulence) | 1.31 ± 1.34 0–4 | |||||
| Having stomach problems that worry me | 1.05 ± 1.13 0–4 | |||||
| Having fullness or heaviness in the stomach | 1.01 ± 1.09 0–4 | |||||
| Having discomfort or pain when eating | 0.94 ± 1.06 0–4 | |||||
| Feeling tired | 0.93 ± 1.11 0–4 | |||||
| Having swelling or cramps in the stomach area | 0.88 ± 1.12 0–4 | |||||
| Having discomfort or pain in the stomach area | 0.89 ± 1.02 0–4 | |||||
| Bothered by reflux or heartburn | 0.72 ± 1.04 0–4 | |||||
| Losing weight | 0.40 ± 0.86 0–4 | |||||
| Loss of appetite | 0.66 ± 1.09 0–4 | |||||
| Having trouble swallowing food | 0.28 ± 0.74 0–4 | |||||
| Having diarrhoea | 0.49 ± 0.84 0–4 | |||||
| Feeling weak all over | 0.63 ± 1.06 0–4 | |||||
| Wu et al., 2024 [43] | PGSAS-45 (7-point Likert scale, where higher scores indicate worse conditions) | 12 months (SOA vs. DTA): | ||||
| Oesophageal reflux subscale a | 3.0 ± 1.2 vs. 4.1 ± 1.3 ↑ | |||||
| Abdominal pain subscale a | 1.7 (1.3, 3.0) vs. 2.0 (1.3, 3.3) | |||||
| Indigestion subscale a | 2.3 (2.3, 3.0) vs. 2.5 (2.3, 3.5) | |||||
| Diarrhoea subscale a | 1.3 (1.3, 2.0) vs. 1.3 (1.7, 2.0) | |||||
| Constipation subscale a | 1.3 (1.3, 1.7) vs. 1.3 (1.3, 1.7) | |||||
| Dumping subscale a | 1.3 (1.3, 1.3) vs. 1.3 (1.3, 1.3) | |||||
| Increased flatus a | 2.5 (2, 3) vs. 4 (3, 5) ↑ | |||||
| Loose stools a | 1 (1, 2) vs. 1 (1, 2) | |||||
| Los Angeles Scale | Reflux oesophagitis a | 2 vs. 9 ↑ | ||||
| Grade A | 2 vs. 5 | |||||
| Grade B | 0 vs. 4 | |||||
| Wu et al., 2024 [44] | PGSAS-45 (7-point Likert scale, where higher scores indicate worse conditions) GERD scale score Los Angeles Scale (1–4, where higher scores indicate worse conditions) | 6 months (modified Kamikawa vs. DTA): | 12 months (modified Kamikawa vs. DTA): | |||
| Oesophageal reflux subscale a | 3.1 ± 1.3 vs. 4.0 ± 1.3 | |||||
| Abdominal pain subscale a | 1.7 (1.3, 4.3) vs. 2.0 (1.3, 4.3) | |||||
| Indigestion subscale a | 2.4 (2.0, 4.0) vs. 2.5 (2.0, 4.8) | |||||
| Diarrhoea subscale a | 1.3 (1.0, 2.7) vs. 1.5 (1.0, 2.7) | |||||
| Constipation subscale a | 1.3 (1.0, 2.3) vs. 1.3 (1.0, 2.3) | |||||
| Dumping subscale a | 1.3 (1.0, 2.3) vs. 1.3 (1.0, 2.3) | |||||
| Increased flatus a | 3.0 (1.0, 6.0) vs. 3.5 (1.0, 6.0) | |||||
| Loose stools a | 1.0 (1.0, 2.0) vs. 1.0 (1.0, 2.0) | |||||
| Gastroesophageal reflux disease a | 3.0 (2.0–4.0) vs. 3.0 (2.0–4.0) | 3.0 (2.0–4.0) vs. 2.5 (2.0–4.0) | ||||
| Grade B reflux esophagitis a | 1 vs. 2 | |||||
| Wu et al., 2024 [45] | 6 months: | 12 months: | ||||
| GERD scale | Gastroesophageal reflux disease | 3 (2–4) | 3 (2–4) | |||
| Los Angeles Scale | Reflux esophagitis | 0 | ||||
| Yang et al., 2024 [35] | - | Reflux esophagitis | Postoperative: (laparoscopic vs. Da Vinci robotic esophagogastric anastomosis): | |||
| 1 case of reflux in the laparoscopic surgery group. Nil in the Da Vinci robotic group |
