Myokine Circulating Levels in Postmenopausal Women with Overweight or Obesity: Effects of Resistance Training and/or DHA-Rich n-3 PUFA Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention Design
2.3. Resistance Training Program
2.4. Evaluation of Body Composition
LST = arms lean mass “[kg]” + legs lean mass “[kg]”,
ALST: Appendicular lean soft tissue.
2.5. Evaluation of Serum Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Basal Characteristics
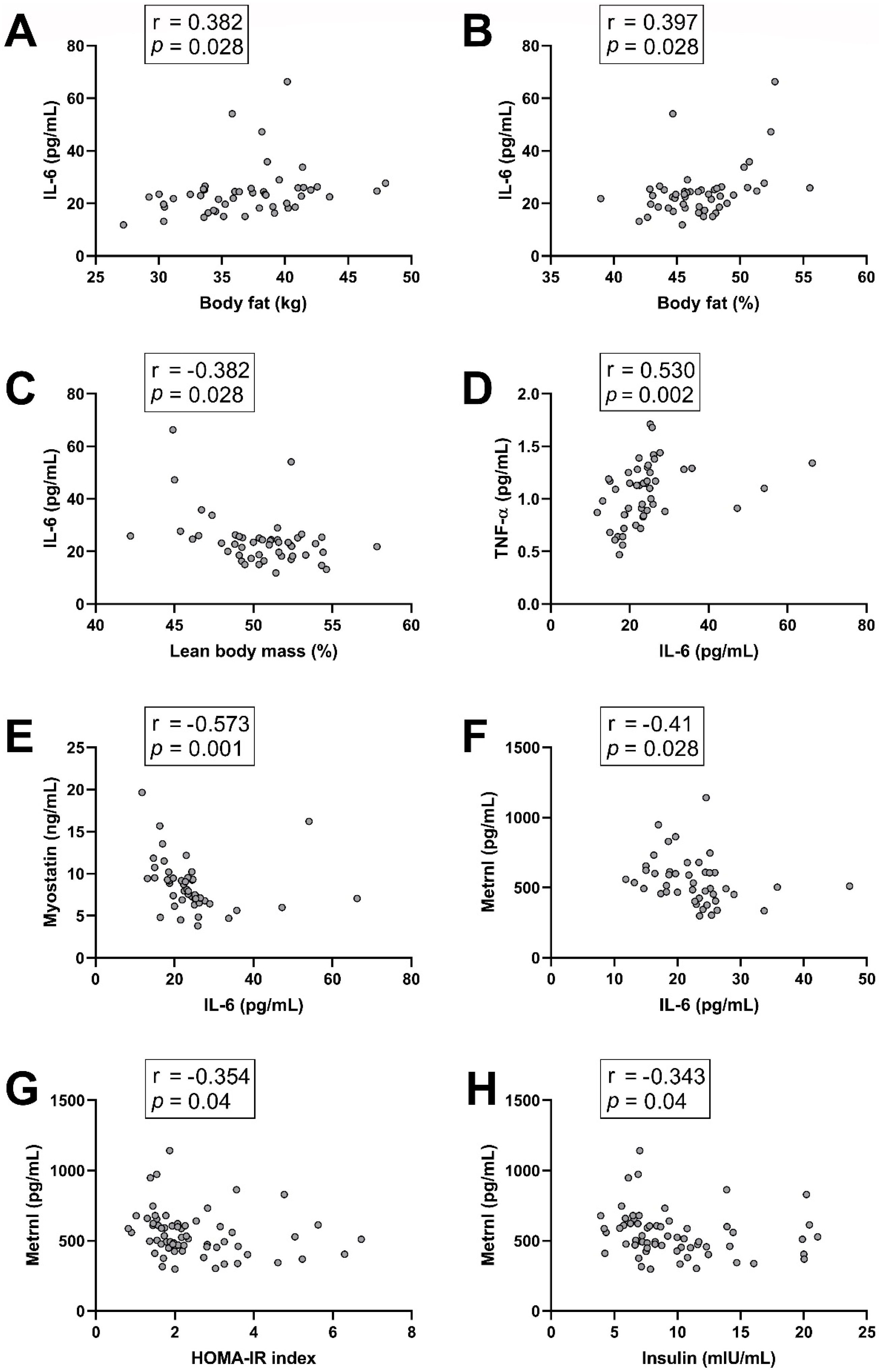
3.2. Correlations Between Myokine/Cytokine Levels and Basal Characteristics of the Subjects
3.3. Baseline Anthropometric and Biochemical Characteristics of the Different Experimental Groups
3.4. Effects of the 16-Week Intervention with DHA-Rich n-3 PUFA Supplementation and/or Resistance Training in Postmenopausal Women with Overweight/Obesity
3.4.1. Effects on Myokines/Cytokines
3.4.2. Effects on Muscle Function and Serum Biochemical Parameters
3.4.3. Correlations Between Changes in Myokine Levels and Changes in Biochemical Parameters After the Intervention
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Health Topics: Obesity. 2021. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 2 November 2024).
