Genetic Variants Influencing Individual Vitamin D Status
Abstract
1. Introduction
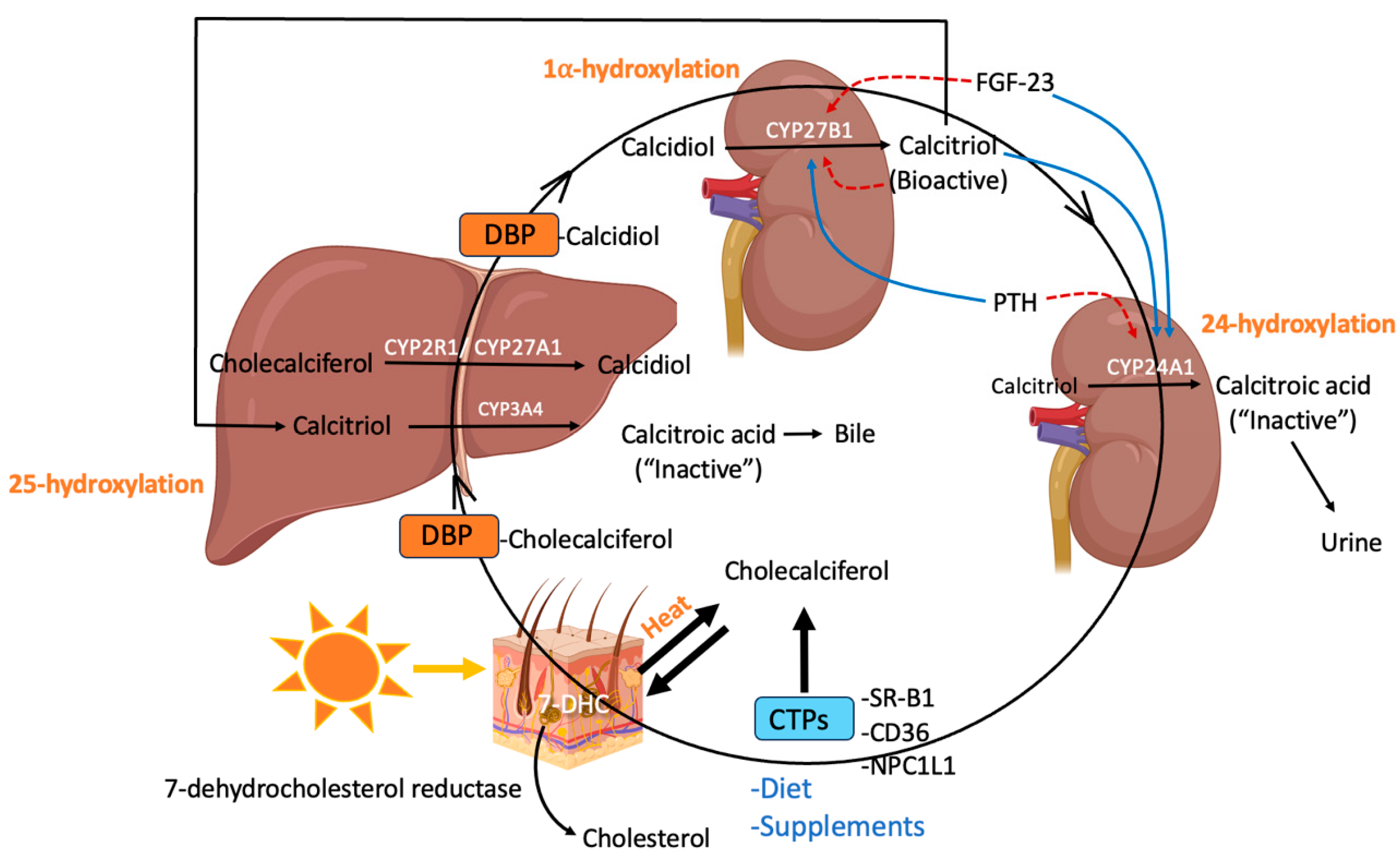
2. Synthesis, Regulation, and Metabolism of Vitamin D
3. Genetic Variants Affecting Vitamin D Status
3.1. Candidate Gene and Genome-Wide Association Studies
3.2. 7-Dehydrocholesterol Reductase (DHCR7)
3.3. Cholesterol-Transport Proteins (CTPs)
3.4. GC
3.5. Cytochrome P450 Genes
3.5.1. Cyp2r1
3.5.2. Cyp27a1
3.5.3. Cyp27b1
3.5.4. Cyp24a1
3.5.5. Cyp3a4
3.5.6. Cyp11a1
4. Summary of Genetic Findings
5. Clinical Implications
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hribar, M.; Hristov, H.; Gregorič, M.; Blaznik, U.; Zaletel, K.; Oblak, A.; Osredkar, J.; Kušar, A.; Žmitek, K.; Rogelj, I.; et al. Nutrihealth Study: Seasonal Variation in Vitamin D Status Among the Slovenian Adult and Elderly Population. Nutrients 2020, 12, 1838. [Google Scholar] [CrossRef]
- Lehmann, B.; Meurer, M. Vitamin D Metabolism. Dermatol. Ther. 2010, 23, 2–12. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of Vitamin D Toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Overcoming Infections Including COVID-19, by Maintaining Circulating 25(OH)D Concentrations Above 50 Ng/mL. Pathol. Lab. Med. Int. 2022, 14, 37–60. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections—Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association Between Preoperative 25-Hydroxyvitamin D Level and Hospital-Acquired Infections Following Roux-En-Y Gastric Bypass Surgery. JAMA Surg. 2014, 149, 112–118. [Google Scholar] [CrossRef]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 Ng/mL 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R.Z. Vitamin D: Evidence-Based Health Benefits and Recommendations for Population Guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. Randomized Controlled Trials of Vitamin D and Cancer Incidence: A Modeling Study. PLoS ONE 2017, 12, e0176448. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Revisiting Vitamin D Guidelines: A Critical Appraisal of the Literature. Endocr. Pract. 2024, 30, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast Cancer Risk Markedly Lower with Serum 25-Hydroxyvitamin D Concentrations ≥60 vs. <20 Ng/Ml (150 vs. 50 Nmol/L): Pooled Analysis of Two Randomized Trials and a Prospective Cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O’Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early Pregnancy Vitamin D Status and Risk of Preeclampsia. J. Clin. Investig. 2016, 126, 4702–4715. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Benedik, E. Sources of Vitamin D for Humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Tsiaras, W.G.; Weinstock, M.A. Factors influencing vitamin D status. Acta Derm Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Tian, X.Q.; Chen, T.C.; Matsuoka, L.Y.; Wortsman, J.; Holick, M.F. Kinetic and Thermodynamic Studies of the Conversion of Previtamin D3 to Vitamin D3 in Human Skin. J. Biol. Chem. 1993, 268, 14888–14892. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Janjetovic, Z.; Slominski, R.M.; Li, W.; Jetten, A.M.; Indra, A.K.; Mason, R.S.; Tuckey, R.C. Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin. J. Investig. Dermatol. 2024, 144, 2145–2161. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism and Function in the Skin. Mol. Cell Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.V.; Luu, W.; Sharpe, L.J.; Brown, A.J. Cholesterol-Mediated Degradation of 7-Dehydrocholesterol Reductase Switches the Balance from Cholesterol to Vitamin D Synthesis. J. Biol. Chem. 2016, 291, 8363–8373. [Google Scholar] [CrossRef]