| Author | Malnutrition Risk | Malnutrition Assessment | Body Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tool | Score | Tool | Score | Weight (kg) | BMI (kg/m2) (Mean ± SD) | Muscle/Lean Body Mass | Fat Mass | Muscle Strength | |
| Akad et al., 2024 [34] | NRS-2002 Score < 3—no nutrition risk; >3—nutrition risk (n (%)) | Both groups: | PG-SGA (n (%)) (Stage A = well nourished Stage B = suspected or moderate malnutrition Stage C = severe malnutrition) | Both groups: | - | - | - | - | |
| Score 0: 44 (88.2%) Score 1: 1 (2%) | |||||||||
| Stage A: 15 (29.4%) | |||||||||
| Stage B: 30 (58.8%) | |||||||||
| Score 2: 3 (5.9%) | Stage C: 6 (11.8%) | ||||||||
| Score 3: 2 (3.9%) | |||||||||
| Fu et al., 2023 [39] | NRS-2002 Score < 3—no nutrition risk; >3—nutrition risk (n (%)) | (RG vs. LG) | PNI (higher scores indicate better nutrition) | (RG vs. LG) | (RG vs. LG) | (RG vs. LG) | - | - | |
| Score < 3 Entire cohort: 69 (65.1) vs. 179 (59.1) | |||||||||
| Preoperative a: 422.7 ± 75.3 vs. 437.02 ± 81.2 | Preoperative a: 63.2 ± 9.7 vs. 62.9 ± 9.4 | Preoperative a: 24.69 ± 4.01 vs. 25.15 ± 3.14 | |||||||
| Score ≥ 3 Entire cohort: 37 (34.9) vs. 124 (40.9) | 3 months a: 362.1 ± 61.4 vs. 369.5 ± 57.6 | 3 months a: 57.8 ± 6.5 vs. 56.1 ± 6.1 | |||||||
| 6 months a: 370.4 ± 53.5 vs. 373.6 ± 55.8 | 6 months a: 58.2 ± 6.8 vs. 57.3 ± 6.3 | ||||||||
| 1 year a: 379.5 ± 51.2 vs. 383.4 ± 51.4 | 1 year a: 58.6 ± 7.2 vs. 57.8 ± 6.9 | ||||||||
| Gharagozlian et al., 2020 [37] | - | - | SGA (n (%)) (SGA-A = well nourished SGA-B = suspected or moderate malnutrition) SGA-C = severe malnutrition) | SGA-A = 15 (72%) | Weight loss (%): 12.8 ± 11.6 | Preoperative BMI: 26.0 ± 4.8 | BIA: (mean ±SD) | - | HGS: |
| SGA-B = 5 (24%) | > 10% loss of current weight: 9 (45%) | Postoperative BMI: 22.2 ± 3.3 | ASMI: Females (kg/m2) = 3.2 ± 0.60 Males (kg/m2): 4.4 ± 0.51 (low scores are ≤7.0 kg/m2 in men and ≤5.5 kg/m2 in women) | Females (kg) = 23.6 ± 5.5 Males (kg) = 43.1 ± 9.3 (low strength defined as <27 kg for males and <16 kg for females) | |||||
| SGA-C = 1 (5%) | |||||||||
| EWGSOP: (n (%)) Pre-sarcopenia: 20 (100.0) Sarcopenia: 1 (5.0) | |||||||||
| Huang et al., 2024 [40] | - | - | PG-SGA score b (mean ± SD) (higher scores indicate greater severity of malnutrition) | Pre-CCRT: 6.2 ± 3.3 | Pre-CCRT b: 65.5 ± 12.6 | - | BIA: (pre-CCRT vs. post CCRT) | BF (%) b: (pre-CCRT vs. post-CCRT) 23.0 ± 6.7 vs. 22.3 ± 6.4 | HGS (kg) b: 41.7 ± 7.9 vs. 39.6 ± 8.6 ↓ |
| 6–8 weeks post-CCRT: 3.6 ± 3.3 ↓ (improved nutrition status) | Post-CCRT b: 65.4 ± 11.9 | ||||||||
| ASMI b (kg/m2): 7.63 ± 0.97 vs. 7.64 ± 1.04 | |||||||||
| BCM (kg) b: 32.7 ± 5.5 vs. 32.6 ± 5.7 | |||||||||
| FFM (kg) b: 49.9 ± 8.6 vs. 50.4 ± 8.6 | |||||||||
| LBM (kg) b: 46.9 ± 8.8 vs. 47.8 ± 8.2 | |||||||||
| PARA b: 6.0 ± 0.8 vs. 5.5 ± 0.8 ↓ | |||||||||
| PALA b: 5.9 ± 0.8 vs. 5.5 ± 0.9 ↓ | |||||||||
| PATR b: 8.6 ± 1.7 vs. 8.0 ± 1.5 ↓ | |||||||||
| PARL b: 6.2 ± 0.9 vs. 5.6 ± 1.0 ↓ | |||||||||
