Long-Term Outcomes of the Dietary Approaches to Stop Hypertension (DASH) Intervention in Nonobstructive Coronary Artery Disease: Follow-Up of the DISCO-CT Study
Abstract
1. Introduction
2. Materials and Methods
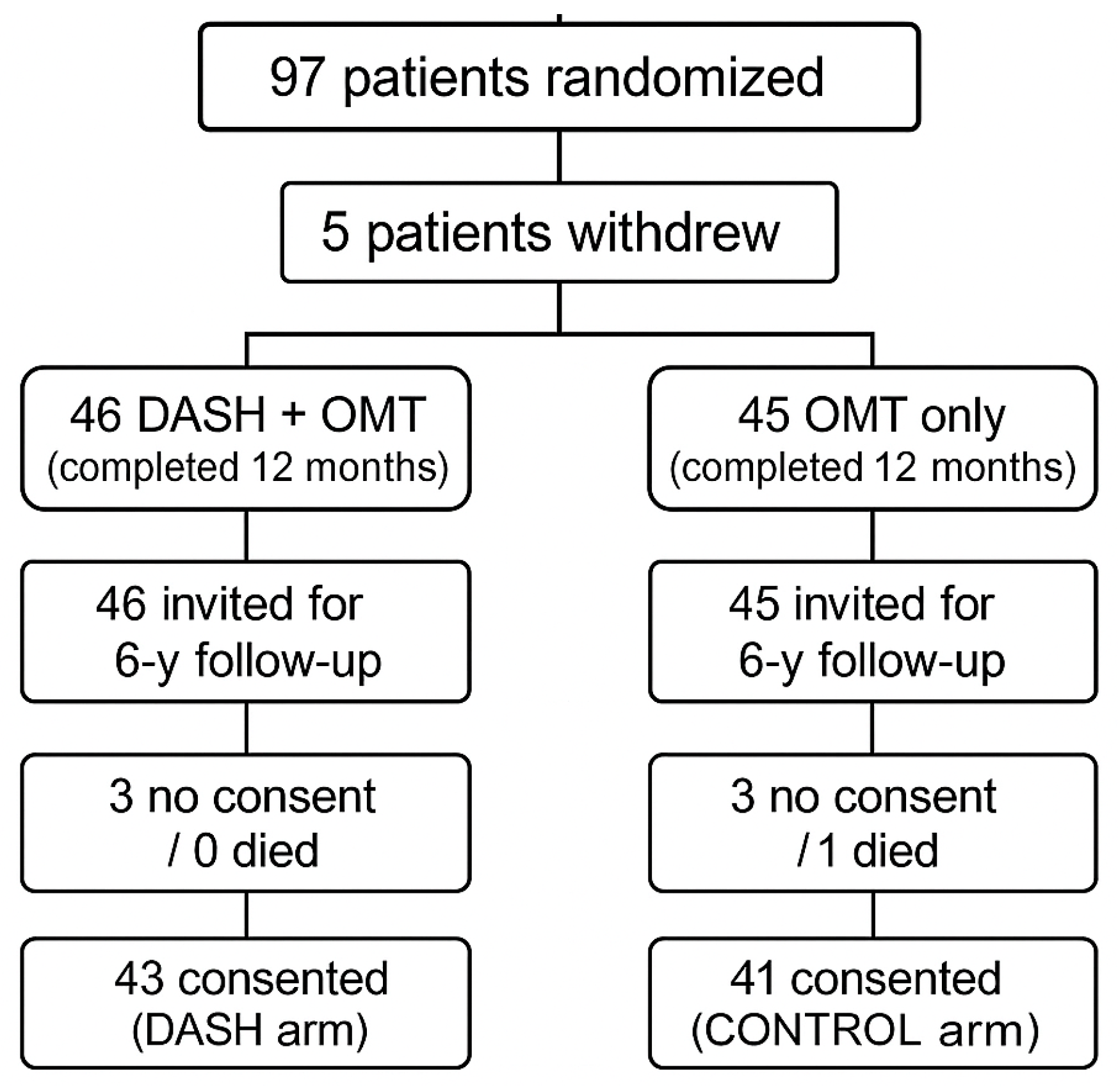
2.1. Study Design and Population
2.2. Data Collection Procedures
2.2.1. Biochemical Analyses
2.2.2. Dietary Adherence and Lifestyle Assessment
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Body Weight and Composition
3.2. Dietary Intake and DASH Adherence
3.3. Physical Activity
3.4. Diet and Biochemical Markers
3.4.1. Lipid Profile
3.4.2. Inflammatory Markers
3.4.3. Homocysteine
3.5. Correlation Analysis
3.5.1. Correlation Between Dietary Changes and Body Composition
3.5.2. Correlations Between Dietary Changes and Biochemical Parameters
3.5.3. Inter-Marker Correlations
3.5.4. The Impact of Baseline Age on Changes to Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-I | angiotensin-converting enzyme inhibitor |
| ACE | angiotensin-converting enzyme |
| ARB | angiotensin II receptor blocker |
| ASA | acetylsalicylic acid |
| BMI | body mass index |
| CAD | coronary artery disease |
| CABG | coronary artery bypass grafting |
| CD163 | cluster of differentiation 163 |
| CXCL4 | C-X-C motif chemokine ligand 4 |
| DASH | Dietary Approaches to Stop Hypertension |
| DISCO-CT | Dietary Intervention to Stop Coronary Atherosclerosis in Computed Tomography |
| ELISA | enzyme-linked immunosorbent assay |
| ESC | European Society of Cardiology |
| FFQ | food frequency questionnaire |
| HDL-C | high-density lipoprotein cholesterol |
| hs-CRP | high-sensitivity C-reactive protein |
| IBM SPSS | International Business Machines Statistical Package for the Social Sciences |
| IL-6 | interleukin-6 |
| LDL-C | low-density lipoprotein cholesterol |
| MACE | major adverse cardiovascular events |
| MCP-1 | monocyte chemoattractant protein-1 |
| MI | myocardial infarction |
| NHANES | National Health and Nutrition Examination Survey |
| non-HDL-C | non-high-density lipoprotein cholesterol |
| PCI | percutaneous coronary intervention |
| RANTES | Regulated on Activation, Normal T Cells Expressed and Secreted |
| SAFA | saturated fatty acids |
| SMM | skeletal muscle mass |
| TBF | total body fat |
| TNF-α | tumour necrosis factor-alpha |
| VFA | visceral fat area |
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Global Burden of Cardiovascular Diseases and Risks Collaborators. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2374–2390. [Google Scholar] [CrossRef] [PubMed]
