Protein Substitute Absorption: A Randomised Controlled Trial Comparing CGMP vs. Amino Acids vs. Micellar Casein in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Selection
2.2. Sample Size/Power Calculation
2.3. Study Products
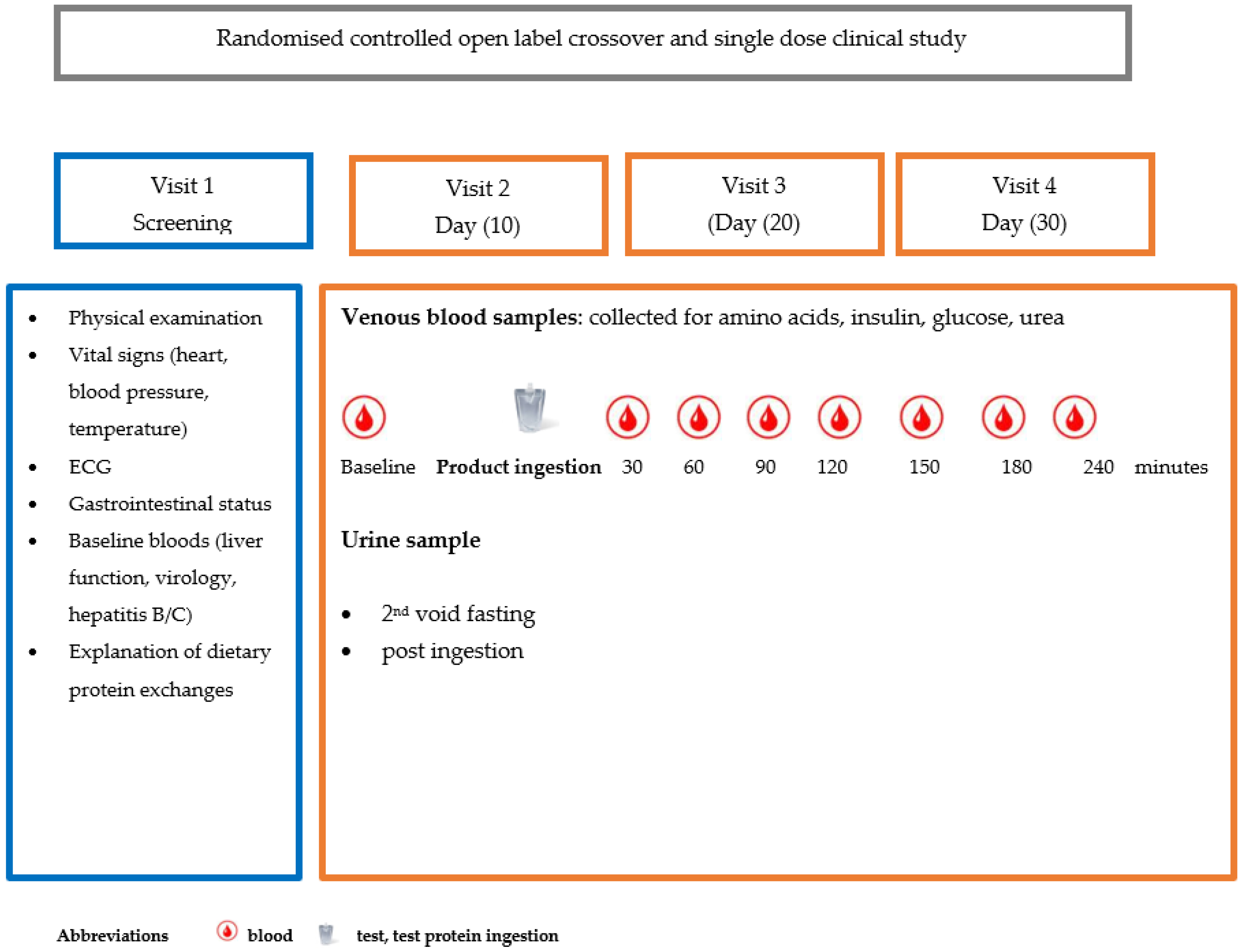
2.4. Study Design
2.5. Screening Visit
- Visits 2, 3 and 4
2.6. Randomisation
- Intervention visits 2, 3 and 4
2.7. Measurement of Blood Biomarkers
2.8. Processing of Biomarkers
2.9. Statistical Analysis
2.10. Ethical Approval
3. Results
3.1. Subjects
3.1.1. Cmax and Tmax
3.1.2. Area Under the Curve (Table 2)
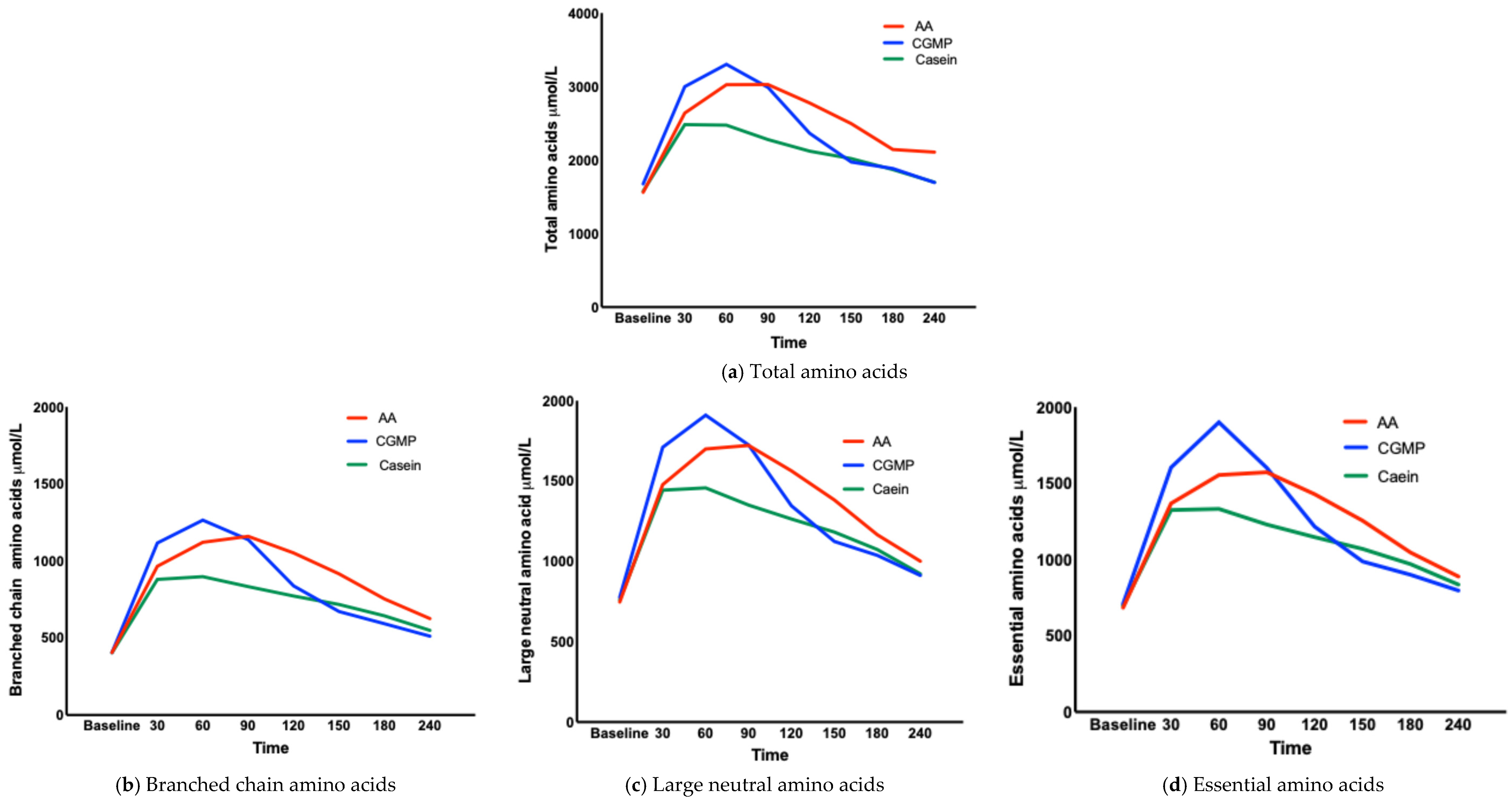
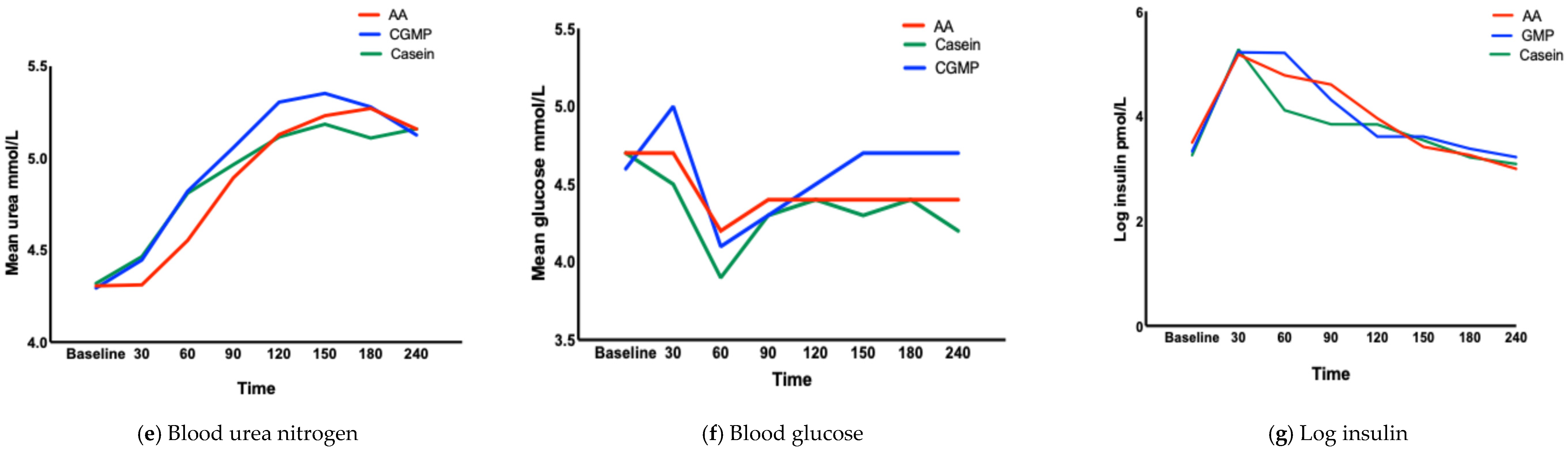
3.1.3. Concentration of AAs at Each Time Point (TMax) (Table 3, Figure 2a–g)
| TAAs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time/Measure μmol/L | Baseline | 30 | 60 | 90 | 120 | 150 | 180 | 240 |
| Mean L-AA | 1562 | 2640 | 3025 | 3027 | 2777 | 2496 | 2144 | 2108 |
| Mean CGMP | 1677 | 2999 | 3302 | 2988 | 2365 | 1973 | 1885 | 1695 |
| Mean Casein | 1577 | 2482 | 2475 | 2278 | 2122 | 2020 | 1871 | 1698 |
| BCAAs | ||||||||
| Mean L-AA | 406 | 966 | 1121 | 1160 | 1052 | 916 | 754 | 626 |
| MeanCGMP | 406 | 1117 | 1265 | 1138 | 838 | 672 | 593 | 512 |
| Mean Casein | 404 | 880 | 898 | 834 | 773 | 718 | 644 | 550 |
| LNAAs | ||||||||
| Mean L-AA | 748 | 1478 | 1700 | 1722 | 1564 | 1382 | 1166 | 1001 |
| Mean CGMP | 777 | 1710 | 1910 | 1725 | 1346 | 1124 | 1038 | 913 |
| Mean Casein | 761 | 1443 | 1458 | 1351 | 1264 | 1183 | 1074 | 923 |
| EAAs | ||||||||
| Mean L-AA | 685 | 1368 | 1555 | 1573 | 1429 | 1255 | 1047 | 889 |
| Mean CGMP | 710 | 1605 | 1901 | 1601 | 1216 | 987 | 901 | 797 |
| Mean Casein | 698 | 1325 | 1332 | 1230 | 1147 | 1070 | 970 | 836 |
| Amino Acid Profile | % Change from Baseline to 30 Minutes | ||
|---|---|---|---|
| L-AA | CGMP | Casein | |
| TAAs | 5 | 7 | 5 |
| BCAAs | 8 | 11 | 8 |
| LNAAs | 7 | 9 | 7 |
| EAAs | 7 | 9 | 7 |
3.1.4. Change of Total, Branched Chain, Large Neutral and Essential Amino Acids Concentrations over Time (Table 4)
3.1.5. Time Above the 90th Centile for Total, Branched Chain, Large Neutral and Essential Amino Acids (Table 5)
