Antimicrobial and Anticancer Activities of Lactiplantibacillus plantarum Probio87 Isolated from Human Breast Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Properties
2.1.1. Strains and Culture Conditions
2.1.2. Cell-Free Supernatant (CFS) Preparation
2.1.3. Carbohydrate Utilization
2.1.4. Acid Tolerance and Bile Tolerance
2.1.5. Antibiotic Susceptibility
2.1.6. Prebiotic Utilization
2.1.7. Adherence to Mucin
2.2. Antimicrobial Properties
2.2.1. Inhibition Against Common Pathogens
2.2.2. Inhibition Against Pathogenic Candida
2.2.3. Symbiosis Properties
2.3. Anticancer Assays
2.3.1. Cancer Cell Cultivation
2.3.2. MTT Array
- ODt: OD measured after 48 h;
- OD0: OD measured before the treatment;
- ODblank: OD of media with no cells at 0 h or after 48 h.
2.3.3. Angiogenesis Array
2.3.4. Cancer Pathway and Dual Luciferase Test
2.3.5. Reverse Transcription Quantitative Real-Time PCR
2.4. Statistical Analysis
3. Results
3.1. Probiotic Properties
3.1.1. Carbohydrate Utilization
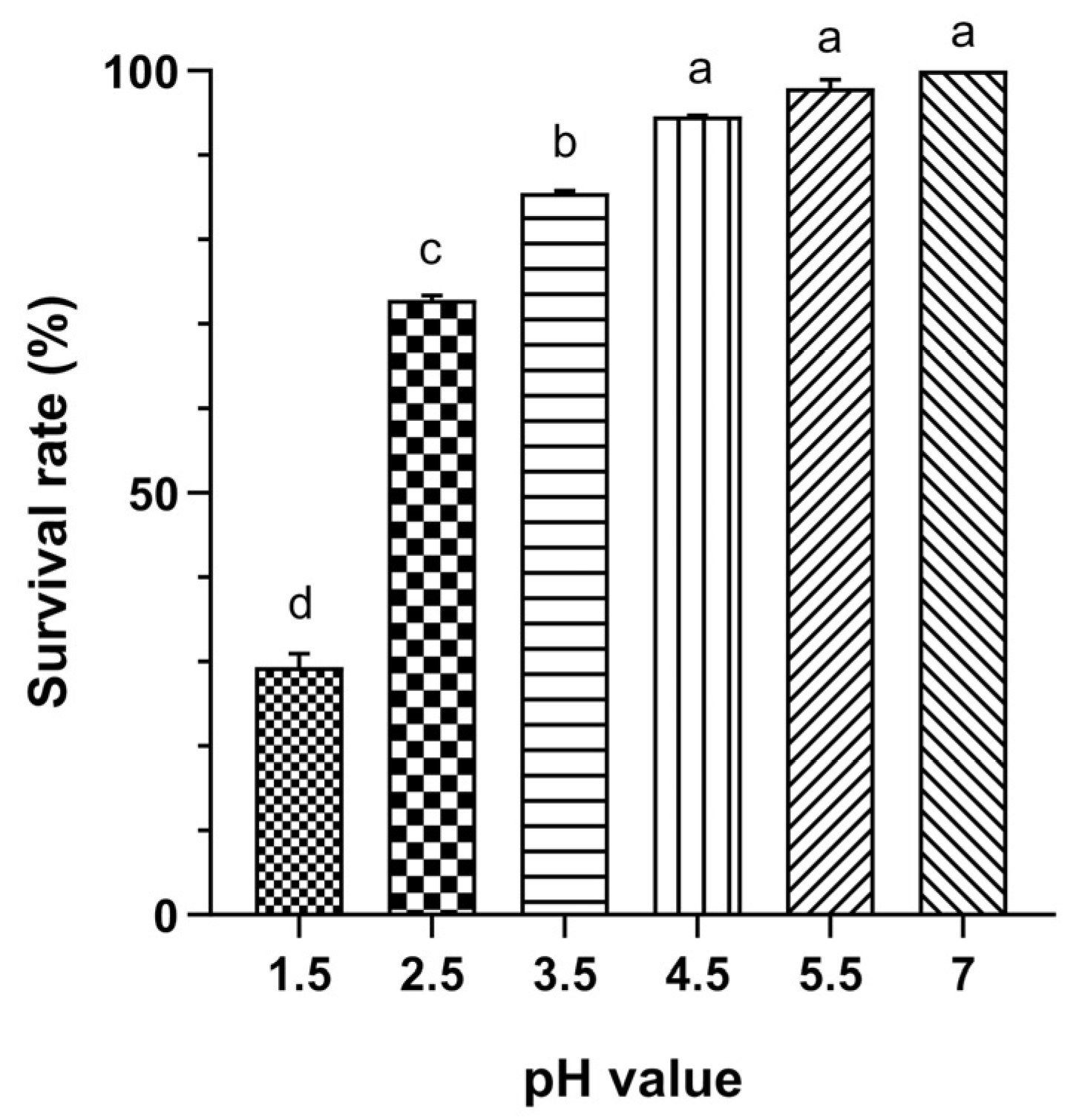
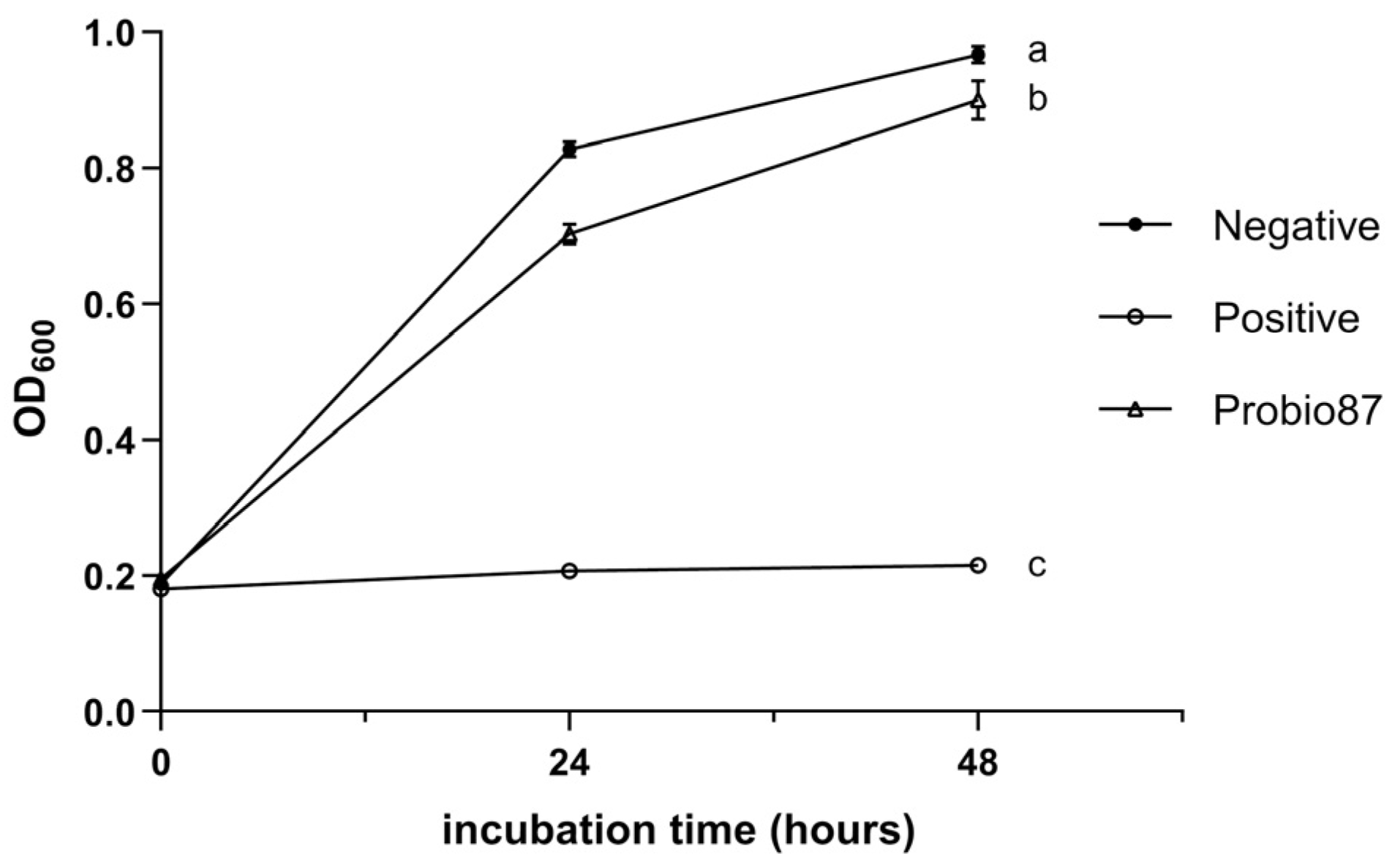
3.1.2. Acid Tolerance
3.1.3. Bile Tolerance
3.1.4. Antibiotic Resistance
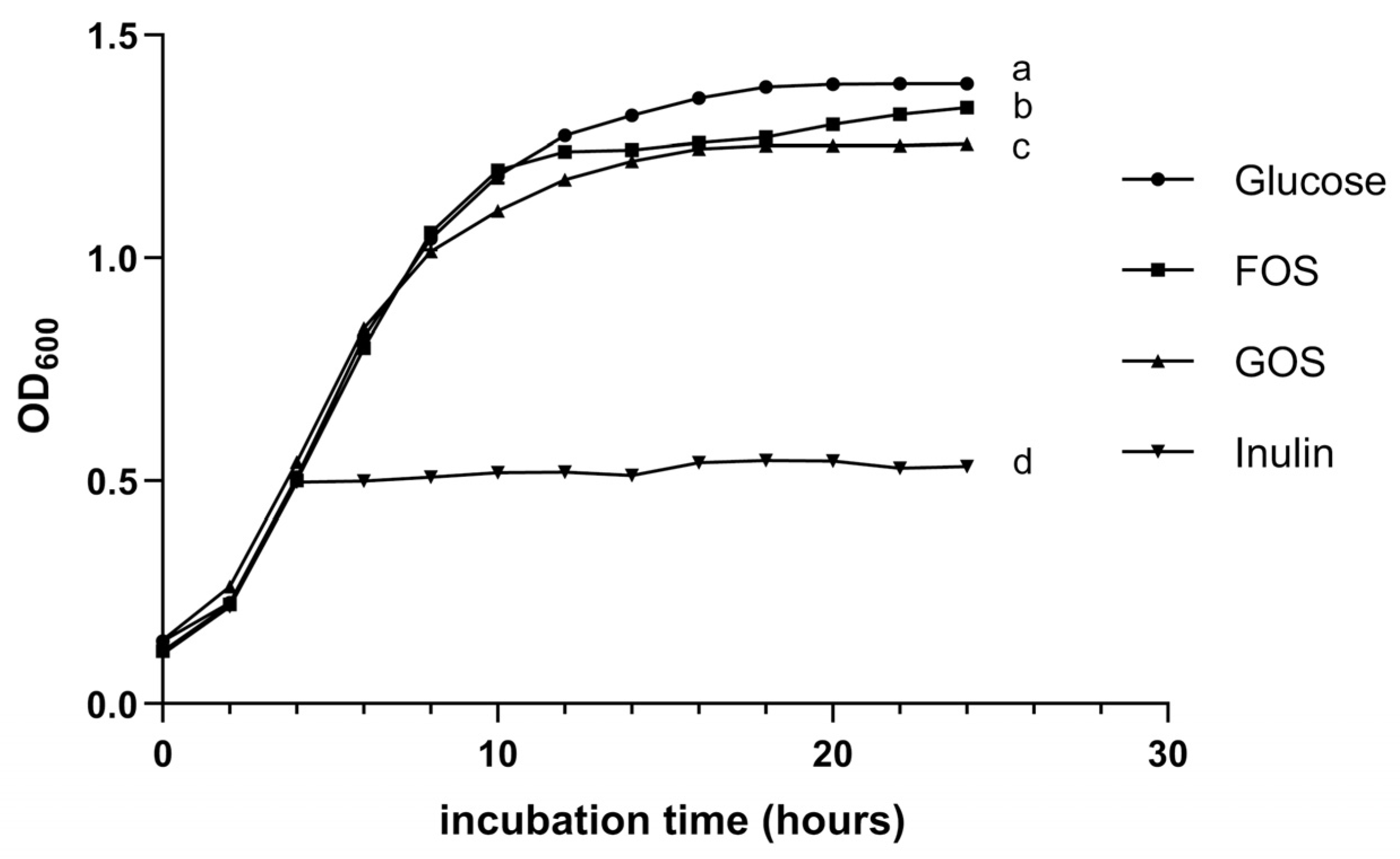
3.1.5. Prebiotic Utilization
3.1.6. Adherence to Mucin
3.2. Antimicrobial Properties
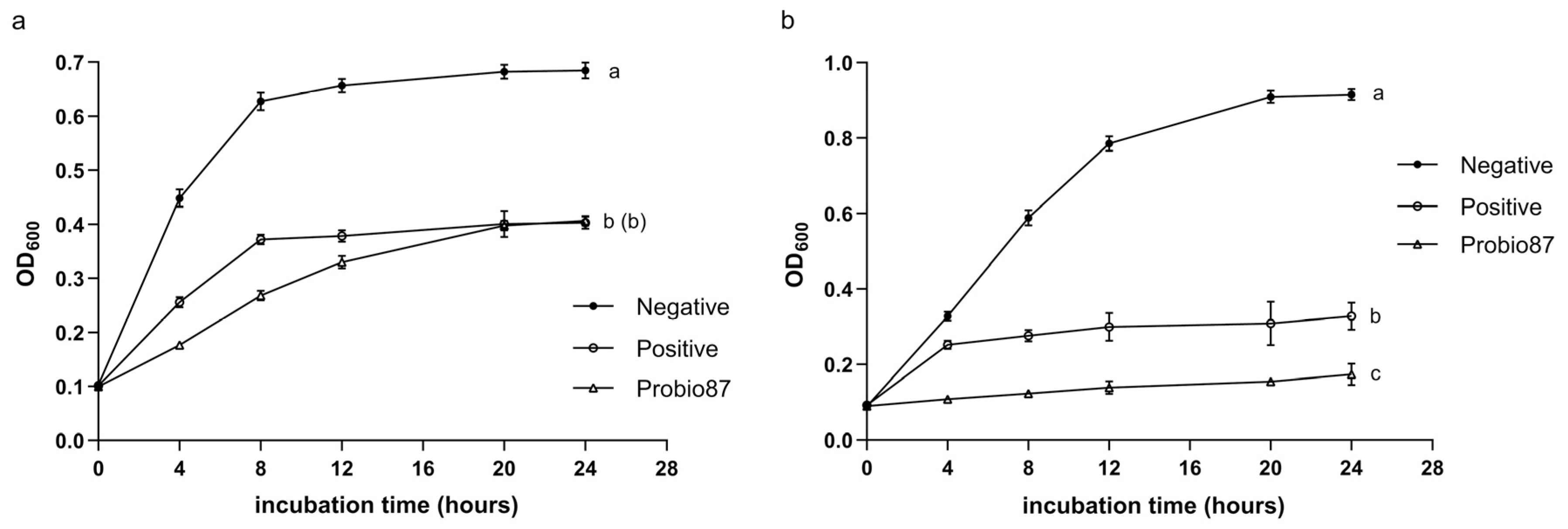
3.2.1. Inhibition Against Common Pathogens
3.2.2. Inhibition Against Pathogenic Candida
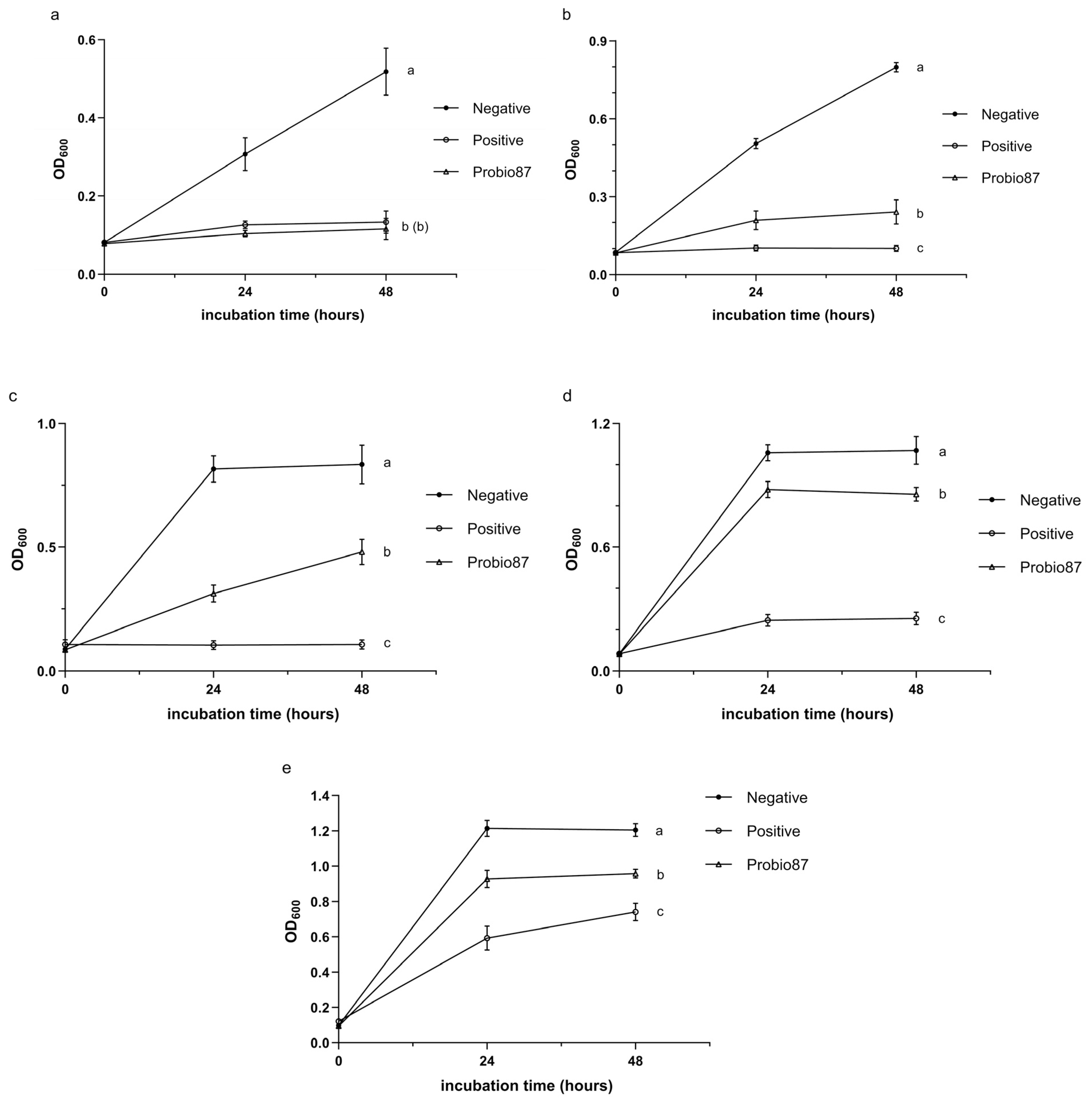
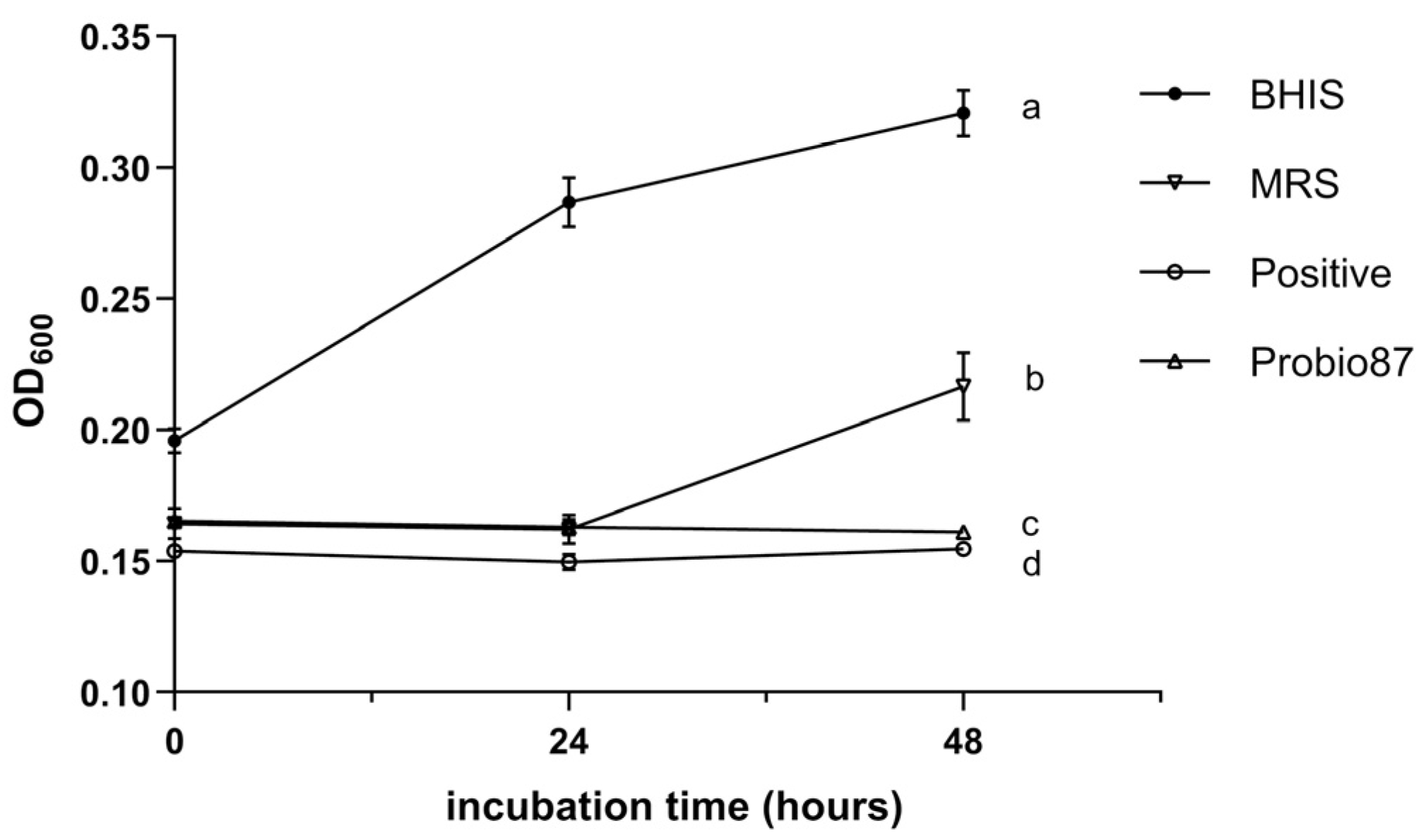
3.2.3. Symbiosis Properties
3.3. Anticancer Assays
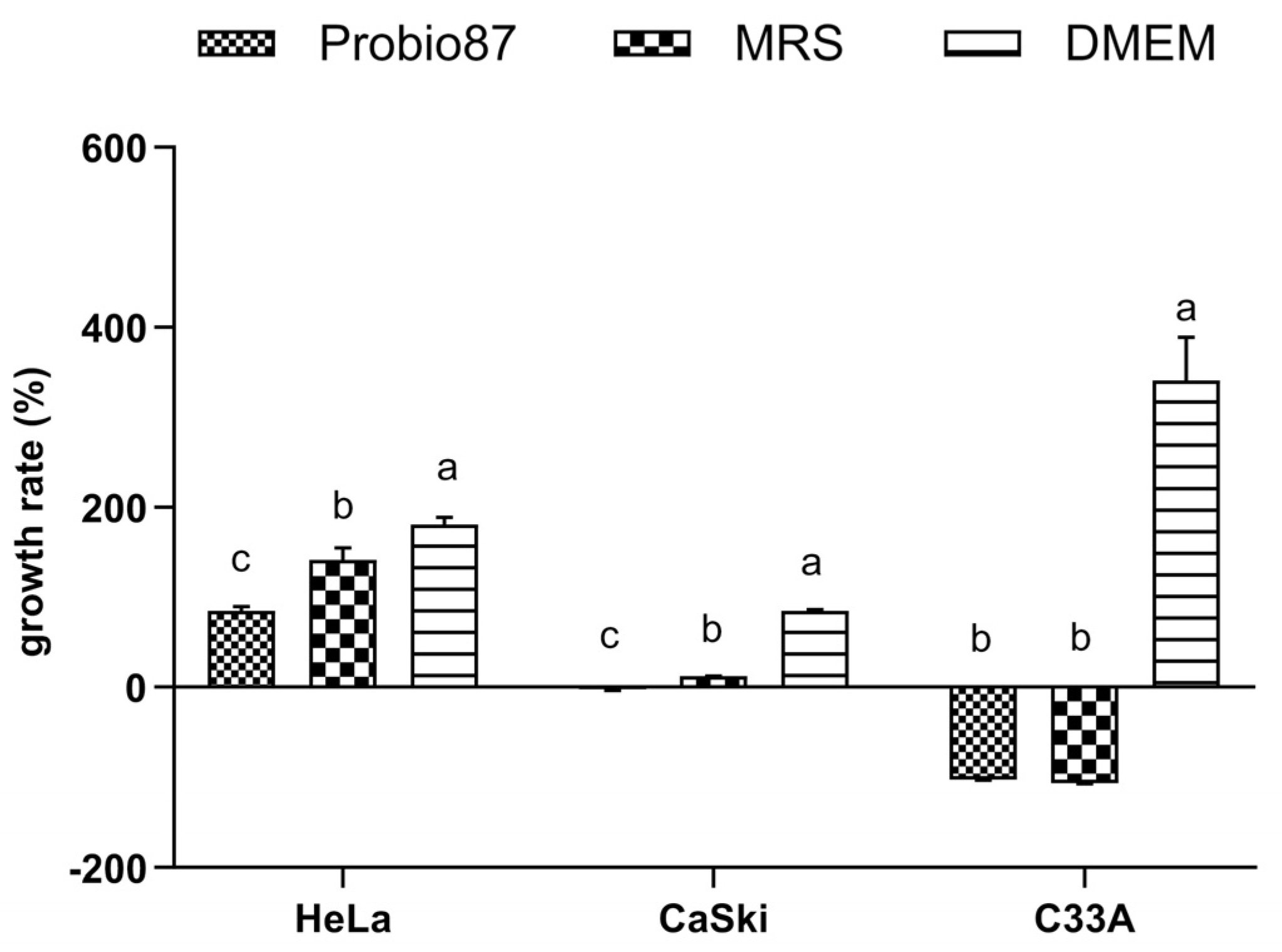
3.3.1. MTT Assay
3.3.2. Angiogenesis Array
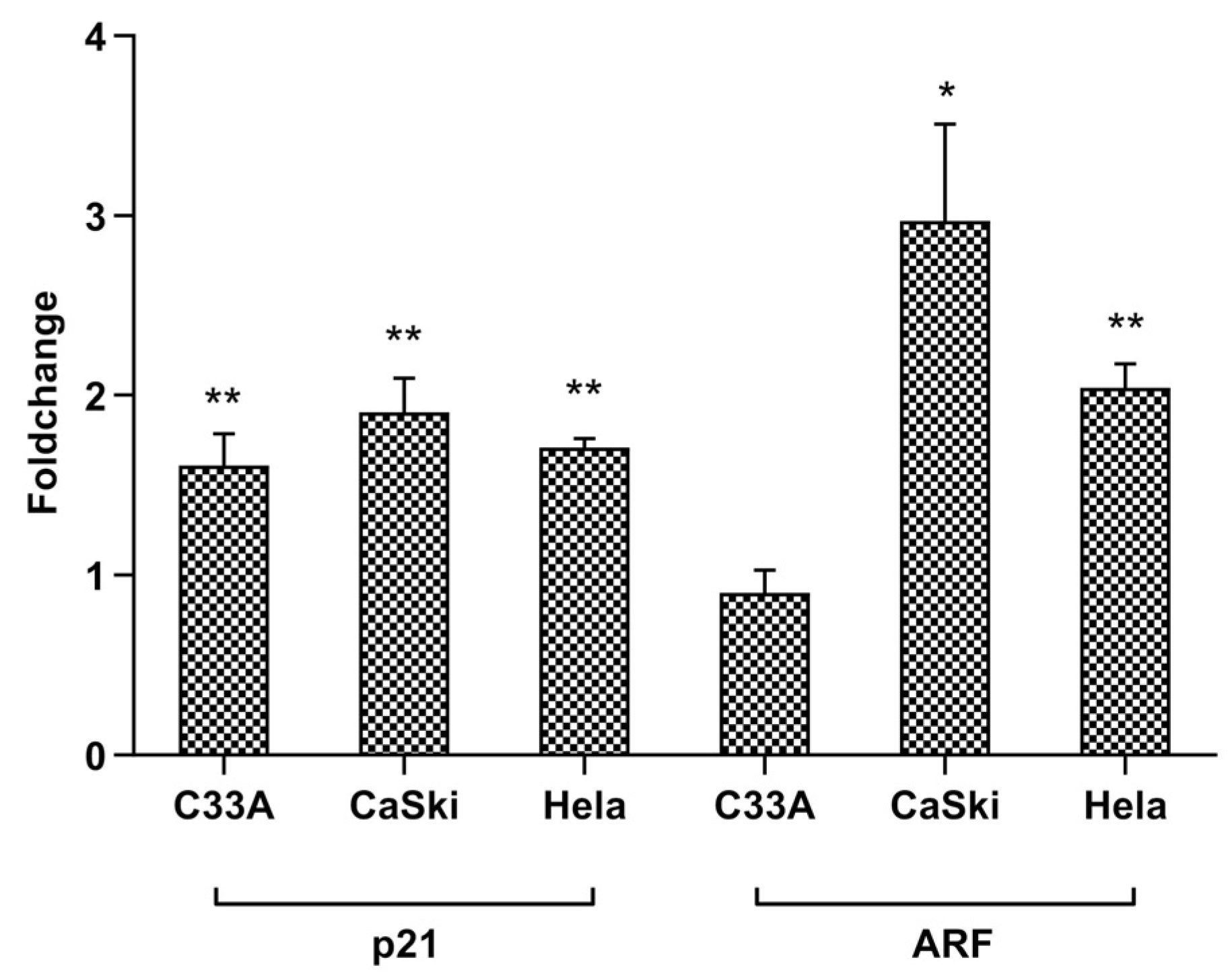
3.3.3. Cancer Pathway and RT-qPCR Test
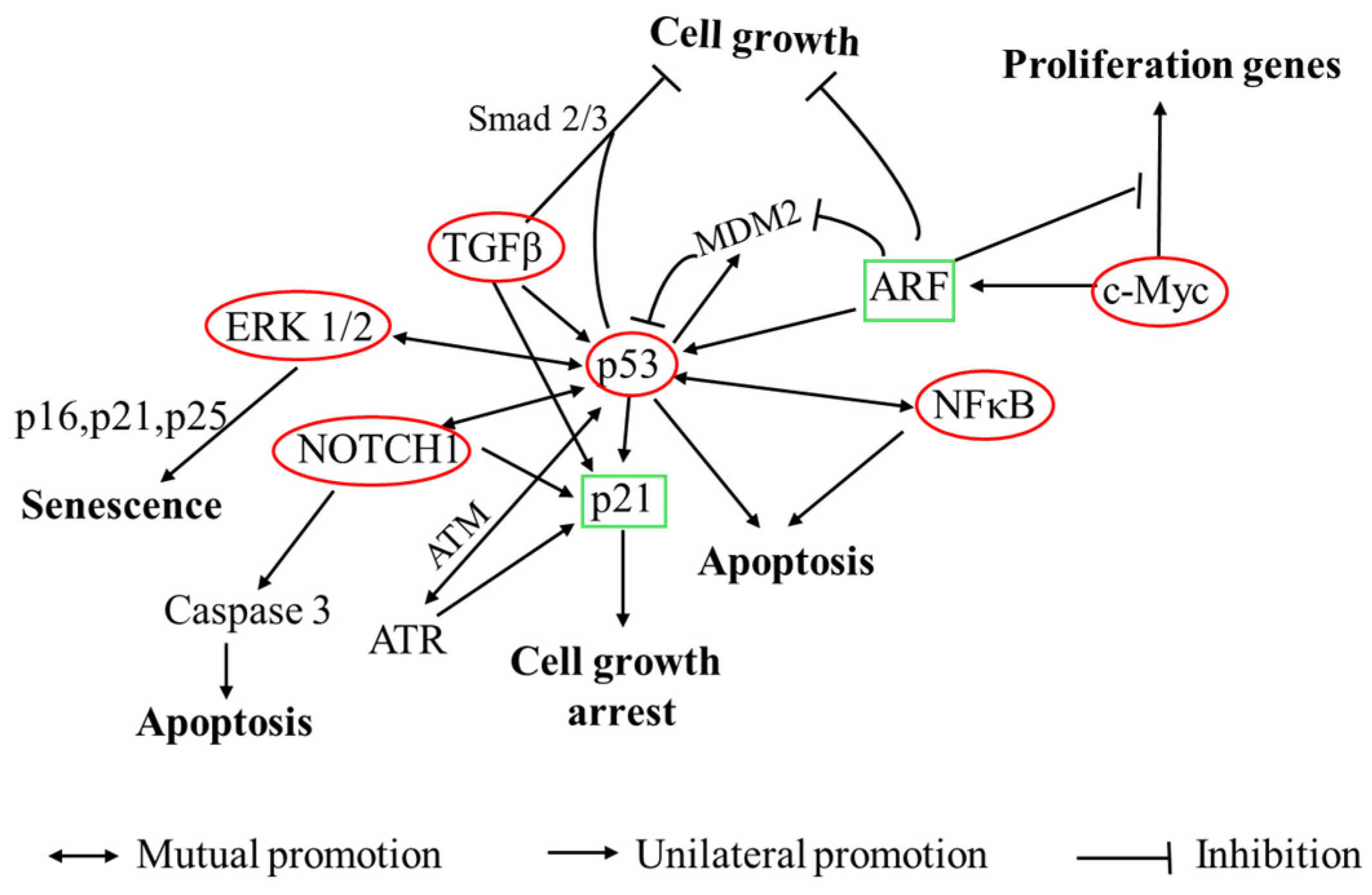
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANG-2 | angiopoietin-2 |
| API | Analytical Profile Index |
| ARF | alternate reading frame (tumor suppressor gene) |
| BHIs | Brain–Heart IUnfusion Supplemented Medium |
| CFS | cell-free supernatant |
| CFU | colony-forming unit |
| CIN | cervical intraepithelial neoplasia |
| CLSI | Clinical and Laboratory Standards Institute |
| C-33A | HPV-negative cervical cancer cell line |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EFSA | European Food Safety Authority |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FBS | Fetal Bovine Serum |
| FOS | fructooligosaccharide |
| GIT | gastrointestinal tract |
| GAL | gene array list |
| GOS | galactooligosaccharide |
| HPV | Human Papillomavirus |
| HUSM | Hospital Universiti Sains Malaysia |
| LAB | lactic acid bacteria |
| LSM | LAB Susceptibility Test Medium |
| MRS | de Man, Rogosa, and Sharpe Medium |
| MIC | minimum inhibitory concentration |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| OD | optical density |
| PBS | phosphate buffered saline |
| qPCR | quantitative polymerase chain reaction |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| SD | standard deviation |
| TGF-β | transforming growth factor beta |
| TSB | Tryptic Soy Broth |
| VEGF | Vascular Endothelial Growth Factor |
| VC | vehicle control |
| VM | vaginal microbiota |
| YEPD | yeast extract peptone dextrose |
References
- Evaluation of Health and Nutritional Properties of Powder Milk and Live Lactic Acid Bacteria. Available online: https://www.iqb.es/digestivo/pdfs/probioticos.pdf (accessed on 6 July 2025).
