Abstract
Background/Objectives: Accurate dietary assessment is crucial for nutritional epidemiology, but tools like 24 h recalls (24HRs) face challenges with missing or implausible data. The Automated Self-Administered 24 h Dietary Assessment Tool (ASA24) facilitates large-scale data collection, but its lack of interviewer input may lead to implausible dietary recalls (IDRs), affecting data integrity. Multiple imputation (MI) is commonly used to handle missing data, but its effectiveness in high-variability dietary data is uncertain. This study aims to assess MI’s accuracy in estimating nutrient intake under varying levels of missing data. Methods: Data from 24HRs completed by 743 adolescents (ages 13–18) in Ontario, Canada, were used. Implausible recalls were excluded based on nutrient thresholds, creating a cleaned reference dataset. Missing data were simulated at 10%, 20%, and 40% deletion rates. MI via chained equations was applied, incorporating demographic and psychosocial variables as predictors. Imputed values were compared to actual values using Spearman’s correlation and accuracy within ±10% of true values. Results: Spearman’s rho values between the imputed and actual nutrient intakes were weak (mean ρ ≈ 0.24). Accuracy within ±10% was low for most nutrients (typically < 25%), with no clear trend by missingness level. Diet quality scores showed slightly higher accuracy, but values were still under 30%. Conclusions: MI performed poorly in estimating individual nutrient intake in this adolescent sample. While MI may preserve sample characteristics, it is unreliable for accurate nutrient estimates and should be used cautiously. Future studies should focus on improving data quality and exploring better imputation methods.
1. Introduction
Accurately measuring dietary intake in individuals is inherently challenging. Common dietary assessment methods include food diaries, food frequency questionnaires [1], and emerging technologies such as photographic records [2]. Among these, the 24 h dietary recall (24HR) is one of the most widely used tools and is designed to provide a detailed account of all foods and beverages consumed on the previous day [1].
Unlike other dietary assessment methods, the 24HR typically relies on a trained interviewer to elicit detailed information. For instance, when a participant reports consuming “a bagel,” the interviewer may follow up with specific questions about the type (e.g., whole grain vs. refined flour), flavour (e.g., plain vs. cinnamon raisin), brand, preparation method (e.g., toasted or not), condiments (e.g., butter, cream cheese), accompanying beverages, and portion size [1]. Although this level of detail allows for more accurate nutrient analysis [3], it also increases respondent burden and the cognitive demand of recall.
While trained interviewers enhance the accuracy of dietary recalls, they also represent a key limitation of traditional 24HRs [4]. Since these interviews are typically conducted one-on-one [3], larger-scale studies must hire multiple interviewers or extend the study duration to accommodate all participants. Each 24HR can take up to an hour to complete [3], making the use of 24HRs a resource-intensive method. As a result, researchers often turn to alternatives such as food frequency questionnaires (FFQs), which are self-administered, less time-consuming, and require minimal training [5]. However, despite their practicality, FFQs are less accurate than 24HRs in estimating specific nutrient intakes [4], limiting their suitability for certain types of analyses.
To address the time and resource burden associated with traditional 24HRs, the National Cancer Institute developed the Automated Self-Administered 24 h Dietary Assessment Tool (ASA24) [6]. The ASA24 utilizes the Automated Multiple-Pass Method (AMPM) to collect detailed information about respondents’ dietary intake from the previous day. Conducted entirely online, ASA24 is well-suited for large-scale nutrition research [6]. By eliminating the need for trained interviewers, ASA24 overcomes many logistical challenges of traditional 24HRs, enabling multiple participants to complete recalls simultaneously and making it feasible for use in large epidemiological studies.
Despite its advantages, the absence of an interviewer in ASA24 introduces new limitations. Participants may misreport their intake—either unintentionally, through typographical errors or misinterpretation of prompts, or intentionally, through selective reporting—resulting in implausible dietary recalls (IDRs) [7]. Many of these errors might have been identified and corrected during interviewer-led 24HRs, but in automated settings, they can distort both mean and variability estimates of nutrient intake. Although ASA24 incorporates standardized portion size prompts and facilitates anomaly detection during data cleaning [8], the removal or exclusion of IDRs still represents a loss of potentially valuable data.