- Crimmins, E.M. Lifespan and healthspan: Past, present, and promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef]
- United Nations (UN). 2019 Revision of World Population Prospects. 2019. Available online: https://population.un.org/wpp/ (accessed on 3 June 2024).
- Bollheimer, L.C.; Buettner, R.; Pongratz, G.; Brunner-Ploss, R.; Hechtl, C.; Banas, M.; Singler, K.; Hamer, O.; Stroszczyynski, C.; Sieber, C.; et al. Sarcopenia in the aging high-fat fed rat: A pilot study for modeling sarcopenic obesity in rodents. Biogerontology 2012, 13, 609–620. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 221 Pt 13, jeb163840. [Google Scholar] [CrossRef]
- Abildgaard, J.; Tingstedt, J.; Zhao, Y.; Hartling, H.J.; Pedersen, A.T.; Lindegaard, B.; Dam Nielsen, S. Increased systemic inflammation and altered distribution of T-cell subsets in postmenopausal women. PLoS ONE 2020, 15, e0235174. [Google Scholar] [CrossRef] [PubMed]
- Samargandy, S.; Matthews, K.A.; Brooks, M.M.; Barinas-Mitchell, E.; Magnani, J.W.; Janssen, I.; Kazlauskaite, R.; El Khoudary, S. Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: The SWAN heart study. Menopause 2021, 28, 626–633. [Google Scholar] [CrossRef]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet Neuronal Interact 2009, 9, 186–197. [Google Scholar]
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef]
- Lovejoy, J.C. The menopause and obesity. Prim. Care Clin. Off. Pract. 2003, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Volaklis, K.A.; Halle, M.; Meisinger, C. Muscular strength as a strong predictor of mortality: A narrative review. Eur. J. Intern. Med. 2015, 26, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Yang, J. Enhanced skeletal muscle for effective glucose homeostasis. Prog. Mol. Biol. Transl. Sci. 2014, 121, 133–163. [Google Scholar]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.; Galis, Z.; Gao, Y.; Haus, J.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Catoire, M.; Kersten, S. The search for exercise factors in humans. FASEB J. 2015, 29, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Goodpaster, B.H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Isenmann, E.; Kaluza, D.; Havers, T.; Elbeshausen, A.; Geisler, S.; Hofmann, K.; Flenker, U.; Diel, P.; Gavanda, S. Resistance training alters body composition in middle-aged women depending on menopause-A 20-week control trial. BMC Women’s Health 2023, 23, 526. [Google Scholar] [CrossRef]
- Félix-Soriano, E.; Martínez-Gayo, A.; Cobo, M.J.; Pérez-Chávez, A.; Ibáñez-Santos, J.; Palacios Samper, N.; Goikoetxea Galarza, I.; Cuervo, M.; García-Unciti, M.; González-Muniesa, P.; et al. Effects of DHA-rich n-3 fatty acid supplementation and/or resistance training on body composition and cardiometabolic biomarkers in overweight and obese post-menopausal women. Nutrients 2021, 13, 2465. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, A.P.; Nahas-Neto, J.; Orsatti, C.L.; Dias, F.B.; Poloni, P.F.; Schmitt, E.B.; Nahas, E. Effects of omega-3 on metabolic markers in postmenopausal women with metabolic syndrome. Climacteric 2015, 18, 290–298. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Bojar, I.; Mlak, R.; Homa-Mlak, I.; Prendecka, M.; Owoc, A.; Małecka-Massalska, T. Association between myostatin serum concentration and body fat level in peri- and postmenopausal women. Arch. Med. Sci. AMS 2022, 18, 365–375. [Google Scholar] [CrossRef]
- Falsetti, I.; Palmini, G.; Donati, S.; Aurilia, C.; Iantomasi, T.; Brandi, M.L. Irisin and its role in postmenopausal osteoporosis and sarcopenia. Biomedicines 2024, 12, 928. [Google Scholar] [CrossRef]
- Parkin, R.A.; Murray, A.J. The therapeutic potential of irisin to mitigate the risk of metabolic syndrome in postmenopausal women. Front. Reprod. Health 2024, 6, 1355922. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Men, Ageing and Health: Achieving Health Across the Life Span; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Aranceta Bartrina, J.; Grupo Colaborativo de la Sociedad Española de Nutrición Comunitaria (SENC); Arija Val, V.; Maíz Aldalur, E.; Martínez de Victoria Muñoz, E.; Ortega Anta, R.M.; Pérez-Rodrigo, C.; Quiles Izquierdo, J.; Rodríguez Martín, A.; Román Villas, B.; et al. Dietary Guidelines for the Spanish population (SENC, diciembre 2016); the new graphic icon of healthy food. Nutr. Hosp. 2016, 33 (Suppl. 8), 1–48. [Google Scholar]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Arrillaga, C.; Ruiz, Z.V.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Munro, I.A.; Garg, M.L. Dietary supplementation with n-3 PUFA does not promote weight loss when combined with a very-low-energy diet. Br. J. Nutr. 2012, 108, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.A.; Garg, M.L. Dietary supplementation with long chain omega-3 polyunsaturated fatty acids and weight loss in obese adults. Obes. Res. Clin. Pract. 2013, 7, e173–e181. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.A.; Garg, M.L. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: A double-blinded randomised controlled trial. Food Funct. 2013, 4, 650–658. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Qualified Health Claims: Letters of Enforcement Discretion. Omega-3 fatty Acids and Reduced Risk of Coronary Heart Disease (Martek Petition). Available online: https://www.fda.gov/food/food-labeling-nutrition/qualified-health-claims-letters-enforcement-discretion (accessed on 13 October 2024).