| TAA Minutes | BCAA Minutes | LNNA Minutes | EAA Minutes | Insulin Minutes | Glucose Minutes | Urea Minutes | |
|---|---|---|---|---|---|---|---|
| ≥90th centile (range) | |||||||
| L-AA | 69 (44, 69) | 66 (40, 66) | 72 (53, 72) | 69 (45, 69) | 35 (17, 35) | 11 (6, 11) | 36 (22, 36) |
| CGMP | 25 (11, 25) | 42 (31, 42) | 37 (28, 37) | 37 (28, 37) | 19 (14, 19) | 13 (9, 13) | 46 (32, 46) |
| Casein | 29 (12, 29) | 53 (22, 53) | 56 (23, 56) | 57 (22, 57) | 10 (8, 10) | 24 (11, 24) | 21 (17, 21) |
| Est (95% CI); p value | |||||||
| L-AA vs. CGMP | 44 | 24 | 36 | 32 | 16 | −2 | −6 |
| (72, 16) | (53, −5) | (67, 5) | (61, 3) | (36, −4) | (−5, 1) | (−31, 19) | |
| 0.001 * | 0.050 | 0.011 * | 0.015 * | 0.059 | 0.079 | 0.516 | |
| L-AA vs. Casein | 40 | 9 | 12 | 11 | 25 | −13 | 15 |
| (65, 15) | (42, −24) | (42, −18) | (41, −19) | (38, 12) | (−29, 3) | (−13, 31) | |
| 0.001 * | 0.499 | 0.322 | 0.337 | <0.001 * | 0.056 | 0.964 | |
| CGMP vs. Casein | 4 | −11 | 19 | −20 | 9 | −2 | 8 |
| (−78, 86) | (−35, 13) | (50, −12) | (−55, 15) | (20, 1) | (0, −4) | (−16, 32) | |
| 0.538 | 0.186 | 0.113 | 0.131 | 0.047 * | 0.976 | 0.458 | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef]
- Blau, N. Sapropterin dihydrochloride for phenylketonuria and tetrahydrobiopterin deficiency. Expert Rev. Endocrinol. Metab. 2010, 5, 483–494. [Google Scholar] [CrossRef]
- Burton, B.K.; Clague, G.E.; Harding, C.O.; Kucuksayrac, E.; Levy, D.G.; Lindstrom, K.; Longo, N.; Maillot, F.; Muntau, A.C.; Rutsch, F.; et al. Long-term comparative effectiveness of pegvaliase versus medical nutrition therapy with and without sapropterin in adults with phenylketonuria. Mol. Genet. Metab. 2024, 141, 108114. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, O.; Daha, M.; Longo, N.; Knol, E.; Müller, I.; Northrup, H.; Brockow, K. Pegvaliase: Immunological profile and recommendations for the clinical management of hypersensitivity reactions in patients with phenylketonuria treated with this enzyme substitution therapy. Mol. Genet. Metab. 2019, 128, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Metges, C.C.; El-Khoury, A.E.; Selvaraj, A.B.; Tsay, R.H.; Atkinson, A.; Regan, M.M.; Bequette, B.J.; Young, V.R. Kinetics of L-[1-(13)C]leucine when ingested with free aminuteso acids, unlabeled or intrinsically labeled casein. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1000–E1009. [Google Scholar] [CrossRef]
- Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: An update. Nutr. Res. Rev. 2024, 37, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Anthony, T.G.; Rasmussen, B.B.; Adams, S.H.; Lynch, C.J.; Brinkworth, G.D.; Davis, T.A. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. 2015, 101, 1330S–1338S. [Google Scholar] [CrossRef]
- Tome, D. Efficiency of Free Amino Acids in Supporting Muscle Protein Synthesis. J. Nutr. 2022, 152, 3–4. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.; de Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.; Van Loon, L.J.C. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef]
- van Rijn, M.; Hoeksma, M.; Sauer, P.; Szczerbak, B.; Gross, M.; Reijngoud, D.J.; van Spronsen, F. Protein metabolism in adult patients with phenylketonuria. Nutrition 2007, 23, 445–453. [Google Scholar] [CrossRef]
- Boulier, A.; Denis, S.; Henry, G.; Guerin, S.; Alric, M.; Meunier, N.; Blot, A.; Pereira, B.; Malpuech-Brugere, C.; Remond, D.; et al. Casein structures differently affect postprandial amino acid delivery through their intra-gastric clotting properties. Food Chem. 2023, 415, 135779. [Google Scholar] [CrossRef]
- Sakata, Y.; Yago, T.; Mori, S.; Seto, N.; Matsunaga, Y.; Nakamura, H.; Tominaga, T.; Miyaji, K.; Takeda, Y. Time Courses of Gastric Volume and Content after Different Types of Casein Ingestion in Healthy Men: A Randomized Crossover Study. J. Nutr. 2022, 152, 2367–2375. [Google Scholar] [CrossRef]
- Gropper, S.S.; Acosta, P.B. Effect of simultaneous ingestion of L-amino acids and whole protein on plasma amino acid and urea nitrogen concentrations in humans. J. Parenter. Enter. Nutr. 1991, 15, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Weijzen, M.E.G.; van Gassel, R.J.J.; Kouw, I.W.K.; Trommelen, J.; Gorissen, S.H.M.; van Kranenburg, J.; Goessens, J.P.B.; van de Poll, M.C.G.; Verdijk, L.B.; van Loon, L.J.C. Ingestion