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 27 April 2024).
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The Impacts of Antimicrobial and Antifungal Activity of Cell-Free Supernatants from Lactic Acid Bacteria in Vitro and Foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Mehboudi, N.; Rahimi, H.R.; Bakhtiari, H.A.; Alimardani, M.; Jalili, A. The Impact of Probiotic Cell-Free Metabolites in MDR Pseudomonas Aeruginosa: Antibacterial Properties and Effect on Antibiotic Resistance Genes Expression. Lett. Appl. Microbiol. 2023, 76, ovad111. [Google Scholar] [CrossRef]
- Shi, L.H.; Balakrishnan, K.; Thiagarajah, K.; Mohd Ismail, N.I.; Yin, O.S. Beneficial Properties of Probiotics. Trop. Life Sci. Res. 2016, 27, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C.; Srigopalram, S. In Vitro Importance of Probiotic Lactobacillus Plantarum Related to Medical Field. Saudi J. Biol. Sci. 2016, 23, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus Plantarum DR7 Alleviates Stress and Anxiety in Adults: A Randomised, Double-Blind, Placebo-Controlled Study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Cubie, H.A. Diseases Associated with Human Papillomavirus Infection. Virology 2013, 445, 21–34. [Google Scholar] [CrossRef]
- Uehara, K.; Tanabe, Y.; Hirota, S.; Higa, S.; Toyoda, Z.; Kurima, K.; Kina, S.; Nakasone, T.; Arasaki, A.; Kinjo, T. Co-Expression of Low-Risk HPV E6/E7 and EBV LMP-1 Leads to Precancerous Lesions by DNA Damage. BMC Cancer 2021, 21, 688. [Google Scholar] [CrossRef]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human Papillomavirus Type Distribution in 30,848 Invasive Cervical Cancers Worldwide: Variation by Geographical Region, Histological Type and Year of Publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef]
- Ahmed, H.G.; Bensumaidea, S.H.; Alshammari, F.D.; Alenazi, F.S.H.; ALmutlaq, B.A.; Alturkstani, M.Z.; Aladani, I.A. Prevalence of Human Papillomavirus Subtypes 16 and 18 among Yemeni Patients with Cervical Cancer. Asian Pac. J. Cancer Prev. 2017, 18, 1543–1548. [Google Scholar] [CrossRef]
- Wang, Y.; Moon, A.; Huang, J.; Sun, Y.; Qiu, H.-J. Antiviral Effects and Underlying Mechanisms of Probiotics as Promising Antivirals. Front. Cell. Infect. Microbiol. 2022, 12, 928050. [Google Scholar] [CrossRef]
- Vanderpool, C.; Yan, F.; Polk, D.B. Mechanisms of Probiotic Action: Implications for Therapeutic Applications in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on Immunity. Am. J. Clin. Nutr. 2001, 73, 444S–450S. [Google Scholar] [CrossRef]
- Moon, E.C.; Park, M.S.; Lim, T.; Kim, R.H.; Ji, G.E.; Kim, S.Y.; Hwang, K.T. Antibacterial Effect of Cell-Free Supernatant Fraction from Lactobacillus Paracasei CH88 against Gardnerella Vaginalis. Sci. Rep. 2022, 12, 4763. [Google Scholar] [CrossRef]
- Li, Y.; Yu, T.; Yan, H.; Li, D.; Yu, T.; Yuan, T.; Rahaman, A.; Ali, S.; Abbas, F.; Dian, Z.; et al. Vaginal Microbiota and HPV Infection: Novel Mechanistic Insights and Therapeutic Strategies. Infect. Drug Resist. 2020, 13, 1213–1220. [Google Scholar] [CrossRef]
- Nami, Y.; Vaseghi Bakhshayesh, R.; Mohammadzadeh Jalaly, H.; Lotfi, H.; Eslami, S.; Hejazi, M.A. Probiotic Properties of Enterococcus Isolated From Artisanal Dairy Products. Front. Microbiol. 2019, 10, 300. [Google Scholar] [CrossRef]