- Shin, M.H.; Lee, Y.; Kim, M.-K.; Lee, D.H.; Chung, J.H. UV Increases Skin-Derived 1α,25-Dihydroxyvitamin D3 Production, Leading to MMP-1 Expression by Altering the Balance of Vitamin D and Cholesterol Synthesis from 7-Dehydrocholesterol. J. Steroid Biochem. Mol. Biol. 2019, 195, 105449. [Google Scholar] [CrossRef]
- Zou, L.; Porter, T.D. Rapid Suppression of 7-Dehydrocholesterol Reductase Activity in Keratinocytes by Vitamin D. J. Steroid Biochem. Mol. Biol. 2015, 148, 64–71. [Google Scholar] [CrossRef]
- Diehl, K.; Krippaehne, E.; Minasyan, M.; Banh, M.; Yumeen, S.; Mirza, F.N.; Hajjar, K.; Ng, J.; Wais, N.; Goulding, A.; et al. Exploring Skin Pigmentation Adaptation: A Systematic Review on the Vitamin D Adaptation Hypothesis. Cutis 2024, 113, E15–E21. [Google Scholar] [CrossRef] [PubMed]
- Lucock, M.D. The Evolution of Human Skin Pigmentation: A Changing Medley of Vitamins, Genetic Variability, and UV Radiation during Human Expansion. Am. J. Biol. Anthropol. 2023, 180, 252–271. [Google Scholar] [CrossRef]
- Chao, Y.-S.; Brunel, L.; Faris, P.; Veugelers, P.J. Vitamin D Status of Canadians Employed in Northern Latitudes. Occup. Med. (Lond) 2013, 63, 485–493. [Google Scholar] [CrossRef]
- Leary, P.F.; Zamfirova, I.; Au, J.; McCracken, W.H. Effect of Latitude on Vitamin D Levels. J. Am. Osteopath. Assoc. 2017, 117, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Meza-Meza, M.R.; Ruiz-Ballesteros, A.I.; de la Cruz-Mosso, U. Functional Effects of Vitamin D: From Nutrient to Immunomodulator. Crit. Rev. Food Sci. Nutr. 2022, 62, 3042–3062. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.-J.; Reboul, E. Fat-Soluble Vitamin Intestinal Absorption: Absorption Sites in the Intestine and Interactions for Absorption. Food Chem. 2015, 172, 155–160. [Google Scholar] [CrossRef]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.-F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D Intestinal Absorption Is Not a Simple Passive Diffusion: Evidences for Involvement of Cholesterol Transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. (Lausanne) 2019, 10, 317. [Google Scholar] [CrossRef]
- Carlberg, C. Genomic Signaling of Vitamin D. Steroids 2023, 198, 109271. [Google Scholar] [CrossRef] [PubMed]
- Żmijewski, M.A. Nongenomic Activities of Vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef]
- Chen, T.C.; Persons, K.S.; Lu, Z.; Mathieu, J.S.; Holick, M.F. An Evaluation of the Biologic Activity and Vitamin D Receptor Binding Affinity of the Photoisomers of Vitamin D3 and Previtamin D3. J. Nutr. Biochem. 2000, 11, 267–272. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Li, W.; Yi, A.-K.; Postlethwaite, A.; Tuckey, R.C. The Role of CYP11A1 in the Production of Vitamin D Metabolites and Their Role in the Regulation of Epidermal Functions. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Lee, S.M.; Carlson, A.H.; Benkusky, N.A.; Kaufmann, M.; Jones, G.; Pike, J.W. A Chromatin-Based Mechanism Controls Differential Regulation of the Cytochrome P450 Gene Cyp24a1 in Renal and Non-Renal Tissues. J. Biol. Chem. 2019, 294, 14467–14481. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Pike, J.W. Mechanistic Homeostasis of Vitamin D Metabolism in the Kidney through Reciprocal Modulation of Cyp27b1 and Cyp24a1 Expression. J. Steroid Biochem. Mol. Biol. 2020, 196, 105500. [Google Scholar] [CrossRef]