- Laffond, A.; Rivera-Picón, C.; Rodríguez-Muñoz, P.M.; Juárez-Vela, R.; Ruiz de Viñaspre-Hernández, R.; Navas-Echazarreta, N.; Sánchez-González, J.L. Mediterranean Diet for Primary and Secondary Prevention of Cardiovascular Disease and Mortality: An Updated Systematic Review. Nutrients 2023, 15, 3356. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Nishi, S.K.; Khan, T.A.; Braunstein, C.R.; Glenn, A.J.; Mejia, S.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Jenkins, D.J.A.; et al. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-Analysis of Controlled Trials. Prog. Cardiovasc. Dis. 2018, 61, 43–53. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., III; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the DASH Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.C.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Lari, A.; Sohouli, M.H.; Fatahi, S.; Cerqueira, H.S.; Santos, H.O.; Pourrajab, B.; Rezaei, M. The Effects of the Dietary Approaches to Stop Hypertension (DASH) Diet on Metabolic Risk Factors in Patients with Chronic Disease: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2766–2778. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Yang, Z.; Duan, M.L. DASH Diet and Risk of Coronary Artery Disease: A Meta-Analysis of Prospective Cohort Studies. Int. J. Food Sci. Nutr. 2019, 70, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, D.B.; Levitan, E.B.; Åkesson, A.; Gigante, B.; Wolk, A. The DASH Diet Is Associated with a Lower Risk of Heart Failure: A Cohort Study. Eur. J. Prev. Cardiol. 2022, 29, 1114–1123. [Google Scholar] [CrossRef]
- Bensaaud, A.; Seery, S.; Gibson, I.; Jones, J.; Flaherty, G.; McEvoy, J.W.; Jordan, F.; Tawfick, W.; Sultan, S.A. Dietary Approaches to Stop Hypertension (DASH) for the Primary and Secondary Prevention of Cardiovascular Diseases. Cochrane Database Syst. Rev. 2025, 5, CD013729. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) Diet on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary Patterns and Biomarkers of Oxidative Stress and Inflammation: A Systematic Review of Observational and Intervention Studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Makarewicz-Wujec, M.; Henzel, J.; Kruk, M.; Kępka, C.; Wardziak, Ł.; Trochimiuk, P.; Parzonko, A.; Demkow, M.; Dzielińska, Z.; Kozłowska-Wojciechowska, M. The Effect of Intensive Dietary Intervention Lowers RANTES and CXCL4 in Non-Obstructive Coronary Disease: A Randomised Study. Biology 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, C.A.; Shaked, I.; Little, K.M.; Ley, K.; Randolph, G.J. CXCL4 Down-Regulates the Atheroprotective Hemoglobin Scavenger Receptor CD163 in Human Macrophages. Circ. Res. 2010, 106, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Domschke, G.; Gleissner, C.A. CXCL4-Induced Macrophages in Human Atherosclerosis. Cytokine 2019, 122, 154141. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Appel, L.J.; Funk, K.; Craddick, S.; Chen, C.; Elmer, P.; McBurnie, M.A.; Champagne, C. The PREMIER Intervention Helps Participants Follow the Dietary Approaches to Stop Hypertension Dietary Pattern and the Current Dietary Reference Intakes Recommendations. J. Am. Diet. Assoc. 2007, 107, 1541–1551. [Google Scholar] [CrossRef]
- Henzel, J.; Kępka, C.; Kruk, M.; Makarewicz-Wujec, M.; Wardziak, Ł.; Trochimiuk, P.; Dzielińska, Z.; Demkow, M. High-Risk Coronary Plaque Regression after Intensive Lifestyle Intervention in Non-Obstructive Coronary Disease: A Randomised Study. JACC Cardiovasc. Imaging 2021, 14, 1192–1202. [Google Scholar] [CrossRef]
- Makarewicz-Wujec, M.; Henzel, J.; Kępka, C.; Kruk, M.; Wardziak, Ł.; Trochimiuk, P.; Parzonko, A.; Dzielińska, Z.; Demkow, M.; Kozłowska-Wojciechowska, M. Usefulness of MCP-1 Chemokine in Monitoring Patients with Coronary Artery Disease Subjected to Intensive Dietary Intervention: A Pilot Study. Nutrients 2021, 13, 3047. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.L.; Liese, A.D.; Bell, R.A.; Dabelea, D.; Lawrence, J.M.; Rodriguez, B.L.; Standiford, D.A.; Mayer-Davis, E.J. Association between the Dietary Approaches to Hypertension Diet and Hypertension in Youth with Diabetes Mellitus. Hypertension 2009, 53, 6–12. [Google Scholar] [CrossRef]
- Bonekamp, N.E.; Cruijsen, E.; Geleijnse, J.M.; Winkels, R.M.; Visseren, F.L.J.; Morris, P.B.; Koopal, C. Diet in Secondary Prevention: The Effect of Dietary Patterns on Cardiovascular Risk Factors in Patients with Cardiovascular Disease—A Systematic Review and Network Meta-Analysis. Nutr. J. 2024, 23, 18. [Google Scholar] [CrossRef]
- Cesaro, A.; De Michele, G.; Fimiani, F.; Acerbo, V.; Scherillo, G.; Signore, G.; Rotolo, F.P.; Scialla, F.; Raucci, G.; Panico, D.; et al. Visceral Adipose Tissue and Residual Cardiovascular Risk: A Pathological Link and New Therapeutic Options. Front. Cardiovasc. Med. 2023, 10, 1187735. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.; Sińska, B.; Panczyk, M.; Szostak-Węgierek, D.; Raciborski, F.; Samoliński, B.; Borowicz, J.; Wronka, L.; Traczyk, I. Diet and Selected Elements of Lifestyle in the Polish Population before and during the COVID-19 Pandemic—A Population Study. Ann. Agric. Environ. Med. 2023, 30, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wyleżoł, M.; Sińska, B.I.; Kucharska, A.; Panczyk, M.; Raciborski, F.; Szostak-Węgierek, D.; Milewska, M.; Samoliński, B.; Frączek, M.; Traczyk, I. The Influence of Obesity on Nutrition and Physical Activity during the COVID-19 Pandemic: A Case-Control Study. Nutrients 2022, 14, 2236. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Chitsazi, M.J.; Salehi-Abargouei, A. The Effect of Dietary Approaches to Stop Hypertension on Serum Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomised Trials. Clin. Nutr. 2018, 37, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Sachais, B.S.; Turrentine, T.; Dawicki-McKenna, J.M. Elimination of platelet factor 4 from platelets reduces atherosclerosis in ApoE-/- mice. Thromb. Haemost. 2007, 98, 1108–1113. [Google Scholar]
- von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.; Hackeng, T.M.; Weber, C. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005, 105, 924–930. [Google Scholar] [CrossRef]
- Versteylen, M.O.; Manca, M.; Joosen, I.A.; Schmidt, D.E.; Das, M.; Hofstra, L.; Crijns, H.J.; Biessen, E.A.; Kietselaer, B.L. CC Chemokine Ligands in Patients Presenting with Stable Chest Pain: Association with Atherosclerosis and Future Cardiovascular Events. Neth. Heart J. 2016, 24, 722–729. [Google Scholar] [CrossRef][Green Version]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Gao, H.K. Impact of Different Types of Tree Nut, Peanut, and Soy Nut Consumption on Serum C-Reactive Protein: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Medicine 2016, 95, e5165. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Y.; Fang, C.; Jia, Y.; Chen, M.; Chen, X.; Jia, J. The Associations between Dietary Fibres Intake and Systemic Immune and Inflammatory Biomarkers: A Multi-Cycle Study of NHANES 2015–2020. Front. Nutr. 2023, 10, 1242115. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, B.; Lin, T.; Greissinger, K.; Rottner, L.; Rillig, A.; Zimmerling, S. The Beneficial Effects of Cardiac Rehabilitation. Cardiol. Ther. 2020, 9, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cobo, C.; Brezmes, L.; Altadill, M.; García-García, H.; Gheorghe, L.L.; Gutiérrez-Barrios, A.; Cañadas, D.; Tur, J.A.; Vázquez-García, R.; Santi-Cano, M.J. Efficacy of a Mobile Health App (eMOTIVA) Regarding Compliance with Cardiac Rehabilitation Guidelines in Coronary Artery Disease: Randomised Controlled Trial. JMIR mHealth uHealth 2024, 12, e55421. [Google Scholar] [CrossRef] [PubMed]
- Nishio, R.; Dohi, T.; Yokoyama, M.; Nakade, T.; Takahashi, N.; Chikata, Y.; Endo, H.; Nishiyama, H.; Okai, I.; Iwata, H.; et al. Wearable Devices in Remote Cardiac Rehabilitation with and without Weekly Online Coaching for Coronary Artery Disease: Randomised Controlled Trial. JMIR mHealth uHealth 2025, 13, e63797. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Final | Follow-Up | p-Value | ||||