- Lee, J.-E.; Lee, N.-K.; Paik, H.-D. Antimicrobial and Anti-Biofilm Effects of Probiotic Lactobacillus Plantarum KU200656 Isolated from Kimchi. Food Sci. Biotechnol. 2020, 30, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Klare, I.; Konstabel, C.; Werner, G.; Huys, G.; Vankerckhoven, V.; Kahlmeter, G.; Hildebrandt, B.; Müller-Bertling, S.; Witte, W.; Goossens, H. Antimicrobial Susceptibilities of Lactobacillus, Pediococcus and Lactococcus Human Isolates and Cultures Intended for Probiotic or Nutritional Use. J. Antimicrob. Chemother. 2007, 59, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Tham, C.S.-C.; Peh, K.-K.; Bhat, R.; Liong, M.-T. Probiotic Properties of Bifidobacteria and Lactobacilli Isolated from Local Dairy Products. Ann. Microbiol. 2012, 62, 1079–1087. [Google Scholar] [CrossRef]
- Carasi, P.; Ambrosis, N.M.; De Antoni, G.L.; Bressollier, P.; Urdaci, M.C.; de los Angeles Serradell, M. Adhesion Properties of Potentially Probiotic Lactobacillus kefiri to Gastrointestinal Mucus. J. Dairy Res. 2014, 81, 16–23. [Google Scholar] [CrossRef]
- Hütt, P.; Lapp, E.; Štšepetova, J.; Smidt, I.; Taelma, H.; Borovkova, N.; Oopkaup, H.; Ahelik, A.; Rööp, T.; Hoidmets, D.; et al. Characterisation of Probiotic Properties in Human Vaginal Lactobacilli Strains. Microb. Ecol. Health Dis. 2016, 27, 30484. [Google Scholar] [CrossRef] [PubMed]
- Pabari, K.; Pithva, S.; Kothari, C.; Purama, R.K.; Kondepudi, K.K.; Vyas, B.R.M.; Kothari, R.; Ambalam, P. Evaluation of Probiotic Properties and Prebiotic Utilization Potential of Weissella Paramesenteroides Isolated From Fruits. Probiotics Antimicrob. Proteins 2020, 12, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.Y.; Liong, M.T. Use of Extracellular Extracts of Lactic Acid Bacteria and Bifidobacteria for the Inhibition of Dermatological Pathogen Staphylococcus Aureus. Dermatol. Sin. 2014, 32, 141–147. [Google Scholar] [CrossRef]
- Turner, S.A.; Butler, G. The Candida Pathogenic Species Complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef]
- Yang, X.; Chang, T.; Yuan, Q.; Wei, W.; Wang, P.; Song, X.; Yuan, H. Changes in the Composition of Gut and Vaginal Microbiota in Patients with Postmenopausal Osteoporosis. Front. Immunol. 2022, 13, 930244. [Google Scholar] [CrossRef]
- Nisaa, A.A.; Oon, C.-E.; Sreenivasan, S.; Balakrishnan, V.; Tan, J.J.; Teh, C.S.-J.; Sany, S.; Todorov, S.D.; Liu, G.; Park, Y.-H.; et al. Breast Milk from Healthy Women Has Higher Anti-Candida Properties than Women with Vaginal Infections during Pregnancy. Food Sci. Biotechnol. 2023, 32, 471–480. [Google Scholar] [CrossRef]
- Yoshimura, K.; Ogawa, M.; Saito, M. In Vitro Characteristics of Intravaginal Lactobacilli; Why Is L. iners Detected in Abnormal Vaginal Microbial Flora? Arch. Gynecol. Obstet. 2020, 302, 671–677. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus Iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Happel, A.-U.; Kullin, B.; Gamieldien, H.; Wentzel, N.; Zauchenberger, C.Z.; Jaspan, H.B.; Dabee, S.; Barnabas, S.L.; Jaumdally, S.Z.; Dietrich, J.; et al. Exploring Potential of Vaginal Lactobacillus Isolates from South African Women for Enhancing Treatment for Bacterial Vaginosis. PLoS Pathog. 2020, 16, e1008559. [Google Scholar] [CrossRef]