- Milan, K.L.; Ramkumar, K.M. Regulatory Mechanisms and Pathological Implications of CYP24A1 in Vitamin D Metabolism. Pathol. Res. Pract. 2024, 264, 155684. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Pandey, M.; Adomat, H.; Guns, E.S.T. Cytochrome P450 3A-Mediated Microsomal Biotransformation of 1α,25-Dihydroxyvitamin D3 in Mouse and Human Liver: Drug-Related Induction and Inhibition of Catabolism. Drug Metab. Dispos. 2012, 40, 907–918. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Y.S.; Zheng, X.E.; Senn, T.; Hashizume, T.; Scian, M.; Dickmann, L.J.; Nelson, S.D.; Baillie, T.A.; Hebert, M.F.; et al. An Inducible Cytochrome P450 3A4-Dependent Vitamin D Catabolic Pathway. Mol. Pharmacol. 2012, 81, 498–509. [Google Scholar] [CrossRef]
- Wang, Z.; Schuetz, E.G.; Xu, Y.; Thummel, K.E. Interplay between Vitamin D and the Drug Metabolizing Enzyme CYP3A4. J. Steroid Biochem. Mol. Biol. 2013, 136, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.B.; Arnold, L.A. Calcitroic Acid-A Review. ACS Chem. Biol. 2016, 11, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Horst, R.; Prapong, S.; Reinhardt, T.; Koszewski, N.; Knutson, J.; Bishop, C. Comparison of the Relative Effects of 1,24-Dihydroxyvitamin D(2) [1, 24-(OH)(2)D(2)], 1,24-Dihydroxyvitamin D(3) [1,24-(OH)(2)D(3)], and 1,25-Dihydroxyvitamin D(3) [1,25-(OH)(2)D(3)] on Selected Vitamin D-Regulated Events in the Rat. Biochem. Pharmacol. 2000, 60, 701–708. [Google Scholar] [CrossRef]
- Houghton, L.A.; Vieth, R. The Case against Ergocalciferol (Vitamin D2) as a Vitamin Supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar] [CrossRef]
- Araya, Z.; Tang, W.; Wikvall, K. Hormonal Regulation of the Human Sterol 27-Hydroxylase Gene CYP27A1. Biochem. J. 2003, 372, 529–534. [Google Scholar] [CrossRef]
- Li, T.; Chen, W.; Chiang, J.Y.L. PXR Induces CYP27A1 and Regulates Cholesterol Metabolism in the Intestine. J. Lipid Res. 2007, 48, 373–384. [Google Scholar] [CrossRef]
- Meyer, M.B.; Benkusky, N.A.; Kaufmann, M.; Lee, S.M.; Onal, M.; Jones, G.; Pike, J.W. A Kidney-Specific Genetic Control Module in Mice Governs Endocrine Regulation of the Cytochrome P450 Gene Cyp27b1 Essential for Vitamin D3 Activation. J. Biol. Chem. 2017, 292, 17541–17558. [Google Scholar] [CrossRef]
- Meyer, M.B.; Lee, S.M.; Towne, J.M.; Cichanski, S.R.; Kaufmann, M.; Jones, G.; Pike, J.W. In Vivo Contribution of Cyp24a1 Promoter Vitamin D Response Elements. Endocrinology 2024, 165, bqae134. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Walton, R.T.; Griffiths, C.J.; Martineau, A.R. Single Nucleotide Polymorphisms in the Vitamin D Pathway Associating with Circulating Concentrations of Vitamin D Metabolites and Non-Skeletal Health Outcomes: Review of Genetic Association Studies. J. Steroid Biochem. Mol. Biol. 2016, 164, 18–29. [Google Scholar] [CrossRef]