|---|---|---|---|---|---|---|---|
| DASH n = 43 | Control n = 41 | DASH n = 43 | Control n = 41 | DASH n = 43 | Control n = 41 | ||
| Age, years (mean ± SD) | 58.8 ± 7.7 | 59.9 ± 7.8 | 60.0 ± 7.8 | 61.7 ± 4.9 | 65.81 ± 7.79 | 67.54 ± 7.45 | 0.304 |
| Women, n (%) | 14 (32.6%) | 18 (43.9%) | 14 (32.6%) | 18 (43.9%) | 14 (32.6%) | 18 (43.9%) | 0.369 |
| BMI, kg·m−2 | 30.0 ± 3.65 | 29.0 ± 3.83 | 28.8 ± 3.36 | 29.79 ± 4.42 | 29.36 ± 3.50 | 28.48 ± 3.52 | 0.260 |
| Current smoker, n (%) | 5 (11.6%) | 8 (19.5%) | 3 (7.0%) | 5 (12.2%) | 3 (7.0%) | 6 (14.6%) | 0.257 |
| Physical activity, n (%) | |||||||
| Regular | 22 (51.2%) | 12 (29.3%) | 36 (83.7%) | 20 (48.7%) | 20 (46.5%) | 13 (31.7%) | 0.473 |
| Irregular | 12 (27.9%) | 19 (46.3%) | 5 (11.6%) | 13 (30.2%) | 14 (32.5%) | 19(19.5%) | 0.425 |
| Rare/seldom | 9 (20.9%) | 8 (19.5%) | 2 (4.7%) | 8 (19.5%) | 8 (18.6%) | 6 (48.7%) | 0.640 |
| Hypertension, n (%) | 39 (90.7%) | 35 (85.4%) | 33 (7.7%) | 32 (78.0%) | 33 (76.7%) | 32 (78.7%) | >0.999 |
| MI, n (%) * | n/a | n/a | 0 | 0 | 0 (0.0%) | 2 (4.4%) | 0.981 |
| Stroke, n (%) * | n/a | n/a | 0 | 0 | 1 (2.3%) | 1 (2.2%) | >0.999 |
| MACE, n (%) * | n/a | n/a | 0 | 0 | 1 (2.3%) | 4 (8.9%) | 0.575 |
| PCI, n (%) * | n/a | n/a | 0 | 0 | 1 (2.3%) | 2 (4.4%) | 0.966 |
| CABG, n (%) * | n/a | n/a | 0 | 0 | 0 | 1 (4.4%) | 0.981 |
| Diabetes mellitus type 2, n (%) | 1 (2.3%) | 0 (0.0%) | 1 (2.3%) | 0 (0.0%) | 6 (14.0%) | 2 (4.9%) | 0.26 |
| Prediabetes, n (%) | 4 (9.3%) | 3 (7.3%) | 4 (9.3%) | 3 (7.3%) | 9 (20.9%) | 14 (34.1%) | 0.409 |
| Statin therapy, n (%) | 28 (65.1%) | 29 (70.7%) | 34 (79.1%) | 32 (78.0%) | 30 (69.8%) | 30 (73.2%) | 0.811 |
| High-intensity statin, n (%) | 7 (16.3%) | 7 (17.1%) | 9 (20.9%) | 8 (19.5%) | 12 (27.9%) | 10 (2.0%) | 0.804 |
| Other lipid-lowering drugs | 3 (7.0%) | 3 (7.3%) | 2 (4.7%) | 2 (4.9%) | 4 (9.3%) | 8 (19.5%) | 0.222 |
| Calcium-channel blocker, n (%) | 12 (27.9%) | 12 (29.3%) | 11 (25.6%) | 14 (34.1%) | 12 (27.9%) | 19 (46.3%) | 0.113 |
| β-blocker, n (%) | 23 (53.5%) | 26 (63.4%) | 24 (55.8%) | 26 (63.4%) | 22 (51.2%) | 26 (63.4%) | 0.376 |
| ACE-I/ARB, n (%) | 27 (62.8%) | 31 (75.6%) | 26 (60.5%) | 31 (75.6%) | 25 (58.1%) | 29 (70.0%) | 0.260 |
| ASA, n (%) | 25 (58.1%) | 24 (58.5%) | 30 (69.8%) | 29 (70.7%) | 27 (62.8%) | 22 (53.7%) | 0.432 |
| Parameter | Group | Baseline | Final | 6-Year Follow-Up | Δ (Mean ± SD) | Δ* (Mean ± SD | p-Value | p-Value * | p-Value Δ | p-Value Δ* |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight, kg | DASH | 85.94 ± 14.89 | 82.32 ± 13.37 | 84.01 ± 13.73 | −3.62 ± 4.20 | 1.69 ± 5.14 | <0.001 | 0.036 | 0.002 | 0.068 |