- Petrina, M.A.B.; Cosentino, L.A.; Rabe, L.K.; Hillier, S.L. Susceptibility of Bacterial Vaginosis (BV)-Associated Bacteria to Secnidazole Compared to Metronidazole, Tinidazole and Clindamycin. Anaerobe 2017, 47, 115–119. [Google Scholar] [CrossRef]
- Li, X.J.; Yue, L.Y.; Guan, X.F.; Qiao, S.Y. The Adhesion of Putative Probiotic Lactobacilli to Cultured Epithelial Cells and Porcine Intestinal Mucus. J. Appl. Microbiol. 2008, 104, 1082–1091. [Google Scholar] [CrossRef]
- Martín, V.; Cárdenas, N.; Ocaña, S.; Marín, M.; Arroyo, R.; Beltrán, D.; Badiola, C.; Fernández, L.; Rodríguez, J.M. Rectal and Vaginal Eradication of Streptococcus Agalactiae (GBS) in Pregnant Women by Using Lactobacillus Salivarius CECT 9145, A Target-Specific Probiotic Strain. Nutrients 2019, 11, 810. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, X.; Jiang, R.; Zhao, A.; Yan, J.; Zheng, X.; Huang, F.; Liu, X.; Panee, J.; Rajani, C.; et al. Dysregulated Bile Acid Signaling Contributes to the Neurological Impairment in Murine Models of Acute and Chronic Liver Failure. EBioMedicine 2018, 37, 294–306. [Google Scholar] [CrossRef]
- Yuan, W.; Seng, Z.J.; Kohli, G.S.; Yang, L.; Yuk, H.-G. Stress Resistance Development and Genome-Wide Transcriptional Response of Escherichia Coli O157:H7 Adapted to Sublethal Thymol, Carvacrol, and Trans-Cinnamaldehyde. Appl. Environ. Microbiol. 2018, 84, e01616-18. [Google Scholar] [CrossRef] [PubMed]
- Gómez, N.C.; Ramiro, J.M.P.; Quecan, B.X.V.; de Melo Franco, B.D.G. Use of Potential Probiotic Lactic Acid Bacteria (LAB) Biofilms for the Control of Listeria Monocytogenes, Salmonella Typhimurium, and Escherichia Coli O157:H7 Biofilms Formation. Front. Microbiol. 2016, 7, 863. [Google Scholar] [CrossRef]
- Cheon, M.-J.; Lim, S.-M.; Lee, N.-K.; Paik, H.-D. Probiotic Properties and Neuroprotective Effects of Lactobacillus Buchneri KU200793 Isolated from Korean Fermented Foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef]
- Gilliland, S.E.; Staley, T.E.; Bush, L.J. Importance of Bile Tolerance of Lactobacillus Acidophilus Used as a Dietary Adjunct. J. Dairy Sci. 1984, 67, 3045–3051. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, E.H.; Ibrahim, M.I.A. Production and Characterization of Exopolysaccharide From Newly Isolated Marine Probiotic Lactiplantibacillus Plantarum EI6 With in Vitro Wound Healing Activity. Front. Microbiol. 2022, 13, 903363. [Google Scholar] [CrossRef]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile Resistance Mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, M.; Gao, F.; Lu, M.; Chen, G. Effects of Dietary Probiotic Supplementation on the Growth, Gut Health and Disease Resistance of Juvenile Nile Tilapia (Oreochromis Niloticus). Anim. Nutr. 2020, 6, 69–79. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Yang, L.; Jiang, L.; Zhang, J.; Yu, X.; Chen, G.; Zhu, H.; Tang, W.; Li, Y.; et al. Spatial and Temporal Persistence of Fluorescent Lactiplantibacillus Plantarum RS-09 in Intestinal Tract. Front. Microbiol. 2022, 13, 843650. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Liu, Y.; Liu, Y.; Wang, S.; Liu, Q.; Hao, H.; Yi, H. Screening and Probiotic Potential Evaluation of Bacteriocin-Producing Lactiplantibacillus Plantarum In Vitro. Foods 2022, 11, 1575. [Google Scholar] [CrossRef]
