Chewing Matters: Masticatory Function, Oral Microbiota, and Gut Health in the Nutritional Management of Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria: Studies Were Selected Based on the Following Inclusion Criteria
2.3. Exclusion Criteria Included Studies Focusing on Non-Human Subjects, Research That Did Not Directly Address Aging Populations, or Studies That Lacked Clear Methodological Reporting
2.4. Data Extraction
2.5. Synthesis of Findings
2.6. Statistical Methods
2.7. Quality Assessment
2.8. Limitations
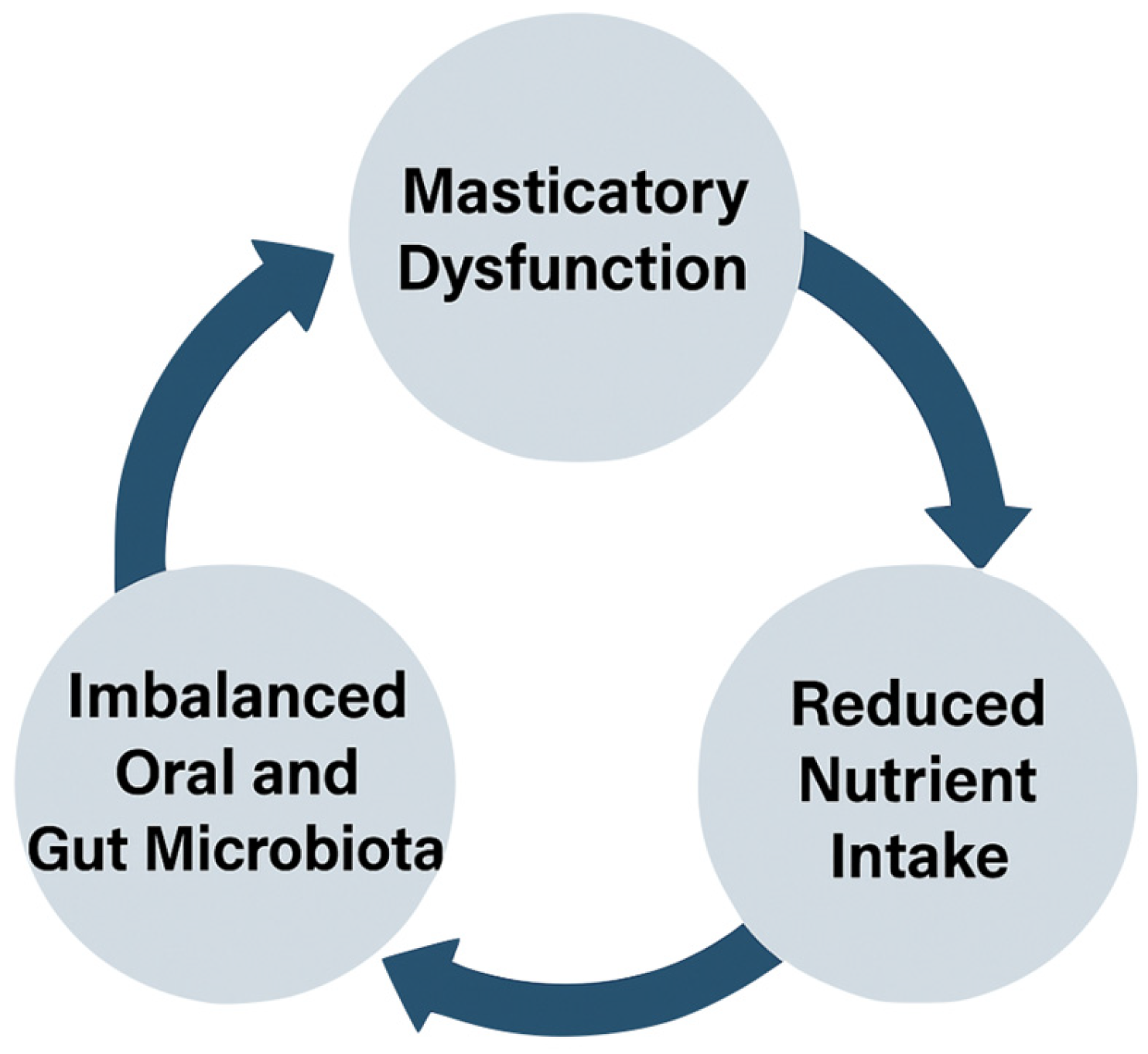
3. Results and Discussion
3.1. Impaired Masticatory Function and Nutritional Status
3.2. Oral Microbiota and Gut Health
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamamoto, Y.; Yamamoto, T.; Miyamoto, N.; Kinoshita, K.; Nishikawa, S.; Adachi, T.; Takizawa, S.; Inoue, R.; Matoba, S. Oral function and the oral microbiome in the elderly in the Kyotango area. Dent. J. 2024, 12, 16. [Google Scholar] [CrossRef]
- Matsuo, T.; Kurokawa, Y.; Minakuchi, S.; Yamamoto, T.; Takeuchi, K. Mastication and nutritional status in community-dwelling older adults: A systematic review. CoDAS 2024, 36, e20230209. [Google Scholar]
- Flores, L.; León, M.; Ruiz, F.; Molina, H.; Sánchez, E. Oral microbiota shifts following tooth loss affect gut health. Int. J. Mol. Sci. 2025, 26, 1120. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Y.; Yuan, Q.; Shen, Y. Masticatory dysfunction in older adults: A scoping review. J. Oral Rehabil. 2023, 50, 850–862. [Google Scholar] [CrossRef]
- Cifuentes-Suazo, G.; Alarcón-Apablaza, J.; Jarpa-Parra, M.; Venegas, C.; Marinelli, F.; Fuentes, R. Dietary counseling as an intervention for malnutrition and masticatory deficiency in complete denture wearers: A scoping review. Nutrients 2025, 17, 141. [Google Scholar] [CrossRef]
- Smith, A.; Jones, B.; Miller, C.; Williams, D.; Patel, R. The oral–gut microbiota axis: A link in cardiometabolic diseases. NPJ Biofilms Microbiomes 2025, 1, 56. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Tran, H.A.; Vu, C.T.; Le, Q.H.; Pham, T.T. The gut microbiota and aging: Interactions, implications, and interventions. Front. Aging 2025, 6, 1452917. [Google Scholar] [CrossRef]
- Shiraki, H.; Kakuta, S.; Kimura, Y.; Iwasaki, M.; Masaki, C.; Wada, T.; Matsubayashi, K.; Ishimoto, Y.; Fujisawa, M.; Okumiya, K.; et al. Relationship between masticatory function and sarcopenic obesity in community-dwelling older adults aged 75 or older: A cross-sectional study. BMC Geriatr. 2025, 25, 191. [Google Scholar] [CrossRef]