| Control | 83.41 ± 15.38 | 82.35 ± 15.46 | 82.04 ± 14.43 | −1.06 ± 2.86 | −0.31 ± 4.80 | 0.023 | 0.679 | |||
| BMI, kg/m2 | DASH | 30.09 ± 3.65 | 28.86 ± 3.36 | 29.36 ± 3.51 | −1.24 ± 1.36 | 0.56 ± 1.80 | <0.001 | 0.052 | 0.006 | 0.022 |
| Control | 29.09 ± 3.83 | 29.73 ± 4.42 | 28.48 ± 3.53 | 0.64 ± 3.95 | −1.04 ± 3.92 | 0.308 | 0.100 | |||
| TBF, kg | DASH | 29.20 ± 9.03 | 25.01 ± 7.52 | 28.34 ± 6.83 | −4.18 ± 4.78 | 3.33 ± 5.55 | <0.001 | <0.001 | <0.001 | 0.111 |
| Control | 26.68 ± 7.90 | 26.98 ± 8.16 | 28.09 ± 8.59 | 0.30 ± 4.09 | 1.22 ± 6.31 | 0.644 | 0.230 | |||
| SMM, kg | DASH | 31.65 ± 7.10 | 31.91 ± 6.74 | 31.42 ± 6.21 | 0.26 ± 2.63 | −0.48 ± 1.91 | 0.520 | 0.104 | 0.018 | 0.177 |
| Control | 31.50 ± 7.15 | 30.51 ± 7.35 | 31.91 ± 10.28 | −1.00 ± 2.10 | 1.33 ± 8.16 | 0.004 | 0.309 | |||
| VFA, cm2 | DASH | 114.54 ± 44.88 | 82.32 ± 13.37 | 175.81 ± 74.33 | −32.22 ± 41.03 | 93.49 ± 69.42 | <0.001 | <0.001 | 0.167 | 0.691 |
| Control | 103.26 ± 37.47 | 82.35 ± 15.46 | 169.93 ± 72.31 | −20.91 ± 33.02 | 87.53 ± 66.85 | <0.001 | <0.001 | |||
| Total Body Water, L | DASH | 41.60 ± 8.40 | 41.57 ± 8.18 | 41.29 ± 8.14 | −0.01 ± 1.83 | −0.29 ± 12.37 | 0.986 | 0.880 | 0.145 | 0.950 |
| Control | 41.23 ± 8.63 | 40.37 ± 8.61 | 40.37 ± 8.74 | −0.86 ± 3.16 | −0.11 ± 13.86 | 0.090 | 0.962 |
| DASH n = 43 | p-Value | p-Value * | Control n = 41 | p-Value | p-Value * | p-Value Δ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Follow-Up | Baseline | Final | Follow-Up | ||||||
| Daily nutrients intake | |||||||||||
| Energy, kcal | 1998.8 ± 669.5 | 1654.4 ± 442 | 1804.6 ± 551.9 | <0.001 | 0.084 | 1898 ± 669.6 | 2000.2 ± 745 | 1936 ± 586.6 | 0.456 | 0.490 | 0.094 |
| Fat energy, % | 32.3 ± 6.9 | 28.7 ± 5.8 | 28.74 ± 5.58 | 0.01 | 0.917 | 31.3 ± 8.4 | 32.3 ± 8.15 | 29.63 ± 5.05 | 0.815 | 0.072 | 0.161 |
| SAFA, g | 21.4 ± 9.7 | 18.2 ± 6.2 | 21.28 ± 9.64 | 0.042 | 0.002 | 22.8 ± 12.9 | 24.17 ± 8.84 | 27.54 ± 12.58 | 0.605 | 0.004 | 0.85 |
| Vit. A, µg | 604.8 ± 35.7 | 633.3 ± 494.4 | 645.44 ± 509.63 | 0.809 | 0.003 | 1002.18 ± 206.8 | 714.79 ± 285.1 | 700.2 ± 283.6 | 0.146 | 0.749 | 0.479 |
| Vit. E, mg | 7.95 ± 4.62 | 12.62 ± 7.03 | 14.43 ± 13.2 | <0.001 | 0.352 | 10.07 ± 5.34 | 11.27 ± 7.24 | 10.74 ± 7.12 | 0.417 | 0.267 | 0.231 |
| Folic Acid, µg | 192.1 ± 0.4 | 253.27 ± 13.2 | 242.5 ± 114.9 | 0.003 | 0.382 | 253.58 ± 58.79 | 244.56 ± 79.21 | 241.26 ± 79.39 | 0.76 | 0.556 | 0.619 |
| Vit. C, mg | 72.31 ± 8.9 | 93.4 ± 63.4 | 96.91 ± 78.82 | 0.021 | 0.863 | 66.05 ± 34.03 | 83.55 ± 61.58 | 76.63 ± 68.59 | 0.122 | 0.306 | 0.592 |
| Fibre, g | 21.44 ± 0.63 | 26.62 ± 7.36 | 24.96 ± 9.97 | <0.001 | 0.25 | 22.99 ± 9.42 | 22.10 ± 9.57 | 23.32 ± 11.98 | 0.595 | 0.827 | 0.472 |