- Mataix-Verdú, J.; Mañas Almendros, M.; Llopis González, J.; Martínez de Victoria, E. Tablas de Composición de Alimentos; Universidad de Granada: Granada, Spain, 1993. [Google Scholar]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, J.; Izquierdo, M.; Argüelles, I.; Forga, L.; Larrión, J.L.; García-Unciti, M.; Idoate, F.; Gorostiaga, E. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 2005, 28, 662–667. [Google Scholar] [CrossRef]
- Ibáñez, J.; Gorostiaga, E.M.; Alonso, A.M.; Forga, L.; Argüelles, I.; Larrión, J.L.; Izquierdo, M. Lower muscle strength gains in older men with type 2 diabetes after resistance training. J. Diabetes Its Complicat. 2008, 22, 112–118. [Google Scholar] [CrossRef]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Schoenfeld, B.J.; Souza, M.F.; Tomeleri, C.M.; Venturini, D.; Barbosa, D.S.; Cyrino, E. Traditional and pyramidal resistance training systems improve muscle quality and metabolic biomarkers in older women: A randomized crossover study. Exp. Gerontol. 2016, 79, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Huerta, A.E.; Navas-Carretero, S.; Prieto-Hontoria, P.L.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity 2015, 23, 313–321. [Google Scholar] [CrossRef]
- Kim, J.; Heshka, S.; Gallagher, D.; Kotler, D.P.; Mayer, L.; Albu, J.; Shen, W.; Freda, P.; Heymsfield, S. Intermuscular adipose tissue-free skeletal muscle mass: Estimation by dual-energy X-ray absorptiometry in adults. J. Appl. Physiol. 2004, 97, 655–660. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Fernández-Macías, J.C.; Ochoa-Martínez, A.C.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Atherogenic index of plasma: Novel predictive biomarker for cardiovascular illnesses. Arch. Med. Res. 2019, 50, 285–294. [Google Scholar] [CrossRef]
- Huerta, A.E.; Prieto-Hontoria, P.L.; Fernández-Galilea, M.; Sáinz, N.; Cuervo, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Circulating irisin and glucose metabolism in overweight/obese women: Effects of α-lipoic acid and eicosapentaenoic acid. J. Physiol. Biochem. 2015, 71, 547–558. [Google Scholar] [CrossRef]
- Yoon, H.; Jeon, D.J.; Park, C.E.; You, H.S.; Moon, A.E. Relationship between homeostasis model assessment of insulin resistance and beta cell function and serum 25-hydroxyvitamin D in non-diabetic Korean adults. J. Clin. Biochem. Nutr. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- DeFina, L.F.; Marcoux, L.G.; Devers, S.M.; Cleaver, J.P.; Willis, B.L. Effects of omega-3 supplementation in combination with diet and exercise on weight loss and body composition. Am. J. Clin. Nutr. 2011, 93, 455–462. [Google Scholar] [CrossRef]
- Hill, A.M.; Buckley, J.D.; Murphy, K.J.; Howe, P.R.C. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am. J. Clin. Nutr. 2007, 85, 1267–1274. [Google Scholar] [CrossRef]
- Vancampfort, D.; Sánchez, C.P.R.; Hallgren, M.; Schuch, F.; Firth, J.; Rosenbaum, S.; Van Damme, T.; Stubbs, B. Dropout from exercise randomized controlled trials among people with anxiety and stress-related disorders: A meta-analysis and meta-regression. J. Affect. Disord. 2021, 282, 996–1004. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Gruman, C.; King, M.B.; Wolfson, L.I. Attrition in an exercise intervention: A comparison of early and later dropouts. J. Am. Geriatr. Soc. 2000, 48, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Batterham, M.; Tapsell, L.C.; Charlton, K.E. Predicting dropout in dietary weight loss trials using demographic and early weight change characteristics: Implications for trial design. Obes. Res. Clin. Pract. 2016, 10, 189–196. [Google Scholar] [CrossRef]
