The Natural Compound CalebinA Suppresses Gemcitabine Resistance and Tumor Progression by Inhibiting Angiogenesis and Invasion Through NF-κB Signaling in Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines and Cell Culture
2.3. Development of Gemcitabine-Resistant Pancreatic Cancer Cell Lines
2.4. Cell Viability Assay
2.5. Nuclear Fraction Preparation and NF-κB p65 Activity Measurement
2.6. Matrigel Invasion Assay
2.7. ELISA
2.8. Tube Formation Assay for Angiogenesis
2.9. Animals
2.10. Experimental Protocol
2.11. Histopathological Analysis
2.12. Statistical Analysis
3. Results
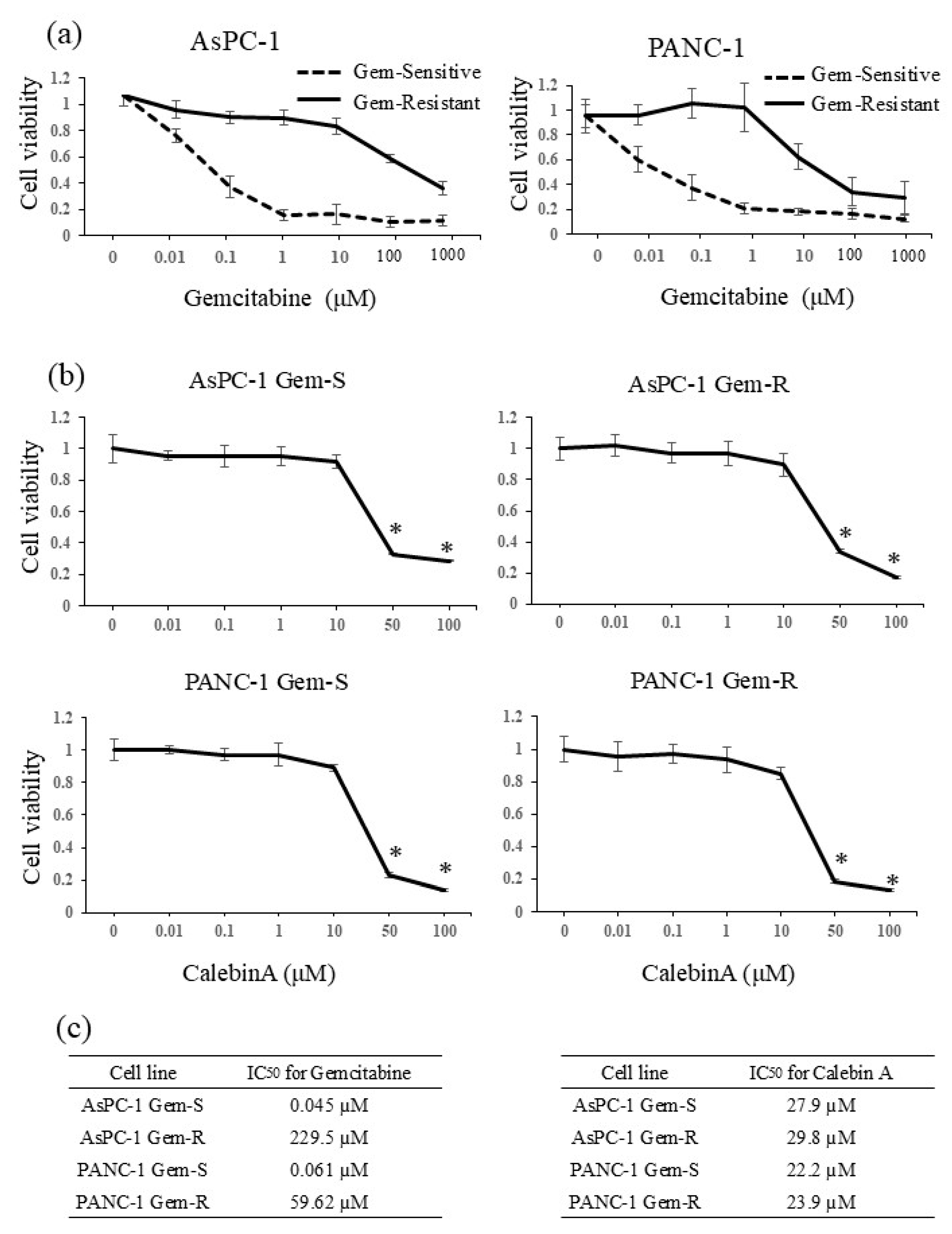
3.1. IC50 Values of Gemcitabine and CalebinA in Pancreatic Cancer Cell Lines
3.2. CalebinA Suppressed NF-κB Activation in Pancreatic Cancer Cells
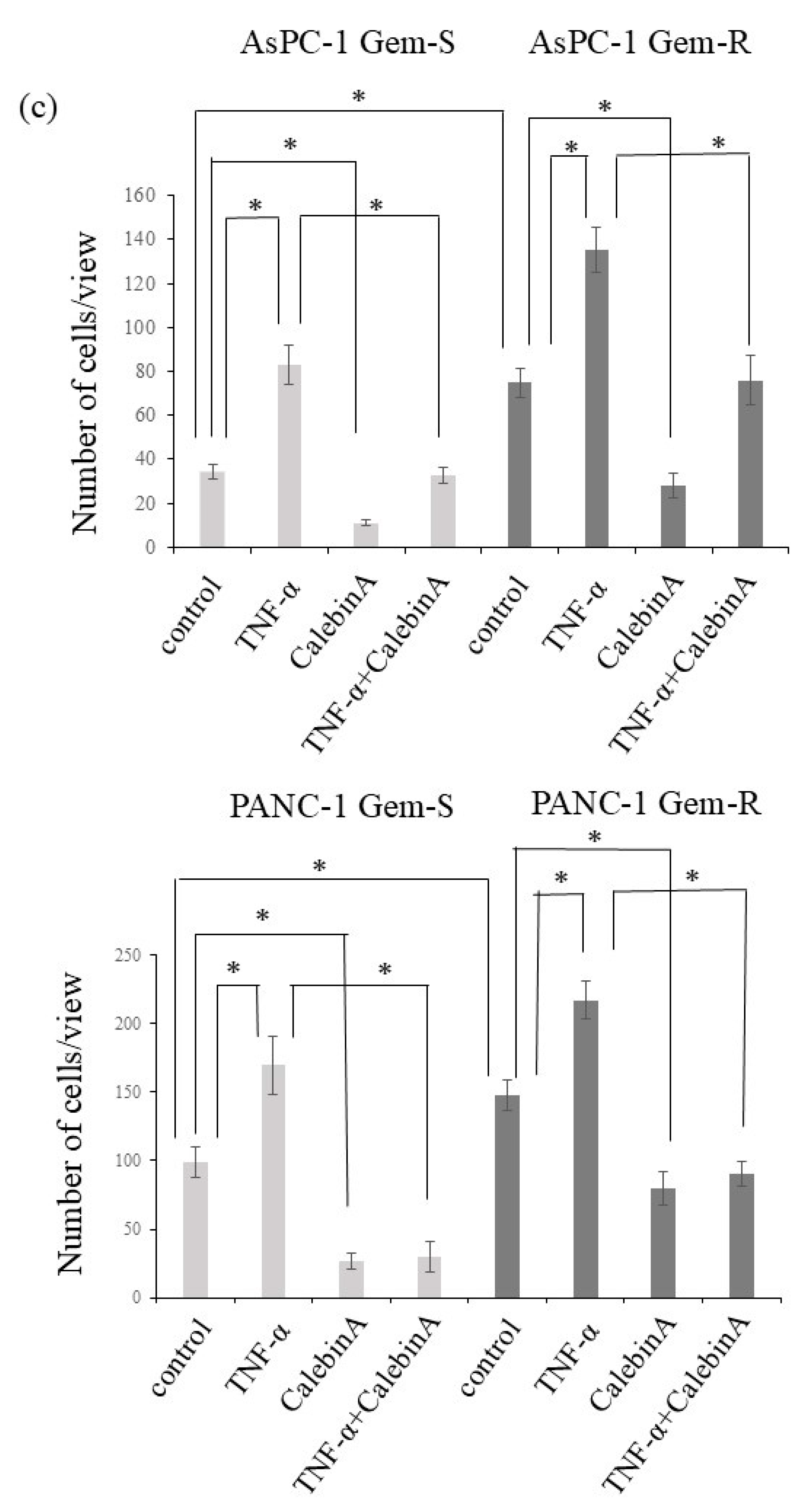
3.3. CalebinA Suppressed the Invasive Ability of Pancreatic Cancer Cells
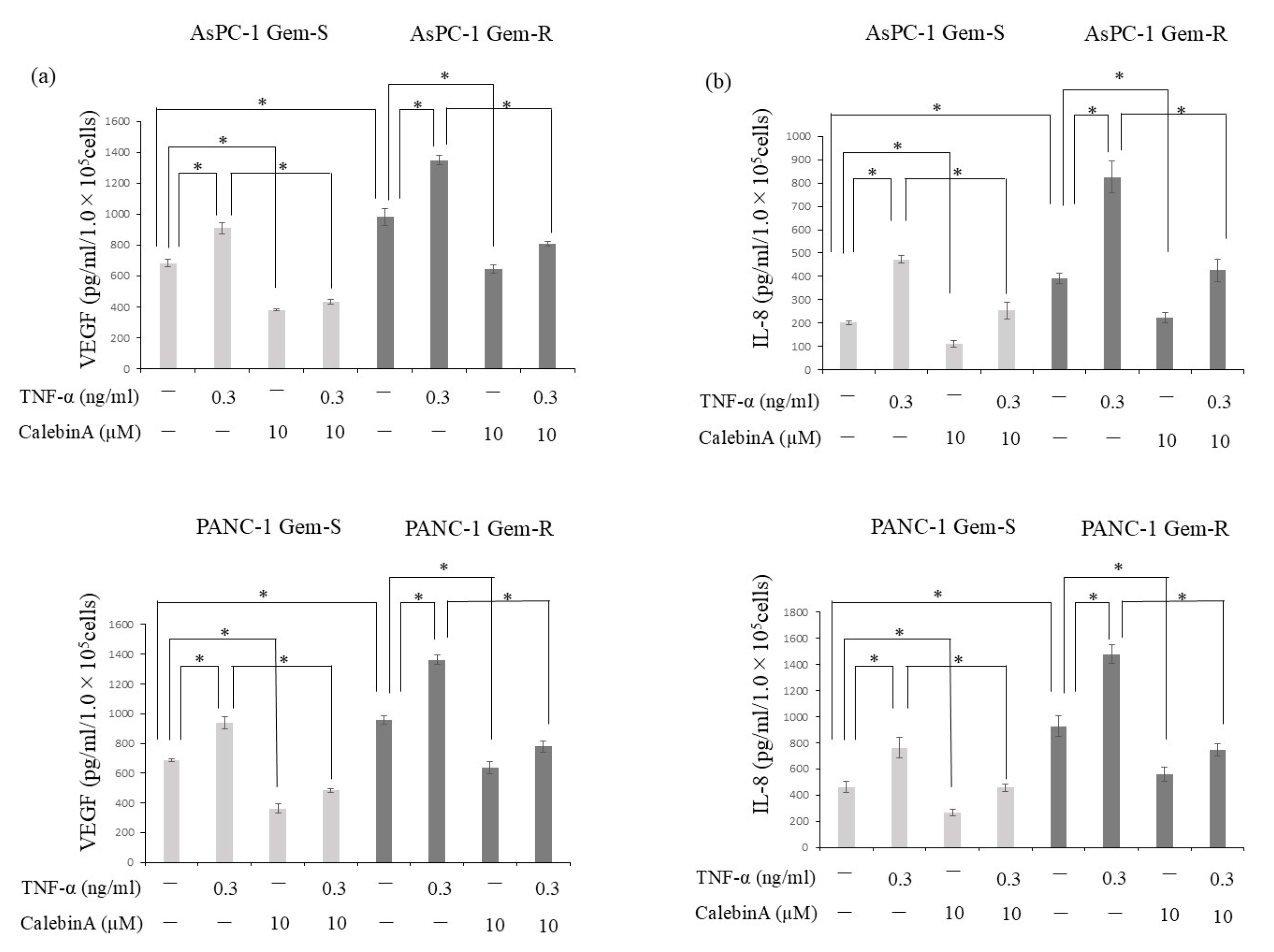
3.4. CalebinA Suppressed the Secretion of VEGF and IL-8 from Pancreatic Cancer Cells
3.5. Angiogenesis in Pancreatic Cancer Is Enhanced by Gemcitabine Resistance but Suppressed by CalebinA
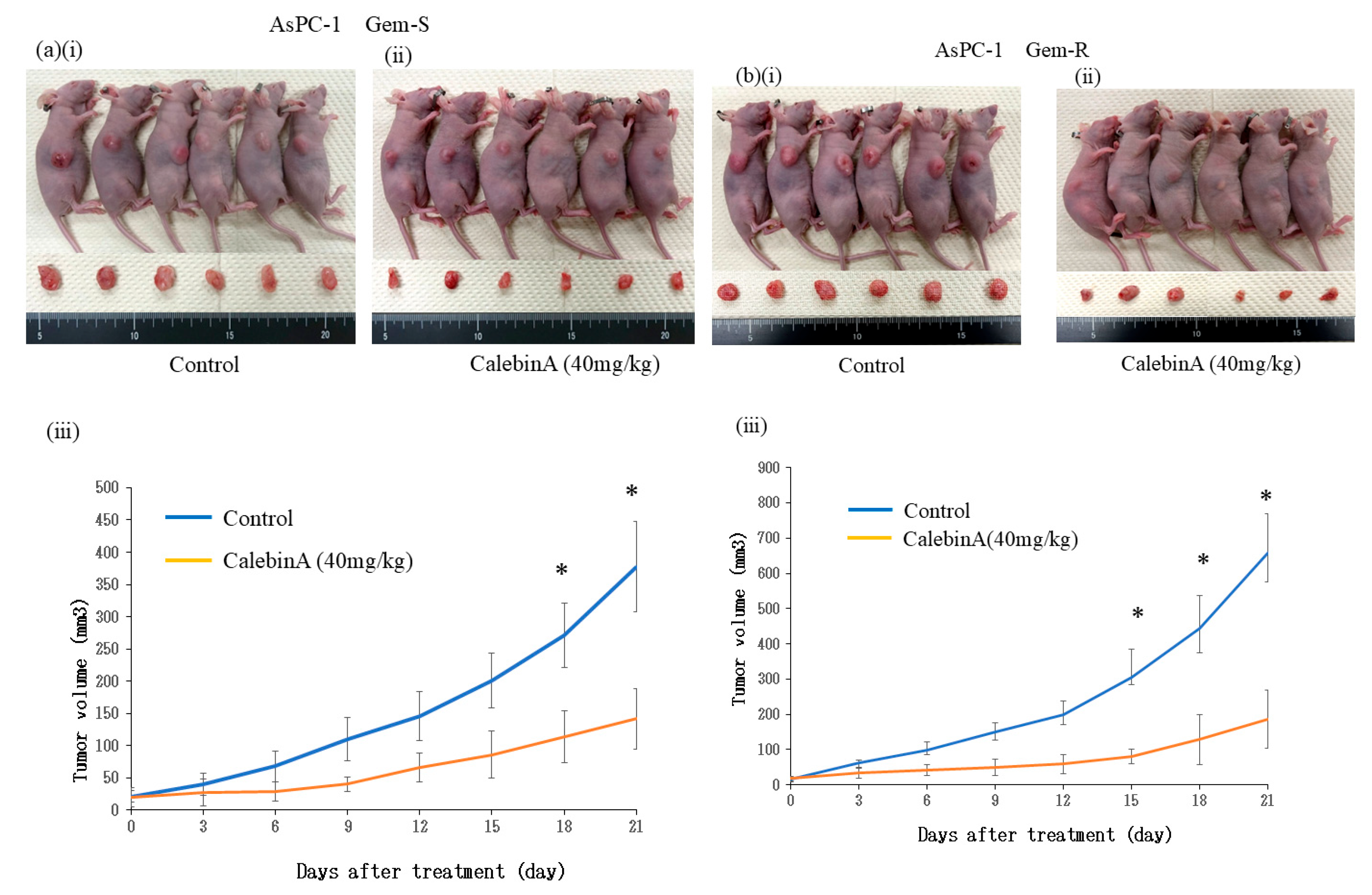
3.6. CalebinA Suppressed Tumor Growth in a Subcutaneous Xenograft Model
3.7. CalebinA Suppressed Proliferation and Angiogenesis in Tumor Tissue by Downregulating the Expression of Ki-67, CD31, and NF-κB p65
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NF-κB | Nuclear factor κB |
| TNFα | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| IL8 | Interleukin-8 |
| Gem-S | Gemcitabine-sensitive cells |