- Ke, A.; Parreira, V.R.; Farber, J.M.; Goodridge, L. Inhibition of Cronobacter Sakazakii in an Infant Simulator of the Human Intestinal Microbial Ecosystem Using a Potential Synbiotic. Front. Microbiol. 2022, 13, 947624. [Google Scholar] [CrossRef] [PubMed]
- Suissa, R.; Oved, R.; Maan, H.; Hadad, U.; Gilhar, O.; Meijler, M.M.; Koren, O.; Kolodkin-Gal, I. Context-Dependent Differences in the Functional Responses of Lactobacillaceae Strains to Fermentable Sugars. Front. Microbiol. 2022, 13, 949932. [Google Scholar] [CrossRef]
- Succi, M.; Tremonte, P.; Pannella, G.; Tipaldi, L.; Cozzolino, A.; Romaniello, R.; Sorrentino, E.; Coppola, R. Pre-Cultivation with Selected Prebiotics Enhances the Survival and the Stress Response of Lactobacillus Rhamnosus Strains in Simulated Gastrointestinal Transit. Front. Microbiol. 2017, 8, 1067. [Google Scholar] [CrossRef]
- Dong, Y.; Han, M.; Fei, T.; Liu, H.; Gai, Z. Utilization of Diverse Oligosaccharides for Growth by Bifidobacterium and Lactobacillus Species and Their in Vitro Co-Cultivation Characteristics. Int. Microbiol. 2024, 27, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Sugiyama, M.; Mukai, T. Adhesion Properties of Lactic Acid Bacteria on Intestinal Mucin. Microorganisms 2016, 4, 34. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Igbinosa, I.H.; Aighewi, I.T. Detection of Potential Pathogenic Bacteria in Selected Public Swimming Pools: Public Health Implications. Niger. J. Life Sci. 2022, 6, 45–50. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Wang, Z.; Xu, Y.; Lin, R.; Jiao, T.; Ouyang, Q.; Chen, Q. ZnO@Ag-Functionalized Paper-Based Microarray Chip for SERS Detection of Bacteria and Antibacterial and Photocatalytic Inactivation. Anal. Chem. 2023, 95, 18415–18425. [Google Scholar] [CrossRef]
- Haindongo, E.H.; Ndakolo, D.; Hedimbi, M.; Vainio, O.; Hakanen, A.; Vuopio, J. Antimicrobial Resistance Prevalence of Escherichia Coli and Staphylococcus Aureus amongst Bacteremic Patients in Africa: A Systematic Review. J. Glob. Antimicrob. Resist. 2023, 32, 35–43. [Google Scholar] [CrossRef]
- Komesu, Y.M.; Dinwiddie, D.L.; Richter, H.E.; Lukacz, E.S.; Sung, V.W.; Siddiqui, N.Y.; Zyczynski, H.M.; Ridgeway, B.; Rogers, R.G.; Arya, L.A.; et al. Defining the Relationship Between Vaginal and Urinary Microbiomes. Am. J. Obstet. Gynecol. 2020, 222, 154.e1–154.e10. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial Communities in Women with Bacterial Vaginosis: High Resolution Phylogenetic Analyses Reveal Relationships of Microbiota to Clinical Criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of Vaginal Probiotics Lactobacillus Crispatus Chen-01 in Women with High-Risk HPV Infection: A Prospective Controlled Pilot Study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef]
- The Role of L. iners in Vaginal Health: Insights and Controversies. Available online: https://ipa-biotics.org/the-role-of-l-iners-in-vaginal-health-insights-and-controversies/ (accessed on 1 August 2025).