- Santos, H.O.; Cerqueira, H.S.; Tinsley, G.M. The effects of dietary supplements, nutraceutical agents, and physical exercise on myostatin levels: Hope or hype? Metabolites 2022, 12, 1146. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, X.; Xu, L.; Huang, G. Irisin: Circulating levels in serum and its relation to gonadal axis. Endocrine 2022, 75, 663–671. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, Z.Y.; Zhang, Z.; Wang, D.S.; Xu, J.; Miao, C.Y. Evaluation of two commercial enzyme-linked immunosorbent assay kits for the detection of human circulating metrnl. Chem. Pharm. Bull. 2018, 66, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Sadeghi Bahmani, D.; Brand, S. Serum and plasma tumor necrosis factor alpha levels in individuals with obstructive sleep apnea syndrome: A meta-analysis and meta-regression. Life 2020, 10, 87. [Google Scholar] [CrossRef]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.; Ruas, J.; Wrann, C.; Lo, J.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Alizadeh, A. Association of meteorin-like hormone with insulin resistance and body composition in healthy Iranian adults. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 881–885. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. 2), S3. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grade inflammation and its markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- El-Mikkawy, D.M.E.; EL-Sadek, M.A.; EL-Badawy, M.A.; Samaha, D. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt. Rheumatol. Rehabil. 2020, 47, 7. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Mikó, A.; Pótó, L.; Mátrai, P.; Hegyi, P.; Füredi, N.; Garami, A.; Illés, A.; Solymár, M.; Vincze, Á.; Balaskó, M.; et al. Gender difference in the effects of interleukin-6 on grip strength-a systematic review and meta-analysis. BMC Geriatr. 2018, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The health ABC study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Netea, M.G.; van Riel, P.L.; van der Meer, J.W.; Stalenhoef, A.F. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Dadmanesh, M.; Aghajani, H.; Fadaei, R.; Ghorban, K. Lower serum levels of meteorin-like/subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PLoS ONE 2018, 13, e0204180. [Google Scholar] [CrossRef]
- Alizadeh, H. Meteorin-like protein (Metrnl): A metabolic syndrome biomarker and an exercise mediator. Cytokine 2022, 157, 155952. [Google Scholar] [CrossRef]
- Ding, X.; Chang, X.; Wang, J.; Bian, N.; An, Y.; Wang, G.; Liu, J. Serum metrnl levels are decreased in subjects with overweight or obesity and are independently associated with adverse lipid profile. Front. Endocrinol. 2022, 13, 938341. [Google Scholar] [CrossRef]
- Du, Y.; Ye, X.; Lu, A.; Zhao, D.; Liu, J.; Cheng, J.; Yang, T. Inverse relationship between serum metrnl levels and visceral fat obesity (VFO) in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2020, 161, 108068. [Google Scholar] [CrossRef]
- Choi, K.; Jang, H.Y.; Ahn, J.M.; Hwang, S.H.; Chung, J.W.; Choi, Y.S.; Kim, J.; Jang, E.; Choi, G.; Jeong, S. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 492–505. [Google Scholar] [CrossRef]
- Wu, Q.; Dan, Y.L.; He, Y.S.; Xiang, K.; Hu, Y.Q.; Zhao, C.N.; Zhong, X.; Wang, D.; Pan, H. Circulating meteorin-like levels in patients with type 2 diabetes mellitus: A meta-analysis. Curr. Pharm. Des. 2020, 26, 5732–5738. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, S.H.; Kim, H.C.; Bang, J.S.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Shin, Y.; Jeong, J. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Schmid, A.; Karrasch, T.; Schäffler, A. Meteorin-like protein (metrnl) in obesity, during weight loss and in adipocyte differentiation. J. Clin. Med. 2021, 10, 4338. [Google Scholar] [CrossRef] [PubMed]
- Pellitero, S.; Piquer-Garcia, I.; Ferrer-Curriu, G.; Puig, R.; Martínez, E.; Moreno, P.; Tarascó, J.; Balibrea, J.; Lerin, C.; Puig-Domingo, M.; et al. Opposite changes in meteorin-like and oncostatin m levels are associated with metabolic improvements after bariatric surgery. Int. J. Obes. 2018, 42, 919–922. [Google Scholar] [CrossRef]
- Fadaei, R.; Dadmanesh, M.; Moradi, N.; Ahmadi, R.; Shokoohi Nahrkhalaji, A.; Aghajani, H.; Ghorban, K. Serum levels of subfatin in patients with type 2 diabetes mellitus and its association with vascular adhesion molecules. Arch. Physiol. Biochem. 2020, 126, 335–340. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, H.M.; Selim, F.O.; Hosny, T.A.M.; Almassry, H.N. Association of low serum meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res. Clin. Pract. 2019, 150, 57–63. [Google Scholar] [CrossRef]
- Ferns, G.A.; Fekri, K.; Shahini Shams Abadi, M.; Banitalebi Dehkordi, M.; Arjmand, M.H. A meta-analysis of the relationship between serums metrnl-like protein/subfatin and risk of type 2 diabetes mellitus and coronary artery disease. Arch. Physiol. Biochem. 2023, 129, 1084–1090. [Google Scholar] [CrossRef]
