Claudin Proteins: Their Potential Role in Obesity and Adipose Tissue Signaling, Physiology and Disease
Abstract
1. Introduction
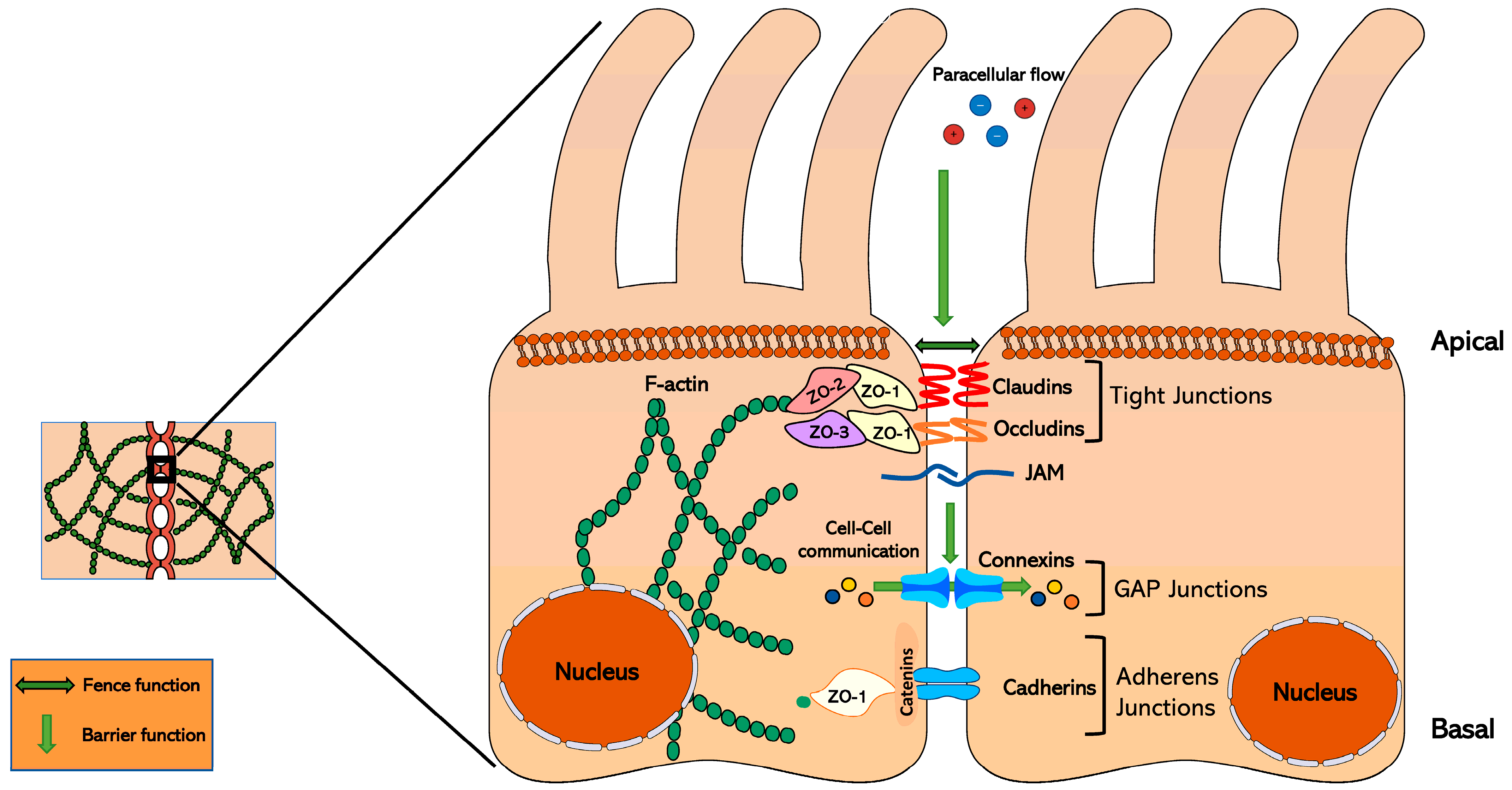
2. Tight Junction Proteins: Junctional Adhesion Molecule, Occludin, and Claudin Structures and Functions
2.1. Junctional Adhesion Molecules and Occludins
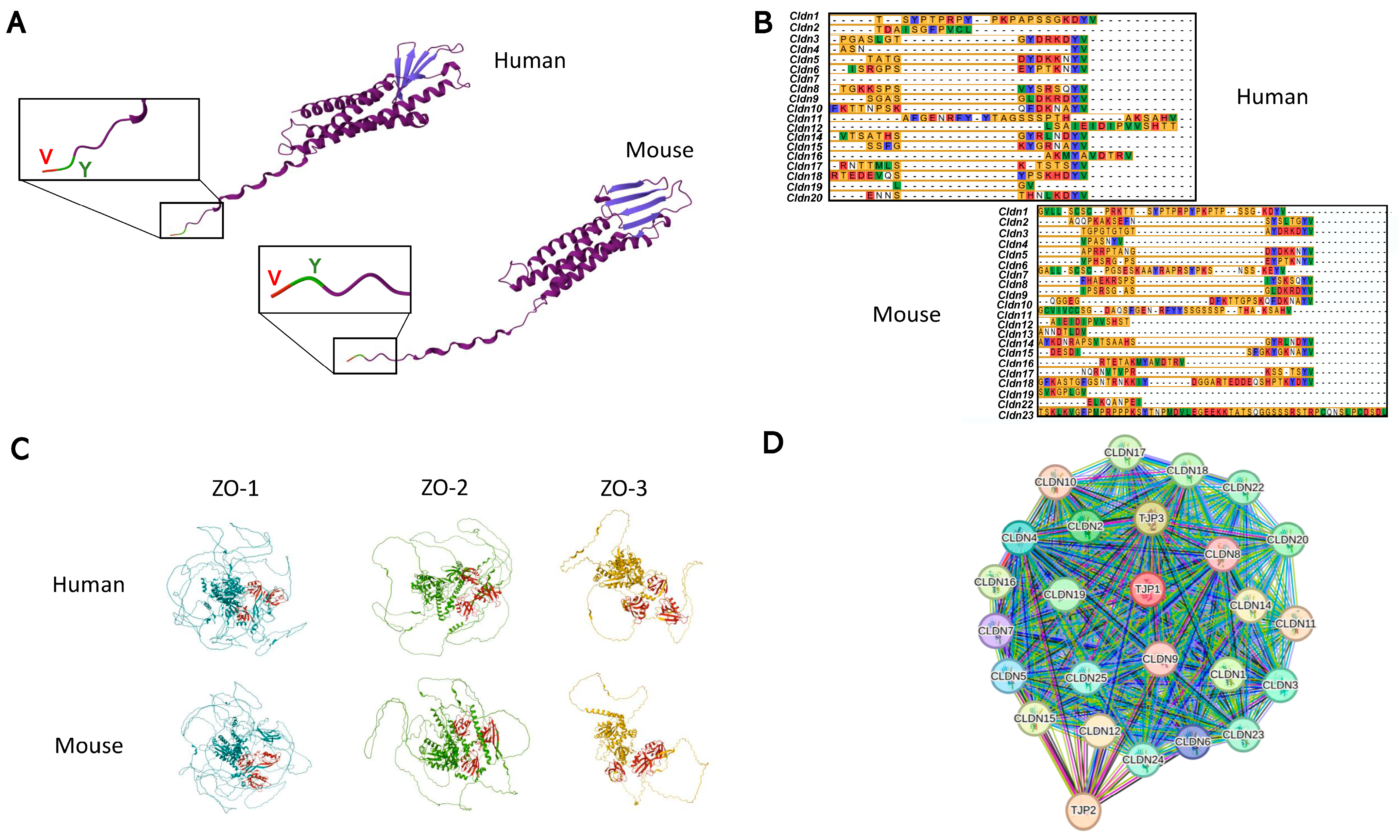
2.2. Claudins
3. Claudins and Cell Signaling
Signaling Hub Locations
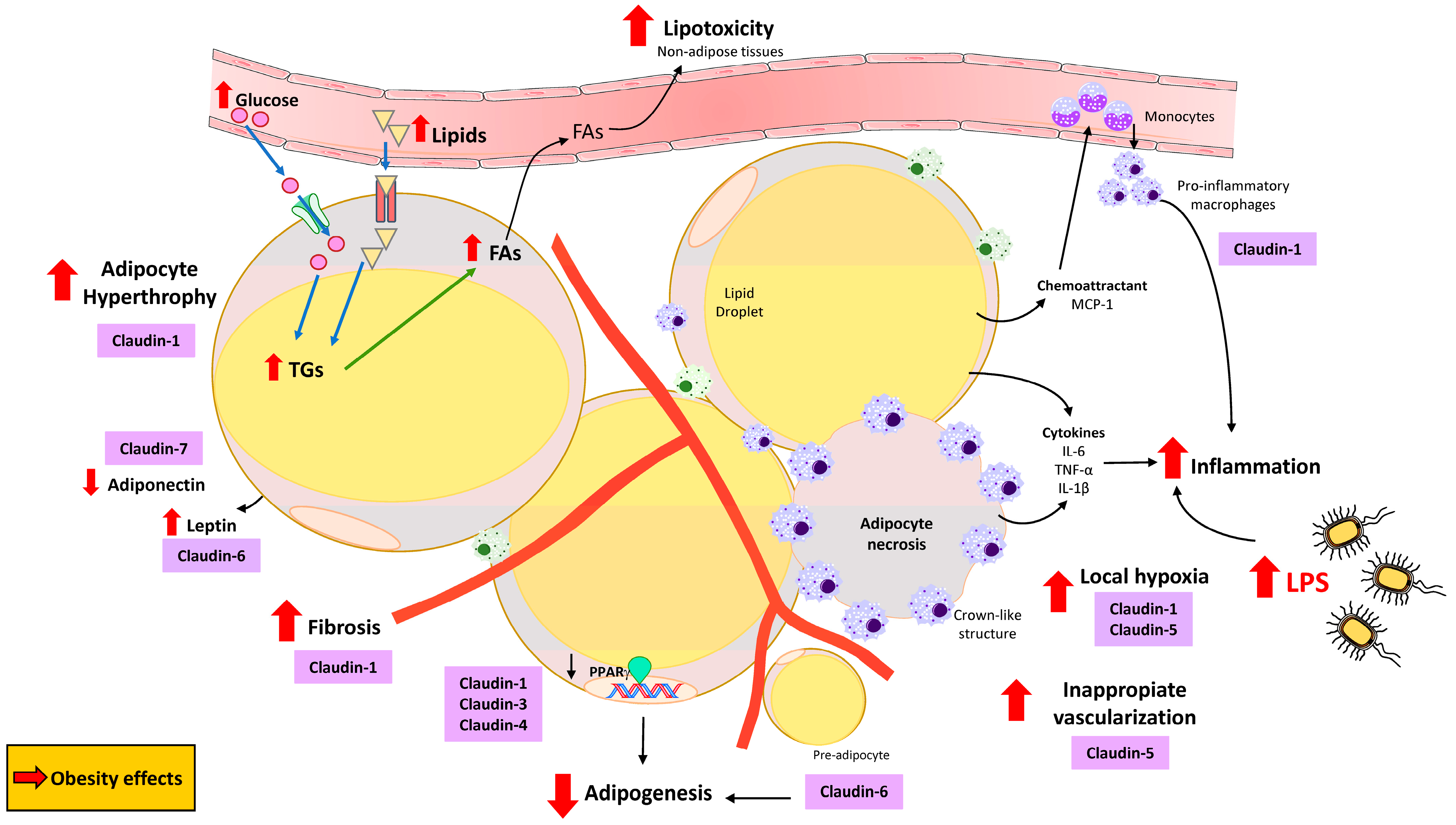
4. The Potential Role of Claudins in Obesity and Adipose Tissue Physiology and Disease
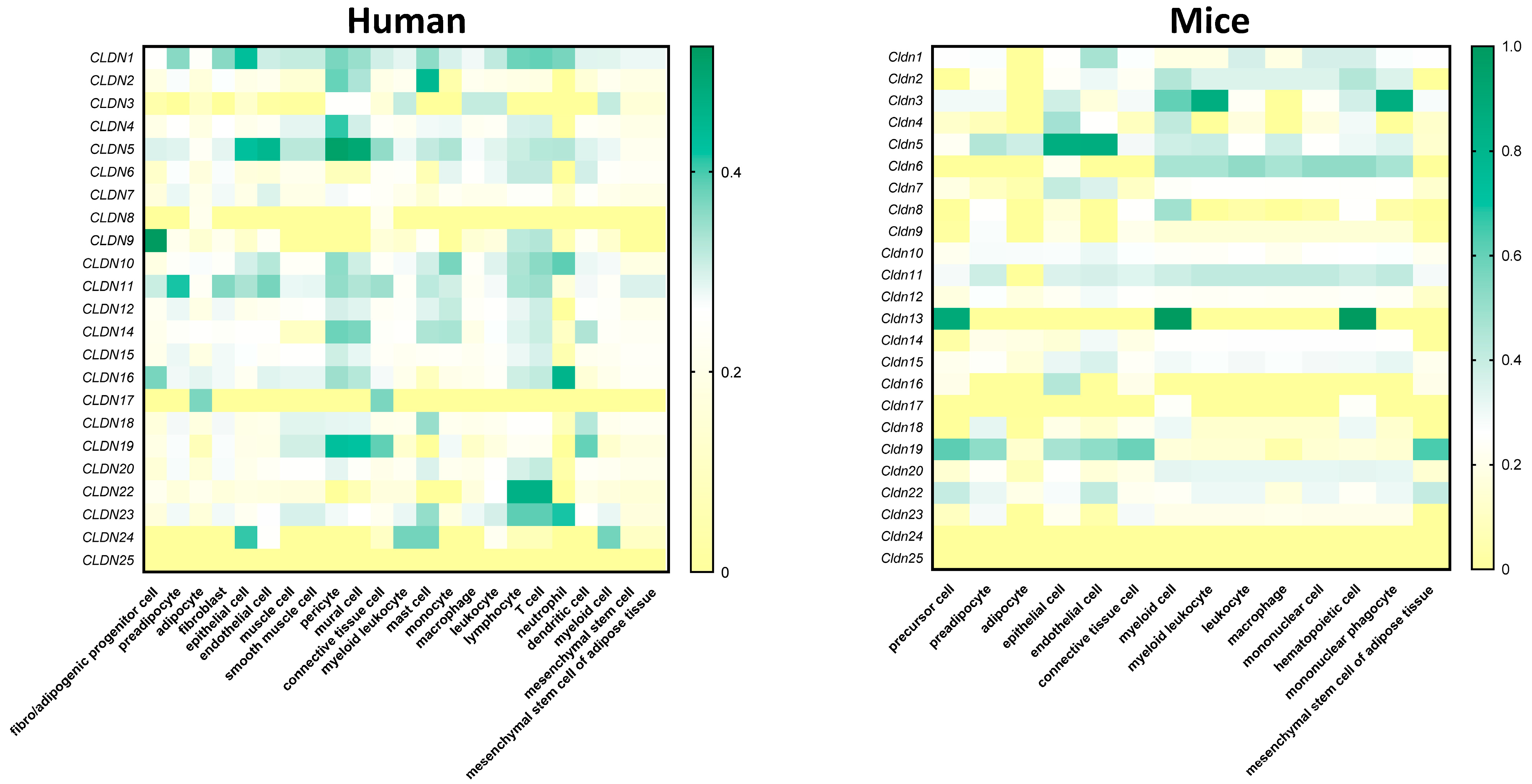
4.1. Claudins Within the Adipose Tissue
4.2. Claudins in Several Adipose Tissue-Related Signaling Pathways
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Segula, D. Complications of obesity in adults: A short review of the literature. Malawi Med. J. 2014, 26, 20–24. [Google Scholar] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Müller, G. Let’s shift lipid burden—From large to small adipocytes. Eur. J. Pharmacol. 2011, 656, 1–4. [Google Scholar] [CrossRef]
- Burke, S.; Nagajyothi, F.; Thi, M.M.; Hanani, M.; Scherer, P.E.; Tanowitz, H.B.; Spray, D.C. Adipocytes in both brown and white adipose tissue of adult mice are functionally connected via gap junctions: Implications for Chagas disease. Microbes Infect. 2014, 16, 893–901. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Fontana, F.; Sugatani, T.; Castillo, I.P.; Leanza, G.; Coler-Reilly, A.; Civitelli, R. Connexin43 in mesenchymal lineage cells regulates body adiposity and energy metabolism in mice. JCI Insight 2024, 9, e170016. [Google Scholar] [CrossRef]
- Nagasawa, K.; Chiba, H.; Fujita, H.; Kojima, T.; Saito, T.; Endo, T.; Sawada, N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell Physiol. 2006, 208, 123–132. [Google Scholar] [CrossRef]
- Franke, W.W. Discovering the molecular components of intercellular junctions—A historical view. Cold Spring Harb Perspect. Biol. 2009, 1, a003061. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, S.; Balda, M.S.; Matter, K. Tight junctions: Molecular architecture and function. Int. Rev. Cytol. 2006, 248, 261–298. [Google Scholar]
- Citi, S.; Guerrera, D.; Spadaro, D.; Shah, J. Epithelial junctions and Rho family GTPases: The zonular signalosome. Small GTPases 2014, 5, e973760-15. [Google Scholar] [CrossRef]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef]
- Takano, K.; Kojima, T.; Sawada, N.; Himi, T. Role of tight junctions in signal transduction: An update. EXCLI J. 2014, 13, 1145. [Google Scholar]
- Hagen, S.J. Non-canonical functions of claudin proteins: Beyond the regulation of cell-cell adhesions. Tissue Barriers 2017, 5, e1327839. [Google Scholar] [CrossRef]
- Roehlen, N.; Saviano, A.; El Saghire, H.; Crouchet, E.; Nehme, Z.; Del Zompo, F.; Jühling, F.; Oudot, M.A.; Durand, S.C.; Duong, F.H.T.; et al. A monoclonal antibody targeting nonjunctional claudin-1 inhibits fibrosis in patient-derived models by modulating cell plasticity. Sci. Transl. Med. 2022, 14, eabj4221. [Google Scholar] [CrossRef]
- Mak, S.; Hammes, A. Canonical and Non-Canonical Localization of Tight Junction Proteins during Early Murine Cranial Development. Int. J. Mol. Sci. 2024, 25, 1426. [Google Scholar] [CrossRef]
- Ikari, A.; Watanabe, R.; Sato, T.; Taga, S.; Shimobaba, S.; Yamaguchi, M.; Yamazaki, Y.; Endo, S.; Matsunaga, T.; Sugatani, J. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Muller, M.; Nehme, Z.; Crouchet, E.; Jühling, F.; Del Zompo, F.; Cherradi, S.; Duong, F.H.; Almeida, N.; Saviano, A.; et al. Treatment of HCC with Claudin-1 specific antibodies suppresses carcinogenic signaling and reprograms the tumor microenvironment. J. Hepatol. 2023, 78, 343–355. [Google Scholar] [CrossRef]
- Bauer, H.C.; Traweger, A.; Zweimueller-Mayer, J.; Lehner, C.; Tempfer, H.; Krizbai, I.; Wilhelm, I. New aspects of the molecular constituents of tissue barriers. J. Neural Transm. 2011, 118, 7–21. [Google Scholar] [CrossRef]