of Free Amino Acids Compared with an Equivalent Amount of Intact Protein Results in More Rapid Amino Acid Absorption and Greater Postprandial Plasma Amino Acid Availability Without Affecting Muscle Protein Synthesis Rates in Young Adults in a Double-Blind Randomized Trial. J. Nutr. 2022, 152, 59–67. [Google Scholar] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef]
- Bohe, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552 Pt 1, 315–324. [Google Scholar] [CrossRef]
- van Calcar, S.C.; MacLeod, E.L.; Gleason, S.T.; Etzel, M.R.; Clayton, M.K.; Wolff, J.A.; Ney, D.M. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am. J. Clin. Nutr. 2009, 89, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Ney, D.M.; Stroup, B.M.; Clayton, M.K.; Murali, S.G.; Rice, G.M.; Rohr, F.; Levy, H.L. Glycomacropeptide for nutritional management of phenylketonuria: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2016, 104, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Ahring, K.K.; Lund, A.M.; Jensen, E.; Jensen, T.G.; Brondum-Nielsen, K.; Pedersen, M.; Bardow, A.; Holst, J.J.; Rehfeld, J.F.; Møller, L.B. Comparison of Glycomacropeptide with Phenylalanine Free-Synthetic Amino Acids in Test Meals to PKU Patients: No Significant Differences in Biomarkers, Including Plasma Phe Levels. J. Nutr. Metab. 2018, 2018, 6352919. [Google Scholar] [CrossRef]
- WHO; FAO; UNU. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Holwerda, A.M.; Lenaerts, K.; Bierau, J.; Wodzig, W.K.; van Loon, L.J. Food ingestion in an upright sitting position increases postprandial amino acid availability when compared with food ingestion in a lying down position. Appl. Physiol. Nutr. Metab. 2017, 42, 738–743. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Pennings, B.; Verdijk, L.; Churchward-Venne, T.; Koopman, R.; Burd, N.; Fuchs, C.J.; et al. Protein Type, Protein Dose, and Age Modulate Dietary Protein Digestion and Phenylalanine Absorption Kinetics and Plasma Phenylalanine Availability in Humans. J. Nutr. 2020, 150, 2041–2050. [Google Scholar] [CrossRef]