Missing data can reduce statistical power, increase the risk of Type II errors [9,10,11], and bias study results, particularly when the missing data are systematically different from those observed [12]. While researchers may commonly use listwise deletion to address this issue, this approach can exacerbate bias [13]. A more sophisticated approach is multiple imputation (MI), which replaces missing values with plausible estimates derived from fitted models across multiple newly created datasets [14]. MI is particularly effective when data are Missing Completely at Random (MCAR) or Missing at Random (MAR)—that is, when the probability of missingness is unrelated or explainable by observed variables [15].
Nevertheless, all imputation methods—including MI—share a fundamental limitation: they involve estimation without knowing the true values. While MI is more robust than simpler approaches, its accuracy in the context of high-variability outcomes like daily nutrient intake remains unclear [15,16]. Given the day-to-day fluctuation in dietary behaviours (and subsequent nutrient intake), it is essential to understand how well MI can reconstruct missing dietary data, particularly in large-scale nutrition studies that use ASA24.
To date, no study has directly evaluated the accuracy of MI in estimating missing nutrient intake data derived from 24HRs. Therefore, the overall goal of this study was to assess the performance of MI in accurately reconstructing nutrient intake values under conditions of simulated missingness. To do so, the following specific objectives were identified:
- To assess the correlation between imputed and true values using Spearman’s rho at 10%, 20%, and 40% levels of simulated missing data.
- To evaluate the accuracy of imputed values, defined as being within ± 10% of the actual value for each nutrient.
- To examine trends in correlation strength and accuracy across increasing proportions of missing data.
2. Materials and Methods
2.1. Study Design and Data Source
Data for this study were drawn from the SmartAPPetite for Youth Study, a cluster-randomized controlled trial conducted in Southwestern Ontario, Canada, among adolescents aged 13–18 years from 2017 to 2020. This age range was selected because it corresponds to the standard age span for high school students in Ontario, Canada, which was the intended target population for the SmartAPPetite intervention. That study aimed to evaluate a smartphone application (“SmartAPPetite”) intended to improve food knowledge, food purchasing, and diet quality [17]. Relevant to this current study, the SmartAPPetite for Youth participants completed two tools at three time points—baseline, post-intervention, and follow-up. The tools were (1) a 24 h dietary recall using ASA24, and (2) a “youth survey” that assessed dietary habits and related psychosocial factors.
A formal sample size calculation was not performed for this secondary analysis, which was based on pre-existing data collected during the SmartAPPetite for Youth cluster-randomized trial. While the original study was powered to detect intervention effects on dietary outcomes, the current analytic sample of 743 adolescents is sufficiently large to support a robust evaluation of multiple imputation accuracy across varying levels of simulated missingness.
2.2. Measures
2.2.1. ASA24 Dietary Recall
The ASA24 dietary recall aimed to capture participants’ dietary intake for the previous 24 h. This validated tool follows a 7-step AMPM process [18] inquiring about foods consumed and associated mealtimes; a probe for additional foods not previously reported; detailed questions about food items, including preparation method, portion size, brand, and condiments; review and editing of entered data; prompting for commonly forgotten foods (e.g., snacks consumed while commuting or shopping); final confirmation of entries; and a self-assessment of whether the reported intake reflected usual intake. Upon completion, ASA24 automatically calculates nutrient intakes using its internal food composition database.
While ASA24 may slightly underestimate the intake of certain nutrients (e.g., energy, protein) when compared to recovery biomarkers [19], it demonstrates comparable accuracy to traditional 24HRs in estimating nutrient intake [19,20,21]. This makes ASA24 a practical and reliable tool for use in large-scale epidemiological studies.
2.2.2. Youth Survey
The youth survey included questions on demographics (e.g., age, sex, ethnicity), self-reported physical and mental health, food-related behaviours and general eating habits (e.g., allergies, cooking frequency, meal skipping), perceived importance of healthy eating, and food purchasing behaviours. Participants were also asked to provide their primary residence’s postal code, which was used to calculate median neighbourhood income. A food knowledge quiz, adapted from two validated instruments [22,23], was administered at the end of the survey.
2.2.3. Nutrient and Diet Quality Measures
From the full nutrient output generated by ASA24, the following 21 nutrients were selected for analysis based on their relevance and previous epidemiologic research: calories (kcal), protein (g), total fat (g), saturated fat (g), carbohydrates (g), total sugars (g), fibre (g), calcium (mg), iron (mg), magnesium (mg), potassium (mg), sodium (mg), zinc (mg), vitamin C (mg), thiamin (mg), riboflavin (mg), niacin (mg), folate (mcg), vitamin B12 (mcg), and vitamin A (mcg, RAE). Two composite diet quality scores were also calculated: Healthy Eating Index-2015 (HEI-2015) [24,25] and Nutrient Rich Foods Index 9.3 (NRF 9.3) [26].