- Otani, T.; Nguyen, T.P.; Tokuda, S.; Sugihara, K.; Sugawara, T.; Furuse, K.; Miura, T.; Ebnet, K.; Furuse, M. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J. Cell Biol. 2019, 218, 3372–3396. [Google Scholar] [CrossRef]
- Martìn-Padura, I.; Lostaglio, S.; Schneemann, M.; Williams, L.; Romano, M.; Fruscella, P.; Panzeri, C.; Stoppacciaro, A.; Ruco, L.; Villa, A.; et al. Junctional Adhesion Molecule, a Novel Member of the Immunoglobulin Superfamily That Distributes at Intercellular Junctions and Modulates Monocyte Transmigration. J. Cell Biol. 1998, 142, 117–127. [Google Scholar] [CrossRef]
- Mandell, K.J.; Parkos, C.A. The JAM family of proteins. Adv. Drug Deliv. Rev. 2005, 57, 857–867. [Google Scholar] [CrossRef]
- Furuse, M.; Sasaki, H.; Fujimoto, K.; Tsukita, S. A Single Gene Product, Claudin-1 or-2, Reconstitutes Tight Junction Strands and Recruits Occludin in Fibroblasts. J. Cell Biol. 1998, 143, 391–401. [Google Scholar] [CrossRef]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Occludin: A Novel Integral Membrane Protein Localizing at Tight Junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.-D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S.; Nelson, W.J. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Schlingmann, B.; Overgaard, C.E.; Molina, S.A.; Lynn, K.S.; Mitchell, L.A.; White, S.D.; Mattheyses, A.L.; Guidot, D.M.; Capaldo, C.T.; Koval, M. Regulation of claudin/zonula occludens-1 complexes by hetero-claudin interactions. Nat. Commun. 2016, 7, 12276. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.O.; He, Y. Allosterism in the PDZ Family. Int. J. Mol. Sci. 2022, 23, 1454. [Google Scholar] [CrossRef]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Krug, S.M.; Günzel, D.; Conrad, M.P.; Lee, I.F.M.; Amasheh, S.; Fromm, M.; Yu, A.S. Charge-selective claudin channels. Ann. N. Y. Acad. Sci. 2012, 1257, 20–28. [Google Scholar] [CrossRef]
- Meoli, L.; Günzel, D. The role of claudins in homeostasis. Nat. Rev. Nephrol. 2023, 19, 587–603. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Furuse, M. Knockout animals and natural mutations as experimental and diagnostic tool for studying tight junction functions in vivo. Biochim. Biophys. Acta Biomembr. 2009, 1788, 813–819. [Google Scholar] [CrossRef]
- Rosenthal, R.; Günzel, D.; Krug, S.M.; Schulzke, J.D.; Fromm, M.; Yu, A.S. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017, 219, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Günzel, D.; Piontek, J.; Krug, S.M.; Ayala-Torres, C.; Hempel, C.; Theune, D.; Fromm, M. Claudin-15 forms a water channel through the tight junction with distinct function compared to claudin-2. Acta Physiol. 2019, 228, e13334. [Google Scholar] [CrossRef]
- Hou, J.; Renigunta, A.; Yang, J.; Waldegger, S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. USA 2010, 107, 18010–18015. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Nagidi, S.H.; Collett, K.; Mckell, J.; Mizrachi, D. Calcium regulates the interplay between the tight junction and epithelial adherens junction at the plasma membrane. FEBS Lett. 2022, 596, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Danziger, J.; Zeidel, M.L. Osmotic homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 852–862. [Google Scholar] [CrossRef]
- Tokuda, S.; Yu, A.S.L. Regulation of Epithelial Cell Functions by the Osmolality and Hydrostatic Pressure Gradients: A Possible Role of the Tight Junction as a Sensor. Int. J. Mol. Sci. 2019, 20, 3513. [Google Scholar] [CrossRef]
- Hou, J.; Renigunta, A.; Gomes, A.S.; Hou, M.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA 2009, 106, 15350–15355. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Rogan, S.; Yu, A.; Vidal, L.S.; Holmes, J.; Anderson, J.M. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Ren. Physiol. 2006, 291, 1288–1299. [Google Scholar] [CrossRef]
- GünZel, D.; Stuiver, M.; Kausalya, P.J.; Haisch, L.; Krug, S.M.; Rosenthal, R.; Meij, I.C.; Hunziker, W.; Fromm, M.; MülLer, D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J. Cell Sci. 2009, 122, 1507–1517. [Google Scholar] [CrossRef]