- Ten Have, G.A.; Engelen, M.P.; Luiking, Y.C.; Deutz, N.E. Absorption kinetics of amino acids, peptides, and intact proteins. Int. J. Sport Nutr. Exerc. Metab. 2007, 17 (Suppl. l), S23–S36. [Google Scholar] [CrossRef]
- Chevalier, S.; Gougeon, R.; Kreisman, S.H.; Cassis, C.; Morais, J.A. The hyperinsulinemic amino acid clamp increases whole-body protein synthesis in young subjects. Metabolism 2004, 53, 388–396. [Google Scholar] [CrossRef]
- MacLeod, E.L.; Clayton, M.K.; van Calcar, S.C.; Ney, D.M. Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria. Mol. Genet. Metab. 2010, 100, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Castellino, P.; DeFronzo, R.A. Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Diabetes 1996, 45, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; Weijzen, M.E.G.; van Kranenburg, J.; Ganzevles, R.A.; Beelen, M.; Verdijk, L.B.; van Loon, L.J.C. Casein Protein Processing Strongly Modulates Post-Prandial Plasma Amino Acid Responses In Vivo in Humans. Nutrients 2020, 12, 2299. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Traylor, D.A.; Gorissen, S.H.M.; Hopper, H.; Prior, T.; McGlory, C.; Phillips, S.M. Aminoacidemia following ingestion of native whey protein, micellar casein, and a whey-casein blend in young men. Appl. Physiol. Nutr. Metab. 2019, 44, 103–106. [Google Scholar] [CrossRef] [PubMed]
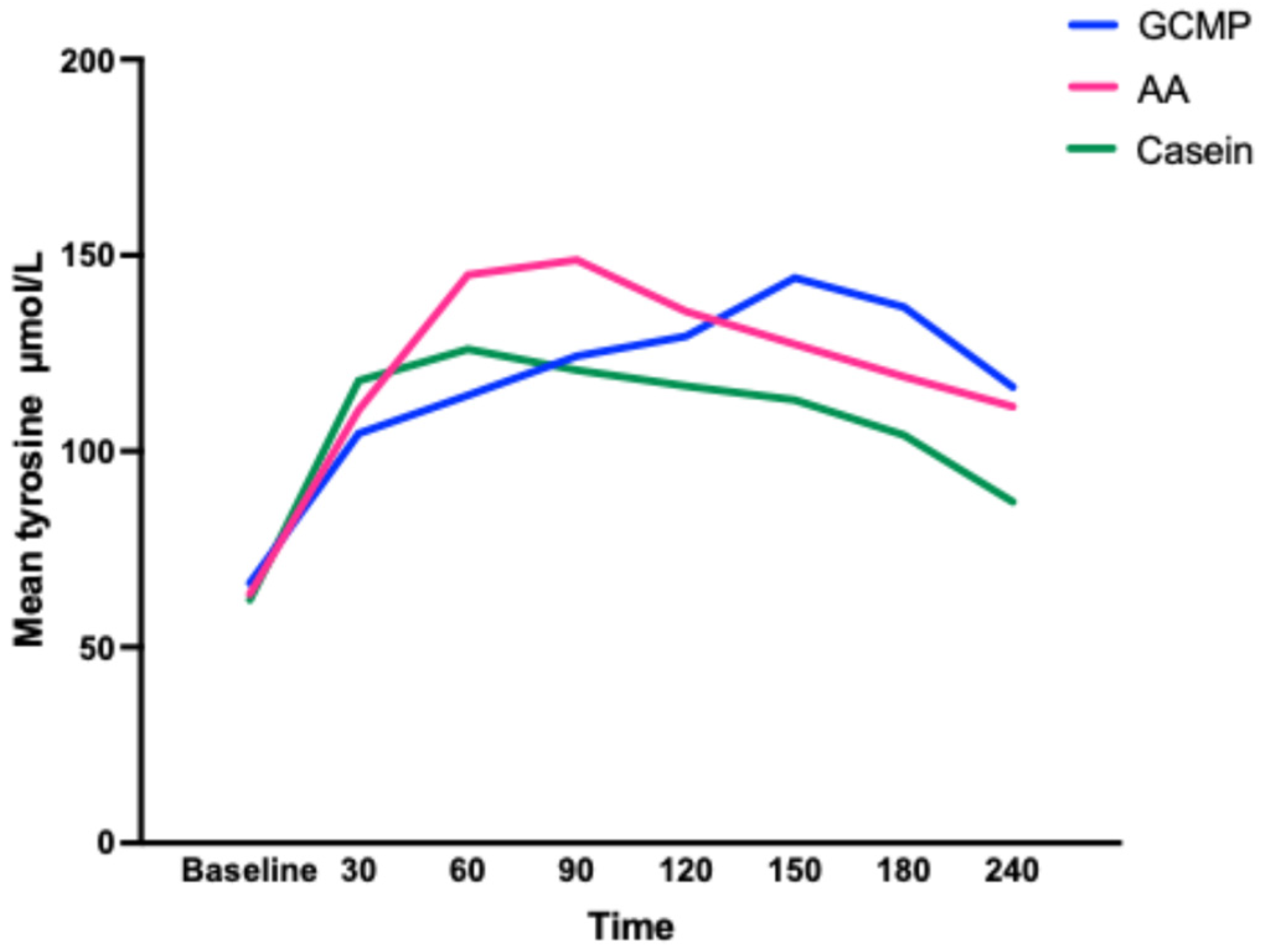
- Ney, D.M.; Murali, S.G.; Stroup, B.M.; Nair, N.; Sawin, E.A.; Rohr, F.; Levy, H.L. Metabolomic changes demonstrate reduced bioavailability of tyrosine and altered metabolism of tryptophan via the kynurenine pathway with ingestion of medical foods in phenylketonuria. Mol. Genet. Metab. 2017, 121, 96–103. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Chahal, S.; Santra, S.; Pinto, A.; Gingell, C.; Rocha, J.C.; van Spronsen, F.; Jackson, R.; MacDonald, A. The Effect of Glycomacropeptide versus Amino Acids on Phenylalanine and Tyrosine Variability over 24 Hours in Children with PKU: A Randomized Controlled Trial. Nutrients 2019, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Darmaun, D. Role of nutrients in the regulation of in vivo protein metabolism in humans. Acta Paediatr. Suppl. 1999, 88, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, N.; Gallina, G.; Panzeri, V.; Frangi, A.; Canobbio, A.; Reiner, G. A New Phe-Free Protein Substitute Engineered to Allow a Physiological Absorption of Free Amino Acids for Phenylketonuria. J. Inborn Errors Metab. Screen. 2018, 6, 2326409818783780. [Google Scholar] [CrossRef]
