Tea Consumption and Liver Cancer: A Population-Based Case–Control Study in Eastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Assessment of Tea Consumption
2.5. Assessment of Covariates
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HBsAg | Hepatitis B surface antigen |
| EGCG | Epigallocatechin gallate |
| ECG | Epicatechin gallate |
| EGC | Epigallocatechin |
| EC | Epicatechin |
| HCC | Hepatocellular carcinoma |
| ELISA | Enzyme-linked immunosorbent assay |
| BMI | Body mass index |
| SB | Semi-Bayes |
| RERI | Relative excess risk due to interaction |
| AP | Attributable proportion due to interaction |
| S | Synergy index |
| ROR | Ratio of the odds ratios |
| CKB | China Kadoorie Biobank |
| GCG | Gallocatechin gallate |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The Relationship between the Structure and Biological Actions of Green Tea Catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xia, G.; Luo, Z.; Liu, S. UHPLC Analysis of Major Functional Components in Six Types of Chinese Teas: Constituent Profile and Origin Consideration. LWT 2019, 102, 52–57. [Google Scholar] [CrossRef]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of High Oral Dose (−)-Epigallocatechin-3-Gallate in Mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef]
- Kim, T.L.; Jeong, G.H.; Yang, J.W.; Lee, K.H.; Kronbichler, A.; van der Vliet, H.J.; Grosso, G.; Galvano, F.; Aune, D.; Kim, J.Y.; et al. Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2020, 11, 1437–1452. [Google Scholar] [CrossRef]
- Yu, J.; Liang, D.; Li, J.; Liu, Z.; Zhou, F.; Wang, T.; Ma, S.; Wang, G.; Chen, B.; Chen, W. Coffee, Green Tea Intake, and the Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Cancer 2023, 75, 1295–1308. [Google Scholar] [CrossRef]
- Li, X.; Yu, C.; Guo, Y.; Bian, Z.; Shen, Z.; Yang, L.; Chen, Y.; Wei, Y.; Zhang, H.; Qiu, Z.; et al. Association between Tea Consumption and Risk of Cancer: A Prospective Cohort Study of 0.5 Million Chinese Adults. Eur. J. Epidemiol. 2019, 34, 753–763. [Google Scholar] [CrossRef]
- Zhao, J.-K.; Wu, M.; Kim, C.H.; Jin, Z.-Y.; Zhou, J.-Y.; Han, R.-Q.; Yang, J.; Zhang, X.-F.; Wang, X.-S.; Liu, A.-M.; et al. Jiangsu Four Cancers Study: A Large Case-Control Study of Lung, Liver, Stomach, and Esophageal Cancers in Jiangsu Province, China. Eur. J. Cancer Prev. 2017, 26, 357–364. [Google Scholar] [CrossRef]