- Ryan, A.S.; Hurlbut, D.E.; Lott, M.E.; Ivey, F.M.; Fleg, J.; Hurley, B.F.; Goldberg, A. Insulin action after resistive training in insulin resistant older men and women. J. Am. Geriatr. Soc. 2001, 49, 247–253. [Google Scholar] [CrossRef]
- Hanson, E.D.; Srivatsan, S.R.; Agrawal, S.; Menon, K.S.; Delmonico, M.J.; Wang, M.Q.; Hurley, B. Effects of strength training on physical function: Influence of power, strength, and body composition. J. Strength Cond. Res. 2009, 23, 2627–2637. [Google Scholar] [CrossRef]
- Häkkinen, K.; Pakarinen, A.; Kraemer, W.J.; Newton, R.U.; Alen, M. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. J. Gerontol. Biol. Sci. Med. Sci. 2000, 55, B95–B105. [Google Scholar]
- Sillanpää, E.; Laaksonen, D.E.; Häkkinen, A.; Karavirta, L.; Jensen, B.; Kraemer, W.J.; Nyman, K.; Häkkinen, K. Body composition, fitness, and metabolic health during strength and endurance training and their combination in middle-aged and older women. Eur. J. Appl. Physiol. 2009, 106, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ahtiainen, J.P.; Sallinen, J.; Häkkinen, K.; Sillanpää, E. Inter-individual variation in response to resistance training in cardiometabolic health indicators. Scand. J. Med. Sci. Sports 2020, 30, 1040–1053. [Google Scholar] [CrossRef]
- Strandberg, E.; Edholm, P.; Ponsot, E.; Wåhlin-Larsson, B.; Hellmén, E.; Nilsson, A.; Engfeldt, P.; Cederholm, T.; Risérus, U.; Kadi, F. Influence of combined resistance training and healthy diet on muscle mass in healthy elderly women: A randomized controlled trial. J. Appl. Physiol. 2015, 119, 918–925. [Google Scholar] [CrossRef]
- Sardeli, A.V.; Tomeleri, C.M.; Cyrino, E.S.; Fernhall, B.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Effect of resistance training on inflammatory markers of older adults: A meta-analysis. Exp. Gerontol. 2018, 111, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Traylor, D.A.; Weijs, P.J.M.; Phillips, S.M. Defining anabolic resistance: Implications for delivery of clinical care nutrition. Curr. Opin. Crit. Care 2018, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Ferrando, A.A.; Wolfe, R.R. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am. J. Physiol. Endocrinol. Metab. 1999, 276, E118–E124. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Phillips, S.M.; Libardi, C.A.; Vechin, F.C.; Lixandrão, M.E.; Jannig, P.R.; Costa, L.; Bacurau, A.; Snijders, T.; Parise, G.; et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016, 594, 5209–5222. [Google Scholar] [CrossRef]
- Tipton, K.D.; Phillips, S.M. Dietary protein for muscle hypertrophy. Limits Hum. Endur. 2013, 76, 73–84. [Google Scholar]
- Bosse, J.D.; Dixon, B.M. Dietary protein to maximize resistance training: A review and examination of protein spread and change theories. J. Int. Soc. Sports Nutr. 2012, 9, 42. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.; Ferrando, A.; Arent, S.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.; Carbone, J.; Combs, G.; Young, A. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef] [PubMed]
- Mettler, S.; Mitchell, N.; Tipton, K.D. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med. Sci. Sports Exerc. 2010, 42, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I. The effects of dietary omega-3s on muscle composition and quality in older adults. Curr. Nutr. Rep. 2016, 5, 99–105. [Google Scholar] [CrossRef]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef]
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle 2017, 8, 245–250. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Caserotti, P.; Aagaard, P.; Larsen, J.B.; Puggaard, L. Explosive heavy-resistance training in old and very old adults: Changes in rapid muscle force, strength and power. Scand. J. Med. Sci. Sports 2008, 18, 773–782. [Google Scholar] [CrossRef]
- Phillips, M.D.; Patrizi, R.M.; Cheek, D.J.; Wooten, J.S.; Barbee, J.J.; Mitchell, J.B. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med. Sci. Sports Exerc. 2012, 44, 2099–2110. [Google Scholar] [CrossRef]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A. Diet and its relationship with grip strength in community-dwelling older men and women: The Hertfordshire cohort study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef]