- Bonnardel, F.; Haslam, S.M.; Dell, A.; Feizi, T.; Liu, Y.; Tajadura-Ortega, V.; Akune, Y.; Sykes, L.; Bennett, P.R.; MacIntyre, D.A.; et al. Proteome-Wide Prediction of Bacterial Carbohydrate-Binding Proteins as a Tool for Understanding Commensal and Pathogen Colonisation of the Vaginal Microbiome. NPJ Biofilms Microbiomes 2021, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, S.; Shruthi, B.; Vanitha, P.R.; Sreenivasa, M.Y. Probiotics and Their Postbiotics for the Control of Opportunistic Fungal Pathogens: A Review. Biotechnol. Rep. 2023, 38, e00800. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.C.; Rossoni, R.D.; de Barros, P.P.; Santos, J.D.; Fugisaki, L.R.O.; Leão, M.P.V.; Junqueira, J.C. Action Mechanisms of Probiotics on Candida Spp. and Candidiasis Prevention: An Update. J. Appl. Microbiol. 2020, 129, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, M.-J.; Kumar, A.; Lee, H.-W.; Yang, Y.; Kim, Y. Vascular Endothelial Growth Factor Signaling in Health and Disease: From Molecular Mechanisms to Therapeutic Perspectives. Signal Transduct. Target. Ther. 2025, 10, 170. [Google Scholar] [CrossRef]
- Hashizume, H.; Falcón, B.L.; Kuroda, T.; Baluk, P.; Coxon, A.; Yu, D.; Bready, J.V.; Oliner, J.D.; McDonald, D.M. Complementary Actions of Inhibitors of Angiopoietin-2 and VEGF on Tumor Angiogenesis and Growth. Cancer Res. 2010, 70, 2213–2223. [Google Scholar] [CrossRef]
- Li, S.; Hu, G. Angiogenin-Mediated rRNA Transcription in Cancer and Neurodegeneration. Int. J. Biochem. Mol. Biol. 2010, 1, 26–35. [Google Scholar] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kon, N.; Zhong, J.; Zhang, P.; Yu, L.; Gu, W. Differential Effects on ARF Stability by Normal versus Oncogenic Levels of C-Myc Expression. Mol. Cell 2013, 51, 46–56. [Google Scholar] [CrossRef]
- Datta, A.; Nag, A.; Pan, W.; Hay, N.; Gartel, A.L.; Colamonici, O.; Mori, Y.; Raychaudhuri, P. Myc-ARF (Alternate Reading Frame) Interaction Inhibits the Functions of Myc. J. Biol. Chem. 2004, 279, 36698–36707. [Google Scholar] [CrossRef]
- Elston, R.; Inman, G.J. Crosstalk between P53 and TGF-β Signalling. J. Signal Transduct. 2012, 2012, 294097. [Google Scholar] [CrossRef] [PubMed]
- Adorno, M.; Cordenonsi, M.; Montagner, M.; Dupont, S.; Wong, C.; Hann, B.; Solari, A.; Bobisse, S.; Rondina, M.B.; Guzzardo, V.; et al. A Mutant-P53/Smad Complex Opposes P63 to Empower TGFβ-Induced Metastasis. Cell 2009, 137, 87–98. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Rutecki, S.; Ksiazek, K. The Functional Multipotency of Transforming Growth Factor β Signaling at the Intersection of Senescence and Cancer. Cell. Mol. Life Sci. 2022, 79, 196. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Dupont, S.; Maretto, S.; Insinga, A.; Imbriano, C.; Piccolo, S. Links between Tumor Suppressors: P53 Is Required for TGF-β Gene Responses by Cooperating with Smads. Cell 2003, 113, 301–314. [Google Scholar] [CrossRef]
- Huang, R.-F.S.; Wei, Y.-J.; Inbaraj, B.S.; Chen, B.-H. Inhibition of Colon Cancer Cell Growth by Nanoemulsion Carrying Gold Nanoparticles and Lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar] [CrossRef]
- Dey, A.; Tergaonkar, V.; Lane, D.P. Double-Edged Swords as Cancer Therapeutics: Simultaneously Targeting P53 and NF-kappaB Pathways. Nat. Rev. Drug Discov. 2008, 7, 1031–1040. [Google Scholar] [CrossRef]
- Wu, H.; Lozano, G. NF-Kappa B Activation of P53. A Potential Mechanism for Suppressing Cell Growth in Response to Stress. J. Biol. Chem. 1994, 269, 20067–20074. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Schmidt, C.; Sclabas, G.M.; Li, Z.; Pelicano, H.; Peng, B.; Yao, A.; Niu, J.; Zhang, W.; Evans, D.B.; et al. Stabilization of P53 Is a Novel Mechanism for Proapoptotic Function of NF-kappaB. J. Biol. Chem. 2004, 279, 27549–27559. [Google Scholar] [CrossRef]