- Hyppönen, E.; Vimaleswaran, K.S.; Zhou, A. Genetic Determinants of 25-Hydroxyvitamin D Concentrations and Their Relevance to Public Health. Nutrients 2022, 14, 4408. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo de Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. CD36 and SR-BI Are Involved in Cellular Uptake of Provitamin A Carotenoids by Caco-2 and HEK Cells, and Some of Their Genetic Variants Are Associated with Plasma Concentrations of These Micronutrients in Humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Vitamin E Metabolic Effects and Genetic Variants: A Challenge for Precision Nutrition in Obesity and Associated Disturbances. Nutrients 2018, 10, 1919. [Google Scholar] [CrossRef]
- Zumaraga, M.P.; Borel, P.; Gleize, B.; Nowicki, M.; Ould-Ali, D.; Landrier, J.-F.; Desmarchelier, C. Genetic Factors Contributing to Interindividual Variability of α-Tocopherol Levels in Subcutaneous Adipose Tissue among Healthy Adult Males. Nutrients 2024, 16, 2556. [Google Scholar] [CrossRef]
- Kashiwabara, Y.; Kobayashi, Y.; Koba, S.; Kohyama, N.; Ohbayashi, M.; Murayama, J.-I.; Hirano, T.; Kobayashi, Y.; Yamamoto, T. Gene Polymorphism and Frequencies of the NPC1L1 Gene (Rs2072183, Rs217434 and Rs217428) in Japanese Patients with Dyslipidemia. J. Clin. Pharm. Ther. 2014, 39, 551–554. [Google Scholar] [CrossRef]
- Miao, L.; Yin, R.-X.; Hu, X.-J.; Wu, D.-F.; Cao, X.-L.; Li, Q.; Yan, T.-T.; Aung, L.H.H.; Wu, J.-Z.; Lin, W.-X. Association of Rs2072183 SNP and Serum Lipid Levels in the Mulao and Han Populations. Lipids Health Dis. 2012, 11, 61. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hwang, J.-J.; Tsai, C.-T.; Su, Y.-N.; Hsueh, C.-H.; Shen, M.J.; Lai, L.-P. The g.-762T>C Polymorphism of the NPC1L1 Gene Is Common in Chinese and Contributes to a Higher Promoter Activity and Higher Serum Cholesterol Levels. J. Hum. Genet. 2009, 54, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Elkum, N.; Alkayal, F.; Noronha, F.; Ali, M.M.; Melhem, M.; Al-Arouj, M.; Bennakhi, A.; Behbehani, K.; Alsmadi, O.; Abubaker, J. Vitamin D Insufficiency in Arabs and South Asians Positively Associates with Polymorphisms in GC and CYP2R1 Genes. PLoS ONE 2014, 9, e113102. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-J.; Fan, H.-Z.; Tian, T.; Wu, M.-P.; Xie, C.-N.; Huang, P.; Yu, R.-B.; Yi, H.-G.; Zhang, Y.; Wang, J. Impact of CYP2R1, CYP27A1 and CYP27B1 Genetic Polymorphisms Controlling Vitamin D Metabolism on Susceptibility to Hepatitis C Virus Infection in a High-Risk Chinese Population. Arch. Virol. 2019, 164, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Yoon, J.W.; Lee, Y.; Choi, H.J.; Yun, J.W.; Bae, E.; Kwon, S.-H.; Ahn, S.E.; Do, A.-R.; Jin, H.; et al. Unveiling Genetic Variants Underlying Vitamin D Deficiency in Multiple Korean Cohorts by a Genome-Wide Association Study. Endocrinol. Metab. (Seoul) 2021, 36, 1189–1200. [Google Scholar] [CrossRef]
- Dong, A.N.; Tan, B.H.; Pan, Y.; Ong, C.E. The CYP2R1 Enzyme: Structure, Function, Enzymatic Properties and Genetic Polymorphism. J. Pharm. Pharm. Sci. 2021, 24, 94–112. [Google Scholar] [CrossRef]
- Slater, N.A.; Rager, M.L.; Havrda, D.E.; Harralson, A.F. Genetic Variation in CYP2R1 and GC Genes Associated With Vitamin D Deficiency Status. J. Pharm. Pract. 2017, 30, 31–36. [Google Scholar] [CrossRef]
- Hu, Z.; Tao, S.; Liu, H.; Pan, G.; Li, B.; Zhang, Z. The Association between Polymorphisms of Vitamin D Metabolic-Related Genes and Vitamin D3 Supplementation in Type 2 Diabetic Patients. J. Diabetes Res. 2019, 2019, 8289741. [Google Scholar] [CrossRef]