- van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [CrossRef]
- Calbet, J.A.; MacLean, D.A. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar] [CrossRef]
- Rasmussen, B.; Gilbert, E.; Turki, A.; Madden, K.; Elango, R. Determination of the safety of leucine supplementation in healthy elderly men. Amino Acids 2016, 48, 1707–1716. [Google Scholar] [CrossRef]
- Nakayama, K.; Sanbongi, C.; Ikegami, S. Effects of Whey Protein Hydrolysate Ingestion on Postprandial Aminoacidemia Compared with a Free Amino Acid Mixture in Young Men. Nutrients 2018, 10, 507. [Google Scholar] [CrossRef]
- Alexander, D.D.; Yan, J.; Bylsma, L.C.; Northington, R.S.; Grathwohl, D.; Steenhout, P.; Erdmann, P.; Spivey-Krobath, E.; Haschke, F. Growth of infants consuming whey-predominant term infant formulas with a protein content of 1.8 g/100 kcal: A multicenter pooled analysis of individual participant data. Am. J. Clin. Nutr. 2016, 104, 1083–1092. [Google Scholar] [CrossRef]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Clinical relevance of intestinal peptide uptake. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Hogler, W.; Crabtree, N.; Shaw, N.; Evans, S.; Pinto, A.; Jackson, R.; Strauss, B.J.; Wilcox, G.; Rocha, J.C.; et al. Growth and Body Composition in PKU Children-A Three-Year Prospective Study Comparing the Effects of L-Amino Acid to Glycomacropeptide Protein Substitutes. Nutrients 2021, 13, 1323. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A. How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. J. Int. Soc. Sports Nutr. 2018, 15, 10. [Google Scholar] [CrossRef] [PubMed]
| Nutrient Intake per 28 g Protein Ingested | L-AA Cooler (Vitaflo Ltd.) | CGMP Sphere (Vitaflo Ltd.) | Casein (Holland and Barrett) |
| Energy Kcal | 183 | 166 + 3.8 mL calogen * 183 | 136 + 10.4 mL calogen * 183 |