- Carrà, G.; Lingua, M.; Maffeo, B.; Taulli, R.; Morotti, A. P53 vs NF-κB: The Role of Nuclear Factor-Kappa B in the Regulation of P53 Activity and Vice Versa. Cell Mol. Life Sci. 2020, 77, 4449–4458. [Google Scholar] [CrossRef]
- Barkett, M.; Gilmore, T.D. Control of Apoptosis by Rel/NF-kappaB Transcription Factors. Oncogene 1999, 18, 6910–6924. [Google Scholar] [CrossRef]
- Yin, L.; Yu, X. Arsenic-Induced Apoptosis in the P53-Proficient and P53-Deficient Cells through Differential Modulation of NFkB Pathway. Food Chem. Toxicol. 2018, 118, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Pan, W.-W.; Liu, S.-B.; Shen, Z.-F.; Xu, Y.; Hu, L.-L. ERK/MAPK Signalling Pathway and Tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, B.; Liu, Y.; Yu, X.; Cheng, G. Dual Effects of Active ERK in Cancer: A Potential Target for Enhancing Radiosensitivity (Review). Oncol. Lett. 2020, 20, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Lei, T.; Guo, P.; Yu, J.; Xu, Q.; Luo, Y.; Ke, R.; Huang, D. Mechanisms Shaping the Role of ERK1/2 in Cellular Senescence (Review). Mol. Med. Rep. 2019, 19, 759–770. [Google Scholar] [CrossRef]
- Ussar, S.; Voss, T. MEK1 and MEK2, Different Regulators of the G1/S Transition. J. Biol. Chem. 2004, 279, 43861–43869. [Google Scholar] [CrossRef]
- Lin, A.W.; Barradas, M.; Stone, J.C.; van Aelst, L.; Serrano, M.; Lowe, S.W. Premature Senescence Involving P53 and P16 Is Activated in Response to Constitutive MEK/MAPK Mitogenic Signaling. Genes Dev. 1998, 12, 3008–3019. [Google Scholar] [CrossRef]
- Cammarano, M.S.; Nekrasova, T.; Noel, B.; Minden, A. Pak4 Induces Premature Senescence via a Pathway Requiring p16INK4/p19ARF and Mitogen-Activated Protein Kinase Signaling. Mol. Cell. Biol. 2005, 25, 9532–9542. [Google Scholar] [CrossRef]
- Zhuang, D.; Mannava, S.; Grachtchouk, V.; Tang, W.-H.; Patil, S.; Wawrzyniak, J.A.; Berman, A.E.; Giordano, T.J.; Prochownik, E.V.; Soengas, M.S.; et al. C-MYC Overexpression Is Required for Continuous Suppression of Oncogene-Induced Senescence in Melanoma Cells. Oncogene 2008, 27, 6623–6634. [Google Scholar] [CrossRef]
- Ntziachristos, P.; Lim, J.S.; Sage, J.; Aifantis, I. From Fly Wings to Targeted Cancer Therapies: A Centennial for Notch Signaling. Cancer Cell 2014, 25, 318–334. [Google Scholar] [CrossRef]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch Signaling Pathway in Cancer: From Mechanistic Insights to Targeted Therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-L.; Wu, Q.; Vega, V.B.; Chiu, K.P.; Ng, P.; Zhang, T.; Shahab, A.; Yong, H.C.; Fu, Y.; Weng, Z.; et al. A Global Map of P53 Transcription-Factor Binding Sites in the Human Genome. Cell 2006, 124, 207–219. [Google Scholar] [CrossRef]
- Lefort, K.; Mandinova, A.; Ostano, P.; Kolev, V.; Calpini, V.; Kolfschoten, I.; Devgan, V.; Lieb, J.; Raffoul, W.; Hohl, D.; et al. Notch1 Is a P53 Target Gene Involved in Human Keratinocyte Tumor Suppression through Negative Regulation of ROCK1/2 and MRCKalpha Kinases. Genes Dev. 2007, 21, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Yugawa, T.; Handa, K.; Narisawa-Saito, M.; Ohno, S.; Fujita, M.; Kiyono, T. Regulation of Notch1 Gene Expression by P53 in Epithelial Cells. Mol. Cell Biol. 2007, 27, 3732–3742. [Google Scholar] [CrossRef]