- Cornely, R.M.; Schlingmann, B.; Shepherd, W.S.; Chandler, J.D.; Neujahr, D.C.; Koval, M. Two common human CLDN5 alleles encode different open reading frames but produce one protein isoform. Ann. N. Y. Acad. Sci. 2017, 1397, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Koval, M. Claudins—Key pieces in the tight junction puzzle. Cell Commun. Adhes. 2006, 13, 127–138. [Google Scholar] [CrossRef]
- Gehne, N.; Lamik, A.; Lehmann, M.; Haseloff, R.F.; Andjelkovic, A.V.; Blasig, I.E.; Brandner, J.M. Cross-over endocytosis of claudins is mediated by interactions via their extracellular loops. PLoS ONE 2017, 12, e0182106. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Nakagawa, T.; Shimizu, S. Mitochondrial membrane permeability transition and cell death. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Liu, H.; Chen, B.; Liu, H.; Abdel-Latif, A.; Kitakaze, M.; Wang, X.; Wu, Y.; Chou, D.; Kim, J.K. A Novel Role of Claudin-5 in Prevention of Mitochondrial Fission Against Ischemic/Hypoxic Stress in Cardiomyocytes. Can. J. Cardiol. 2021, 37, 1593–1606. [Google Scholar] [CrossRef]
- Citi, S.; Fromm, M.; Furuse, M.; González-Mariscal, L.; Nusrat, A.; Tsukita, S.; Turner, J.R. A short guide to the tight junction. J Cell Sci. 2024, 137, jcs261776. [Google Scholar] [CrossRef]
- Nehme, Z.; Roehlen, N.; Dhawan, P.; Baumert, T.F. Tight Junction Protein Signaling and Cancer Biology. Cells 2023, 12, 243. [Google Scholar] [CrossRef]
- Saviano, A.; Roehlen, N.; Baumert, T.F. Tight Junction Proteins as Therapeutic Targets to Treat Liver Fibrosis and Hepatocellular Carcinoma. Semin. Liver Dis. 2024, 44, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Mannan, P.; Lu, M.; Udey, M.C. Epithelial cell adhesion molecule (EpCAM) regulates claudin dynamics and tight junctions. J. Biol. Chem. 2013, 288, 12253–12268. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Yan, J.; Wang, X.; Xu, M.; Wang, M.; Fan, B.; Liu, J.; Lin, N.; Wang, X.; et al. Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease. Nat. Commun. 2022, 13, 1600. [Google Scholar] [CrossRef]
- Xing, T.; Benderman, L.J.; Sabu, S.; Parker, J.; Yang, J.; Lu, Q.; Ding, L.; Chen, Y.-H. Tight Junction Protein Claudin-7 Is Essential for Intestinal Epithelial Stem Cell Self-Renewal and Differentiation. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.; Shiou, S.-R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef]
- Sugimoto, K.; Chiba, H. The claudin—transcription factor signaling pathway. Tissue Barriers 2021, 9, 1908109. [Google Scholar] [CrossRef]
- Martínez-Estrada, O.M.; Cullerés, A.; Soriano, F.X.; Peinado, H.; Bolós, V.; Martínez, F.O.; Reina, M.; Cano, A.; Fabre, M.; Vilaró, S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem. J. 2006, 394, 449–457. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ichikawa-Tomikawa, N.; Kashiwagi, K.; Endo, C.; Tanaka, S.; Sawada, N.; Watabe, T.; Higashi, T.; Chiba, H. Cell adhesion signals regulate the nuclear receptor activity. Proc. Natl. Acad. Sci. USA 2019, 116, 24600–24609. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, Y.; Tao, C.; Shao, M.; Zhao, S.; Huang, W.; Yao, T.; Johnson, J.A.; Liu, T.; Cypess, A.M.; et al. Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell Metab. 2016, 24, 420–433. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Wang, J.; Bäcker, T.; Krueger, M.; Zamani, S.; Rosowski, S.; Gruber, T.; Onogi, Y.; Feuchtinger, A.; Schulz, T.J.; et al. Active integrins regulate white adipose tissue insulin sensitivity and brown fat thermogenesis. Mol. Metab. 2021, 45, 101147. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-L.; Teo, Z.; Chong, H.C.; Zhu, P.; Tan, M.J.; Tan, C.K.; Lam, C.R.I.; Sng, M.K.; Leong, D.T.W.; Tan, S.M.; et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 2011, 118, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Gu, Y.H.; Kanazawa, M.; Tsubota, Y.; Hawkins, B.T.; Spatz, M.; Milner, R.; Del Zoppo, G.J. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by β1-integrins. J. Cereb. Blood Flow Metab. 2011, 31, 1972–1985. [Google Scholar] [CrossRef] [PubMed]