- An, R.; Wilms, E.; Masclee, A.A.M.; Smidt, H. Age-dependent changes in gastrointestinal physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef]
- Sadovska, D.; Freimane, L.; Kivrane, A.; Bortkevica, S.; Zayakin, P.; Kimsis, J.; Igumnova, V.; Kazarina, A.; Kuzmicka, J.; Namina, A.; et al. Oral microbiome variations related to ageing: Possible implications beyond oral health. Arch. Microbiol. 2023, 205, 116. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in older adults—Recent advances and remaining challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.B.; Pettersson, S.; Melby, M.K.; Bosch, T.C.G. The microbiome mediates environmental effects on aging. BioEssays 2019, 41, e1800257. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Miura, H. Association of mastication and factors affecting masticatory function with obesity in adults: A systematic review. BMC Oral Health 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Moutsopoulos, N.M. Oral microbiota: A repository for periodontal pathobionts. Nat. Rev. Microbiol. 2022, 20, 273–284. [Google Scholar] [CrossRef]
- Barnaba, P.; Rosa, A.; Gargari, M.; Martelli, M. The Use of Bioceramics in Retrograde Apicectomy: A Systematic Review of Clinical Applications and Outcomes. Aust. Endod. J. 2025. [Google Scholar] [CrossRef]
- Okada, M.; Hama, Y.; Futatsuya, R.; Sasaki, Y.; Noritake, K.; Yamaguchi, K.; Matsuzaki, M.; Kubota, C.; Hosoda, A.; Minakuchi, S. Association between Masticatory Performance, Nutritional Intake, and Frailty in Japanese Older Adults. Nutrients 2023, 15, 5075. [Google Scholar] [CrossRef]
- Rosa, A.; Pujia, A.M.; Arcuri, C. The Protective Role Antioxidant of Vitamin C in the Prevention of oral Disease: A Scoping Review of Current Literature. Eur. J. Dent. 2024, 18, 965–970. [Google Scholar] [CrossRef]
- Larson, P.J.; Zhou, W.; Santiago, A.; Driscoll, S.; Fleming, E.; Voigt, A.Y.; Chun, O.K.; Grady, J.J.; Kuchel, G.A.; Robison, J.T.; et al. Associations of the skin, oral and gut microbiome with aging, frailty and infection risk reservoirs in older adults. Nat. Aging 2022, 2, 941–955. [Google Scholar] [CrossRef]
- Iwauchi, M.; Horigome, A.; Ishikawa, K.; Mikuni, A.; Nakano, M.; Xiao, J.Z.; Odamaki, T.; Hironaka, S. Relationship between oral and gut microbiota in elderly people. Immun. Inflamm. Dis. 2019, 7, 229–236. [Google Scholar] [CrossRef]
- Lexomboon, D.; Kumar, A.; Freyland, S.; Xu, W.; Sandborgh-Englund, G. Is poor chewing ability a risk factor for malnutrition? A six-year longitudinal study of older adults in Sweden. J. Nutr. Health Aging 2025, 29, 100554. [Google Scholar] [CrossRef] [PubMed]