| Fat g | 2.2 | 2.3 CGMP + 1.9 Calogen | 0.83 Casein + 5.2 Calogen |
| Carbohydrate g | 12.4 | 3.5 | 4.1 |
| Protein equivalent g | 28 | 28 | 28 |
| L-phenylalanine g | - | 0.052 | 1.35 |
| Amino Acid (g) | L-AA PKU Cooler (Vitaflo Ltd.) | CGMP Sphere (Vitaflo Ltd.) | Casein (Holland and Barrett) |
| L-Alanine | 1.29 | 1.18 | 0.78 |
| L-Arginine | 2.11 | 1.37 | 1.02 |
| L-Aspartic acid | 0.85 | 1.87 | 1.88 |
| L-Cystine | 3.29 | 0.35 | 0.10 |
| L-Glutamine | - | 3.86 | 5.85 |
| Glycine | 3.29 | 1.01 | 0.60 |
| L-Histidine | 1.29 | 1.01 | 0.81 |
| L-Isoleucine | 2.27 | 2.03 | 1.55 |
| L-Leucine | 3.56 | 4.31 | 2.52 |
| L-Lysine | 2.34 | 1.36 | 2.15 |
| L-Methionine | 0.63 | 0.40 | 0.76 |
| L-Phenylalanine | 0.36 | ||
| L-Proline | 2.37 | 2.29 | 2.86 |
| L-Serine | 1.46 | 1.44 | 1.65 |
| L-Threonine | 2.27 | 3.28 | 1.23 |
| L-Tryptophan | 0.71 | 0.58 | 0.35 |
| L-Tyrosine | 3.34 | 3.22 | 1.00 |
| L-Valine | 2.51 | 1.64 | 1.90 |
| L-Carnitine | 0.032 | - | - |
| Taurine | 0.061 | - | - |
| Total L-AAs excluding carnitine and taurine | 33.6 | 31.2 | 27 |
| Test Product | AUC μmol/L minutes | Test Product | Model Est. (95% CI) | p-Value | |
|---|---|---|---|---|---|
| TAAs | L-AA | 706,116 (650,643, 706,116) | L-AA vs. CGMP | −21,401 (−104,860, 62,058) | 0.617 |
| CGMP | 659,856 (588,521, 659,856) | L-AA vs. Casein | −115,163 (−197,631, −32,695) | 0.008 * | |
| Casein | 563,634 (492,437, 563,634) | CGMP vs. Casein | 21,401 (−62,058, 104,860) | 0.026 * | |
| BCAAs | L-AA | 226,804 (206,987, 226,804) | L-AA vs. CGMP | −7670 (−30,666, 15,327) | 0.516 |
| CGMP | 213,680 (200,919, 213,680) | L-AA vs. Casein | −46,054 (−69,050, −23,057) | <0.001 * | |
| Casein | 175,279 (156,044, 175,279) | CGMP vs. Casein | 7670 (−15,327, 30,666) | 0.002 * | |
| LNAAs | L-AA | 381,375 (351,077, 381,375) | L-AA vs. CGMP | 168 (−38,958, 39,293) | 0.993 |
| CGMP | 381,062 (322,099, 381,062) | L-AA vs. Casein | −36,918 (−76,043, 2208) | 0.070 | |
| Casein | 333,029 (277,647, 333,029) | CGMP vs. Casein | −168 (−39,293, 38,958) | 0.068 | |
| EAAs | L-AA | 355,626 (322,746, 355,626) | L-AA vs. CGMP | 2054 (−36,405, 40,513) | 0.917 |
| CGMP | 353,123 (296,958, 353,123) | L-AA vs. Casein | −33,026 (−71,485, 5433) | 0.098 | |
