High-Fructose-Induced Salt-Sensitive Hypertension: The Potential Benefit of SGLT4 or SGLT5 Modulation
Abstract
1. Introduction
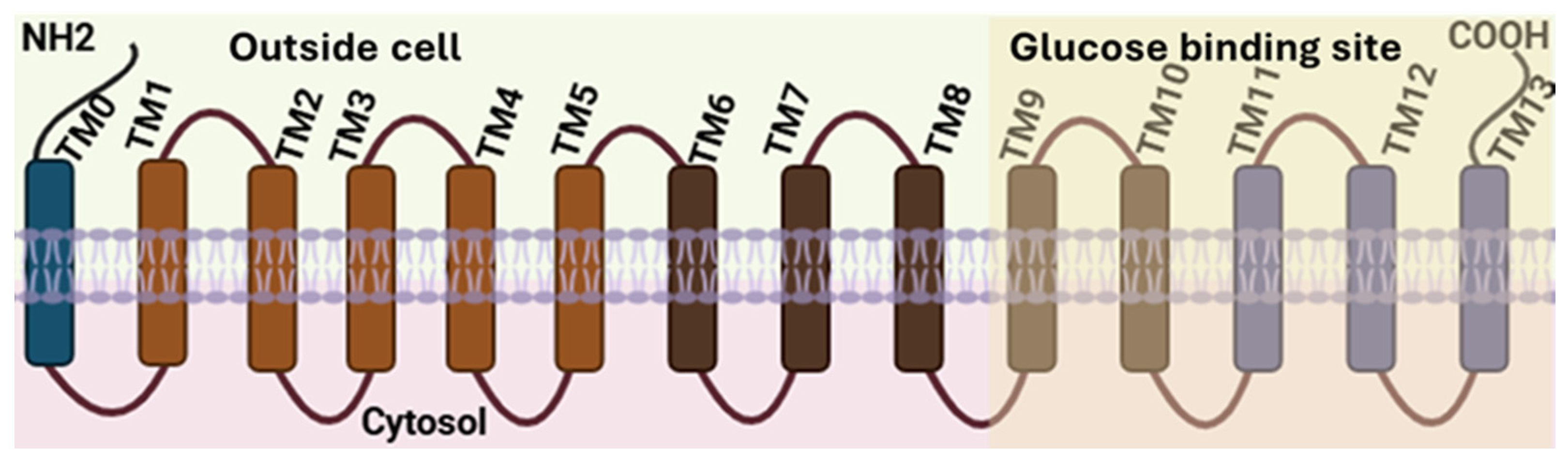
2. Molecular Structure and Function of SGLTs
| SGLT1 | SGLT2 | SGLT4 | SGLT5 | GLUT2 | GLUT5 | |
|---|---|---|---|---|---|---|
| D-glucose | 0.5–1.8 | 5 | 7.7 | 5 | 17 | N |
| D-fructose | N | >100 | ~100 | 0.6 | 76 | 6 |
| D-mannose | N | >100 | 0.08–0.15 | 0.4–1.73 | 125 | N |
| D-galactose | 6 | >100 | N | 8 | 92 | N |
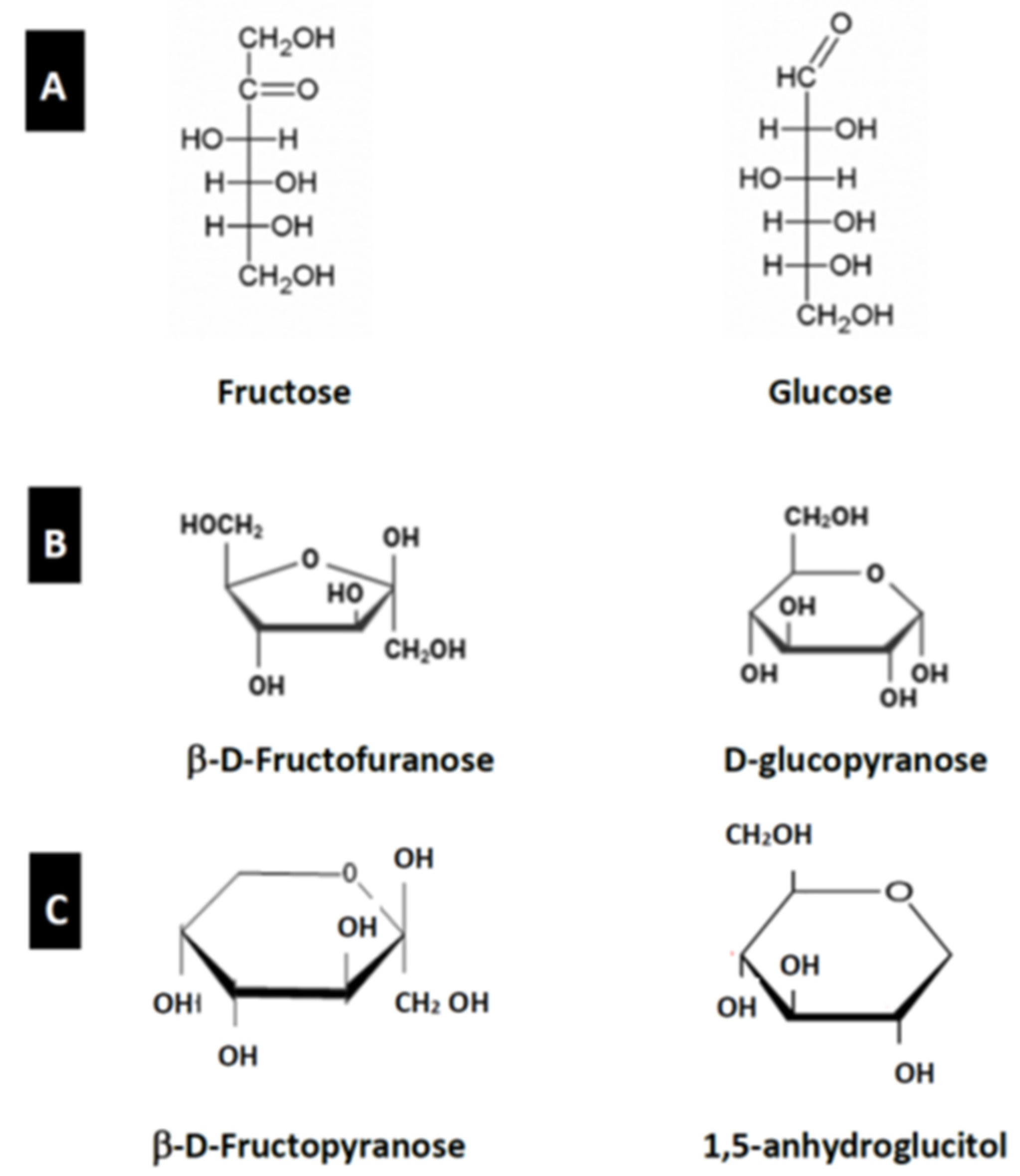
3. Structure and Function of Glucose and Fructose
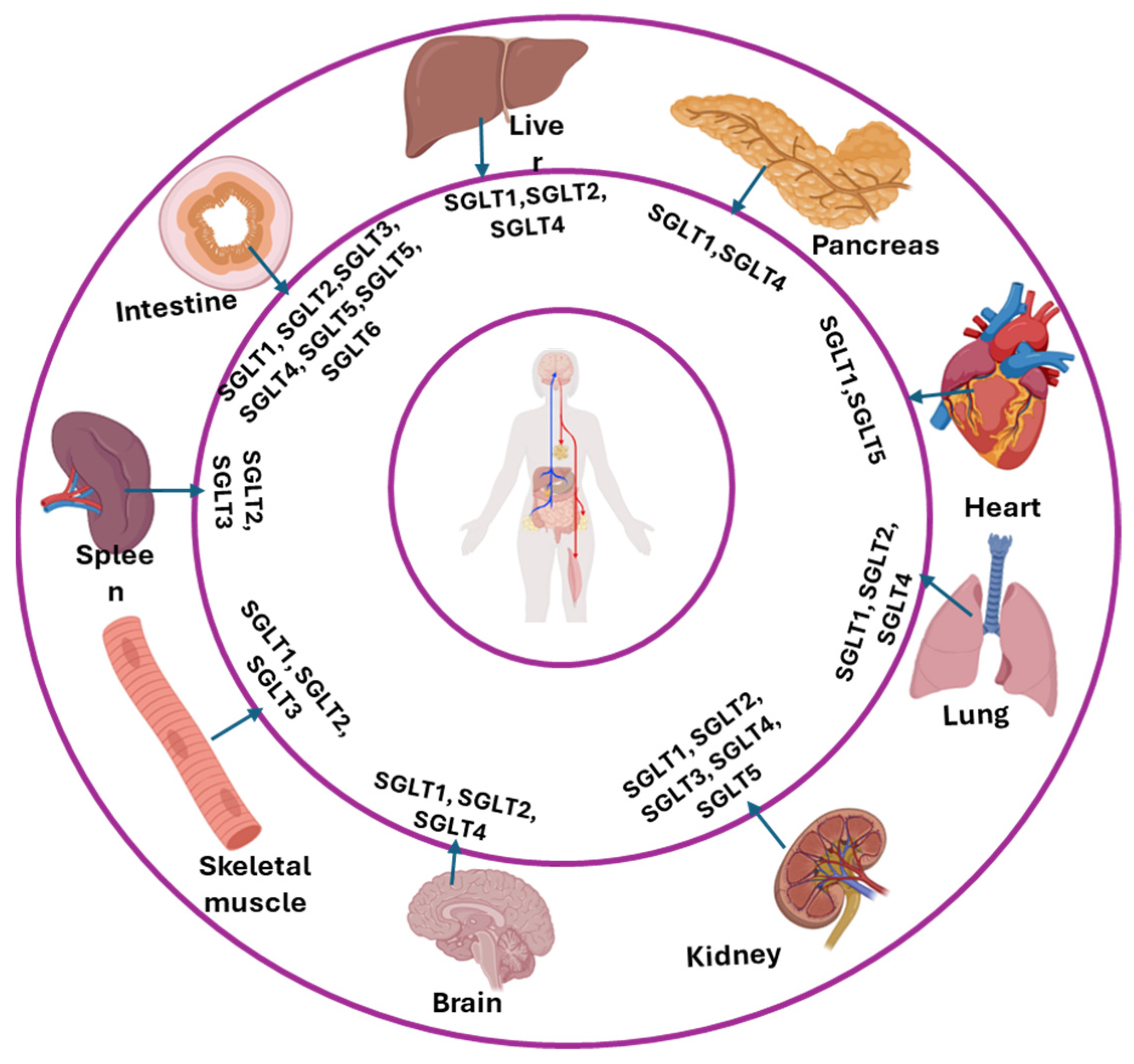
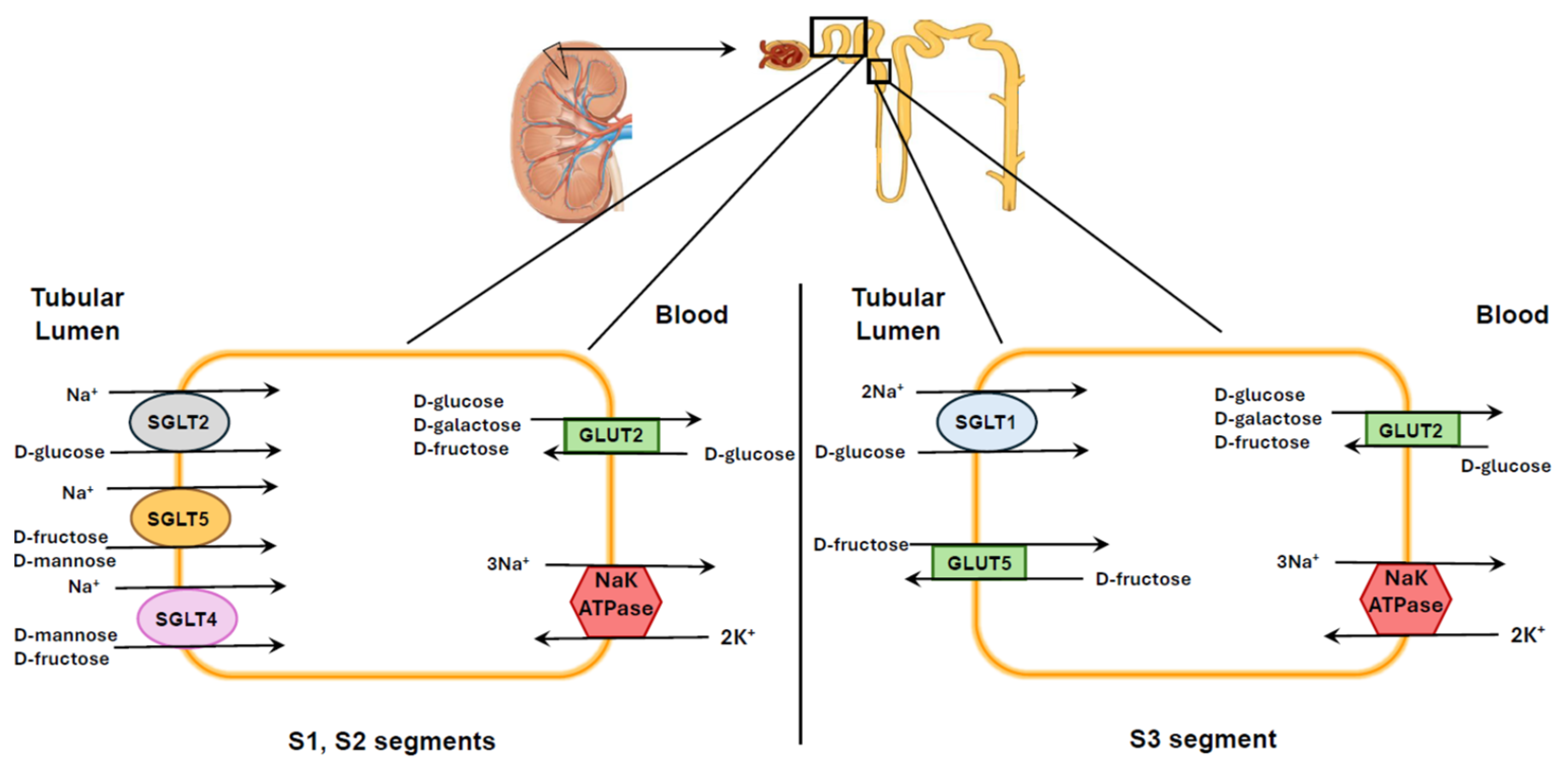
4. Localization of Different SGLTs
| SGLTs | Location | Protein/mRNA | Authors | Reference |
|---|---|---|---|---|
| SGLT1 (Slc5a1) | Intestinal epithelial cell | Protein/mRNA | Vrhovac et al., 2015 | [77] |
| S3 segment proximal tubule | Protein/mRNA | Vrhovac et al., 2015 | [77] | |
| Salivary gland | Protein | Sabino-Silva et al., 2013 | [76] | |
| Liver | Protein/mRNA | Vrhovac et al., 2015, Liang et al., 2020 | [77,79] | |
| Lung | Protein/mRNA | Vrhovac et al., 2015 | [77] | |
| Skeletal muscle | Madunić et al., 2017 | [78] | ||
| Heart | Protein/mRNA | Vrhovac et al., 2015, Liang et al., 2020 | [77,79] | |
| Brain | mRNA | Madunić et al., 2017 | [78] | |
| Pancreatic α-cells | mRNA | Madunić et al., 2017 | [78] | |
| SGLT2 (Scl5a2) | S1,S2 segments proximal tubule | Protein/mRNA | Ghezzi et al., 2018 | [83] |
| Mammary glands | mRNA | Zhao et al., 2005 | [82] | |
| Testis | Protein | Kosinski et al., 2024 | [84] | |
| Liver | Wright et al., 2011 | [31] | ||
| Lung | mRNA | Chen et al., 2010 | [23] | |
| Intestine, | mRNA | Chen et al., 2010 | [23] | |
| Skeletal muscle | mRNA | Wright et al., 2011, Zhao et al., 2005 | [31,82] | |
| Spleen | mRNA | Zhao et al., 2005 | [82] | |
| Cerebellum | mRNA | Wright et al., 2011 | [31] | |
| SGLT3 (Scl5a4) | Intestine | mRNA | Soták et al., 2021 | [88] |
| Spleen | mRNA | Diez-Sampedro et al., 2003 | [87] | |
| Kidney | mRNA | Diez-Sampedro et al., 2003 | [87] | |
| Skeletal muscle | Protein/mRNA | Diez-Sampedro et al., 2003 | [87] | |
| Cholinergic neurons | mRNA | Diez-Sampedro et al., 2003 | [87] | |
| SGLT4 (Scl5a9) | Small intestine | mRNA | Chen et al., 2010; Tazawa et al., 2005 | [23,42] |
| Kidneys | mRNA | Tazawa et al., 2005, Liang et al., 2020 | [42,79] | |
| Liver | mRNA | Liang et al., 2020 | [79] | |
| Lung | mRNA | Wright et al., 2011 | [31] | |
| Skeletal muscle | mRNA | Chen et al., 2010, Liang et al., 2020 | [23,79] | |
| Brain | mRNA | Liang et al., 2020 | [79] | |
| Trachea | mRNA | Tazawa et al., 2005 | [42] | |
| Pancreas | mRNA | Gatto et al., 2020 | [94] | |
| SGLT5 (Scl5a10) | S1,S2 segment proximal tubule | Protein/mRNA | Chen et al., 2010; Grempler et al., 2012; Fukuzawa et al., 2013; Gonzalez-Vicente et al., 2019 | [22,23,26,91] |
| Heart | mRNA | Chen et al., 2010 | [23] | |
| SGLT6 (Scl5a11) | Small intestine | Protein | Baader-Pagler et al., 2018 | [93] |
| Brain | Protein | Baader-Pagler et al., 2018 | [93] |
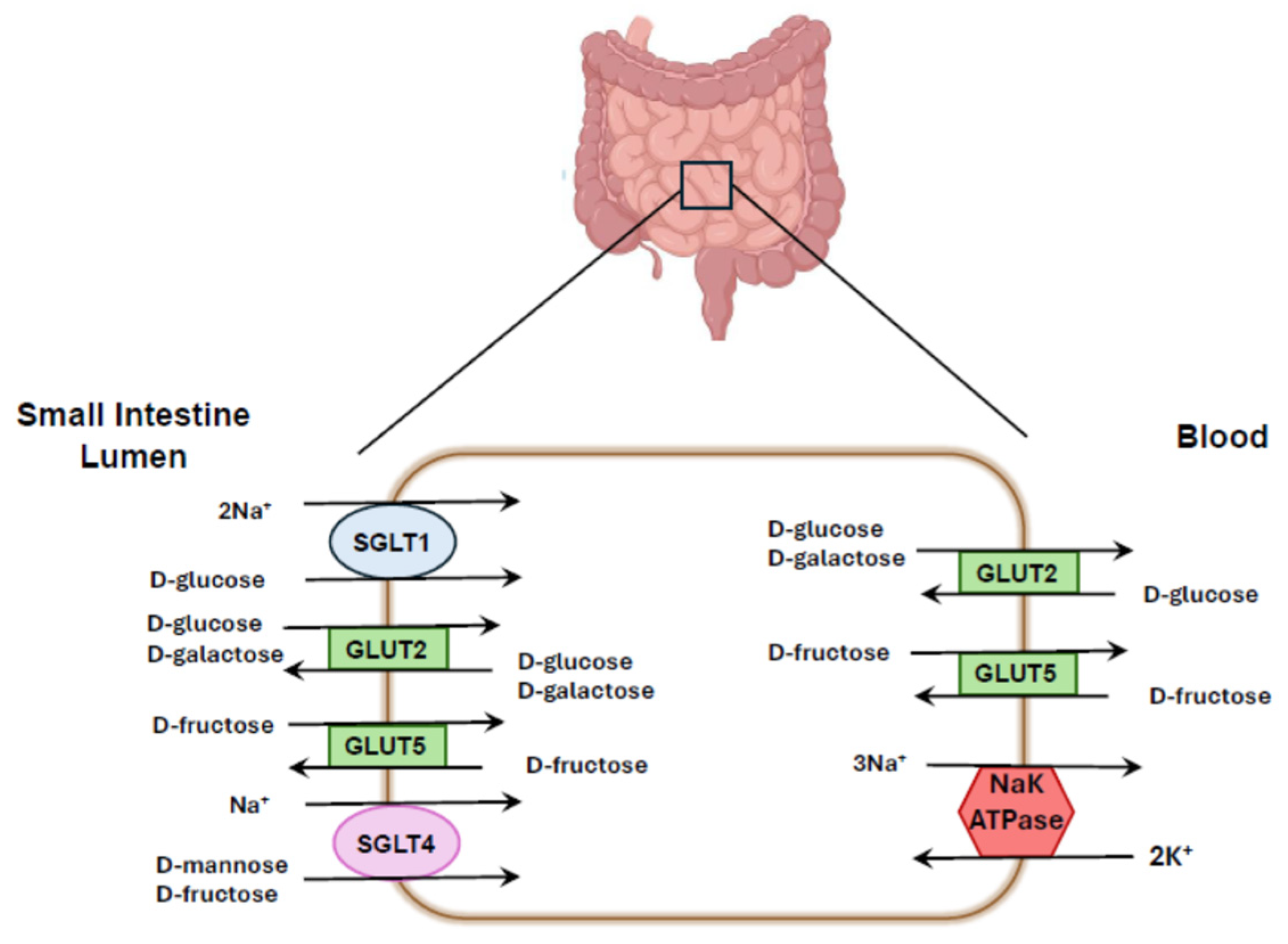
5. Fructose, Salt-Sensitive Hypertension, SGLT4 and SGLT5
6. Conclusions
7. Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1,5-AG | 1,5-anhydroglucitol |
| APC | Amino acid-polyamine-organocation |
| ENaC | Epithelial sodium channel |
| GLUT | Glucose transporter |
| NHE3 | Sodium-hydrogen exchanger 3 |
| PAT1 | Putative anion transporter 1 |
| RAS | Renin–angiotensin system |
| SGLT | Sodium-dependent glucose linked cotransporter |
| TM | Transmembrane |
| SMIT2 | Sodium/myo-inositol transporter 2 |
References
- Riby, J.E.; Fujisawa, T.; Kretchmer, N. Fructose absorption. Am. J. Clin. Nutr. 1993, 58, S748–S753. [Google Scholar] [CrossRef]
- Hallfrisch, J. Metabolic effects of dietary fructose. FASEB J. 1990, 4, 2652–2660. [Google Scholar] [CrossRef]