| DASH index components | |||||||||||
| Grains | 5.30 ± 2.44 | 8.26 ± 1.62 | 7.81 ± 1.80 | <0.001 | 0.024 | 5.49 ± 2.19 | 5.51 ± 2.20 | 5.29 ± 2.15 | 0.173 | 0.095 | 0.334 |
| Vegetables | 4.53 ± 1.97 | 7.07 ± 1.88 | 6.77 ± 1.91 | <0.001 | 0.217 | 4.63 ± 1.81 | 5.20 ± 1.94 | 5.15 ± 1.90 | 0.026 | 0.847 | 0.469 |
| Fruits | 4.16 ± 2.35 | 6.42 ± 3.09 | 5.72 ± 2.64 | <0.001 | 0.007 | 3.90 ± 2.45 | 4.12 ± 2.33 | 4.56 ± 2.16 | 0.071 | 0.018 | <0.001 |
| Diary | 5.72 ± 2.71 | 7.42 ± 2.59 | 6.98 ± 2.60 | 0.001 | 0.024 | 5.63 ± 2.67 | 6.05 ± 2.60 | 5.63 ± 2.42 | 0.058 | 0.011 | 0.912 |
| Meat | 4.16 ± 3.34 | 8.60 ± 1.93 | 8.47 ± 2.09 | <0.0001 | 0.183 | 4.54 ± 3.29 | 5.22 ± 3.12 | 4.98 ± 2.86 | 0.001 | 0.133 | 0.584 |
| Nuts | 4.16 ± 3.34 | 8.60 ± 1.93 | 8.47 ± 2.09 | <0.0001 | 0.183 | 1.07 ± 2.44 | 1.71 ± 2.62 | 1.80 ± 2.63 | 0.003 | 0.16 | 0.863 |
| Fat | 6.00 ± 3.77 | 8.65 ± 1.78 | 8.33 ± 2.04 | <0.001 | 0.333 | 6.02 ± 3.64 | 6.29 ± 3.56 | 6.17 ± 3.60 | 0.147 | 0.168 | 0.605 |
| Sweets | 3.07 ± 3.78 | 7.49 ± 3.19 | 6.65 ± 3.37 | <0.0001 | 0.21 | 6.63 ± 5.65 | 4.02 ± 2.20 | 4.39 ± 5.65 | <0.0001 | 0.803 | 0.337 |
| DASH index | 33.70 ± 4.64 | 59.67 ± 8.92 | 55.65 ± 9.99 | 0.0001 | 0.004 | 34.93 ± 3.28 | 38.12 ± 2.20 | 37.98 ± 11.91 | <0.0001 | 0.803 | <0.001 |
| Study Group | Baseline | Final | Follow-Up | Change (Δ) | Change (Δ) * | p-Value | p-Value * | p-Value Δ | p-Value Δ* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total cholesterol, mg/dL | DASH | 177.68 ± 44.21 | 167.14 ± 31.97 | 177.42 ± 44.66 | −22.44 ± 36.11 | 14.58 ± 49.56 | <0.001 | 0.06 | 0.162 | 0.209 |
| control | 180.14 ± 42.93 | 165.68 ± 8.62 | 170.10 ± 36.04 | −10.54 ± 40.87 | 2.96 ± 33.36 | 0.106 | 0.573 | |||
| LDL-C, mg/dL | DASH | 109.27 ± 0.75 | 91.11 ± 31.84 | 103.07 ± 39.66 | −18.16 ± 33.13 | 11.96 ± 42.92 | 0.001 | 0.075 | 0.27 | 0.046 |
| control | 109.11 ± 40.59 | 99.70 ± 29.47 | 95.51 ± 30.62 | −9.41 ± 38.67 | −4.19 ± 28.91 | 0.127 | 0.359 | |||
| HDL-C, mg/dL | DASH | 54.55 ± 15.32 | 57.13 ± 16.02 | 58.72 ± 13.89 | 2.58 ± 8.52 | 1.59 ± 11.37 | 0.053 | 0.364 | 0.328 | 0.823 |
| control | 57.90 ± 14.11 | 58.77 ± 15.38 | 60.90 ± 15.84 | 0.87 ± 7.43 | 2.13 ± 10.65 | 0.459 | 0.208 | |||
| Triglycerides, mg/dL | DASH | 88.36 ± 16.68 | 131.76 ± 242.17 | 114.48 ± 56.12 | 43.40 ± 140.01 | −19.17 ± 50.87 | 0.048 | 0.623 | 0.086 | 0.859 |
| control | 114.09 ± 1.06 | 113.94 ± 64.72 | 101.83 ± 38.39 | −0.16 ± 83.23 | −12.11 ± 6.00 | 0.99 | 0.174 | |||
| hs-CRP, mg/L | DASH | 0.23 ± 0.24 | 0.12 ± 0.13 | 0.21 ± 0.16 | −0.09 ± 0.22 | 0.09 ± 0.14 | 0.01 | <0.001 | 0.188 | 0.237 |