- Greenland, S. Bayesian Perspectives for Epidemiological Research. II. Regression Analysis. Int. J. Epidemiol. 2007, 36, 195–202. [Google Scholar] [CrossRef]
- Andersson, T.; Alfredsson, L.; Källberg, H.; Zdravkovic, S.; Ahlbom, A. Calculating Measures of Biological Interaction. Eur. J. Epidemiol. 2005, 20, 575–579. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Lu, X.; Min, H.; Wu, Q.-Q.; Shi, X.-T.; Bian, K.-Q.; Zou, X.-P. Green Tea and Liver Cancer Risk: A Meta-Analysis of Prospective Cohort Studies in Asian Populations. Nutrition 2016, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Cancer and Metastasis: Prevention and Treatment by Green Tea. Cancer Metastasis Rev. 2010, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-M. Cancer Prevention by Green Tea: Evidence from Epidemiologic Studies. Am. J. Clin. Nutr. 2013, 98, 1676S–1681S. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green Tea (Camellia sinensis) for the Prevention of Cancer. Cochrane Database Syst. Rev. 2020, 3, CD005004. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, J.; Kim, E.M.; Kim, K.H.; Kang, S.; Lee, H.; Lee, J. Comprehensive Investigation of the Effects of Brewing Conditions in Sample Preparation of Green Tea Infusions. Molecules 2019, 24, 1735. [Google Scholar] [CrossRef]
- Saklar, S.; Ertas, E.; Ozdemir, I.S.; Karadeniz, B. Effects of Different Brewing Conditions on Catechin Content and Sensory Acceptance in Turkish Green Tea Infusions. J. Food Sci. Technol. 2015, 52, 6639–6646. [Google Scholar] [CrossRef]
- Kelebek, H. LC-DAD-ESI-MS/MS Characterization of Phenolic Constituents in Turkish Black Tea: Effect of Infusion Time and Temperature. Food Chem. 2016, 204, 227–238. [Google Scholar] [CrossRef]
- Bharrhan, S.; Koul, A.; Chopra, K.; Rishi, P. Catechin Suppresses an Array of Signalling Molecules and Modulates Alcohol-Induced Endotoxin Mediated Liver Injury in a Rat Model. PLoS ONE 2011, 6, e20635. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, H.J.; Boo, Y.C. Effect of Green Tea and (-)-Epigallocatechin Gallate on Ethanol-Induced Toxicity in HepG2 Cells. Phytother. Res. PTR 2008, 22, 669–674. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.-C.; Goldstein, B.Y.; Scheider, W.L.; Cai, L.; You, N.-C.Y.; Tarleton, H.P.; Ding, B.; Zhao, J.; Wu, M.; et al. Green Tea Consumption, Inflammation and the Risk of Primary Hepatocellular Carcinoma in a Chinese Population. Cancer Epidemiol. 2011, 35, 362–368. [Google Scholar] [CrossRef]