- Tabariès, S.; Dong, Z.; Annis, M.G.; Omeroglu, A.; Pepin, F.; Ouellet, V.; Russo, C.; Hassanain, M.; Metrakos, P.; Diaz, Z.; et al. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene 2010, 30, 1318–1328. [Google Scholar] [CrossRef]
- Lu, Z.; Kim, D.H.; Fan, J.; Lu, Q.; Verbanac, K.; Ding, L.; Renegar, R.; Chen, Y.-H. A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment. Mol. Cancer 2015, 14, 120. [Google Scholar] [CrossRef]
- Zhong, J.; Zareifi, D.; Weinbrenner, S.; Hansen, M.; Klingelhuber, F.; Nankam, P.A.N.; Frendo-Cumbo, S.; Bhalla, N.; Cordeddu, L.; Barbosa, T.d.C.; et al. adiposetissue.org: A knowledge portal integrating clinical and experimental data from human adipose tissue. Cell Metab. 2025, 37, 566–569. [Google Scholar] [CrossRef]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jørgensen, A.M.; Dashti, H.; Stefek, A.; et al. A single-cell atlas of human and mouse white adipose tissue. Nature 2022, 603, 926–933. [Google Scholar] [CrossRef]
- Vázquez-Liébanas, E.; Mocci, G.; Li, W.; Laviña, B.; Reddy, A.; O’cOnnor, C.; Hudson, N.; Elbeck, Z.; Nikoloudis, I.; Gaengel, K.; et al. Mosaic deletion of claudin-5 reveals rapid non-cell-autonomous consequences of blood-brain barrier leakage. Cell Rep. 2024, 43, 113911. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Förster, C.Y. Claudin-5 as a novel estrogen target in vascular endothelium. Arter. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef]
- Shan, S.; Alanazi, A.H.; Han, Y.; Zhang, D.; Liu, Y.; Narayanan, S.P.; Somanath, P.R. Pro-Inflammatory Characteristics of Extracellular Vesicles in the Vitreous of Type 2 Diabetic Patients. Biomedicines 2024, 12, 2053. [Google Scholar] [CrossRef]
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2021, 11, 624903. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Adipose Tissue-Endothelial Cell Interactions in Obesity-Induced Endothelial Dysfunction. Front. Cardiovasc. Med. 2021, 8, 681581. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ding, L.; Jiang, X.; Zhang, S.; Yan, M.; Yang, G.; Tian, X.; Wang, Q. Single-nucleus RNA transcriptome profiling reveals murine adipose tissue endothelial cell proliferation gene networks involved in obesity development. Arch. Biochem. Biophys. 2024, 757, 110029. [Google Scholar] [CrossRef]
- Eguchi, J.; Wada, J.; Hida, K.; Zhang, H.; Matsuoka, T.; Baba, M.; Hashimoto, I.; Shikata, K.; Ogawa, N.; Makino, H. Identification of adipocyte adhesion molecule (ACAM), a novel CTX gene family, implicated in adipocyte maturation and development of obesity. Biochem. J. 2005, 387, 343–353. [Google Scholar] [CrossRef]
- Murakami, K.; Eguchi, J.; Hida, K.; Nakatsuka, A.; Katayama, A.; Sakurai, M.; Choshi, H.; Furutani, M.; Ogawa, D.; Takei, K.; et al. Antiobesity Action of ACAM by Modulating the Dynamics of Cell Adhesion and Actin Polymerization in Adipocytes. Diabetes 2016, 65, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Raschperger, E.; Engstrom, U.; Pettersson, R.F.; Fuxe, J. CLMP, a Novel Member of the CTX Family and a New Component of Epithelial Tight Junctions. J. Biol. Chem. 2004, 279, 796–804. [Google Scholar] [CrossRef]
- Hernandez, L.; Ward, L.J.; Arefin, S.; Ebert, T.; Laucyte-Cibulskiene, A.; Collaborators, G.-F.; Pilote, L.; Norris, C.M.; Raparelli, V.; Kautzky-Willer, A.; et al. Blood-brain barrier and gut barrier dysfunction in chronic kidney disease with a focus on circulating biomarkers and tight junction proteins. Sci. Rep. 2022, 12, 4414. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; De Angelis, M.; Calabrese, F.M.; D’amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef]
- Shemtov, S.J.; Emani, R.; Bielska, O.; Covarrubias, A.J.; Verdin, E.; Andersen, J.K.; Winer, D.A. The intestinal immune system and gut barrier function in obesity and ageing. FEBS J. 2023, 290, 4163–4186. [Google Scholar] [CrossRef]