- Pereira, R.M.; Botezelli, J.D.; da Cruz Rodrigues, K.C.; Mekary, R.A.; Cintra, D.E.; Pauli, J.R.; da Silva, A.S.R.; Ropelle, E.R.; de Moura, L.P. Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients 2017, 9, 405. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investg. 2018, 128, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef] [PubMed]
- Busnatu, S.S.; Salmen, T.; Pana, M.A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Pitpitan, R.; Boychev, B.; Komnenov, D.; Rossi, N.F. Impact of inhibition of the renin-angiotensin system on early cardiac and renal abnormalities in Sprague Dawley rats fed short-term high fructose plus high salt diet. Front. Nutr. 2024, 11, 1436958. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: What do we really know? Adv. Nutr. 2013, 4, 236–245. [Google Scholar] [CrossRef]
- Singh, A.K.; Amlal, H.; Haas, P.J.; Dringenberg, U.; Fussell, S.; Barone, S.L.; Engelhardt, R.; Zuo, J.; Seidler, U.; Soleimani, M. Fructose-induced hypertension: Essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Fussell, S.L.; Singh, A.K.; Lucas, F.; Xu, J.; Kim, C.; Wu, X.; Yu, Y.; Amlal, H.; Seidler, U.; et al. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J. Biol. Chem. 2009, 284, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M. Dietary fructose, salt absorption and hypertension in metabolic syndrome: Towards a new paradigm. Acta Physiol. 2011, 201, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Alborzi, P. The role of salt in the pathogenesis of fructose-induced hypertension. Int. J. Nephrol. 2011, 2011, 392708. [Google Scholar] [CrossRef]
- Queiroz-Leite, G.D.; Crajoinas, R.O.; Neri, E.A.; Bezerra, C.N.; Girardi, A.C.; Rebouças, N.A.; Malnic, G. Fructose acutely stimulates NHE3 activity in kidney proximal tubule. Kidney Blood Press. Res. 2012, 36, 320–334. [Google Scholar] [CrossRef]
- Cabral, P.D.; Hong, N.J.; Hye Khan, M.A.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Daugherty, A.M.; Komnenov, D.; Rossi, N.F. Dietary fructose and high salt in young male Sprague Dawley rats induces salt-sensitive changes in renal function in later life. Physiol. Rep. 2022, 10, e15456. [Google Scholar] [CrossRef]
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol. Rep. 2017, 5, e13162. [Google Scholar] [CrossRef]
- Klein, A.V.; Kiat, H. The mechanisms underlying fructose-induced hypertension: A review. J. Hypertens. 2015, 33, 912–920. [Google Scholar] [CrossRef]
- Soncrant, T.; Komnenov, D.; Beierwaltes, W.H.; Chen, H.; Wu, M.; Rossi, N.F. Bilateral renal cryodenervation decreases arterial pressure and improves insulin sensitivity in fructose-fed Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R529–R538. [Google Scholar] [CrossRef]
- Eren, O.C.; Ortiz, A.; Afsar, B.; Covic, A.; Kuwabara, M.; Lanaspa, M.A.; Johnson, R.J.; Kanbay, M. Multilayered Interplay Between Fructose and Salt in Development of Hypertension. Hypertension 2019, 73, 265–272. [Google Scholar] [CrossRef]
- Fukuzawa, T.; Fukazawa, M.; Ueda, O.; Shimada, H.; Kito, A.; Kakefuda, M.; Kawase, Y.; Wada, N.A.; Goto, C.; Fukushima, N.; et al. SGLT5 reabsorbs fructose in the kidney but its deficiency paradoxically exacerbates hepatic steatosis induced by fructose. PLoS ONE 2013, 8, e56681. [Google Scholar] [CrossRef]
- Chen, J.; Williams, S.; Ho, S.; Loraine, H.; Hagan, D.; Whaley, J.M.; Feder, J.N. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010, 1, 57–92. [Google Scholar] [CrossRef]