2.3. Additional Covariates
Variables from the youth survey included in the analysis were sex, age, ethnicity (White/Caucasian: yes/no), self-rated physical and mental health, number of physically active days in the past week (0–7 days), perceived importance of eating healthy, and total food knowledge score (ranging from 0 (minimum)–50 (maximum)). Additionally, a proxy for socioeconomic status was considered by incorporating a variable for neighbourhood-level median household income, as calculated by linking each participant’s primary residence’s postal code to 2016 Canadian census data at the dissemination area level [27,28]. Information on how each question from the youth survey was asked can be found in Appendix A.
2.4. Data Cleaning and Identification of Implausible Dietary Recalls
To identify IDRs, thresholds were applied to ASA24-derived nutrient intakes based on established guidelines [8]. Specifically, records were set to “missing” if any of the following nutrient values fell outside plausible ranges (Table 1):

Table 1.
Classifying implausible dietary recalls by sex.
These thresholds were derived from the upper and lower 5% bounds of National Health and Nutrition Examination Survey (NHANES) data distributions [8].
2.5. Simulation of Missing Data
Following the creation of a cleaned dataset—one with no missing entries and no implausible nutrient values—a simulation procedure was implemented to artificially introduce missing data. A random number generator was used to randomly select dietary records to be set as missing. For each selected case, the corresponding dietary intake data were exported and stored separately as the reference “true” values. These original values were then removed from the dataset to simulate realistic patterns of missingness.
The dataset was subsequently prepared for MI. An MI model using chained equations was fitted, generating 200 imputed datasets. Predictor variables included in the imputation model were sex, age, white/Caucasian ethnicity (yes/no), self-reported physical health, self-reported mental health, number of physically active days in the past week, perceived importance of healthy eating, total food knowledge score, and neighbourhood-level median household income.
Once imputation was completed, the average of the 200 values was calculated for each nutrient and used as the “final” imputed estimate. The original (true) values were then reinserted into the dataset for comparative analysis.
2.6. Analysis of Imputation Data
To assess the performance of the imputation model, comparisons were made between the imputed values and their corresponding true values on an intra-individual basis. Descriptive statistics, including means and standard deviations, were computed for both actual and imputed values. Spearman’s rho was used to calculate correlation coefficients between true and imputed nutrient values, with corresponding p-values to determine statistical significance. A p-value < 0.05 was considered statistically significant.
To evaluate practical accuracy, a ±10% threshold was applied. Specifically, for each nutrient, an imputed value was considered accurate if it fell within 10% of the individual’s true value. For instance, for a participant with an actual energy intake of 2000 kcal, any imputed value between 1800 and 2200 kcal would be classified as accurate. The proportion of imputed values meeting this criterion was then calculated.
This entire process was repeated across three levels of simulated missing data: 10%, 20%, and 40%. These levels were chosen to reflect pragmatic real-world scenarios. A missing data level under 10% might not substantially affect results, while missingness exceeding 40% [29] may undermine study validity regardless of the missing data handling method (e.g., listwise deletion or multiple imputation).
3. Results
3.1. Descriptive Statistics
Table 2 presents the demographic and dietary characteristics of the sample (n = 743). Participants had a mean age of 15.6 (SD = 1.2) years, with 62.6% identifying as female and 68.1% identifying as White. Mean calorie intake was 1735.4 (SD = 712.2) kcal/day, with notable variability in macronutrient and micronutrient intake.

Table 2.
Demographic and nutrient intake information of sample (n = 743).
3.2. Correlation Between Imputed and Actual Values
Across all three levels of missing data (10%, 20%, 40%), Spearman’s rho (ρ) values between the imputed and actual nutrient values were consistently low (Table 3, Table 4 and Table 5). At 10% missingness, correlations were weakest, with rho values ranging from −0.10 to 0.31 and few statistically significant correlations. At 20% and 40% missingness, correlations modestly improved, with most nutrients demonstrating statistically significant yet weak correlations (mean ρ ≈ 0.24).

Table 3.