- Ahmad, R.; Rah, B.; Bastola, D.; Dhawan, P.; Singh, A.B. Obesity-induces Organ and Tissue Specific Tight Junction Restructuring and Barrier Deregulation by Claudin Switching. Sci. Rep. 2017, 7, 5125. [Google Scholar] [CrossRef]
- AlMarzooqi, S.K.; Almarzooqi, F.; Sadida, H.Q.; Jerobin, J.; Ahmed, I.; Abou-Samra, A.B.; Fakhro, K.A.; Dhawan, P.; Bhat, A.A.; Al-Shabeeb Akil, A.S. Deciphering the complex interplay of obesity, epithelial barrier dysfunction, and tight junction remodeling: Unraveling potential therapeutic avenues. Obes. Rev. 2024, 25, e13766. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Claudin switching: Physiological plasticity of the Tight Junction. Semin. Cell Dev. Biol. 2015, 42, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.G.; Møller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Kobayashi, K.; Oyama, S.; Numata, A.; Rahman, M.M.; Kumura, H. Lipopolysaccharide Disrupts the Milk-Blood Barrier by Modulating Claudins in Mammary Alveolar Tight Junctions. PLoS ONE 2013, 8, e62187. [Google Scholar] [CrossRef]
- Wu, J.; He, C.; Bu, J.; Luo, Y.; Yang, S.; Ye, C.; Yu, S.; He, B.; Yin, Y.; Yang, X. Betaine attenuates LPS-induced downregulation of Occludin and Claudin-1 and restores intestinal barrier function. BMC Vet. Res. 2020, 16, 75. [Google Scholar] [CrossRef]
- Shen, W.; Li, Y.; Zou, Y.; Cao, L.; Cai, X.; Gong, J.; Xu, Y.; Zhu, W. Mesenteric Adipose Tissue Alterations in Crohn’s Disease Are Associated with the Lymphatic System. Inflamm. Bowel Dis. 2019, 25, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Hishikawa, D.; Miyahara, H.; Nishimura, Y.; Tsuzuki, H.; Gotoh, C.; Iga, T.; Suzuki, Y.; Song, S.-H.; Choi, K.-C.; et al. Up-Regulation of the Claudin-6 Gene in Adipogenesis. Biosci. Biotechnol. Biochem. 2005, 69, 2117–2121. [Google Scholar] [CrossRef]
- Dubois, S.G.; Heilbronn, L.K.; Smith, S.R.; Albu, J.B.; Kelley, D.E.; Ravussin, E.; The Look AHEAD Adipose Research Group. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity 2006, 14, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.; Laforest, S.; Pelletier, M.; Leboeuf, M.; Blackburn, L.; Tchernof, A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte 2014, 3, 197–205. [Google Scholar] [CrossRef]
- Li, E.; Li, C.; Horn, N.; Ajuwon, K.M. PPARγ activation inhibits endocytosis of claudin-4 and protects against deoxynivalenol-induced intestinal barrier dysfunction in IPEC-J2 cells and weaned piglets. Toxicol. Lett. 2023, 375, 8–20. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K.; et al. The Mechanisms by Which Both Heterozygous Peroxisome Proliferator-activated Receptor γ (PPARγ) Deficiency and PPARγ Agonist Improve Insulin Resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef]
- Belalcazar, L.M.; Papandonatos, G.D.; McCaffery, J.M.; Peter, I.; Pajewski, N.M.; Erar, B.; Allred, N.D.; Balasubramanyam, A.; Bowden, D.W.; Brautbar, A.; et al. A common variant in the CLDN7/ELP5 locus predicts adiponectin change with lifestyle intervention and improved fitness in obese individuals with diabetes. Physiol. Genom. 2015, 47, 215–224. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.M.; Clément, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef]
- Cereijido, M.; Rendón, J.M. A historical and Evolutionary View of Tight Junctions. In Tight Junctions; Springer International Publishing: Berlin, Germany, 2022; pp. 1–334. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, C.; Lu, F.; Liao, Y.; Cai, J.; Gao, J. Challenges and opportunities in obesity: The role of adipocytes during tissue fibrosis. Front. Endocrinol. 2024, 15, 1365156. [Google Scholar] [CrossRef]
- Lappi-Blanco, E.; Lehtonen, S.T.; Sormunen, R.; Merikallio, H.M.; Soini, Y.; Kaarteenaho, R.L. Divergence of tight and adherens junction factors in alveolar epithelium in pulmonary fibrosis. Hum. Pathol. 2013, 44, 895–907. [Google Scholar] [CrossRef]