- Forester, B.R.; Zhang, R.; Schuhler, B.; Brostek, A.; Gonzalez-Vicente, A.; Garvin, J.L. Knocking Out Sodium Glucose-Linked Transporter 5 Prevents Fructose-Induced Renal Oxidative Stress and Salt-Sensitive Hypertension. Hypertension 2024, 81, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Takayanagi, K.; Shimizu, T.; Iwashita, T.; Ikari, A.; Maeshima, A.; Hasegawa, H. Possible involvement of up-regulated salt-dependent glucose transporter-5 (SGLT5) in high-fructose diet-induced hypertension. Hypertens. Res. 2025, 48, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Grempler, R.; Augustin, R.; Froehner, S.; Hildebrandt, T.; Simon, E.; Mark, M.; Eickelmann, P. Functional characterisation of human SGLT-5 as a novel kidney-specific sodium-dependent sugar transporter. FEBS Lett. 2012, 586, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Pilic, L.; Pedlar, C.R.; Mavrommatis, Y. Salt-sensitive hypertension: Mechanisms and effects of dietary and other lifestyle factors. Nutr. Rev. 2016, 74, 645–658. [Google Scholar] [CrossRef]
- Prince, P.D.; Lanzi, C.R.; Toblli, J.E.; Elesgaray, R.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Dietary (-)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2016, 90, 35–46. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Chung, C.S.; Komnenov, D.; Rossi, N.F. Fructose plus High-Salt Diet in Early Life Results in Salt-Sensitive Cardiovascular Changes in Mature Male Sprague Dawley Rats. Nutrients 2021, 13, 3129. [Google Scholar] [CrossRef]
- Baud, G.; Raverdy, V.; Bonner, C.; Daoudi, M.; Caiazzo, R.; Pattou, F. Sodium glucose transport modulation in type 2 diabetes and gastric bypass surgery. Surg. Obes. Relat. Dis. 2016, 12, 1206–1212. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Lin, X.; Ma, L.; Fitzgerald, R.L.; Ostlund, R.E.J. Human sodium/inositol cotransporter 2 (SMIT2) transports inositols but not glucose in L6 cells. Arch. Biochem. Biophys. 2009, 481, 197–201. [Google Scholar] [CrossRef]
- Poulsen, S.B.; Fenton, R.A.; Rieg, T. Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 2015, 24, 463–469. [Google Scholar] [CrossRef]
- Bebernitz, G. 7.13-Sodium–Glucose Cotransporters. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 491–511. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Speight, P.; Silverman, M. Reanalysis of structure/function correlations in the region of transmembrane segments 4 and 5 of the rabbit sodium/glucose cotransporter. Biochem. Biophys. Res. Commun. 2009, 378, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Faham, S.; Watanabe, A.; Besserer, G.M.; Cascio, D.; Specht, A.; Hirayama, B.A.; Wright, E.M.; Abramson, J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 2008, 321, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Niu, Y.; Sun, Z.; Liu, R.; Chen, L. Structures of human SGLT in the occluded state reveal conformational changes during sugar transport. Nat. Commun. 2023, 14, 2920. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, R.; Guan, C.; Zhang, Y.; Chen, Z.; Hoerer, S.; Nar, H.; Chen, L. Structural basis of inhibition of the human SGLT2-MAP17 glucose transporter. Nature 2022, 601, 280–284. [Google Scholar] [CrossRef]
- Rentsch, D.; Schmidt, S.; Tegeder, M. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett. 2007, 581, 2281–2289. [Google Scholar] [CrossRef]
- Han, L.; Qu, Q.; Aydin, D.; Panova, O.; Robertson, M.J.; Xu, Y.; Dror, R.O.; Skiniotis, G.; Feng, L. Structure and mechanism of the SGLT family of glucose transporters. Nature 2022, 601, 274–279. [Google Scholar] [CrossRef]
- Tazawa, S.; Yamato, T.; Fujikura, H.; Hiratochi, M.; Itoh, F.; Tomae, M.; Takemura, Y.; Maruyama, H.; Sugiyama, T.; Wakamatsu, A.; et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life Sci. 2005, 76, 1039–1050. [Google Scholar] [CrossRef]
- Harada, N.; Inagaki, N. Role of sodium-glucose transporters in glucose uptake of the intestine and kidney. J. Diabetes Investig. 2012, 3, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Kamitori, K.; Shirota, M.; Fujiwara, Y. Structural Basis of the Selective Sugar Transport in Sodium-Glucose Cotransporters. J. Mol. Biol. 2022, 434, 167464. [Google Scholar] [CrossRef] [PubMed]