- Reinders, I.; Song, X.; Visser, M.; Eiriksdottir, G.; Gudnason, V.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Brouwer, I.; Harris, T.; et al. Plasma phospholipid PUFAs are associated with greater muscle and knee extension strength but not with changes in muscle parameters in older adults. J. Nutr. 2015, 145, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.H.; Kleppinger, A.; Kenny, A.M. Self-reported dietary intake of omega-3 fatty acids and association with bone and lower extremity function. J. Am. Geriatr. Soc. 2009, 57, 1781–1788. [Google Scholar] [CrossRef]
- Rodacki, C.L.N.; Rodacki, A.L.F.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef]
- Ren, J.; Grundy, S.M.; Liu, J.; Wang, W.; Wang, M.; Sun, J.; Liu, J.; Li, Y.; Wu, Z.; Zhao, D. Long-term coronary heart disease risk associated with very-low-density lipoprotein cholesterol in Chinese: The results of a 15-Year Chinese Multi-Provincial Cohort Study (CMCS). Atherosclerosis 2010, 211, 327–332. [Google Scholar] [CrossRef]
- Johansen, M.Ø.; Nielsen, S.F.; Afzal, S.; Vedel-Krogh, S.; Davey Smith, G.; Nordestgaard, B.G. Very low-density lipoprotein cholesterol may mediate a substantial component of the effect of obesity on myocardial infarction risk: The Copenhagen general population study. Clin. Chem. 2021, 67, 276–287. [Google Scholar] [CrossRef]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.; Khaw, K.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FERHDL). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic index of plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran 2015, 29, 240. [Google Scholar]
- Nwagha, U.I.; Ikekpeazu, E.J.; Ejezie, F.E.; Neboh, E.E.; Maduka, I.C. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr. Health Sci. 2010, 10, 248–252. [Google Scholar]
- Jensen, J.; Nilas, L.; Christiansen, C. Influence of menopause on serum lipids and lipoproteins. Maturitas 1990, 12, 321–331. [Google Scholar] [CrossRef]
- Monteiro-Junior, R.S.; de Tarso Maciel-Pinheiro, P.; da Matta Mello Portugal, E.; da Silva Figueiredo, L.F.; Terra, R.; Carneiro, L.S.F.; Rodrigues, V.; Nascimento, O.; Deslandes, A.; Laks, J. Effect of exercise on inflammatory profile of older persons: Systematic review and meta-analyses. J. Phys. Act. Health 2018, 15, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Wongwarawipat, T.; Papageorgiou, N.; Bertsias, D.; Siasos, G.; Tousoulis, D. Olive oil-related anti-inflammatory effects on atherosclerosis: Potential clinical implications. Endocr. Metab. Immune Disord.-Drug Targets 2018, 18, 51–62. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Glaser, R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav. Immun. 2011, 25, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Toft, A.D.; Jensen, L.B.; Bruunsgaard, H.; Ibfelt, T.; Halkjaer-Kristensen, J.; Febbraio, M.; Pedersen, B. Cytokine response to eccentric exercise in young and elderly humans. Am. J. Physiol.-Cell Physiol. 2002, 283, C289–C295. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Stupin, M.; Kibel, A.; Stupin, A.; Selthofer-Relatić, K.; Matić, A.; Mihalj, M.; Mihaljević, Z.; Jukić, I.; Drenjančević, I. The Physiological Effect of n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) Intake and Exercise on Hemorheology, Microvascular Function, and Physical Performance in Health and Cardiovascular Diseases; Is There an Interaction of Exercise and Dietary n-3 PUFA Intake? Front. Physiol. 2019, 10, 1129. [Google Scholar] [CrossRef]
- Khalafi, M.; Habibi Maleki, A.; Symonds, M.E.; Rosenkranz, S.K.; Ehsanifar, M.; Mohammadi Dinani, S. The combined effects of omega-3 polyunsaturated fatty acid supplementation and exercise training on body composition and cardiometabolic health in adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2025, 66, 151–159. [Google Scholar] [CrossRef]
- Tayebi, S.M.; Golmohammadi, M.; Eslami, R.; Shakiba, N.; Costa, P.B. The effects of eight weeks of circuit resistance training on serum metrnl levels and insulin resistance in individuals with type 2 diabetes. Diabetes Metab. Disord. 2023, 22, 1151–1158. [Google Scholar] [CrossRef]