- Inoue, M.; Kurahashi, N.; Iwasaki, M.; Shimazu, T.; Tanaka, Y.; Mizokami, M.; Tsugane, S.; Japan Public Health Center-Based Prospective Study Group. Effect of Coffee and Green Tea Consumption on the Risk of Liver Cancer: Cohort Analysis by Hepatitis Virus Infection Status. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1746–1753. [Google Scholar] [CrossRef]
- Iso, H.; Kubota, Y.; Japan Collaborative Cohort Study for Evaluation of Cancer. Nutrition and Disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac. J. Cancer Prev. 2007, 8, 35–80. [Google Scholar]
- Nagano, J.; Kono, S.; Preston, D.L.; Mabuchi, K. A Prospective Study of Green Tea Consumption and Cancer Incidence, Hiroshima and Nagasaki (Japan). Cancer Causes Control 2001, 12, 501–508. [Google Scholar] [CrossRef]
| Characteristics | Case (n, %) | Tea Consumption (%) | Control (n, %) | Tea Consumption (%) | χ2 | p * | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Never | Former | Current | Never | Former | Current | |||||
| Total | 2011 | 80.6 | 9.2 | 10.2 | 7933 | 76.4 | 2.2 | 21.5 | ||
| County of residence | 42.271 | <0.001 | ||||||||
| Dafeng | 632 (31.4) | 80.5 | 8.7 | 10.8 | 2534 (31.9) | 73.1 | 3.1 | 23.8 | ||
| Ganyu | 390 (19.4) | 55.6 | 21.5 | 22.8 | 2010 (25.3) | 58.6 | 2.4 | 39.0 | ||
| Chuzhou | 301 (15.0) | 81.0 | 10.0 | 9.0 | 1134 (14.3) | 77.7 | 2.8 | 19.5 | ||
| Tongshan | 688 (34.2) | 94.6 | 2.2 | 3.2 | 2255 (28.5) | 95.3 | 0.6 | 4.2 | ||
| Sex | 15.441 | <0.001 | ||||||||
| Male | 1534 (76.3) | 75.7 | 11.6 | 12.7 | 5705 (71.9) | 70.3 | 2.7 | 26.9 | ||
| Female | 477 (23.7) | 96.2 | 1.3 | 2.5 | 2228 (28.1) | 91.7 | 0.8 | 7.5 | ||
| Age group (years) | 338.90 | <0.001 | ||||||||
| <50 | 471 (23.4) | 84.1 | 8.3 | 7.6 | 872 (11.0) | 78.0 | 0.7 | 21.3 | ||
| 50–59 | 603 (30.0) | 79.9 | 8.8 | 11.3 | 1773 (22.4) | 74.9 | 2.4 | 22.7 | ||
| 60–69 | 515 (25.6) | 78.6 | 10.9 | 10.5 | 2542 (32.0) | 76.2 | 1.9 | 22.0 | ||
| 70–79 | 322 (16.0) | 77.6 | 10.3 | 12.1 | 2168 (27.3) | 75.5 | 3.0 | 21.5 | ||
| 80+ | 100 (5.0) | 88.0 | 3.0 | 9.0 | 578 (7.3) | 82.4 | 2.3 | 15.4 | ||
| In marriage | 22.886 | <0.001 | ||||||||
| Yes | 1717 (86.0) | 80.6 | 9.3 | 10.1 | 6426 (81.5) | 75.4 | 2.2 | 22.4 | ||
| No | 279 (14.0) | 81.0 | 7.9 | 11.1 | 1463 (18.5) | 80.4 | 2.0 | 17.6 | ||
| Missing | 15 | 44 | ||||||||
| Education level | 81.962 | <0.001 | ||||||||