- Diederich, J.; Mounkoro, P.; Tirado, H.A.; Chevalier, N.; Van Schaftingen, E.; Veiga-da-Cunha, M. SGLT5 is the renal transporter for 1, 5-anhydroglucitol, a major player in two rare forms of neutropenia. Cell. Mol. Life Sci. 2023, 80, 259. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.; Stephenne, X.; Diederich, J.; Mounkoro, P.; Chevalier, N.; Ferster, A.; Van Schaftingen, E.; Veiga-da-Cunha, M. Successful use of empagliflozin to treat neutropenia in two G6PC3-deficient children: Impact of a mutation in SGLT5. J. Inherit. Metab. Dis. 2022, 45, 759–768. [Google Scholar] [CrossRef]
- Li, M.; Maruthur, N.M.; Loomis, S.J.; Pietzner, M.; North, K.E.; Mei, H.; Morrison, A.C.; Friedrich, N.; Pankow, J.S.; Nauck, M.; et al. Genome-wide association study of 1,5-anhydroglucitol identifies novel genetic loci linked to glucose metabolism. Sci. Rep. 2017, 7, 2812. [Google Scholar] [CrossRef]
- Loomis, S.J.; Köttgen, A.; Li, M.; Tin, A.; Coresh, J.; Boerwinkle, E.; Gibbs, R.; Muzny, D.; Pankow, J.; Selvin, E.; et al. Rare variants in SLC5A10 are associated with serum 1,5-anhydroglucitol (1,5-AG) in the Atherosclerosis Risk in Communities (ARIC) Study. Sci. Rep. 2019, 9, 5941. [Google Scholar] [CrossRef]
- Long, T.; Hicks, M.; Yu, H.C.; Biggs, W.H.; Kirkness, E.F.; Menni, C.; Zierer, J.; Small, K.S.; Mangino, M.; Messier, H.; et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 2017, 49, 568–578. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Odeh, M.A.; Rastogi, P.K. Chapter 5-Sodium-glucose cotransporter-2 (SGLT2) inhibitors (gliflozins) in diabetes mellitus: Mechanisms and therapeutic insights. In Diabetes Mellitus; Nidhar, M., Tewari, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 79–107. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, Y.; Li, D.; Pu, J.; Ding, H.; Zhang, X. Mechanisms of SGLT2 Inhibitors in Heart Failure and Their Clinical Value. J. Cardiovasc. Pharmacol. 2023, 81, 4–14. [Google Scholar] [CrossRef]
- Udell, J.A.; Jones, W.S.; Petrie, M.C.; Harrington, J.; Anker, S.D.; Bhatt, D.L.; Hernandez, A.F.; Butler, J. Sodium Glucose Cotransporter-2 Inhibition for Acute Myocardial Infarction: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 2058–2068. [Google Scholar] [CrossRef]
- Maxson, R.; Starr, J.; Sewell, J.; Lyas, C. SGLT2 Inhibitors to Slow Chronic Kidney Disease Progression: A Review. Clin. Ther. 2024, 46, e23–e28. [Google Scholar] [CrossRef] [PubMed]
- Hummel, C.S.; Lu, C.; Loo, D.D.; Hirayama, B.A.; Voss, A.A.; Wright, E.M. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am. J. Physiol. Cell Physiol. 2011, 300, C14–C21. [Google Scholar] [CrossRef] [PubMed]
- Uldry, M.; Ibberson, M.; Hosokawa, M.; Thorens, B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002, 524, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Burant, C.F.; Takeda, J.; Brot-Laroche, E.; Bell, G.I.; Davidson, N.O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [CrossRef]
- Shendurse, A.; Khedkar, C. Glucose: Properties and analysis. Encycl. Food Health 2016, 3, 239–247. [Google Scholar]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; Leon, A.d. Analysis of the structure and vibrational spectra of glucose and fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Mai, B.H.; Yan, L.J. The negative and detrimental effects of high fructose on the liver, with special reference to metabolic disorders. Diabetes Metab. Syndr. Obes. 2019, 12, 821–826. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef]
- Agarwal, V.; Das, S.; Kapoor, N.; Prusty, B.; Das, B. Dietary Fructose: A Literature Review of Current Evidence and Implications on Metabolic Health. Cureus 2024, 16, e74143. [Google Scholar] [CrossRef]
- Barry, C.P.; Honeyman, J. Fructose and its derivatives. Adv. Carbohydr. Chem. 1952, 7, 53–98. [Google Scholar] [CrossRef]