- Selahvarzi, H.; Kamdideh, M.; Vahabi, M.; Dezhgir, A.; Houshmand, M.; Sadeghi, S. Association of Single Nucleotide Polymorphisms in the VDR and CYP27B1 Genes with Risk of Developing Vitamin D3 Deficiency. J. Pure Appl. Microbiol. 2021, 15, 201–211. [Google Scholar] [CrossRef]
- Jarrar, Y.; Alhammadin, G.; Lee, S.-J. Genetic Polymorphisms in Cytochrome P450 Enzymes Involved in Vitamin D Metabolism and the Vitamin D Receptor: Their Clinical Relevance. J. Pers. Med. 2025, 15, 128. [Google Scholar] [CrossRef]
- Usama; Khan, A.; Raza, M.K.; Abbas, K.; Mansour, L.; Ali, A.; Imran, M. Genetic insights into vitamin D deficiency: A case-control study of GC and CYP24A1 gene polymorphism. Steroids 2025, 218, 109607. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Cervera, A.; Jiménez-Ortega, R.F.; Aparicio-Bautista, D.I.; López-Pérez, T.V.; Patiño, N.; Castillejos-López, M.; Hidalgo-Bravo, A.; Denova-Gutiérrez, E.; Salmerón, J.; Rivera-Paredez, B.; et al. Genetic Variants in Vitamin D Metabolism-Related Genes Are Associated with Vitamin D Status and Adiposity Markers. Nutr. Res. 2025, 136, 105–119. [Google Scholar] [CrossRef]
- Lai, H.J.; Song, J.; Lu, Q.; Murali, S.G.; Gajapathy, M.; Wilk, B.M.; Brown, D.M.; Worthey, E.A.; Farrell, P.M.; FIRST Study Group. Genetic Factors Help Explain the Variable Responses of Young Children with Cystic Fibrosis to Vitamin D Supplements. Clin. Nutr. ESPEN 2022, 51, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Gotoh-Saito, S.; Wada, R.; Nishimura, T.; Kawaji, H. Drug-Induced Cis-Regulatory Elements in Human Hepatocytes Affect Molecular Phenotypes Associated with Adverse Reactions. Nat. Commun. 2025, 16, 3851. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, D.; Szatko, A.; Latocha, J.; Kochman, M.; Duchnowska, M.; Wójcicka, A.; Misiorowski, W.; Zgliczyníski, W.; Glinicki, P. Persistent Hypercalcaemia Associated with Two Pathogenic Variants in the CYP24A1 Gene and a Parathyroid Adenoma-a Case Report and Review. Front. Endocrinol. (Lausanne) 2024, 15, 1355916. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.D.; Li, D.; O’Lear, L.; Javaid, M.K.; Shaw, N.J.; Ebeling, P.R.; Nguyen, H.H.; Rodda, C.P.; Thummel, K.E.; Thacher, T.D.; et al. CYP3A4 Mutation Causes Vitamin D-Dependent Rickets Type 3. J. Clin. Investig. 2018, 128, 1913–1918. [Google Scholar] [CrossRef]
- Jones, P.; Lucock, M.; Chaplin, G.; Jablonski, N.G.; Veysey, M.; Scarlett, C.; Beckett, E. Distribution of Variants in Multiple Vitamin D-Related Loci (DHCR7/NADSYN1, GC, CYP2R1, CYP11A1, CYP24A1, VDR, RXRα and RXRγ) Vary between European, East-Asian and Sub-Saharan African-Ancestry Populations. Genes Nutr. 2020, 15, 5. [Google Scholar] [CrossRef]
- Boland, M.R.; Tatonetti, N.P. Investigation of 7-Dehydrocholesterol Reductase Pathway to Elucidate off-Target Prenatal Effects of Pharmaceuticals: A Systematic Review. Pharmacogenomics J. 2016, 16, 411–429. [Google Scholar] [CrossRef]
- Niforou, A.; Konstantinidou, V.; Naska, A. Genetic Variants Shaping Inter-Individual Differences in Response to Dietary Intakes-A Narrative Review of the Case of Vitamins. Front. Nutr. 2020, 7, 558598. [Google Scholar] [CrossRef]