| Illiteracy | 764 (38.2) | 83.3 | 8.5 | 8.3 | 3796 (47.9) | 80.1 | 1.8 | 18.2 | ||
| Primary | 662 (33.1) | 80.4 | 9.5 | 10.1 | 2490 (31.5) | 74.1 | 2.7 | 23.3 | ||
| Middle | 461 (23.1) | 79.6 | 10.0 | 10.4 | 1298 (16.4) | 72.5 | 2.5 | 25.0 | ||
| High | 101 (5.0) | 70.3 | 6.9 | 22.8 | 292 (3.7) | 67.1 | 2.4 | 30.5 | ||
| College | 12 (0.6) | 58.3 | 16.7 | 25.0 | 40 (0.5) | 52.5 | 2.5 | 45.0 | ||
| missing | 11 | 17 | ||||||||
| Per-capita family income 10 years earlier (RMB yuan/year) | 5.849 | 0.119 | ||||||||
| <1000 | 392 (20.0) | 80.6 | 10.2 | 9.2 | 1656 (21.3) | 76.0 | 2.2 | 21.7 | ||
| 1000–1499 | 405 (20.6) | 79.5 | 8.4 | 12.1 | 1512 (19.5) | 78.4 | 1.7 | 20.0 | ||
| 1500–2499 | 487 (24.8) | 82.3 | 10.3 | 7.4 | 2057 (26.5) | 77.8 | 2.2 | 20.0 | ||
| 2500+ | 680 (34.6) | 79.9 | 7.7 | 12.5 | 2542 (32.7) | 74.6 | 2.4 | 23.0 | ||
| missing | 47 | 166 | ||||||||
| BMI | 122.36 | <0.001 | ||||||||
| <18.5 | 211 (10.7) | 81.0 | 7.1 | 11.9 | 452 (5.7) | 79.9 | 1.3 | 18.8 | ||
| 18.5–23.9 | 1315 (66.4) | 80.3 | 10.2 | 9.5 | 4791 (60.7) | 76.2 | 2.1 | 21.7 | ||
| 24.0–27.9 | 379 (19.1) | 83.1 | 7.4 | 9.5 | 2198 (27.9) | 77.1 | 2.3 | 20.6 | ||
| 28.0+ | 75 (3.8) | 65.3 | 8.0 | 26.7 | 449 (5.7) | 71.5 | 3.1 | 25.4 | ||
| missing | 31 | 43 | ||||||||
| HBsAg | 1273.0 | <0.001 | ||||||||
| Negative | 689 (56.8) | 89.3 | 4.5 | 6.2 | 6074 (93.5) | 77.2 | 2.0 | 20.8 | ||
| Positive | 524 (43.2) | 79.4 | 9.4 | 11.3 | 425 (6.5) | 78.6 | 1.7 | 19.8 | ||
| missing | 798 | 1434 | ||||||||
| Ever alcohol drinker | 59.407 | <0.001 | ||||||||
| No | 885 (44.0) | 91.8 | 3.2 | 5.1 | 4254 (53.6) | 88.9 | 1.4 | 9.7 | ||
| Yes | 1126 (56.0) | 71.9 | 13.9 | 14.3 | 3679 (46.4) | 61.8 | 3.1 | 35.0 | ||
| Ever tobacco smoker | 17.231 | <0.001 | ||||||||
| No | 971 (48.3) | 91.7 | 4.3 | 4.0 | 4241 (53.5) | 88.1 | 1.2 | 10.7 | ||
| Yes | 1040 (51.7) | 70.3 | 13.7 | 16.1 | 3692 (46.5) | 62.8 | 3.3 | 33.9 | ||
| Family history of liver cancer | 359.49 | <0.001 | ||||||||
| No | 1735 (86.3) | 80.4 | 9.3 | 10.3 | 7684 (96.9) | 76.5 | 2.2 | 21.3 | ||
| Yes | 276 (13.7) | 81.9 | 8.0 | 10.1 | 249 (3.1) | 73.1 | 1.6 | 25.3 | ||
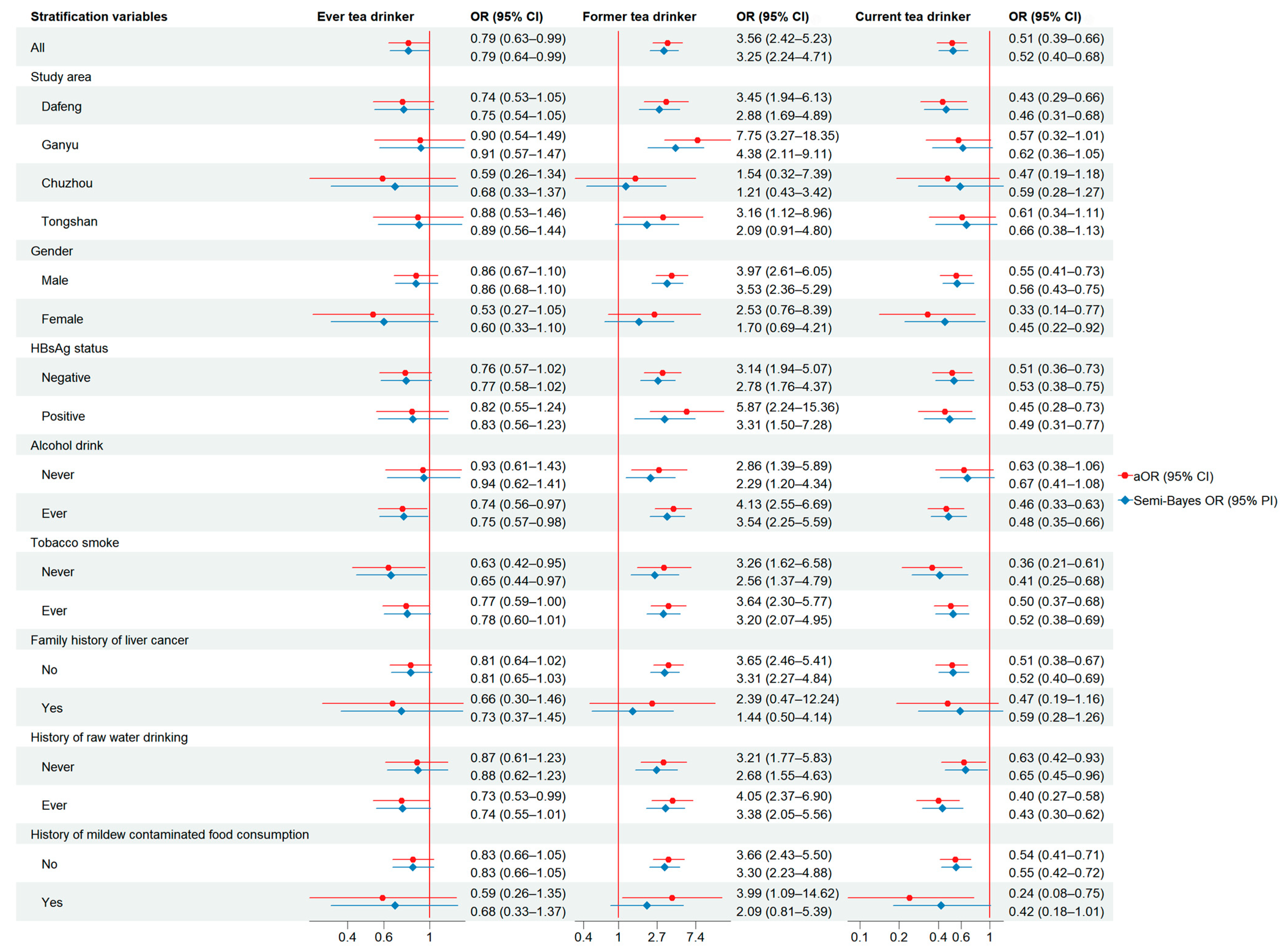
| Tea Drinking Patterns | Cases | Controls | cOR (95% CI) | aOR (95% CI) * | SB-aOR (95% CI) |
|---|---|---|---|---|---|
| Ever tea drinker | |||||