| Gem-R | Gemcitabine-resistant cells |
References
- National Cancer Center Japan. Center for Cancer Control and Information Services. 2023. Available online: https://ganjoho.jp/ reg_stat/statistics/stat/summary.html (accessed on 17 February 2024).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Chowdhury, S.; Zhang, M.; Bajaj, V.; Dhir, M.; Are, C. Trends in the Global Incidence of Pancreatic Cancer and a Brief Review of its Histologic and Molecular Subtypes. J. Gastrointest. Cancer 2025, 56, 71. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Miao, R.; Liu, X.; Liu, C.; Liu, Z. Prognosis and survival analysis of patients with pancreatic cancer: Retrospective experience of a single institution. World J. Surg. Oncol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Katanoda, K.; Hori, M.; Saito, E.; Shibata, A.; Ito, Y.; Minami, T.; Ikeda, S.; Suzuki, T.; Matsuda, T. Updated Trends in Cancer in Japan: Incidence in 1985–2015 and Mortality in 1958–2018-A Sign of Decrease in Cancer Incidence. J. Epidemiol. 2021, 31, 426–450. [Google Scholar] [CrossRef]
- Katsuda, M.; Miyazawa, M.; Ojima, T.; Katanuma, A.; Hakamada, K.; Sudo, K.; Asahara, S.; Endo, I.; Ueno, M.; Hara, K.; et al. A double-blind randomized comparative clinical trial to evaluate the safety and efficacy of dendritic cell vaccine loaded with WT1 peptides (TLP0-001) in combination with S-1 in patients with advanced pancreatic cancer refractory to standard chemotherapy. Trials 2019, 20, 242. [Google Scholar] [CrossRef]