Comparison of 10% imputed values to actual values (n = 74).

Table 4.
Comparison of 20% imputed values to actual values (n = 149).

Table 5.
Comparison of 40% imputed values to actual values (n = 297).
3.3. Accuracy of Imputed Values
Table 6 and Figure 1 summarize the proportion of imputed values falling within ±10% of the actual values. Accuracy was generally poor. HEI-2015 showed the highest proportion of accurate imputation values (approximately 28%) across all levels of missingness. For nutrients, accuracy rarely exceeded 25%, and in many cases, fewer than 15% of values were within the accuracy threshold. No clear pattern of improved accuracy was observed with less missingness.

Table 6.
Number of accurate estimates, by missing percentage.
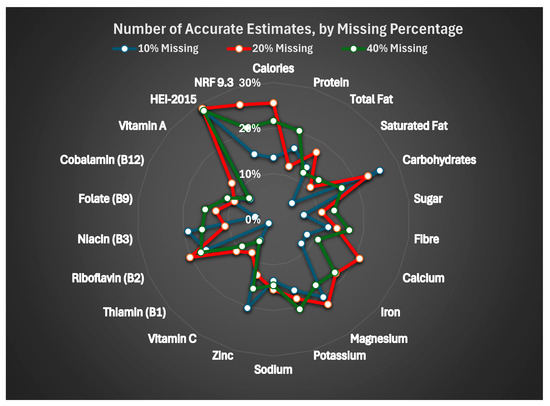
Figure 1.
Number of accurate estimates, by missing percentage.
4. Discussion
This study evaluated the accuracy of MI for estimating missing nutrient intake data among Canadian adolescents, using 24 h dietary recall data from ASA24. To our knowledge, this is the first study to assess imputation accuracy using a reference dataset with known true values.
Across all levels of missingness, correlations between imputed and actual nutrient values were weak. While MI is regarded as a robust method when data are MAR, our findings suggest that its performance may be limited when applied to high-variability outcomes such as nutrient intake. Notably, the modest improvement in correlation coefficients at 20% and 40% missingness could be attributed to increased sample size and statistical power rather than improved model performance.
The accuracy of imputed values, defined as being within 10% of the true value, was low for most nutrients. Furthermore, and contrary to expectations, MI did not become less reliable with more missing data [30]. For example, the correlation coefficient for calories was lowest among the dataset with 10% missing data and increased as the percentage increased, while other nutrients showed no clear pattern of relationship between missing data percentage and coefficient values. Even the most accurately imputed variable (HEI-2015) had correct estimates in less than one-third of cases. These findings suggest that although MI may preserve overall distributions and allow for full sample inclusion, it does not reliably reproduce individual-level nutrient intake data. This is critical, as nutritional epidemiology research often relies on accurate intake values to examine exposure–outcome relationships.
Our findings diverge from previous research that used MI in FFQs or registry data, largely because those studies lacked true values for comparison. For example, studies from Japan [31], Italy [32], and the U.S. [33] assessed MI performance via comparisons to complete-case analyses, rather than direct validation against known intakes. While such approaches may provide insight into relative bias, they cannot assess absolute accuracy, as the missing values remain unknown.
Importantly, this study leveraged a rare opportunity to simulate missingness within a dataset of plausible recalls, thereby enabling direct comparison between imputed and true values. While this design enhances internal validity, it also introduces limitations and potential biases. The study excluded IDRs to establish a known reference, which may not reflect real-world patterns where data are often missing not at random (MNAR) [30]. Moreover, our use of a single 24 h recall per participant limited our ability to estimate usual intake, which likely constrained the imputation model’s performance. This was necessary because the source data came from an intervention study. Using multiple recalls could have altered the “usual intake” over time because of the intervention itself.
Additionally, the classification of implausible recalls based on extreme values in energy and select nutrients, while conservative and consistent with ASA24 guidance [8], may have excluded some valid recalls. We opted for this method as we did not have the necessary data to apply more refined methods such as the Goldberg cutoff [34], which would have required measured body weight or energy expenditure data.
Nevertheless, this study has important strengths. It is the first to assess the absolute accuracy of MI for nutrient data using known true values, across varying levels of missingness. It also adopts an individual-level evaluation approach, offering insights that average-based comparisons cannot. Additionally, because ASA24 allowed for multiple dietary recalls without accompanying interviewer requirements, we were able to collect a large enough sample to examine detailed dietary intakes of participants, which may not have been possible using traditional 24HRs. Our pragmatic use of ASA24 and common demographic covariates makes this study directly applicable to dietitians and other nutrition researchers conducting primary nutrition studies.