| PALL b: 5.9 ± 0.9 vs. 5.4 ± 1.0 ↓ | |||||||||
| Hsu et al., 2024 [41] | - | - | MNA (mean ± SD) (lower scores indicate poorer nutrition status, with a range from 0 to 30) | BIA: skeletal muscle mass (mean ± SD): | BIA: visceral fat mass (mean ± SD): | HGS: | |||
| T0: 23.85 ± 3.63 | T0: 60.46 ± 11.44 | T0: 21.35 ± 5.71 | T0: 2.30 ± 1.37 | T0: 26.13 ± 9.25 | |||||
| T1 b: 22.96 ± 3.37, T1/T0 ↓ | T1 b: 57.62 ± 10.69 ↓ | T1 b: 21.94 ± 6.03, T1/T0 ↓ | T1 b: 1.70 ± 1.13, T1/T0 ↓ | T1 b: 23.20 ± 9.07, T1/T0 ↓ | |||||
| T2 b: 24.59 ± 3.01, T2/T0 | T2 b: 56.81 ± 10.71 ↓ | T2 b: 22.15 ± 5.52, T2/T0 | T2 b: 1.77 ± 1.20, T2/T0 ↓ | T2 b: 24.44 ± 9.99, T2/T0 ↓ | |||||
| T3 b: 25.09 ± 3.57, T3/T0 | T3 b: 57.58 ± 11.46 ↓ | T3 b: 21.91 ± 4.75 T3/T0 | T3 b: 1.87 ± 1.24, T3/T0 ↓ | T3 b: 24.10 ± 9.56 T3/T0 ↓ | |||||
| T0 = before surgery T1 = 3 months after surgery T2 = 6 months after surgery T3 = 12 months after surgery | Lower scores indicate lower fat mass | 30 s sit-to-stand test Lower-limb strength: | |||||||
| T0: 18.46 ± 6.63 | |||||||||
| T1 b: 19.15 ± 7.69, T1/T0 | |||||||||
| T2 b: 20.54 ± 9.04, T2/T0 | |||||||||
| T3 b: 20.44 ± 7.43 T3/T0 ↑ Lower scores indicate lower strength | |||||||||
| Nishibeppu et al., 2024 [36] | - | - | GLIM (n) | 1 month: Severe: 30 Moderate: 24 No malnutrition: 15 | - | Preoperative BMI: | Psoas muscle mass index (PMI) from CT scan: High PMI: 34 participants Low PMI: 35 participants | - | |
| GLIM normal/moderate: 23.7 ± 2.6 | |||||||||
| Severe: 20.4 ± 2.8 ↓ | |||||||||
| 1 year: Severe: 25 Moderate: 35 No malnutrition: 9 | |||||||||
| Wang et al., 2022 [42] | - | - | MNA (n) (lower scores indicate poorer nutrition status, with a range from 0 to 30) | 48 = score > 24, indicating well-nourished | - | - | - | - | |
| 44 = score between 17.5 and 23, indicating risk of malnutrition | |||||||||
| 9 = score < 17, suggesting malnutrition | |||||||||
| Wu et al., 2024 [43] | NRS 2002 (mean (range)) Score < 3—no nutrition risk; >3—nutrition risk | 3 months a SOA: 2 (1.25, 2) DTA: 2 (1, 2) | PG-SGA (mean (range)) (Stage A (1) = well nourished Stage B (2) = suspected or moderate malnutrition Stage C (3) = severe malnutrition) | 3 months a: SOA: 2 (1.25, 2) DTA: 2 (1, 2) | 12 months a: (%) change: SOA: 12.1 ± 4.6 DTA: 12.9 ± 4.3 | 3 months a: SOA: 21.3 ± 2.6 DTA: 21.5 ± 2.6 | - | - | |
| 6 months a: SOA: 2 (2, 2) DTA: 2 (1, 2) | 6 months a: SOA: 2 (1, 2) DTA: 2 (1, 2) | 6 months a: SOA: 21.5 ± 2.9 DTA: 21.4 ± 2.6 | |||||||
| 12 months a: SOA: 1.5 (1, 2) DTA: 1.5 (1, 2) | 12 months a: SOA: 2 (1, 2) DTA: 2 (1, 2) | 12 months a: SOA: 22.0 ± 2.5 DTA: 22.3 ± 2.6 | |||||||
| Wu et al., 2024 [44] | MUST (0 = low, 1 = moderate, and 2 = high) (mean (range)) | 6 months a Modified Kamikawa anastomosis: 1 (1.0–2.0) DTA: 1 (1.0–2.0) | PG-SGA (mean (range)) (Stage A (1) = well nourished Stage B (2) = suspected or moderate malnutrition Stage C (3) = severe malnutrition) | 6 months a Modified Kamikawa anastomosis: 2 (1.0–3.0) DTA: 2 (1.0–3.0) | 12 months a: Δ % weight loss Modified Kamikawa anastomosis: 12.6 ± 4.6 DTA: 13.8 ± 5.1 | Baseline: Modified Kamikawa anastomosis: 22.2 ± 2. DTA: 21.2 ± 3.3 | - | - | |