- Kiourtzidis, M.; Kühn, J.; Brandsch, C.; Stangl, G.I. Vitamin D Status of Mice Deficient in Scavenger Receptor Class B Type 1, Cluster Determinant 36 and ATP-Binding Cassette Proteins G5/G8. Nutrients 2020, 12, 2169. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. (Lausanne) 2020, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.W.; Tung, K.T.S.; Wong, R.S.; Wong, S.Y.; Tung, J.Y.L.; Chua, G.T.; Ho, M.H.K.; Pang, C.P.; Wong, W.H.S.; Chan, G.C.F.; et al. Association of Vitamin D-Binding Protein Polymorphisms and Serum 25(OH)D Concentration Varies among Chinese Healthy Infants of Different VDR-FokI Genotypes: A Multi-Centre Cross-Sectional Study. Nutr. Bull. 2024, 49, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.M.; Fink, S.L.; Bassyouni, H.; Argiropoulos, B.; Brown, L.; Laha, T.J.; Jackson, K.J.; Lewkonia, R.; Ferreira, P.; Hoofnagle, A.N.; et al. Vitamin D-Binding Protein Deficiency and Homozygous Deletion of the GC Gene. N. Engl. J. Med. 2019, 380, 1150–1157. [Google Scholar] [CrossRef]
- Casella, A.; Long, C.; Zhou, J.; Lai, M.; O’Lear, L.; Caplan, I.; Levine, M.A.; Roizen, J.D. Differential Frequency of CYP2R1 Variants Across Populations Reveals Pathway Selection for Vitamin D Homeostasis. J. Clin. Endocrinol. Metab. 2020, 105, 1302–1315. [Google Scholar] [CrossRef]
- Thacher, T.D.; Levine, M.A. CYP2R1 Mutations Causing Vitamin D-Deficiency Rickets. J. Steroid Biochem. Mol. Biol. 2017, 173, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Norlin, M.; Wikvall, K. Enzymatic Activation in Vitamin D Signaling—Past, Present and Future. Arch. Biochem. Biophys. 2023, 742, 109639. [Google Scholar] [CrossRef]
- Tran, T.A.T.; Dien, T.M.; Nguyen, N.L.; Nguyen, K.N.; Can, T.B.N.; Thao, B.P.; Hong, N.T.T.; Tran, V.K.; Tran, T.H.; Khoa, N.X.; et al. Phenotypes and Genotypes of Children with Vitamin D-Dependent Rickets Type 1A: A Single Tertiary Pediatric Center in Vietnam. Diagnostics 2025, 15, 918. [Google Scholar] [CrossRef]
- Prytuła, A.; Cransberg, K.; Raes, A. Drug-Metabolizing Enzymes CYP3A as a Link between Tacrolimus and Vitamin D in Renal Transplant Recipients: Is It Relevant in Clinical Practice? Pediatr. Nephrol. 2019, 34, 1201–1210. [Google Scholar] [CrossRef]
- Alhamoudi, K.M.; Alswailem, M.; Alghamdi, B.; Alashwal, A.; Alzahrani, A.S. A CYP11A1 Homozygous Exonic Variant Inducing an Alternative Splicing, Frameshift and Truncation in a Family with Congenital Adrenal Hyperplasia. Heliyon 2024, 10, e35058. [Google Scholar] [CrossRef] [PubMed]
- Goursaud, C.; Mallet, D.; Janin, A.; Menassa, R.; Tardy-Guidollet, V.; Russo, G.; Lienhardt-Roussie, A.; Lecointre, C.; Plotton, I.; Morel, Y.; et al. Aberrant Splicing Is the Pathogenicity Mechanism of the p.Glu314Lys Variant in CYP11A1 Gene. Front. Endocrinol. (Lausanne) 2018, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, J.; Chen, Y.; Guo, J.; Li, Q.; Dinnyes, A.; Sun, Q.; Liu, X.; He, G.; Zhu, B.; et al. Placenta-Specific CYP11A1 Overexpression Lead to Autism-like Symptom in Offspring with Altered Steroid Hormone Biosynthesis in the Placenta-Brain Axis and Rescued by Vitamin D Intervention. Brain Behav. Immun. 2024, 121, 13–25. [Google Scholar] [CrossRef]
- O’Hearn, K.; Menon, K.; Albrecht, L.; Amrein, K.; Britz-McKibbin, P.; Cayouette, F.; Choong, K.; Foster, J.R.; Fergusson, D.A.; Floh, A.; et al. Rapid Normalization of Vitamin D Deficiency in PICU (VITdALIZE-KIDS): Study Protocol for a Phase III, Multicenter Randomized Controlled Trial. Trials 2024, 25, 619. [Google Scholar] [CrossRef] [PubMed]