5. Conclusions
In conclusion, MI for missing nutrient intake data demonstrated limited accuracy when compared to known values, even when standard modelling techniques and a validated dietary assessment tool were used. These findings highlight the need for caution when using MI for individual-level dietary data and underscore the importance of improving data quality at the point of collection. Future research should explore methods to enhance imputation accuracy, consider repeated recalls for estimating usual intake, and investigate model performance in datasets with true MNAR characteristics.
Author Contributions
Conceptualization, N.W., J.G. and J.A.S.; methodology, N.W. and J.A.S.; software, N.W.; validation, N.W., J.G., L.W.M., C.O., S.S., S.D. and J.A.S.; formal analysis, N.W. and J.A.S.; investigation, N.W.; resources, N.W., J.G. and J.A.S.; data curation, N.W.; writing—original draft preparation, N.W.; writing—review and editing, N.W., J.G., L.W.M., C.O., S.S., S.D. and J.A.S.; visualization, N.W., J.G., L.W.M. and J.A.S.; supervision, J.G., L.W.M. and J.A.S.; project administration, J.G., L.W.M. and J.A.S.; funding acquisition, J.G., C.O., S.S., S.D. and J.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study used SmartAPPetite for Youth data, which has received funding from the Canadian Institutes of Health Research (#399384), Heart & Stroke Canada (G-17-0018327), and the Children’s Health Foundation (2015–2017).
Institutional Review Board Statement
This study used SmartAPPetite for Youth data, which was conducted according to the guidelines of the Declaration of Helsinki and approved by the Non-Medical Research Ethics Board of Western University (protocol code 107034, approved 14 December 2017) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Supporting data may be provided upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 24HRS | 24 h Recalls |
| ASA24 | Automated Self-Administered 24 h Dietary Assessment Tool |
| MI | Multiple Imputation |
| FFQs | Food Frequency Questionnaires |
| AMPM | Automated Multiple-Pass Method |
| IDRs | Implausible Dietary Recalls |
| MCAR | Missing Completely at Random |
| MAR | Missing at Random |
| MNAR | Missing Not at Random |
| HEI-2015 | Healthy Eating Index-2015 |
| NRF 9.3 | Nutrient Rich Foods Index 9.3 |
| NHANES | National Health and Nutrition Examination Survey |
Appendix A
Appendix A presents the youth survey questions used as covariates and how the questions were asked.
Appendix A.1. Covariates and Source Questions
Appendix A.1.1. Sex
Sex was determined by asking respondents to respond to the following: “I am a (insert response),” where responses included “Male” “Female,” and “I identify as (please specify).” No participant who identified as neither male nor female had complete data, and therefore, sex was treated as a binary variable.
Appendix A.1.2. Age
Age was obtained by asking “What is your current age?” with responses being limited to ages 13–18.
Appendix A.1.3. Ethnicity
Ethnicity was determined by asking “What is your ethnicity? (Please select all that apply)” with possible responses of “White/Caucasian”, “South Asian (e.g., East Indian, Pakistani, Sri Lankan)”, “East Asian (e.g., Chinese, Japanese, Korean)”, “Middle Eastern (e.g., Egyptian, Iranian, Lebanese)”, “Latin American (e.g., Mexican, Columbian, Peruvian)”, “Indigenous (i.e., First Nations, Métis, or Inuit)”, “Black (e.g., African, Caribbean)”, and/or “Other (Please Specify)”.
Appendix A.1.4. Physical Health Score
Physical health was determined by asking “In general, how do you rate your own physical health?” with possible responses of “Excellent,” “Very Good,” “Good,” “Fair,” and “Poor.”
Appendix A.1.5. Mental Health Score
Mental health was determined by asking “In general, how do you rate your own mental health?” with possible responses of “Excellent,” “Very Good,” “Good,” “Fair,” and “Poor.”
Appendix A.1.6. Number of Days Physically Active in Previous Week
Physical activity was determined via the following question: “Physical activity is an activity that increases your heart rate and makes you feel out of breath some of the time. Add up all the time you spend engaged in physical activity each day. Some examples of physical activity are running, brisk walking, rollerblading, biking, dancing, skateboarding, swimming, soccer, or basketball.