| control | 0.19 ± 0.17 | 0.26 ± 0.75 | 0.20 ± 0.17 | 0.08 ± 0.78 | −0.06 ± 0.75 | 0.535 | 0.638 | |||
| Homocysteine, umol/L | DASH | 14.13 ± 8.83 | 11.98 ± 2.30 | 12.24 ± 3.16 | −2.09 ± 8.30 | 0.26 ± 4.04 | 0.111 | 0.674 | 0.394 | 0.353 |
| control | 12.67 ± 3.60 | 11.74 ± 3.51 | 12.90 ± 2.92 | −0.93 ± 2.69 | 1.16 ± 4.58 | 0.033 | 0.118 | |||
| RANTES, ng/mL | DASH | 41.66 ± 22.62 | 36.94 ± 18.46 | 40.24 ± 19.98 | –4.72 ± 29.23 | 3.29 ± 27.20 | 0.244 | 0.408 | 0.048 | 0.637 |
| control | 34.60 ± 22.74 | 40.27 ± 19.85 | 41.02 ± 19.02 | 5.67 ± 24.66 | 0.74 ± 22.44 | 0.144 | 0.841 | |||
| CXCL4, ng/mL | DASH | 12.72 ± 4.25 | 8.37 ± 2.42 | 9.85 ± 2.19 | −4.34 ± 3.02 | 1.45 ± 3.24 | <0.001 | 0.008 | <0.001 | <0.001 |
| control | 11.07 ± 4.14 | 13.33 ± 5.02 | 10.16 ± 2.47 | 2.19 ± 5.13 | −3.15 ± 5.03 | 0.008 | <0.001 | |||
| Non-HDL-C mg/dl | DASH | 130.74 ± 40.41 | 105.71 ± 35.13 | 118.70 ± 40.81 | −25.03 ± 37.05 | 12.99 ± 52.69 | <0.001 | 0.114 | 0.108 | 0.19 |
| control | 119.78 ± 41.76 | 108.37 ± 31.26 | 109.20 ± 32.65 | −11.41 ± 39.63 | 0.83 ± 28.52 | 0.073 | 0.854 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarewicz-Wujec, M.; Henzel, J.; Kępka, C.; Kruk, M.; Jakubczak, B.; Wróbel, A.; Dąbrowski, R.; Dzielińska, Z.; Demkow, M.; Czepielewska, E.; et al. Long-Term Outcomes of the Dietary Approaches to Stop Hypertension (DASH) Intervention in Nonobstructive Coronary Artery Disease: Follow-Up of the DISCO-CT Study. Nutrients 2025, 17, 2565. https://doi.org/10.3390/nu17152565
Makarewicz-Wujec M, Henzel J, Kępka C, Kruk M, Jakubczak B, Wróbel A, Dąbrowski R, Dzielińska Z, Demkow M, Czepielewska E, et al. Long-Term Outcomes of the Dietary Approaches to Stop Hypertension (DASH) Intervention in Nonobstructive Coronary Artery Disease: Follow-Up of the DISCO-CT Study. Nutrients. 2025; 17(15):2565. https://doi.org/10.3390/nu17152565
Chicago/Turabian StyleMakarewicz-Wujec, Magdalena, Jan Henzel, Cezary Kępka, Mariusz Kruk, Barbara Jakubczak, Aleksandra Wróbel, Rafał Dąbrowski, Zofia Dzielińska, Marcin Demkow, Edyta Czepielewska, and et al. 2025. "Long-Term Outcomes of the Dietary Approaches to Stop Hypertension (DASH) Intervention in Nonobstructive Coronary Artery Disease: Follow-Up of the DISCO-CT Study" Nutrients 17, no. 15: 2565. https://doi.org/10.3390/nu17152565
APA StyleMakarewicz-Wujec, M., Henzel, J., Kępka, C., Kruk, M., Jakubczak, B., Wróbel, A., Dąbrowski, R., Dzielińska, Z., Demkow, M., Czepielewska, E., & Filipek, A. (2025). Long-Term Outcomes of the Dietary Approaches to Stop Hypertension (DASH) Intervention in Nonobstructive Coronary Artery Disease: Follow-Up of the DISCO-CT Study. Nutrients, 17(15), 2565. https://doi.org/10.3390/nu17152565