- Tok, Ö.; Kişioğlu, S.V.; Ersöz, H.Ö.; Kahveci, B.; Göktaş, Z. Effects of increased physical activity and/or weight loss diet on serum myokine and adipokine levels in overweight adults with impaired glucose metabolism. J. Diabetes Its Complicat. 2021, 35, 107892. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Tannerstedt, J.; Selänne, H.; Kainulainen, H.; Kovanen, V.; Mero, A.A. Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J. Appl. Physiol. 2009, 106, 1720–1729. [Google Scholar] [CrossRef]
- Motahari Rad, M.; Bijeh, N.; Attarzadeh Hosseini, S.R.; Raouf Saeb, A. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2023, 129, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Shabkhiz, F.; Khalafi, M.; Rosenkranz, S.; Karimi, P.; Moghadami, K. Resistance training attenuates circulating FGF-21 and myostatin and improves insulin resistance in elderly men with and without type 2 diabetes mellitus: A randomised controlled clinical trial. Eur. J. Sport Sci. 2021, 21, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Gharakhanloo, R.; Sharghi, S.; Gharaati, M.R.; Larijani, B.; Omidfar, K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol. Cell. Endocrinol. 2010, 317, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Aria, B.; Symonds, M.E.; Rosenkranz, S.K. The effects of resistance training on myostatin and follistatin in adults: A systematic review and meta-analysis. Physiol. Behav. 2023, 269, 114272. [Google Scholar] [CrossRef] [PubMed]
- Haß, U.; Kochlik, B.; Herpich, C.; Rudloff, S.; Norman, K. Effects of an omega-3 supplemented, high-protein diet in combination with vibration and resistance exercise on muscle power and inflammation in old adults: A pilot randomized controlled trial. Nutrients 2022, 14, 4274. [Google Scholar] [CrossRef]
- Fife, E.; Kostka, J.; Kroc, Ł.; Guligowska, A.; Pigłowska, M.; Sołtysik, B.; Kaufman-Szymczyk, A.; Fabianowska-Majewska, K.; Kostka, T. Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr. 2018, 18, 200. [Google Scholar] [CrossRef]
- McPherron, A.C. The ups and downs of exercise and insulin sensitivity: A role for the myokine myostatin in glucose metabolism? Acta Physiol. 2016, 217, 6–10. [Google Scholar] [CrossRef][Green Version]
- Morissette, M.R.; Cook, S.A.; Buranasombati, C.; Rosenberg, M.A.; Rosenzweig, A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am. J. Physiol.-Cell Physiol. 2009, 297, 1124–1132. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol.-Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.; Birkeland, K.; Jensen, J.; Drevon, C. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Shockett, P.; Webb, N.D.; Shah, U.; Castracane, V.D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2014, 46, 150–154. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.; Boström, E.; Choi, J.; Long, J.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Ellefsen, S.; Vikmoen, O.; Slettaløkken, G.; Whist, J.E.; Nygaard, H.; Hollan, I.; Rauk, I.; Vegge, G.; Strand, T.; Raastad, T.; et al. Irisin and FNDC5: Effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur. J. Appl. Physiol. 2014, 114, 1875–1888. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Hofmann, T.; Elbelt, U.; Ahnis, A.; Kobelt, P.; Rose, M.; Stengel, A. Irisin levels are not affected by physical activity in patients with anorexia nervosa. Front. Endocrinol. 2014, 4, 202. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans -results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010, 235, 785–795. [Google Scholar] [CrossRef]
- Pokushalov, E.; Ponomarenko, A.; Bayramova, S.; Garcia, C.; Pak, I.; Shrainer, E.; Voronina, E.; Sokolova, E.; Johnson, M.; Miller, R. Evaluating the Impact of Omega-3 Fatty Acid (SolowaysTM) Supplementation on Lipid Profiles in Adults with PPARG Polymorphisms: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 16, 97. [Google Scholar] [CrossRef]
- Loukil, I.; Mutch, D.M.; Plourde, M. Genetic association between FADS and ELOVL polymorphisms and the circulating levels of EPA/DHA in humans: A scoping review. Genes Nutr. 2024, 19, 11. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. Do Non-Responders to Exercise Exist-and If So, What Should We Do About Them? Sports Med. 2019, 49, 1–7. [Google Scholar] [CrossRef]


| Characteristic | Baseline |
|---|---|
| Age (years) | 58.50 (3.20) |
| Weight (kg) | 78.87 (7.35) |