- Alonso-Fernández, J.; Timson, D.; Szilagyi, A.; Obendorf, R.L.; Abdelmalek, M.; McGrath, M.; Boukraâ, L.; Gastrich, M.; Haukioja, A.; Jensen, J. Dietary Sugars: Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef]
- Kane, S.; Seatter, M.J.; Gould, G.W. Functional studies of human GLUT5: Effect of pH on substrate selection and an analysis of substrate interactions. Biochem. Biophys. Res. Commun. 1997, 238, 503–505. [Google Scholar] [CrossRef]
- Ghezzi, C.; Gorraitz, E.; Hirayama, B.A.; Loo, D.D.; Grempler, R.; Mayoux, E.; Wright, E.M. Fingerprints of hSGLT5 sugar and cation selectivity. Am. J. Physiol. Cell Physiol. 2014, 306, C864–C870. [Google Scholar] [CrossRef]
- Iizuka, K. Recent Progress on Fructose Metabolism-Chrebp, Fructolysis, and Polyol Pathway. Nutrients 2023, 15, 1778. [Google Scholar] [CrossRef]
- Nakagawa, T.; Johnson, R.J.; Andres-Hernando, A.; Roncal-Jimenez, C.; Sanchez-Lozada, L.G.; Tolan, D.R.; Lanaspa, M.A. Fructose Production and Metabolism in the Kidney. J. Am. Soc. Nephrol. 2020, 31, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Bae, H.; Song, W.S.; Jang, C. Dietary Fructose and Fructose-Induced Pathologies. Annu. Rev. Nutr. 2022, 42, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Benoy, M.P.; Elliott, K.A. The metabolism of lactic and pyruvic acids in normal and tumour tissues: Synthesis of carbohydrate. Biochem. J. 1937, 31, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Nakajo, S.; Kamiya, F.; Toyama, Y.; Nishio, T.; Nakagawa, H. Renal net glucose release in vivo and its contribution to blood glucose in rats. J. Clin. Investig. 1978, 62, 721–726. [Google Scholar] [CrossRef]
- Krebs, H.A.; Lund, P. Formation of glucose from hexoses, pentoses, polyols and related substances in kidney cortex. Biochem. J. 1966, 98, 210–214. [Google Scholar] [CrossRef]
- Gladding, M.; Shen, X.; Snyder, M.P.; Havel, P.J.; Adams, S.H. Interindividual Variability in Postprandial Plasma Fructose Patterns in Adults. Nutrients 2024, 16, 3079. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Shinozaki, Y.; Ohta, T. Sodium-glucose cotransporters: Functional properties and pharmaceutical potential. J. Diabetes Investig. 2020, 11, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Silva, R.; Okamoto, M.M.; David-Silva, A.; Mori, R.C.; Freitas, H.S.; Machado, U.F. Increased SGLT1 expression in salivary gland ductal cells correlates with hyposalivation in diabetic and hypertensive rats. Diabetol. Metab. Syndr. 2013, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Vrhovac, I.; Balen Eror, D.; Klessen, D.; Burger, C.; Breljak, D.; Kraus, O.; Radović, N.; Jadrijević, S.; Aleksic, I.; Walles, T.; et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflug. Arch. 2015, 467, 1881–1898. [Google Scholar] [CrossRef]
- Madunić, I.V.; Breljak, D.; Karaica, D.; Koepsell, H.; Sabolić, I. Expression profiling and immunolocalization of Na(+)-D-glucose-cotransporter 1 in mice employing knockout mice as specificity control indicate novel locations and differences between mice and rats. Pflug. Arch. 2017, 469, 1545–1565. [Google Scholar] [CrossRef]
- Liang, X.; Yan, F.; Gao, Y.; Xiong, M.; Wang, H.; Onxayvieng, K.; Tang, R.; Li, L.; Zhang, X.; Chi, W.; et al. Sugar transporter genes in grass carp (Ctenopharyngodon idellus): Molecular cloning, characterization, and expression in response to different stocking densities. Fish. Physiol. Biochem. 2020, 46, 1039–1052. [Google Scholar] [CrossRef]
- Wang, N.; Li, S.; Guo, X.C.; Li, J.Y.; Ren, G.P.; Li, D.S. Fibroblast growth factor 21 improves glucose homeostasis partially via down-regulation of Na(+)-d-glucose cotransporter SGLT1 in the small intestine. Biomed. Pharmacother. 2019, 109, 1070–1077. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Friedrich, A.; Keller, T.; Mann, M.; Koepsell, H. The impact of high-fat diet on metabolism and immune defense in small intestine mucosa. J. Proteome Res. 2015, 14, 353–365. [Google Scholar] [CrossRef]