- Fernández-García, P.; Taxerås, S.D.; Reyes-Farias, M.; González, L.; Soria-Gondek, A.; Pellitero, S.; Tarascó, J.; Moreno, P.; Sumoy, L.; Stephens, J.M.; et al. Claudin-1 as a novel target gene induced in obesity and associated to inflammation, fibrosis and cell differentiation. Eur. J. Endocrinol. 2024, 190, 201–210. [Google Scholar] [CrossRef]
- Priscilla, L.; Yoo, C.; Jang, S.; Park, S.; Lim, G.; Kim, T.; Lee, D.Y. Immunotherapy targeting the obese white adipose tissue microenvironment: Focus on non-communicable diseases. Bioact. Mater. 2024, 35, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013, 18, 478–489. [Google Scholar] [CrossRef]
- Gealekman, O.; Burkart, A.; Chouinard, M.; Nicoloro, S.M.; Straubhaar, J.; Corvera, S. Enhanced angiogenesis in obesity and in response to PPARγ activators through adipocyte VEGF and ANGPTL4 production. Am. J. Physiol. Metab. 2008, 295, E1056–E1064. [Google Scholar] [CrossRef]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Morinaga, H.; Talukdar, S.; Bae, E.J.; Olefsky, J.M. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012, 61, 346–354. [Google Scholar] [CrossRef]
- Markey, G.E.; Ryan, S.; Furuta, G.T.; Menard-Katcher, C.; McNamee, E.N.; Masterson, J.C. Hypoxia-inducible microRNA-155 negatively regulates epithelial barrier in eosinophilic esophagitis by suppressing tight junction claudin-7. FASEB J. 2023, 38, e23358. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bossche, J.; Malissen, B.; Mantovani, A.; De Baetselier, P.; Van Ginderachter, J.A. Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood 2012, 119, 1623–1633. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Kao, D.J.; Kitzenberg, D.A.; Dobrinskikh, E.; Schwisow, K.D.; Masterson, J.C.; Kendrick, A.A.; Kelly, C.J.; Bayless, A.J.; Kominsky, D.J.; et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 2015, 26, 2252–2262. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Zhong, G.; Li, W.; Chen, B.; Zhang, J. Claudin-5 Affects Endothelial Autophagy in Response to Early Hypoxia. Front. Physiol. 2021, 12, 737474. [Google Scholar] [CrossRef] [PubMed]
- Koto, T.; Takubo, K.; Ishida, S.; Shinoda, H.; Inoue, M.; Tsubota, K.; Okada, Y.; Ikeda, E. Hypoxia Disrupts the Barrier Function of Neural Blood Vessels through Changes in the Expression of Claudin-5 in Endothelial Cells. Am. J. Pathol. 2007, 170, 1389–1397. [Google Scholar] [CrossRef]
- Qiao, T.-Y.; Yuan, Z.-M.; Ma, T.-Y.; Hu, H.-Q.; Zhu, Y.-H.; Zhang, W.-Y.; Zhang, Q.; Huang, R.; Tang, Q.-C.; Wang, G.-Y.; et al. Claudin14 promotes colorectal cancer progression via the PI3K/AKT/mTOR pathway. Neoplasma 2021, 68, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Zhou, S.; Liu, X.; Li, G.; Song, B.; Xu, W. Claudin-1/4 as directly target gene of HIF-1α can feedback regulating HIF-1α by PI3K-AKT-mTOR and impact the proliferation of esophageal squamous cell though Rho GTPase and p-JNK pathway. Cancer Gene Ther. 2021, 29, 665–682. [Google Scholar] [CrossRef]
- Luo, J.; Chimge, N.; Zhou, B.; Flodby, P.; Castaldi, A.; Firth, A.L.; Liu, Y.; Wang, H.; Yang, C.; Marconett, C.N.; et al. CLDN18.1 attenuates malignancy and related signaling pathways of lung adenocarcinoma in vivo and in vitro. Int. J. Cancer 2018, 143, 3169–3180. [Google Scholar] [CrossRef]
- Shimobaba, S.; Taga, S.; Akizuki, R.; Hichino, A.; Endo, S.; Matsunaga, T.; Watanabe, R.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; et al. Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of PDK1 and Akt in human lung adenocarcinoma A549 cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, M.; Chaudhuri, S.; Kumar, A. The non-canonical nuclear functions of key players of the PI3K-AKT-MTOR pathway. J. Cell Physiol. 2022, 237, 3181–3204. [Google Scholar] [CrossRef]
- London, E.; Stratakis, C.A. The regulation of PKA signaling in obesity and in the maintenance of metabolic health. Pharmacol. Ther. 2022, 237, 108113. [Google Scholar] [CrossRef]
- Balda, M.S.; Garrett, M.D.; Matter, K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003, 160, 423–432. [Google Scholar] [CrossRef]