| BMI (kg/m2) | 30.61 (2.12) |
| Body fat (kg) | 36.84 (4.83) |
| Body fat (%) | 46.76 (3.29) |
| VAT (kg) | 1.28 (0.46) |
| Lean body mass (kg) | 39.59 (3.82) |
| Lean body mass (%) | 50.42 (3.11) |
| Skeletal muscle mass (kg) | 19.18 (2.54) |
| Muscle quality | 10.73 (1.53) |
| Characteristic | Baseline |
|---|---|
| Fasting glucose (mg/dL) | 104.90 (15.50) |
| Insulin (mIU/mL) | 9.96 (4.60) |
| HOMA-IR index | 2.62 (1.44) |
| HOMA-β index | 93.82 (47.65) |
| Triglycerides (mg/dL) | 105.10 (43.60) |
| Total cholesterol (mg/dL) | 245.60 (37.50) |
| HDL-cholesterol (mg/dL) | 64.28 (14.70) |
| LDL-cholesterol (mg/dL) | 160.36 (34.42) |
| VLDL-cholesterol (mg/dL) | 21.03 (8.72) |
| Atherogenic index | −0.16 (0.23) |
| TNF-α (pg/mL) | 1.02 (0.27) |
| IL-6 (pg/mL) | 24.20 (9.67) |
| METRNL (pg/mL) | 547.70 (162.10) |
| Myostatin (ng/mL) | 9.23 (3.36) |
| Irisin (ng/mL) | 12.68 (1.95) |
| Characteristic | P | n-3 | P+RT | n-3+RT | p Two-Way ANOVA c | ||
|---|---|---|---|---|---|---|---|
| n-3 | RT | n-3xRT | |||||
| Body fat (kg) | |||||||
| Baseline | 36.44 (4.24) | 36.70 (5.09) | 36.67 (5.58) | 37.70 (4.62) | |||
| Change | −2.92 (1.93) a,*** | −2.39 (2.02) a,*** | −2.39 (2.19) a,*** | −2.81 (3.14) a,** | 0.928 | 0.921 | 0.399 |
| Lean body mass (kg) | |||||||
| Baseline | 38.16 (3.50) | 41.28 (3.68) | 38.93 (4.24) | 40.64 (3.10) | |||
| Change | 0.29 (1.35) | −0.25 (0.77) | 0.19 (0.93) | 0.11 (1.11) | 0.233 | 0.629 | 0.373 |
| Skeletal muscle mass (kg) | |||||||
| Baseline | 18.21 (2.22) | 20.31 (2.49) | 18.78 (2.49) | 19.83 (2.63) | |||
| Change | −0.02 (1.25) | −0.29 (0.63) | 0.12 (0.74) | 0.17 (0.94) | 0.618 | 0.182 | 0.485 |
| Muscle quality | |||||||
| Baseline | 11.29 (1.47) | 10.65 (1.89) | 10.92 (1.56) | 9.96 (1.11) | |||
| Change | −0.05 (0.64) | 0.40 (0.50) a,* | 1.18 (0.89) b,*** | 1.77 (0.92) a,*** | 0.011 | <0.001 | 0.730 |
| HOMA-β index | |||||||
| Baseline | 107.75 (44.19) | 101.23 (66.96) | 79.03 (39.60) | 87.92 (36.18) | |||
| Change | −13.28 (51.88) | −0.52 (43.52) | −5.56 (47.02) | −11.05 (30.89) | 0.735 | 0.895 | 0.397 |
| VLDL-cholesterol (mg/dL) | |||||||
| Baseline | 18.53 (5.89) | 23.64 (11.06) | 22.18 (10.33) | 20.27 (6.65) | |||
| Change | 0.39 (5.26) | −5.77 (10.59) b,* | −3.42 (4.76) b,** | −3.78 (5.75) b,* | 0.047 | 0.576 | 0.075 |
| Adjusted change d | 0.58 ± 1.10 | −6.00 ± 1.74 b,** | −3.53 ± 1.50 b,** | −3.67 ± 1.67 b,* | 0.039 | 0.580 | 0.051 |
| Atherogenic index | |||||||
| Baseline | −0.22 (0.21) | −0.10 (0.23) | −0.14 (0.26) | −0.18 (0.19) | |||
| Change | 0.00 (0.11) | −0.11 (0.20) a,* | −0.04 (0.09) | −0.07 (0.15) | 0.040 | 0.917 | 0.204 |
| Adjusted change d | 0.00 ± 0.03 | −0.12 ± 0.04 a,* | −0.04 ± 0.03 | −0.07 ± 0.03 | 0.037 | 0.918 | 0.174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Gayo, A.; Félix-Soriano, E.; Ibáñez-Santos, J.; García-Unciti, M.; González-Muniesa, P.; Moreno-Aliaga, M.J.; on behalf of OBELEX Project. Myokine Circulating Levels in Postmenopausal Women with Overweight or Obesity: Effects of Resistance Training and/or DHA-Rich n-3 PUFA Supplementation. Nutrients 2025, 17, 2553. https://doi.org/10.3390/nu17152553
Martínez-Gayo A, Félix-Soriano E, Ibáñez-Santos J, García-Unciti M, González-Muniesa P, Moreno-Aliaga MJ, on behalf of OBELEX Project. Myokine Circulating Levels in Postmenopausal Women with Overweight or Obesity: Effects of Resistance Training and/or DHA-Rich n-3 PUFA Supplementation. Nutrients. 2025; 17(15):2553. https://doi.org/10.3390/nu17152553
Chicago/Turabian StyleMartínez-Gayo, Alejandro, Elisa Félix-Soriano, Javier Ibáñez-Santos, Marisol García-Unciti, Pedro González-Muniesa, María J. Moreno-Aliaga, and on behalf of OBELEX Project. 2025. "Myokine Circulating Levels in Postmenopausal Women with Overweight or Obesity: Effects of Resistance Training and/or DHA-Rich n-3 PUFA Supplementation" Nutrients 17, no. 15: 2553. https://doi.org/10.3390/nu17152553
APA StyleMartínez-Gayo, A., Félix-Soriano, E., Ibáñez-Santos, J., García-Unciti, M., González-Muniesa, P., Moreno-Aliaga, M. J., & on behalf of OBELEX Project. (2025). Myokine Circulating Levels in Postmenopausal Women with Overweight or Obesity: Effects of Resistance Training and/or DHA-Rich n-3 PUFA Supplementation. Nutrients, 17(15), 2553. https://doi.org/10.3390/nu17152553