Over the past 7 days, how many days were you physically active for a total of at least 60 min per day?”
Appendix A.1.7. Importance of Eating Healthy Score
Importance of eating healthy was determined via a Likert scale, where the following statement was asked to be ranked: “Eating healthy food is important to me,” with the options of “Strongly Agree,” “Agree,” “Neither Agree nor Disagree,” “Disagree,” and “Strongly Disagree.”
References
- Dao, M.C.; Subar, A.F.; Warthon-Medina, M.; Cade, J.E.; Burrows, T.; Golley, R.K.; Forouhi, N.G.; Pearce, M.; Holmes, B.A. Dietary assessment toolkits: An overview. Public Health Nutr. 2019, 22, 404–418. [Google Scholar] [CrossRef]
- Martin, C.K.; Correa, J.B.; Han, H.; Allen, H.R.; Rood, J.C.; Champagne, C.M.; Gunturk, B.K.; Bray, G.A. Validity of the remote food photography method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity 2012, 20, 891–899. [Google Scholar] [CrossRef]
- 24-hour Dietary Recall (24HR) At a Glance|Dietary Assessment Primer. Available online: https://dietassessmentprimer.cancer.gov/profiles/recall/ (accessed on 19 April 2020).
- Thompson, F.E.; Subar, A.F. Dietary Assessment Methodology. In Nutrition in the Prevention and Treatment of Disease, 4th ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Delahanty, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 5–48. Available online: https://www.sciencedirect.com/science/article/pii/B9780128029282000011 (accessed on 1 May 2025).
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.P.; Hull, S.G.; McNutt, S.; Mittl, B.; Islam, N.; Guenther, P.M.; Thompson, F.E.; Potischman, N.A.; Subar, A.F. Challenges in converting an interviewer-administered food probe database to self-administration in the National Cancer Institute automated self-administered 24-hour recall (ASA24). J. Food Compos. Anal. 2009, 22, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Ruth Charrondiere, U.; Bell, W. Measurement Errors in Dietary Assessment Using Self-Reported 24-Hour Recalls in Low-Income Countries and Strategies for Their Prevention. Adv. Nutr. 2017, 8, 980. [Google Scholar] [CrossRef]
- Reviewing & Cleaning ASA24® Data|EGRP/DCCPS/NCI/NIH. Available online: https://epi.grants.cancer.gov/asa24/resources/cleaning.html#guidelines (accessed on 1 May 2025).
- Kang, H. The prevention and handling of the missing data. Korean J. Anesth. 2013, 64, 402. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, H.; Laasanen, H.; Twynstra, J.; Seabrook, J.A. A review of statistical reporting in dietetics research (2010–2019): How is a Canadian journal doing? Can. J. Diet. Pract. Res. 2021, 82, 59–67. [Google Scholar] [CrossRef]
- Seabrook, J.A. How Many Participants Are Needed? Strategies for Calculating Sample Size in Nutrition Research. Can. J. Diet. Pract. Res. 2025, 86, 479–483. [Google Scholar] [CrossRef]
- Howe, C.J.; Cain, L.E.; Hogan, J.W. Are all biases missing data problems? Curr. Epidemiol. Rep. 2015, 2, 162. [Google Scholar] [CrossRef][Green Version]
- Ross, R.K.; Breskin, A.; Westreich, D. When Is a Complete-Case Approach to Missing Data Valid? The Importance of Effect-Measure Modification. Am. J. Epidemiol. 2020, 189, 1583. [Google Scholar] [CrossRef]
- van Buuren, S. Flexible Imputation of Missing Data, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018; pp. 29–62. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9780429492259-2 (accessed on 1 May 2025).
- Li, P.; Stuart, E.A.; Allison, D.B. Multiple Imputation: A Flexible Tool for Handling Missing Data. JAMA 2015, 314, 1966–1967. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK, 2012; pp. 1–552. Available online: https://academic.oup.com/book/27443 (accessed on 1 May 2025).