- French, A.D.; Fiori, J.L.; Camilli, T.C.; Leotlela, P.D.; O’COnnell, M.P.; Frank, B.P.; Subaran, S.; Indig, F.E.; Taub, D.D.; Weeraratna, A.T. PKC and PKA Phosphorylation Affect the Subcellular Localization of Claudin-1 in Melanoma Cells. Int. J. Med. Sci. 2009, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Chen, T.; Zheng, L.; Zou, J.; Zou, J.; Li, W.; Chen, Q.; Cheng, L.; Qian, B. Calcium sensing receptor regulate claudin-14 via PKA-STAT3 pathway in rat model of nephrolithiasis. Front. Pharmacol. 2024, 15, 1477122. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Liang, N.; Guan, L. Claudin-9 enhances the metastatic potential of hepatocytes via Tyk2/Stat3 signaling. Turk. J. Gastroenterol. 2019, 30, 722–731. [Google Scholar] [CrossRef]
- Sun, L.; Feng, L.; Cui, J. Increased expression of claudin-17 promotes a malignant phenotype in hepatocyte via Tyk2/Stat3 signaling and is associated with poor prognosis in patients with hepatocellular carcinoma. Diagn Pathol 2018, 13, 72. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, A.; Li, Q.; Zhou, M.; Li, T. CLDN10 promotes a malignant phenotype of osteosarcoma cells via JAK1/Stat1 signaling. J. Cell Commun. Signal 2019, 13, 395–405. [Google Scholar] [CrossRef]
- Hu, W.; Lv, J.; Han, M.; Yang, Z.; Li, T.; Jiang, S.; Yang, Y. STAT3: The art of multi-tasking of metabolic and immune functions in obesity. Prog. Lipid Res. 2018, 70, 17–28. [Google Scholar] [CrossRef]
- Buettner, C.; Muse, E.D.; Cheng, A.; Chen, L.; Scherer, T.; Pocai, A.; Su, K.; Cheng, B.; Li, X.; Harvey-White, J.; et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 2008, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef]
- Ozaki, K.-I.; Awazu, M.; Tamiya, M.; Iwasaki, Y.; Harada, A.; Kugisaki, S.; Tanimura, S.; Kohno, M. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E643–E651. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Xiang, R.-L.; Cong, X.; Zhang, Y.; Li, J.; Yi, X.; Park, K.; Han, J.-Y.; Wu, L.-L.; Yu, G.-Y. Claudin-3 is required for modulation of paracellular permeability by TNF-α through ERK1/2/slug signaling axis in submandibular gland. Cell Signal 2015, 27, 1915–1927. [Google Scholar] [CrossRef]
- Lu, Y.; Shao, Y.; Xie, Y.; Qu, H.; Qi, D.; Dong, Y.; Jin, Q.; Wang, L.; Wei, J.; Quan, C. CLDN6 inhibits breast cancer cell malignant behavior by suppressing ERK signaling. Cell Signal 2022, 97, 110393. [Google Scholar] [CrossRef]
- Cheng, B.; Rong, A.; Zhou, Q.; Li, W. CLDN8 promotes colorectal cancer cell proliferation, migration, and invasion by activating MAPK/ERK signaling. Cancer Manag. Res. 2019, 11, 3741–3751. [Google Scholar] [CrossRef]
- Ryu, W.-I.; Lee, H.; Bae, H.C.; Jeon, J.; Ryu, H.J.; Kim, J.; Kim, J.H.; Son, J.W.; Kim, J.; Imai, Y.; et al. IL-33 down-regulates CLDN1 expression through the ERK/STAT3 pathway in keratinocytes. J. Dermatol. Sci. 2018, 90, 313–322. [Google Scholar] [CrossRef]
- Amoozadeh, Y.; Dan, Q.; Anwer, S.; Huang, H.H.; Barbieri, V.; Waheed, F.; Maishan, M.; Szászi, K. Tumor Necrosis Factor-α Increases Claudin-1, 4, and 7 Expression in Tubular Cells: Role in Permeability Changes. J. Cell Physiol. 2017, 232, 2210–2220. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Liang, J.; Sun, Z.; Wu, Q.; Liu, Y.; Sun, C. The Mechanism of Leptin on Inhibiting Fibrosis and Promoting Browning of White Fat by Reducing ITGA5 in Mice. Int. J. Mol. Sci. 2021, 22, 12353. [Google Scholar] [CrossRef]
- Morandi, E.M.; Verstappen, R.; Zwierzina, M.E.; Geley, S.; Pierer, G.; Ploner, C. ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells. Sci. Rep. 2016, 6, 28889. [Google Scholar] [CrossRef]
- Bastie, C.C.; Zong, H.; Xu, J.; Busa, B.; Judex, S.; Kurland, I.J.; Pessin, J.E. Integrative Metabolic Regulation of Peripheral Tissue Fatty Acid Oxidation by the Src Kinase Family Member Fyn. Cell Metab. 2007, 5, 371–381. [Google Scholar] [CrossRef]