- Helmeczi, E.; Pandya, H.; O’Hearn, K.; McNally, D.; Britz-McKibbin, P. Treatment Response Variations to a Single Large Bolus of Enteral Cholecalciferol in Vitamin D Deficient Critically Ill Children: Metabolomic Insights for Precision Nutrition. J. Steroid Biochem. Mol. Biol. 2025, 250, 106720. [Google Scholar] [CrossRef]
| Gene | NCBI Functional Consequence | Functional Role | Reference | |
|---|---|---|---|---|
| rs12785878 | DHCR7/NADSYN1 | Intronic | Regulates precursor availability (7-DHC) | [49] |
| rs3829251 | DHCR7/NADSYN1 | Intronic | Regulates precursor availability (7-DHC) | [49] |
| rs7944926 | DHCR7/NADSYN1 | Intronic | Regulates precursor availability (7-DHC) | [49] |
| rs12800438 | DHCR7/NADSYN1 | Intronic | Regulates precursor availability (7-DHC) | [49] |
| rs3794060 | DHCR7/NADSYN1 | Intronic | Regulates precursor availability (7-DHC) | [49] |
| rs4944957 | DHCR7/NADSYN1 | Intronic | Regulates precursor availability (7-DHC) | [49] |
| rs4945008 | DHCR7/NADSYN1 | Unknown | Regulates precursor availability (7-DHC) | [49] |
| rs5888 | SCARB1 | Synonymous | Cholesterol/VD absorption and transport | [51,52] |
| rs11057830 | SCARB1 | Intronic | Cholesterol/VD absorption and transport | [52] |
| rs4238001 | SCARB1 | Missense | Cholesterol/VD absorption and transport | [51,52] |
| rs61932577 | SCARB1 | Intronic | Cholesterol/VD absorption and transport | [51] |
| rs1984112 | CD36 | Intronic | Cholesterol/VD absorption and transport | [51] |
| rs1761667 | CD36 | Intronic | Cholesterol/VD absorption and transport | [51] |
| rs1527479 | CD36 | Intronic | Cholesterol/VD absorption and transport | [51] |
| rs1527483 | CD36 | Intronic | Cholesterol/VD absorption and transport | [51] |
| rs13230419 | CD36 | Unknown | Cholesterol/VD absorption and transport | [51] |
| rs3211958 | CD36 | Intronic | Cholesterol/VD absorption and transport | [53] |
| rs2072183 | NPC1L1 | Synonymous | Cholesterol/VD absorption and transport | [54,55] |
| rs62001882 | NPC1L1 | Missense | Cholesterol/VD absorption and transport | [52] |
| rs141973731 | NPC1L1 | Missense | Cholesterol/VD absorption and transport | [52] |
| rs139659653 | NPC1L1 | Missense | Cholesterol/VD absorption and transport | [52] |
| rs114375162 | NPC1L1 | Stop gained | Cholesterol/VD absorption and transport | [52] |
| g.-762T>C | NPC1L1 | Unknown | Cholesterol/VD absorption and transport | [56] |
| rs4588 | GC | Missense | Affects VD binding protein affinity and levels | [49] |
| rs7041 | GC | Missense | Affects VD binding protein affinity and levels | [49] |
| rs1155563 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs17467825 | GC | Unknown | Affects VD binding protein affinity and levels | [49] |
| rs2070741 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs2298849 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs16846876 | GC | Unknown | Affects VD binding protein affinity and levels | [49] |
| rs842999 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs222035 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs3755967 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs2298850 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs12512631 | GC | Unknown | Affects VD binding protein affinity and levels | [49] |
| rs2282679 | GC | Intronic | Affects VD binding protein affinity and levels | [49] |
| rs705117 | GC | Intronic | Affects VD binding protein affinity and levels | [50] |