- Özsürekci, C.; Kara, M.; Güngör, A.E.; Ayçiçek, G.Ş.; Çalışkan, H.; Doğu, B.B.; Cankurtaran, M.; Halil, M.G. Relationship between chewing ability and malnutrition, sarcopenia, and frailty in older adults. Nutr. Clin. Pract. 2022, 37, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, K.; Mikami, Y.; Shirobe, M.; Edahiro, A.; Ohara, Y.; Iwasaki, M.; Watanabe, Y.; Kawai, H.; Kera, T.; Obuchi, S.; et al. Relationship between chewing ability and nutritional status in Japanese older adults: A cross-sectional study. Int. J. Environ. Res. Public Health 2021, 18, 1216. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor oral health as a determinant of malnutrition and sarcopenia. Nutrients 2019, 11, 2898. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Peña, N.; Kawar, N.; Zhang, A.; Callahan, N.; Robles, S.J.; Griebel, A.; Adami, G.R. Old age and other factors associated with salivary microbiome variation. BMC Oral Health 2021, 21, 490. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Lee, W.-F.; Salamanca, E.; Yao, W.-L.; Su, J.-N.; Wang, S.-Y.; Hu, C.-J.; Chang, W.-J. Oral microbiota changes in elderly patients, an indicator of Alzheimer’s disease. Int. J. Environ. Res. Public Health 2021, 18, 4211. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Zhang, Z.; Chen, N. Distinct gut microbiota signatures in older people with sarcopenic obesity and sarcopenia without obesity. Clin. Nutr. 2025, 49, 77–89. [Google Scholar] [CrossRef]
- DeClercq, V.; Wright, R.J.; Nearing, J.T.; Langille, M.G.I. Oral microbial signatures associated with age and frailty in Canadian adults. Sci. Rep. 2024, 14, 9685. [Google Scholar] [CrossRef]
- Kwon, H.K.; Lee, J.H.; Park, Y.D. Difference in food and nutrient intakes in Korean elderly people according to chewing difficulty. Korean J. Community Nutr. 2017, 22, 127–135. [Google Scholar] [CrossRef]
- Fluitman, K.S.; Davids, M.; Olofsson, L.E.; Wijdeveld, M.; Tremaroli, V.; Keijser, B.J.F.; Visser, M.; Bäckhed, F.; Nieuwdorp, M.; IJzerman, R.G. Gut microbial characteristics in poor appetite and undernutrition: A cohort of older adults and microbiota transfer in germ-free mice. J. Cachexia Sarcopenia Muscle 2022, 13, 2188–2201. [Google Scholar] [CrossRef]
- Picca, A.; Ponziani, F.R.; Calvani, R.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Primiano, A.; Putignani, L.; Del Chierico, F.; et al. Gut Microbial, Inflammatory and Metabolic Signatures in Older People with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2019, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont Colonization and Immunity. Cell Host Microbe 2020, 28, 558–570. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, W.Z.; Li, Y.L.; Bi, C.; Du, L.J.; Liu, Y.; Zhou, L.J.; Liu, T.; Xu, S.; Shi, C.J.; et al. Roles of oral microbiota and oral-gut microbial transmission in hypertension. J. Adv. Res. 2023, 43, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The oral-gut microbiome axis in health and disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Martelli, M.; Hafedh, S.; Maddalena Marrapodi, M.; Di Blasio, M.; Bollero, P.; Cicciù, M. Low-level laser treatment’s ability to reduce dry socket pain. Acta Odontol. Scand. 2024, 83, 631–641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morley, J.E.; Vellas, B.; Van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K.; Iliffe, S. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Avlund, K. Fatigue in older adults: An early indicator of the aging process? Aging Clin. Exp. Res. 2010, 22, 100–115. [Google Scholar] [CrossRef]
- Zengarini, E.; Ruggiero, C.; Pérez-Zepeda, M.U.; Hoogendijk, E.O.; Vellas, B.; Mecocci, P.; Cesari, M. Fatigue: Relevance and implications in the aging population. Exp. Gerontol. 2015, 70, 78–83. [Google Scholar] [CrossRef]
- Ream, E.; Richardson, A. Fatigue: A concept analysis. Int. J. Nurs. Stud. 1996, 33, 519–529. [Google Scholar] [CrossRef]
- Vestergaard, S.; Nayfield, S.G.; Patel, K.V.; Eldadah, B.; Cesari, M.; Ferrucci, L.; Ceresini, G.; Guralnik, J.M. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 76–82. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, M.; Rosa, A.; Spataro, F.; Capria, G.; Barnaba, P.; Gargari, M.; Martelli, M. Chewing Matters: Masticatory Function, Oral Microbiota, and Gut Health in the Nutritional Management of Aging. Nutrients 2025, 17, 2507. https://doi.org/10.3390/nu17152507
Lettieri M, Rosa A, Spataro F, Capria G, Barnaba P, Gargari M, Martelli M. Chewing Matters: Masticatory Function, Oral Microbiota, and Gut Health in the Nutritional Management of Aging. Nutrients. 2025; 17(15):2507. https://doi.org/10.3390/nu17152507
Chicago/Turabian StyleLettieri, Monia, Alessio Rosa, Fabrizio Spataro, Giovanni Capria, Paolo Barnaba, Marco Gargari, and Mirko Martelli. 2025. "Chewing Matters: Masticatory Function, Oral Microbiota, and Gut Health in the Nutritional Management of Aging" Nutrients 17, no. 15: 2507. https://doi.org/10.3390/nu17152507
APA StyleLettieri, M., Rosa, A., Spataro, F., Capria, G., Barnaba, P., Gargari, M., & Martelli, M. (2025). Chewing Matters: Masticatory Function, Oral Microbiota, and Gut Health in the Nutritional Management of Aging. Nutrients, 17(15), 2507. https://doi.org/10.3390/nu17152507