| Casein | 298,301 (255,697, 298,301) | CGMP vs. Casein | −2054 (−40,513, 36,405) | 0.079 | |
| Glucose | L-AA | 1034 (984, 1034) | L-AA vs. CGMP | 19 (−135, 172) | 0.812 |
| CGMP | 1007 (962, 1007) | L-AA vs. Casein | −47 (−199, 105) | 0.547 | |
| Casein | 1033 (948, 1033) | CGMP vs. Casein | −19 (−172, 135) | 0.387 | |
| Insulin log | L-AA | 1001 (880, 1001) | L-AA vs. CGMP | 5824 (−9711, 21,360) | 0.721 |
| CGMP | 998 (931, 998) | L-AA vs. Casein | −2688 (−18,223, 12,847) | 0.417 | |
| Casein | 937 (768, 937) | CGMP vs. Casein | −5825 (−21,360, 9711) | 0.648 | |
| Urea | L-AA | 1061 (983, 1061) | L-AA vs. CGMP | 34 (−183, 252) | 0.759 |
| CGMP | 1113 (950, 1113) | L-AA vs. Casein | 22 (−193, 237) | 0.840 | |
| Casein | 1079 (891, 1079) | CGMP vs. Casein | −34 (−252, 183) | 0.911 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daly, A.; Pinto, A.; Evans, S.; Geberhiwot, T.; Jackson, R.; Rocha, J.C.; Tang, J.C.Y.; MacDonald, A. Protein Substitute Absorption: A Randomised Controlled Trial Comparing CGMP vs. Amino Acids vs. Micellar Casein in Healthy Volunteers. Nutrients 2025, 17, 2671. https://doi.org/10.3390/nu17162671
Daly A, Pinto A, Evans S, Geberhiwot T, Jackson R, Rocha JC, Tang JCY, MacDonald A. Protein Substitute Absorption: A Randomised Controlled Trial Comparing CGMP vs. Amino Acids vs. Micellar Casein in Healthy Volunteers. Nutrients. 2025; 17(16):2671. https://doi.org/10.3390/nu17162671
Chicago/Turabian StyleDaly, Anne, Alex Pinto, Sharon Evans, Tarekegn Geberhiwot, Richard Jackson, Júlio César Rocha, Jonathan C. Y. Tang, and Anita MacDonald. 2025. "Protein Substitute Absorption: A Randomised Controlled Trial Comparing CGMP vs. Amino Acids vs. Micellar Casein in Healthy Volunteers" Nutrients 17, no. 16: 2671. https://doi.org/10.3390/nu17162671
APA StyleDaly, A., Pinto, A., Evans, S., Geberhiwot, T., Jackson, R., Rocha, J. C., Tang, J. C. Y., & MacDonald, A. (2025). Protein Substitute Absorption: A Randomised Controlled Trial Comparing CGMP vs. Amino Acids vs. Micellar Casein in Healthy Volunteers. Nutrients, 17(16), 2671. https://doi.org/10.3390/nu17162671