- Ioka, T.; Ueno, M.; Ueno, H.; Park, J.O.; Chang, H.M.; Sasahira, N.; Kanai, M.; Chung, I.J.; Ikeda, M.; Nakamori, S.; et al. TAS-118 (S-1 plus leucovorin) versus S-1 in patients with gemcitabine-refractory advanced pancreatic cancer: A randomised, open-label, phase 3 study (GRAPE trial). Eur. J. Cancer 2019, 106, 78–88. [Google Scholar] [CrossRef]
- Denda, Y.; Matsuo, Y.; Sugita, S.; Eguchi, Y.; Nonoyama, K.; Murase, H.; Kato, T.; Imafuji, H.; Saito, K.; Morimoto, M.; et al. The Natural Product Parthenolide Inhibits both Angiogenesis and Invasiveness and Improves Gemcitabine Resistance by Suppressing Nuclear Factor κB Activation in Pancreatic Cancer Cell Lines. Nutrients 2024, 16, 705. [Google Scholar] [CrossRef]
- Perko, N.; Mousa, S.A. Management of Pancreatic Cancer and its Microenvironment: Potential Impact of Nano-Targeting. Cancers 2022, 14, 2879. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, K.; Matsuo, Y.; Sugita, S.; Eguchi, Y.; Denda, Y.; Murase, H.; Kato, T.; Imafuji, H.; Saito, K.; Morimoto, M.; et al. Expression of ZKSCAN3 protein suppresses proliferation, migration, and invasion of pancreatic cancer through autophagy. Cancer Sci. 2024, 115, 1964–1978. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef]
- Imafuji, H.; Matsuo, Y.; Ueda, G.; Omi, K.; Hayashi, Y.; Saito, K.; Tsuboi, K.; Morimoto, M.; Koide, S.; Ogawa, R.; et al. Acquisition of gemcitabine resistance enhances angiogenesis via upregulation of IL-8 production in pancreatic cancer. Oncol. Rep. 2019, 41, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Matsuo, Y.; Murase, H.; Aoyama, Y.; Kato, T.; Omi, K.; Hayashi, Y.; Imafuji, H.; Saito, K.; Tsuboi, K.; et al. 10Z-Hymenialdisine inhibits angiogenesis by suppressing NF-kappaB activation in pancreatic cancer cell lines. Oncol. Rep. 2022, 47, 48. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yi, Y.; Han, C.; Shi, B. NF-kappaB signaling pathway in tumor microenvironment. Front. Immunol. 2024, 15, 1476030. [Google Scholar] [CrossRef]
- Melisi, D.; Chiao, P.J. NF-kappa B as a target for cancer therapy. Expert. Opin. Ther. Targets. 2007, 11, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Karashima, T.; Sweeney, P.; Kamat, A.; Huang, S.; Kim, S.J.; Bar-Eli, M.; McConkey, D.J.; Dinney, C.P. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin. Cancer Res. 2003, 9, 2786–2797. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12855659 (accessed on 10 August 2025).
- Zhang, S.; Lin, Z.N.; Yang, C.F.; Shi, X.; Ong, C.N.; Shen, H.M. Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis 2004, 25, 2191–2199. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ahmad, A.; Banerjee, S.; Azmi, A.S.; Kong, D.; Sarkar, F.H. Pancreatic cancer: Understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 27–33. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Khan, A.Q.; Uddin, S. Anticancer Activity of Natural Compounds. Asian Pac. J. Cancer Prev. 2021, 22, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Cozmin, M.; Lungu, I.I.; Gutu, C.; Stefanache, A.; Duceac, L.D.; Soltuzu, B.D.; Damir, D.; Calin, G.; Bogdan Goroftei, E.R.; Grierosu, C.; et al. Turmeric: From spice to cure. A review of the anti-cancer, radioprotective and anti-inflammatory effects of turmeric sourced compounds. Front. Nutr. 2024, 11, 1399888. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, L.; Lei, J.; Wu, Z.; Ma, Q.; Wang, Z. Curcumin inhibits pancreatic cancer cell invasion and EMT by interfering with tumor-stromal crosstalk under hypoxic conditions via the IL-6/ERK/NF-κB axis. Oncol. Rep. 2020, 44, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Gunther, J.R.; Chadha, A.S.; Guha, S.; Raju, G.S.; Maru, D.M.; Munsell, M.F.; Jiang, Y.; Yang, P.; Felix, E.; Clemons, M.; et al. A phase II randomized double blinded trial evaluating the efficacy of curcumin with pre-operative chemoradiation for rectal cancer. J. Gastrointest. Oncol. 2022, 13, 2938–2950. [Google Scholar] [CrossRef]
- Brockmueller, A.; Girisa, S.; Motallebi, M.; Kunnumakkara, A.B.; Shakibaei, M. CalebinA targets the HIF-1α/NF-κB pathway to suppress colorectal cancer cell migration. Front. Pharmacol. 2023, 14, 1203436. [Google Scholar] [CrossRef]
- Buhrmann, C.; Kunnumakkara, A.B.; Popper, B.; Majeed, M.; Aggarwal, B.B.; Shakibaei, M. CalebinA Potentiates the Effect of 5-FU and TNF-β (Lymphotoxin α) against Human Colorectal Cancer Cells: Potential Role of NF-κB. Int. J. Mol. Sci. 2020, 21, 2393. [Google Scholar] [CrossRef]
- Brockmueller, A.; Mueller, A.L.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Multifunctionality of CalebinA in inflammation, chronic diseases and cancer. Front. Oncol. 2022, 12, 962066. [Google Scholar] [CrossRef]
- Saito, K.; Matsuo, Y.; Imafuji, H.; Okubo, T.; Maeda, Y.; Sato, T.; Shamoto, T.; Tsuboi, K.; Morimoto, M.; Takahashi, H.; et al. Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer. Cancer Sci. 2018, 109, 132–140. [Google Scholar] [CrossRef]