| No | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| Yes | 390 | 1876 | 0.78 (0.69–0.88) | 0.79 (0.63–0.99) | 0.79 (0.64–0.99) |
| Regular tea drinker | |||||
| No | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| Former | 184 | 173 | 3.97 (3.21–4.93) | 3.56 (2.42–5.23) | 3.25 (2.24–4.71) |
| Current | 206 | 1703 | 0.45 (0.39–0.53) | 0.51 (0.39–0.66) | 0.52 (0.40–0.68) |
| Tea type | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| Green | 301 | 1434 | 0.78 (0.69–0.90) | 0.80 (0.62–1.04) | 0.81 (0.63–1.04) |
| Black | 37 | 223 | 0.62 (0.44–0.88) | 0.65 (0.39–1.10) | 0.69 (0.42–1.11) |
| Flower | 27 | 81 | 1.25 (0.80–1.93) | 0.96 (0.47–1.96) | 0.97 (0.51–1.83) |
| Oolong | 2 | 22 | 0.34 (0.08–1.45) | 1.34 (0.25–7.11) | 1.13 (0.39–3.28) |
| Other | 19 | 97 | 0.73 (0.45–1.20) | 0.85 (0.39–1.85) | 0.88 (0.45–1.74) |
| Monthly tea-leaf consumption (g) | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| 1–50 | 51 | 406 | 0.47 (0.35–0.63) | 0.62 (0.40–0.96) | 0.65 (0.43–0.98) |
| 51–100 | 103 | 481 | 0.80 (0.64–1.00) | 0.81 (0.56–1.17) | 0.82 (0.58–1.17) |
| 101–200 | 88 | 374 | 0.88 (0.69–1.12) | 0.89 (0.58–1.36) | 0.90 (0.60–1.35) |
| 200+ | 135 | 550 | 0.92 (0.75–1.12) | 0.90 (0.61–1.33) | 0.91 (0.62–1.32) |
| Daily frequency of adding fresh leaves | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| 1 | 106 | 643 | 0.62 (0.50–0.76) | 0.55 (0.38–0.78) | 0.57 (0.40–0.81) |
| 2 | 172 | 766 | 0.84 (0.71–1.00) | 0.98 (0.72–1.32) | 0.98 (0.73–1.32) |
| 3 | 66 | 306 | 0.81 (0.61–1.06) | 0.88 (0.53–1.46) | 0.89 (0.56–1.44) |
| 4+ | 40 | 131 | 1.14 (0.80–1.63) | 1.25 (0.63–2.46) | 1.20 (0.65–2.21) |
| Daily frequency of re-brewing | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| 1~2 | 87 | 404 | 0.81 (0.63–1.02) | 0.62 (0.41–0.94) | 0.64 (0.43–0.96) |
| 3+ | 294 | 1438 | 0.76 (0.67–0.88) | 0.86 (0.67–1.10) | 0.86 (0.68–1.10) |
| Brew strength | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| Light | 61 | 378 | 0.60 (0.46–0.80) | 0.51 (0.31–0.83) | 0.55 (0.35–0.87) |
| Medium | 202 | 974 | 0.78 (0.66–0.91) | 0.73 (0.55–0.98) | 0.74 (0.56–0.98) |
| Strong | 126 | 506 | 0.93 (0.76–1.14) | 1.18 (0.84–1.67) | 1.17 (0.84–1.63) |
| Water temperature | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| Warm | 66 | 353 | 0.70 (0.53–0.91) | 0.88 (0.57–1.37) | 0.89 (0.59–1.35) |
| Spoiled or boil together | 317 | 1499 | 0.79 (0.69–0.90) | 0.78 (0.61–0.99) | 0.79 (0.62–1.00) |