| 12 months a Modified Kamikawa anastomosis: 1 (1.0–2.0) DTA: 1 (1.0–2.0) | 12 months a Modified Kamikawa anastomosis: 2 (1.0–3.0) DTA: 1.5 (1.0–3.0) | 6 months a Modified Kamikawa anastomosis: 22.2 ± 2.7 DTA: 22.8 ± 2.9 | |||||||
| 12 months a Modified Kamikawa anastomosis: 22.4 ± 2.5 DTA: 21.5 ± 2.9 | |||||||||
| Wu et al., 2024 [45] | NRS 2002 Score < 3—no nutrition risk; > 3—nutrition risk (mean (range)) | Preoperative: 2 (1–2) | PG-SGA (mean, (range)) (Stage A (1) = well nourished Stage B (2) = suspected or moderate malnutrition Stage C (3) = severe malnutrition) | Preoperative: 1 (1–3) | - | Baseline: 22.6 ± 3.1 | - | - | |
| 6 months: 2 (1–2) | 6 months: 1 (1–3) | 6 months: 22.6 ± 3.1 | |||||||
| 12 months: 22.6 ± 3.2 | |||||||||
| 12 months: 2 (1–2) | 12 months: 1 (1–3) | ||||||||
| Yang et al., 2024 [35] | NRS2002 Score < 3—no nutrition risk; > 3—nutrition risk (n (%)) | Both groups Combined a: | PG-SGA (n (%)) (Stage A (1) = well nourished Stage B (2) = suspected or moderate malnutrition Stage C (3) = severe malnutrition) | Both groups combined a: | - | Baseline: Laparoscopic: 23.63 ± 2.59 Da Vinci Robot surgery 23.11 ± 2.65 | - | - | |
| Score 1: 18 (51%) | Score 1: 13 (37%) | ||||||||
| Score 2: 14 (40%) | |||||||||
| Score 3: 8 (23%) | |||||||||
| Score 2: 17 (49%) | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McShane, E.; Hanna, L.; Zoanetti, C.; Murnane, L.; Baguley, B.; Furness, K. The Effect of Nutrition Impact Symptoms on Nutrition Status After Completion of Curative-Intent Treatment for Gastric, Oesophageal, and Pancreatic Cancer: A Systematic Review. Nutrients 2025, 17, 2691. https://doi.org/10.3390/nu17162691
McShane E, Hanna L, Zoanetti C, Murnane L, Baguley B, Furness K. The Effect of Nutrition Impact Symptoms on Nutrition Status After Completion of Curative-Intent Treatment for Gastric, Oesophageal, and Pancreatic Cancer: A Systematic Review. Nutrients. 2025; 17(16):2691. https://doi.org/10.3390/nu17162691
Chicago/Turabian StyleMcShane, Emma, Lauren Hanna, Carmel Zoanetti, Lisa Murnane, Brenton Baguley, and Kate Furness. 2025. "The Effect of Nutrition Impact Symptoms on Nutrition Status After Completion of Curative-Intent Treatment for Gastric, Oesophageal, and Pancreatic Cancer: A Systematic Review" Nutrients 17, no. 16: 2691. https://doi.org/10.3390/nu17162691
APA StyleMcShane, E., Hanna, L., Zoanetti, C., Murnane, L., Baguley, B., & Furness, K. (2025). The Effect of Nutrition Impact Symptoms on Nutrition Status After Completion of Curative-Intent Treatment for Gastric, Oesophageal, and Pancreatic Cancer: A Systematic Review. Nutrients, 17(16), 2691. https://doi.org/10.3390/nu17162691