- Dotto, G.P. Crosstalk of Notch with P53 and P63 in Cancer Growth Control. Nat. Rev. Cancer 2009, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Amount (g/L) | Reagent Information |
|---|---|---|
| Peptone | 10 | Bendosen, Selangor Darul Ehsan, Malaysia |
| Meat extract | 8 | Himedia, Mumbai, India |
| Yeast extract | 4 | |
| Ammonium citrate | 2 | |
| Sodium acetate | 3 | Oxoid, Basingstoke, Hampshire, UK |
| Magnesium sulphate | 0.1 | |
| Manganese sulphate | 0.05 | |
| Dipotassium phosphate | 2 | |
| Glu/ | 20 | Bendosen, Selangor Darul Ehsan, Malaysia |
| FOS/GOS/ | NFBC, Yunfu, China | |
| Inulin | Fuji Neihon Seito, Tokyo, Japan |
| Active Ingredients | Reaction | Active Ingredients | Reaction |
|---|---|---|---|
| Control | − | Esculin ferriccitrate | + |
| Glycerol | − | Salicin | + |
| Erythritol | − | D-cellobiose | + |
| D-arabinose | − | D-maltose | + |
| L-arabinose | + | D-lactose (bovine origin) | + |
| D-ribose | + | D-melibiose | + |
| D-xylose | − | D-saccharose | + |
| L-xylose | − | D-trehalose | + |
| D-adonitol | − | Inulin | − |
| Methyl-β-D-xylopyranoside | − | D-melezitose | + |
| D-galactose | + | D-raffinose | + |
| D-glucose | + | Amidon (starch) | − |
| D-fructose | + | Glycogen | − |
| D-mannose | + | Xylitol | − |
| L-sorbose | − | Gentiobiose | + |
| L-rhamnose | − | D-turanose | + |
| Dulcitol | − | D-lyxose | − |
| Inositol | − | D-tagatose | − |
| D-mannitol | + | D-fucose | − |
| D-sorbitol | + | L-fucose | − |
| Methyl-α-D-mannopytanoside | + | D-arabitol | − |
| Methyl-α-D-glucopyranoside | − | L-arabitol | − |
| N-acetylglucosamine | + | Potassium gluconate | + |
| Amygdalin | + | Potassium 2-ketogluconate | − |
| Arbutin | + | Potassium 5-ketogluconate | − |
| Antibiotic | EFSA Cut-Off Values (mg/L) | Probio87 MICs * (mg/L) | Classification |
|---|---|---|---|
| Ampicillin | 2 | 2 | Susceptible |
| Gentamicin | 16 | 1 | Susceptible |
| Kanamycin | 64 | 32 | Susceptible |
| Erythromycin | 1 | 0.06 | Susceptible |
| Clindamycin | 2 | 0.5 | Susceptible |
| Tetracycline | 32 | 32 | Susceptible |
| Chloramphenicol | 8 | 8 | Susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Uma Mageswary, M.; Nisaa, A.A.; Li, X.; Tan, Y.-J.; Oon, C.-E.; Tan, C.-S.; Luo, W.; Liong, M.-T. Antimicrobial and Anticancer Activities of Lactiplantibacillus plantarum Probio87 Isolated from Human Breast Milk. Nutrients 2025, 17, 2554. https://doi.org/10.3390/nu17152554
Xu P, Uma Mageswary M, Nisaa AA, Li X, Tan Y-J, Oon C-E, Tan C-S, Luo W, Liong M-T. Antimicrobial and Anticancer Activities of Lactiplantibacillus plantarum Probio87 Isolated from Human Breast Milk. Nutrients. 2025; 17(15):2554. https://doi.org/10.3390/nu17152554
Chicago/Turabian StyleXu, Pei, Mageswaran Uma Mageswary, Azka Ainun Nisaa, Xiang Li, Yi-Jer Tan, Chern-Ein Oon, Cheng-Siang Tan, Wen Luo, and Min-Tze Liong. 2025. "Antimicrobial and Anticancer Activities of Lactiplantibacillus plantarum Probio87 Isolated from Human Breast Milk" Nutrients 17, no. 15: 2554. https://doi.org/10.3390/nu17152554
APA StyleXu, P., Uma Mageswary, M., Nisaa, A. A., Li, X., Tan, Y.-J., Oon, C.-E., Tan, C.-S., Luo, W., & Liong, M.-T. (2025). Antimicrobial and Anticancer Activities of Lactiplantibacillus plantarum Probio87 Isolated from Human Breast Milk. Nutrients, 17(15), 2554. https://doi.org/10.3390/nu17152554