| Temperature of tea when drink | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| Cold | 8 | 61 | 0.49 (0.23–1.03) | 0.27 (0.07–1.02) | 0.51 (0.19–1.33) |
| Warm | 251 | 1308 | 0.72 (0.62–0.83) | 0.76 (0.59–0.98) | 0.77 (0.60–0.98) |
| Hot/very hot | 127 | 476 | 1.00 (0.81–1.22) | 1.03 (0.70–1.50) | 1.03 (0.71–1.48) |
| Water source | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| Tap or purified | 171 | 792 | 0.81 (0.68–0.96) | 0.82 (0.60–1.13) | 0.83 (0.61–1.13) |
| Deep-well | 75 | 504 | 0.56 (0.43–0.71) | 0.47 (0.31–0.70) | 0.50 (0.34–0.74) |
| Shallow-well | 130 | 475 | 1.02 (0.84–1.25) | 1.59 (1.08–2.33) | 1.54 (1.06–2.23) |
| Pool/ditch/river/rain water | 12 | 79 | 0.57 (0.31–1.04) | 0.33 (0.11–1.02) | 0.51 (0.21–1.21) |
| Total years of tea consumption | |||||
| Never drink | 1621 | 6057 | 1.00 | 1.00 | 1.00 |
| <30 | 214 | 968 | 0.83 (0.71–0.97) | 0.69 (0.53–0.91) | 0.70 (0.54–0.91) |
| 30+ | 171 | 875 | 0.73 (0.61–0.87) | 1.02 (0.74–1.41) | 1.02 (0.74–1.39) |
| Variables | Ever Drink Tea | Case/Control | Crude OR (95% CI) | Adjusted OR (95% CI) * | SB-Adjusted OR (95% PI) | Interaction | |
|---|---|---|---|---|---|---|---|
| HBsAg positive | |||||||
| No | Yes | 74/1387 | 1.00 | 1.00 | 1.00 | ROR | 0.46 (0.29–0.72) |
| No | No | 615/4687 | 2.46 (1.92–3.15) | 1.66 (1.25–2.22) | 1.63 (1.23–2.15) | RERI | −5.09 (−11.70–1.53) |
| Yes | Yes | 108/91 | 22.25 (15.46–32.01) | 18.65 (12.42–28.00) | 14.79 (10.02–21.85) | AP | −0.36 (−0.84–0.12) |
| Yes | No | 416/334 | 23.35 (17.74–30.72) | 14.22 (10.41–19.44) | 12.51 (9.22–16.96) | S | 0.72 (0.50–1.05) |
| Ever drink alcohol | |||||||
| No | Yes | 73/472 | 1.00 | 1.00 | 1.00 | ROR | 1.78 (1.10–2.86) |
| No | No | 812/3782 | 1.39 (1.07–1.80) | 0.86 (0.57–1.31) | 0.87 (0.58–1.30) | RERI | 0.45 (0.14–0.76) |
| Yes | Yes | 317/1404 | 1.46 (1.11–1.92) | 1.20 (0.77–1.87) | 1.18 (0.77–1.80) | AP | 0.20 (0.04–0.35) |
| Yes | No | 809/2275 | 2.30 (1.77–2.98) | 1.83 (1.21–2.77) | 1.74 (1.17–2.59) | S | 1.53 (0.95–2.48) |
| Ever smoke | |||||||
| No | Yes | 81/503 | 1.00 | 1.00 | 1.00 | ROR | 1.11 (0.70–1.75) |
| No | No | 890/3738 | 1.48 (1.16–1.89) | 1.28 (0.86–1.92) | 1.26 (0.85–1.85) | RERI | 0.30 (−0.18–0.78) |
| Yes | Yes | 309/1373 | 1.40 (1.07–1.82) | 1.40 (0.91–2.15) | 1.36 (0.90–2.05) | AP | 0.15 (−0.12–0.42) |
| Yes | No | 731/2319 | 1.96 (1.53–2.51) | 1.98 (1.33–2.95) | 1.88 (1.28–2.76) | S | 1.44 (0.59–3.53) |