- Zhao, F.Q.; McFadden, T.B.; Wall, E.H.; Dong, B.; Zheng, Y.C. Cloning and expression of bovine sodium/glucose cotransporter SGLT2. J. Dairy. Sci. 2005, 88, 2738–2748. [Google Scholar] [CrossRef]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef]
- Kosinski, C.; Papadakis, G.E.; Salamin, O.; Kuuranne, T.; Nicoli, R.; Pitteloud, N.; Zanchi, A. Effects of empagliflozin on reproductive system in men without diabetes. Sci. Rep. 2024, 14, 13802. [Google Scholar] [CrossRef]
- Bolla, A.M.; Butera, E.; Pellegrini, S.; Caretto, A.; Bonfanti, R.; Zuppardo, R.A.; Barera, G.; Cavestro, G.M.; Sordi, V.; Bosi, E. Expression of glucose transporters in duodenal mucosa of patients with type 1 diabetes. Acta Diabetol. 2020, 57, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Akiba, J.; Tsutsumi, T.; Kawaguchi, M.; Yoshida, T.; Koga, H.; Kawaguchi, T. Hepatic expression of sodium-glucose cotransporter 2 (SGLT2) in patients with chronic liver disease. Med. Mol. Morphol. 2022, 55, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Diez-Sampedro, A.; Hirayama, B.A.; Osswald, C.; Gorboulev, V.; Baumgarten, K.; Volk, C.; Wright, E.M.; Koepsell, H. A glucose sensor hiding in a family of transporters. Proc. Natl. Acad. Sci. USA 2003, 100, 11753–11758. [Google Scholar] [CrossRef] [PubMed]
- Soták, M.; Casselbrant, A.; Rath, E.; Zietek, T.; Strömstedt, M.; Adingupu, D.D.; Karlsson, D.; Fritsch Fredin, M.; Ergang, P.; Pácha, J.; et al. Intestinal sodium/glucose cotransporter 3 expression is epithelial and downregulated in obesity. Life Sci. 2021, 267, 118974. [Google Scholar] [CrossRef]
- Bianchi, L.; Diez-Sampedro, A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS ONE 2010, 5, e10241. [Google Scholar] [CrossRef]
- Gyimesi, G.; Pujol-Giménez, J.; Kanai, Y.; Hediger, M.A. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: From molecular discovery to clinical application. Pflug. Arch. 2020, 472, 1177–1206. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Cabral, P.D.; Hong, N.J.; Asirwatham, J.; Saez, F.; Garvin, J.L. Fructose reabsorption by rat proximal tubules: Role of Na(+)-linked cotransporters and the effect of dietary fructose. Am. J. Physiol. Ren. Physiol. 2019, 316, F473–F480. [Google Scholar] [CrossRef]
- Sugawara-Yokoo, M.; Suzuki, T.; Matsuzaki, T.; Naruse, T.; Takata, K. Presence of fructose transporter GLUT5 in the S3 proximal tubules in the rat kidney. Kidney Int. 1999, 56, 1022–1028. [Google Scholar] [CrossRef]
- Baader-Pagler, T.; Eckhardt, M.; Himmelsbach, F.; Sauer, A.; Stierstorfer, B.E.; Hamilton, B.S. SGLT6-A pharmacological target for the treatment of obesity? Adipocyte 2018, 7, 277–284. [Google Scholar] [CrossRef]
- Gatto, F.; Ferreira, R.; Nielsen, J. Pan-cancer analysis of the metabolic reaction network. Metab. Eng. 2020, 57, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, S.P.; Cuthbertson, D.J.; Wilding, J.P. Energy balance and metabolic changes with sodium-glucose co-transporter 2 inhibition. Diabetes Obes. Metab. 2016, 18, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Liu, R.; Staruschenko, A. Gliflozins in hypertension: Basic mechanisms and clinical insights. Am. J. Physiol. Ren. Physiol. 2025, 329, F32–F45. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflug. Arch. 2020, 472, 1345–1370. [Google Scholar] [CrossRef]
- Vasquez-Rios, G.; Nadkarni, G.N. SGLT2 Inhibitors: Emerging Roles in the Protection Against Cardiovascular and Kidney Disease Among Diabetic Patients. Int. J. Nephrol. Renov. Dis. 2020, 13, 281–296. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, Y.; Malik, V.; Li, X.; Peng, X.; Zhang, F.F.; Shan, Z.; Liu, L. Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages and Risk of Cardiovascular Disease: A Meta-Analysis and Systematic Review. Adv. Nutr. 2021, 12, 89–101. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Shoham, D.A.; Durazo-Arvizu, R.; Kramer, H.; Luke, A.; Vupputuri, S.; Kshirsagar, A.; Cooper, R.S. Sugary soda consumption and albuminuria: Results from the National Health and Nutrition Examination Survey, 1999–2004. PLoS ONE 2008, 3, e3431. [Google Scholar] [CrossRef]
- Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt. Int. J. Mol. Sci. 2023, 24, 4294. [Google Scholar] [CrossRef]
- Jayalath, V.H.; Sievenpiper, J.L.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Santaren, I.D.; Blanco Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Total fructose intake and risk of hypertension: A systematic review and meta-analysis of prospective cohorts. J. Am. Coll. Nutr. 2014, 33, 328–339. [Google Scholar] [CrossRef]
- Liu, Q.; Ayoub-Charette, S.; Khan, T.A.; Au-Yeung, F.; Blanco Mejia, S.; de Souza, R.J.; Wolever, T.M.S.; Leiter, L.A.; Kendall, C.W.C.; Sievenpiper, J.L. Important Food Sources of Fructose-Containing Sugars and Incident Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2019, 8, e010977. [Google Scholar] [CrossRef]
- Xu, L.; Hu, G.; Qiu, J.; Fan, Y.; Ma, Y.; Miura, T.; Kohzuki, M.; Ito, O. High Fructose-Induced Hypertension and Renal Damage Are Exaggerated in Dahl Salt-Sensitive Rats via Renal Renin-Angiotensin System Activation. J. Am. Heart Assoc. 2021, 10, e016543. [Google Scholar] [CrossRef]
- Hayasaki, T.; Ishimoto, T.; Doke, T.; Hirayama, A.; Soga, T.; Furuhashi, K.; Kato, N.; Kosugi, T.; Tsuboi, N.; Lanaspa, M.A.; et al. Fructose increases the activity of sodium hydrogen exchanger in renal proximal tubules that is dependent on ketohexokinase. J. Nutr. Biochem. 2019, 71, 54–62. [Google Scholar] [CrossRef]
- Song, J.; Hu, X.; Shi, M.; Knepper, M.A.; Ecelbarger, C.A. Effects of dietary fat, NaCl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am. J. Physiol. Ren. Physiol. 2004, 287, F1204–F1212. [Google Scholar] [CrossRef]
- Sharma, N.; Li, L.; Ecelbarger, C.M. Sex differences in renal and metabolic responses to a high-fructose diet in mice. Am. J. Physiol. Ren. Physiol. 2015, 308, F400–F410. [Google Scholar] [CrossRef] [PubMed]
- Benziane, B.; Demaretz, S.; Defontaine, N.; Zaarour, N.; Cheval, L.; Bourgeois, S.; Klein, C.; Froissart, M.; Blanchard, A.; Paillard, M.; et al. NKCC2 surface expression in mammalian cells: Down-regulation by novel interaction with aldolase B. J. Biol. Chem. 2007, 282, 33817–33830. [Google Scholar] [CrossRef] [PubMed]
- Komnenov, D.; Rossi, N.F. Fructose-induced salt-sensitive blood pressure differentially affects sympathetically mediated aortic stiffness in male and female Sprague-Dawley rats. Physiol. Rep. 2023, 11, e15687. [Google Scholar] [CrossRef] [PubMed]
- Bekhbat, M.; Merrill, L.; Kelly, S.D.; Lee, V.K.; Neigh, G.N. Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: Implications for tissue collection methods. Behav. Brain Res. 2016, 305, 122–125. [Google Scholar] [CrossRef]
- Rauchova, H.; Hojna, S.; Kadlecova, M.; Vaneckova, I.; Chao, Y.M.; Chan, J.; Zicha, J. Sex differences in blood pressure, free radicals and plasma cholesterol fractions in Ren-2 transgenic rats of various ages. Physiol. Res. 2023, 72, 167–175. [Google Scholar] [CrossRef]
- Rauchova, H.; Hojna, S.; Kadlecova, M.; Vaneckova, I.; Zicha, J. Sex differences in blood pressure of aged Ren-2 transgenic rats. Physiol. Res. 2020, 69, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Shao, Y.; Omerovic, E. Influence of anesthetic agent, depth of anesthesia and body temperature on cardiovascular functional parameters in the rat. Lab. Anim. 2014, 48, 6–14. [Google Scholar] [CrossRef] [PubMed]
- DiBona, G.F. Neural regulation of renal tubular sodium reabsorption and renin secretion. Fed. Proc. 1985, 44, 2816–2822. [Google Scholar] [PubMed]
- Bell-Reuss, E.; Trevino, D.L.; Gottschalk, C.W. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J. Clin. Investig. 1976, 57, 1104–1107. [Google Scholar] [CrossRef]