- Gilliland, J.A.; McEachern, L.W.; Cappuccitti, S.; Doherty, S.; O’Connor, C.; Seabrook, J.; Haines, J.; Stranges, S. SmartAPPetite for youth: Development and evaluation of a smartphone app for improving adolescent food literacy and healthy eating. Proc. Nutr. Soc. 2022, 81, E167. Available online: https://www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/smartappetite-for-youth-development-and-evaluation-of-a-smartphone-app-for-improving-adolescent-food-literacy-and-healthy-eating/2FF9AB4D21F9A0764CDCAAF705B3A3E3 (accessed on 1 May 2025). [CrossRef]
- Nci, DCCPS, Egrp. ASA24 Participant Quick Start Guide for Food Records Using ASA24. Available online: https://epi.grants.cancer.gov/asa24/resources/asa24-quick-start-guide-food-record-06062022.pdf (accessed on 30 April 2025).
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.I.; Potischman, N.; Dodd, K.W.; Douglass, D.; Zimmerman, T.P.; Kahle, L.L.; Thompson, F.E.; George, S.M.; Subar, A.F. The Use of Digital Images in 24-Hour Recalls May Lead to Less Misestimation of Portion Size Compared with Traditional Interviewer-Administered Recalls. J. Nutr. 2016, 146, 2567–2573. [Google Scholar] [CrossRef]
- Thompson, F.E.; Dixit-Joshi, S.; Potischman, N.; Dodd, K.W.; Kirkpatrick, S.I.; Kushi, L.H.; Alexander, G.L.; Coleman, L.A.; Zimmerman, T.P.; Sundaram, M.E.; et al. Comparison of Interviewer-Administered and Automated Self-Administered 24-Hour Dietary Recalls in 3 Diverse Integrated Health Systems. Am. J. Epidemiol. 2015, 181, 970–978. [Google Scholar] [CrossRef]
- Anderson, A.S.; Bell, A.; Adamson, A.; Moynihan, P. A questionnaire assessment of nutrition knowledge—validity and reliability issues. Public Health Nutr. 2002, 5, 497–503. [Google Scholar] [CrossRef]
- Vereecken, C.A.; Keukelier, E.; Maes, L. Influence of mother’s educational level on food parenting practices and food habits of young children. Appetite 2004, 43, 93–103. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015 HHS Public Access. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Healthy Eating Index SAS Code|EGRP/DCCPS/NCI/NIH. Available online: https://epi.grants.cancer.gov/hei/sas-code.html (accessed on 8 June 2025).
- Fulgoni, V.L.; Keast, D.R.; Drewnowski, A. Development and Validation of the Nutrient-Rich Foods Index: A Tool to Measure Nutritional Quality of Foods. J. Nutr. 2009, 139, 1549–1554. [Google Scholar] [CrossRef]
- Statistics Canada. 2016 Census of Population—Data Products. 2016. Available online: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/index-eng.cfm (accessed on 19 April 2020).
- Healy, M.A.; Gilliland, J.A. Quantifying the magnitude of environmental exposure misclassification when using imprecise address proxies in public health research. Spat. Spatiotemporal Epidemiol. 2012, 3, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—A practical guide with flowcharts. BMC Med. Res. Methodol. 2017, 17, 162. [Google Scholar] [CrossRef]
- Lee, K.J.; Simpson, J.A. Introduction to multiple imputation for dealing with missing data. Respirology 2014, 19, 162–167. [Google Scholar] [CrossRef]
- Ichikawa, M.; Hosono, A.; Tamai, Y.; Watanabe, M.; Shibata, K.; Tsujimura, S.; Oka, K.; Fujita, H.; Okamoto, N.; Kamiya, M.; et al. Handling missing data in an FFQ: Multiple imputation and nutrient intake estimates. Public Health Nutr. 2019, 22, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Barzi, F.; Woodward, M.; Marfisi, R.M.; Tognoni, G.; Marchioli, R. Analysis of the benefits of a Mediterranean diet in the GISSI-Prevenzione study: A case study in imputation of missing values from repeated measurements. Eur. J. Epidemiol. 2006, 21, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Meadows, R.J.; Paskett, E.D.; Bower, J.K.; Kaye, G.L.; Lemeshow, S.; Harris, R.E. Socio-demographic differences in the dietary inflammatory index from National Health and Nutrition Examination Survey 2005–2018: A comparison of multiple imputation versus complete case analysis. Public Health Nutr. 2024, 27, e184. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E.; Goldberg, G.R.; Jebb, S.A.; Livingstone, M.B.E.; Cole, T.J.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. Eur. J. Clin. Nutr. 1991, 45, 583–599. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).