| Family history of liver cancer | |||||||
| No | Yes | 340/1809 | 1.00 | 1.00 | 1.00 | ROR | 1.21 (0.62–2.37) |
| No | No | 1395/5875 | 1.26 (1.11–1.44) | 1.24 (0.98–1.56) | 1.23 (0.98–1.55) | RERI | 1.55 (−1.00–4.10) |
| Yes | Yes | 50/67 | 3.97 (2.70–5.83) | 3.57 (1.96–6.48) | 2.92 (1.69–5.06) | AP | 0.29 (−0.14–0.72) |
| Yes | No | 226/182 | 6.61 (5.27–8.29) | 5.36 (3.74–7.67) | 4.82 (3.41–6.83) | S | 1.55 (0.69–3.48) |
| Possibility of mildew contamination on food | |||||||
| No | Yes | 344/1672 | 1.00 | 1.00 | 1.00 | ROR | 1.41 (0.66–3.05) |
| No | No | 1414/5534 | 1.24 (1.09–1.41) | 1.21 (0.96–1.53) | 1.20 (0.96–1.51) | RERI | 0.41 (−0.11–0.92) |
| Yes | Yes | 44/181 | 1.18 (0.83–1.68) | 0.92 (0.45–1.87) | 0.94 (0.50–1.76) | AP | 0.22 (−0.04–0.49) |
| Yes | No | 175/465 | 1.83 (1.49–2.26) | 1.57 (1.10–2.23) | 1.53 (1.08–2.15) | S | 1.96 (0.66–5.83) |
| Ever drink raw water | |||||||
| No | Yes | 177/933 | 1.00 | 1.00 | 1.00 | ROR | 1.41 (0.93–2.14) |
| No | No | 535/2595 | 1.09 (0.90–1.31) | 1.01 (0.73–1.39) | 1.01 (0.74–1.38) | RERI | 0.35 (0.10–0.61) |
| Yes | Yes | 204/896 | 1.20 (0.96–1.50) | 0.98 (0.67–1.43) | 0.98 (0.68–1.41) | AP | 0.22 (0.05–0.39) |
| Yes | No | 1034/3320 | 1.64 (1.38–1.96) | 1.40 (1.04–1.90) | 1.38 (1.03–1.85) | S | 2.24 (0.71–7.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Baecker, A.; Wu, M.; Zhou, J.; Jin, Z.; Mu, L.; He, N.; Rao, J.; Lu, Q.-Y.; Li, L.; et al. Tea Consumption and Liver Cancer: A Population-Based Case–Control Study in Eastern China. Nutrients 2025, 17, 2647. https://doi.org/10.3390/nu17162647
Liu X, Baecker A, Wu M, Zhou J, Jin Z, Mu L, He N, Rao J, Lu Q-Y, Li L, et al. Tea Consumption and Liver Cancer: A Population-Based Case–Control Study in Eastern China. Nutrients. 2025; 17(16):2647. https://doi.org/10.3390/nu17162647
Chicago/Turabian StyleLiu, Xing, Aileen Baecker, Ming Wu, Jinyi Zhou, Ziyi Jin, Lina Mu, Na He, Jianyu Rao, Qing-Yi Lu, Liming Li, and et al. 2025. "Tea Consumption and Liver Cancer: A Population-Based Case–Control Study in Eastern China" Nutrients 17, no. 16: 2647. https://doi.org/10.3390/nu17162647
APA StyleLiu, X., Baecker, A., Wu, M., Zhou, J., Jin, Z., Mu, L., He, N., Rao, J., Lu, Q.-Y., Li, L., Zhao, J.-K., & Zhang, Z.-F. (2025). Tea Consumption and Liver Cancer: A Population-Based Case–Control Study in Eastern China. Nutrients, 17(16), 2647. https://doi.org/10.3390/nu17162647