- Omi, K.; Matsuo, Y.; Ueda, G.; Aoyama, Y.; Kato, T.; Hayashi, Y.; Imafuji, H.; Saito, K.; Tsuboi, K.; Morimoto, M.; et al. Escin inhibits angiogenesis by suppressing interleukin-8 and vascular endothelial growth factor production by blocking nuclear factor-κB activation in pancreatic cancer cell lines. Oncol. Rep. 2021, 45, 55. [Google Scholar] [CrossRef]
- Murase, H.; Matsuo, Y.; Denda, Y.; Nonoyama, K.; Kato, T.; Aoyama, Y.; Hayashi, Y.; Imafuji, H.; Saito, K.; Morimoto, M.; et al. Upregulation of integrin-linked kinase enhances tumor progression in gemcitabine-resistant pancreatic cancer. Oncol. Rep. 2023, 50, 164. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Evidence That CalebinA, a Component of Curcuma Longa Suppresses NF-B Mediated Proliferation, Invasion and Metastasis of Human Colorectal Cancer Induced by TNF-beta (Lymphotoxin). Nutrients 2019, 11, 2904. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Tsai, Y.J.; Lin, M.Y.; You, H.L.; Kalyanam, N.; Ho, C.T.; Pan, M.H. CalebinA induced death of malignant peripheral nerve sheath tumor cells by activation of histone acetyltransferase. Phytomedicine 2019, 57, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Lu, Y.Y.; Nagabhushanam, K.; Ho, C.T.; Mei, H.C.; Pan, M.H. CalebinA prevents HFD-induced obesity in mice by promoting thermogenesis and modulating gut microbiota. J. Tradit. Complement. Med. 2023, 13, 119–127. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguchi, Y.; Matsuo, Y.; Ishida, M.; Uehara, Y.; Sugita, S.; Denda, Y.; Nonoyama, K.; Murase, H.; Kato, T.; Saito, K.; et al. The Natural Compound CalebinA Suppresses Gemcitabine Resistance and Tumor Progression by Inhibiting Angiogenesis and Invasion Through NF-κB Signaling in Pancreatic Cancer. Nutrients 2025, 17, 2641. https://doi.org/10.3390/nu17162641
Eguchi Y, Matsuo Y, Ishida M, Uehara Y, Sugita S, Denda Y, Nonoyama K, Murase H, Kato T, Saito K, et al. The Natural Compound CalebinA Suppresses Gemcitabine Resistance and Tumor Progression by Inhibiting Angiogenesis and Invasion Through NF-κB Signaling in Pancreatic Cancer. Nutrients. 2025; 17(16):2641. https://doi.org/10.3390/nu17162641
Chicago/Turabian StyleEguchi, Yuki, Yoichi Matsuo, Masaki Ishida, Yuriko Uehara, Saburo Sugita, Yuki Denda, Keisuke Nonoyama, Hiromichi Murase, Tomokatsu Kato, Kenta Saito, and et al. 2025. "The Natural Compound CalebinA Suppresses Gemcitabine Resistance and Tumor Progression by Inhibiting Angiogenesis and Invasion Through NF-κB Signaling in Pancreatic Cancer" Nutrients 17, no. 16: 2641. https://doi.org/10.3390/nu17162641
APA StyleEguchi, Y., Matsuo, Y., Ishida, M., Uehara, Y., Sugita, S., Denda, Y., Nonoyama, K., Murase, H., Kato, T., Saito, K., Sato, T., Sagawa, H., Yamakawa, Y., Ogawa, R., Takahashi, H., Mitsui, A., & Takiguchi, S. (2025). The Natural Compound CalebinA Suppresses Gemcitabine Resistance and Tumor Progression by Inhibiting Angiogenesis and Invasion Through NF-κB Signaling in Pancreatic Cancer. Nutrients, 17(16), 2641. https://doi.org/10.3390/nu17162641