| rs1352846 | GC | Intronic | Affects VD binding protein affinity and levels | [50] |
| rs10741657 | cyp2r1 | 2KB Upstream | Catalyzes 25-hydroxylation | [49,57] |
| rs2060793 | cyp2r1 | 2KB Upstream | Catalyzes 25-hydroxylation | [49] |
| rs1993116 | cyp2r1 | Intronic | Catalyzes 25-hydroxylation | [49,57] |
| rs7116978 | cyp2r1 | Intronic | Catalyzes 25-hydroxylation | [49,57] |
| rs12794714 | cyp2r1 | Synonymous | Catalyzes 25-hydroxylation | [49,50,57,58] |
| rs10500804 | cyp2r1 | Intronic | Catalyzes 25-hydroxylation | [49,57] |
| rs10766197 | cyp2r1 | Unknown | Catalyzes 25-hydroxylation | [49,58] |
| rs2060793 | cyp2r1 | 2KB Upstream | Catalyzes 25-hydroxylation | [59] |
| rs10741657 | cyp2r1 | 5’UTR | Catalyzes 25-hydroxylation | [58,60,61] |
| rs1562902 | cyp2r1 | Unknown | Catalyzes 25-hydroxylation | [58] |
| 6359T>C | cyp2r1 | Unknown | Catalyzes 25-hydroxylation | [60] |
| rs17470271 | cyp27a1 | Intronic | Catalyzes 25-hydroxylation | [58] |
| rs933994 | cyp27a1 | Intronic | Catalyzes 25-hydroxylation | [58] |
| rs10877012 | cyp27b1 | 2KB Upstream | Converts 25(OH)D to active form (calcitriol) | [49,62] |
| rs118204009 | cyp27b1 | Missense | Converts 25(OH)D to active form (calcitriol) | [49] |
| rs4646536 | cyp27b1 | Intronic | Converts 25(OH)D to active form (calcitriol) | [49,63] |
| rs2248137 | cyp24a1 | Intronic | Inactivates calcitriol via hydroxylation | [64] |
| rs2296241 | cyp24a1 | Synonymous | Inactivates calcitriol via hydroxylation | [64] |
| rs927650 | cyp24a1 | Intronic | Inactivates calcitriol via hydroxylation | [64] |
| rs4809960 | cyp24a1 | Intronic | Inactivates calcitriol via hydroxylation | [65] |
| rs2585428 | cyp24a1 | Intronic | Inactivates calcitriol via hydroxylation | [65] |
| rs6013897 | cyp24a1 | Unknown | Inactivates calcitriol via hydroxylation | [66] |
| rs17216707 | cyp24a1 | Unknown | Inactivates calcitriol via hydroxylation | [67] |
| rs6013892 | cyp24a1 | Unknown | Inactivates calcitriol via hydroxylation | [68] |
| rs158523 | cyp24a1 | Unknown | Inactivates calcitriol via hydroxylation | [68] |
| rs114368325 | cyp24a1 | Missense | Inactivates calcitriol via hydroxylation | [69] |
| rs777676129 | cyp24a1 | Inframe Deletion | Inactivates calcitriol via hydroxylation | [69] |
| c.902T>C | cyp3a4 | Unknown | Alternative inactivation pathway (hepatic) | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karrow, N.A.; Leuschner, S.E.; Shandilya, U.K.; Mallard, B.A.; Wagter-Lesperance, L.; Bridle, B.W. Genetic Variants Influencing Individual Vitamin D Status. Nutrients 2025, 17, 2673. https://doi.org/10.3390/nu17162673
Karrow NA, Leuschner SE, Shandilya UK, Mallard BA, Wagter-Lesperance L, Bridle BW. Genetic Variants Influencing Individual Vitamin D Status. Nutrients. 2025; 17(16):2673. https://doi.org/10.3390/nu17162673
Chicago/Turabian StyleKarrow, Niel A., Spencer E. Leuschner, Umesh K. Shandilya, Bonnie A. Mallard, Lauraine Wagter-Lesperance, and Byram W. Bridle. 2025. "Genetic Variants Influencing Individual Vitamin D Status" Nutrients 17, no. 16: 2673. https://doi.org/10.3390/nu17162673
APA StyleKarrow, N. A., Leuschner, S. E., Shandilya, U. K., Mallard, B. A., Wagter-Lesperance, L., & Bridle, B. W. (2025). Genetic Variants Influencing Individual Vitamin D Status. Nutrients, 17(16), 2673. https://doi.org/10.3390/nu17162673