- Hartig, S.M.; He, B.; Long, W.; Buehrer, B.M.; Mancini, M.A. Homeostatic levels of SRC-2 and SRC-3 promote early human adipogenesis. J. Cell Biol. 2011, 192, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Géhin, M.; Annicotte, J.-S.; Rocchi, S.; Champy, M.-F.; O’MAlley, B.W.; Chambon, P.; Auwerx, J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 2002, 111, 931–941. [Google Scholar] [CrossRef]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct Binding of Three Tight Junction-Associated Maguks, Zo-1, Zo-2, and Zo-3, with the Cooh Termini of Claudins. J. Cell Biol. 1999, 147, 1351–1363. [Google Scholar] [CrossRef]
- Nayler, O. SAF-B protein couples transcription and pre-mRNA splicing to SAR/MAR elements. Nucleic Acids Res. 1998, 26, 3542–3549. [Google Scholar] [CrossRef]
- Debril, M.-B.; Dubuquoy, L.; Feige, J.-N.; Wahli, W.; Desvergne, B.; Auwerx, J.; Gelman, L. Scaffold attachment factor B1 directly interacts with nuclear receptors in living cells and represses transcriptional activity. J. Mol. Endocrinol. 2005, 35, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Y.; Shi, J.; Qi, M.; Li, X.; Yang, Y.; Zhu, C.; Wang, C.; Tang, Z.; Ma, Y.; et al. Osthole relieves skin damage and inhibits chronic itch through modulation of Akt/ZO-3 pathway in atopic dermatitis. Eur. J. Pharmacol. 2023, 947, 175649. [Google Scholar] [CrossRef]
| Claudin | Ion Permeability | Function |
|---|---|---|
| CLDN1 | Ions/Water | Barrier-forming |
| CLDN2 | Cations/Water | Channel-forming |
| CLDN3 | Ions/Water | Barrier-forming |
| CLDN4 | Cations (?) | Barrier/channel-forming |
| CLDN5 | Ions/Water | Barrier-forming |
| CLDN6 | Ions/Water | Barrier-forming |
| CLDN7 | Anions (?) | Barrier/channel forming |
| CLDN8 | Anions/Cations | Barrier/channel forming |
| CLDN9 | Ions/Water | Barrier-forming |
| CLDN10a | Anions | Channel-forming |
| CLDN10b | Cations | Channel-forming |
| CLDN11 | Ions/Water | Barrier-forming |
| CLDN12 | Cations | Barrier/channel forming |
| CLDN14 | Ions/Water | Barrier-forming |
| CLDN15 | Cations/water | Channel-forming |
| CLDN16 | Anions/Cations | Channel-forming |
| CLDN17 | Anions | Channel-forming |
| CLDN18 | Ions/Water | Barrier-forming |
| CLDN19 | Anions/Cations | Barrier-forming |
| Claudin | Cellular Pathways and/or Transcription Factors | Effect | Cell Type |
|---|---|---|---|
| CLDN1 | Ras/Erk; Src/AKT; NF-κβ; PI3K/AKT; Src | ↑ | Intestine, epithelial, colon, liver, esophageal squamous cells |
| CLDN2 | AC; FOXO | ↑ | Epithelial cells, lung adenocarcinoma |
| CLDN3 | ERK | ↑ | Submandibular glands |
| CLDN4 | TGFβ/Smad; PI3K/AKT | ↑ | Lung, esophageal squamous cells |
| CLDN5 | Wnt | ↑ | Podocytes, endothelial cells |
| CLDN6 | ERK | ↓ | Breast cancer |
| CLDN7 | Wnt; Src/AKT | ↑ | Epithelial, intestine |
| CLDN8 | ERK | ↑ | Colorectal cancer |
| CLDN9 | STAT3 | ↑ | Hepatocytes |
| CLDN10 | JAK1/STAT1 | ↑ | Osteosarcoma |
| CLDN11 | Notch | ↑ | Osteoblasts |
| CLDN17 | STAT3 | ↑ | Hepatocytes |
| CLDN18 | PDK1/AKT | ↑ | Lung adenocarcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-García, P.; Villarroya, F.; Sánchez-Infantes, D.; Corrales, P. Claudin Proteins: Their Potential Role in Obesity and Adipose Tissue Signaling, Physiology and Disease. Nutrients 2025, 17, 2611. https://doi.org/10.3390/nu17162611
Fernández-García P, Villarroya F, Sánchez-Infantes D, Corrales P. Claudin Proteins: Their Potential Role in Obesity and Adipose Tissue Signaling, Physiology and Disease. Nutrients. 2025; 17(16):2611. https://doi.org/10.3390/nu17162611
Chicago/Turabian StyleFernández-García, Pablo, Francesc Villarroya, David Sánchez-Infantes, and Patricia Corrales. 2025. "Claudin Proteins: Their Potential Role in Obesity and Adipose Tissue Signaling, Physiology and Disease" Nutrients 17, no. 16: 2611. https://doi.org/10.3390/nu17162611
APA StyleFernández-García, P., Villarroya, F., Sánchez-Infantes, D., & Corrales, P. (2025). Claudin Proteins: Their Potential Role in Obesity and Adipose Tissue Signaling, Physiology and Disease. Nutrients, 17(16), 2611. https://doi.org/10.3390/nu17162611