- Pontes, R.B.; Girardi, A.C.; Nishi, E.E.; Campos, R.R.; Bergamaschi, C.T. Crosstalk between the renal sympathetic nerve and intrarenal angiotensin II modulates proximal tubular sodium reabsorption. Exp. Physiol. 2015, 100, 502–506. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Perecki, N.; Chung, C.S.; Rossi, N.F. Aortic Stiffness and Diastolic Dysfunction in Sprague Dawley Rats Consuming Short-Term Fructose Plus High Salt Diet. Integr. Blood Press. Control 2020, 13, 111–124. [Google Scholar] [CrossRef]
- Forester, B.R.; Brostek, A.; Schuhler, B.; Gonzalez-Vicente, A.; Garvin, J.L. Angiotensin II-stimulated proximal nephron superoxide production and fructose-induced salt-sensitive hypertension. Am. J. Physiol. Ren. Physiol. 2024, 326, F249–F256. [Google Scholar] [CrossRef]
- Ares, G.R.; Kassem, K.M.; Ortiz, P.A. Fructose acutely stimulates NKCC2 activity in rat thick ascending limbs by increasing surface NKCC2 expression. Am. J. Physiol. Ren. Physiol. 2019, 316, F550–F557. [Google Scholar] [CrossRef]
- Bahena-Lopez, J.P.; Rojas-Vega, L.; Chavez-Canales, M.; Bazua-Valenti, S.; Bautista-Perez, R.; Lee, J.H.; Madero, M.; Vazquez-Manjarrez, N.; Alquisiras-Burgos, I.; Hernandez-Cruz, A.; et al. Glucose/Fructose Delivery to the Distal Nephron Activates the Sodium-Chloride Cotransporter via the Calcium-Sensing Receptor. J. Am. Soc. Nephrol. 2023, 34, 55–72. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, S.; Jadhav, D.A.; Kim, N.; Brostek, A.; Forester, B.R.; Shukla, R.; Qu, C.; Kramer, B.; Garvin, J.L.; et al. Abnormal activation of the mineralocorticoid receptor in the aldosterone-sensitive distal nephron contributes to fructose-induced salt-sensitive hypertension. bioRxiv 2024. [Google Scholar] [CrossRef]
- Curry, D.L. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas 1989, 4, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Elliott, S.S.; Tschop, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’Alessio, D.; Havel, P.J. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef]
- Fuster, D.G.; Bobulescu, I.A.; Zhang, J.; Wade, J.; Moe, O.W. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am. J. Physiol. Ren. Physiol. 2007, 292, F577–F585. [Google Scholar] [CrossRef]
- Ito, O.; Kondo, Y.; Takahashi, N.; Kudo, K.; Igarashi, Y.; Omata, K.; Imai, Y.; Abe, K. Insulin stimulates NaCl transport in isolated perfused MTAL of Henle’s loop of rabbit kidney. Am. J. Physiol. 1994, 267, F265–F270. [Google Scholar] [CrossRef]
- Chavez-Canales, M.; Arroyo, J.P.; Ko, B.; Vazquez, N.; Bautista, R.; Castaneda-Bueno, M.; Bobadilla, N.A.; Hoover, R.S.; Gamba, G. Insulin increases the functional activity of the renal NaCl cotransporter. J. Hypertens. 2013, 31, 303–311. [Google Scholar] [CrossRef]
- Blass, G.; Klemens, C.A.; Brands, M.W.; Palygin, O.; Staruschenko, A. Postprandial Effects on ENaC-Mediated Sodium Absorption. Sci. Rep. 2019, 9, 4296. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, S.H.; Rossi, N.F. High-Fructose-Induced Salt-Sensitive Hypertension: The Potential Benefit of SGLT4 or SGLT5 Modulation. Nutrients 2025, 17, 2511. https://doi.org/10.3390/nu17152511
Siddiqui SH, Rossi NF. High-Fructose-Induced Salt-Sensitive Hypertension: The Potential Benefit of SGLT4 or SGLT5 Modulation. Nutrients. 2025; 17(15):2511. https://doi.org/10.3390/nu17152511
Chicago/Turabian StyleSiddiqui, Sharif Hasan, and Noreen F. Rossi. 2025. "High-Fructose-Induced Salt-Sensitive Hypertension: The Potential Benefit of SGLT4 or SGLT5 Modulation" Nutrients 17, no. 15: 2511. https://doi.org/10.3390/nu17152511
APA StyleSiddiqui, S. H., & Rossi, N. F. (2025). High-Fructose-Induced Salt-Sensitive Hypertension: The Potential Benefit of SGLT4 or SGLT5 Modulation. Nutrients, 17(15), 2511. https://doi.org/10.3390/nu17152511






