Cross-Lagged Relationship Between Adiposity and HOMA and Mediating Role of Adiposity Between Lifestyle Factors and HOMA Among in Mexican Health Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic Characteristics and Lifestyle Factors
2.3. Physical Activity
2.4. Sleep Time
2.5. Depressive Symptoms
2.6. Dietary Assessment
2.7. Dietary Inflammatory Index
2.8. Insulin Resistance
2.9. Anthropometric Assessment
2.10. Statistical Analysis
Goodness of Fit
3. Results
3.1. Descriptive Characteristics of the Study Population
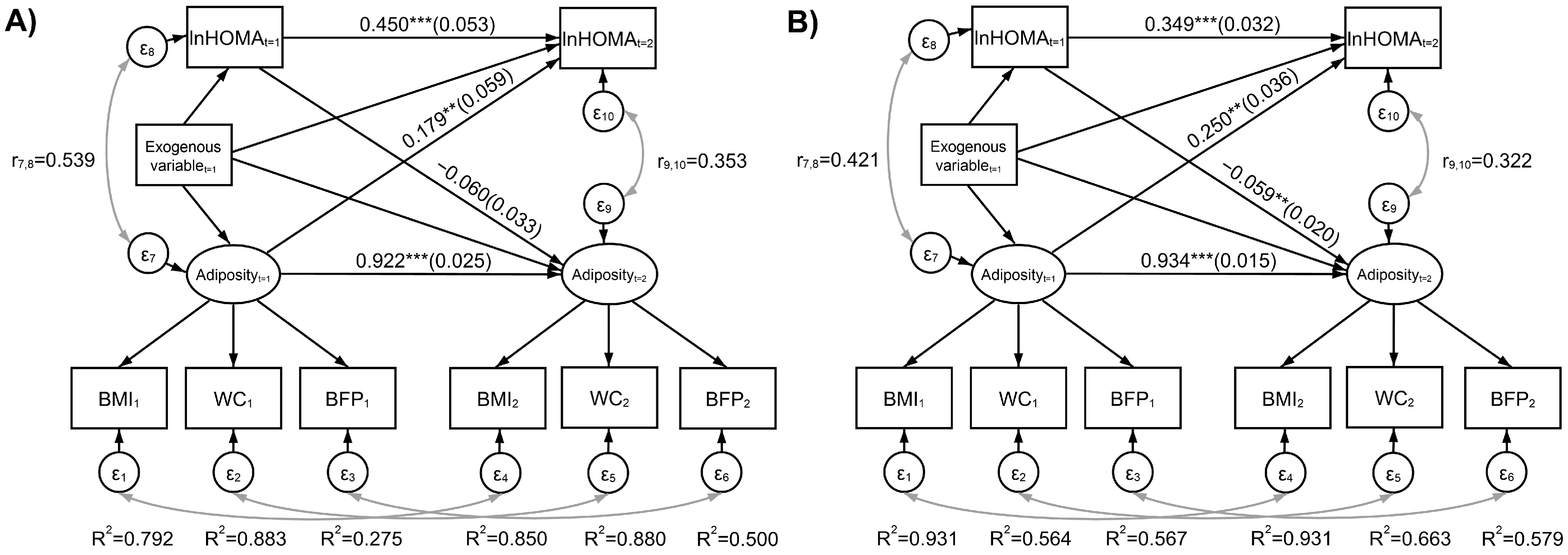
3.2. Adiposity Measurement System and Bidirectional Relationships Between Adiposity and IR
3.3. Mediating Role of Adiposity in the Relationship Between Lifestyle Factors and IR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IR | Insulin Resistance |
| HOMA | Homeostasis model assessment |
| BMI | Body mass index |
| WC | Waist circumference |
| BFP | Body fat proportion |
| T2D | Type 2 diabetes |
| NHANES | National Health and Nutrition Examination Survey |
| SEM | Structural equation modeling |
| AHEI | Alternate healthy eating index |
| ENSANUT | National Health and Nutrition Survey |
| HWCS | Health Workers Cohort Study |
| IMSS | Mexican Social Security Institute |
| PA | Physical activity |
| ST | Sleep time |
| CES-D | Center for Epidemiological Studies Depression Scale |
| DS | Depressive symptoms |
| FFQ | Food frequency questionnaire |
| SNUT | Nutritional Habits and Nutrient Consumption Assessment System |
| DII | Dietary inflammatory index |
| DXA | Dual-energy X-ray absorptiometry |
| CFI | Comparative Fit Index |
| TLI | Tucker-Lewis Index |
| RMSEA | Root Mean Square Error of Approximation |
| 90%CI | 90% confidence interval |
| R2 | Coefficient of determination |
| SD | Standard deviation |
| IQR | Interquartile range |
References
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance; StatPearls: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507839/ (accessed on 10 February 2025).
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Silva, A.A.; Carmo, J.M.; Li, X.; Wang, Z. Role of hyperinsulinemia and insulin resistance in hypertension: Metabolic syndrome revisited. Can. J. Cardiol. 2021, 36, 671–682. [Google Scholar] [CrossRef]
- Feve, B.; Bastard, J.P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 305–311. [Google Scholar] [CrossRef]
- Méndez-Hernández, P.; Dosamantes-Carrasco, L.D.; Siani, C.; Pierlot, R.; Martínez-Gómez, M.; Rivera-Paredez, B.; Cervantes-Popoca, L.; Rojas-Lima, E.; Salazar-Martínez, E.; Flores, Y.N.; et al. Mealtime habits and risk of developing the metabolic syndrome or insulin resistance among Mexican adults. Br. J. Nutr. 2016, 116, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Parcha, V.; Heindl, B.; Kalra, R.; Li, P.; Gower, B.; Arora, G.; Arora, P. Insulin Resistance and Cardiometabolic Risk Profile Among Nondiabetic American Young Adults: Insights From NHANES. J. Clin. Endocrinol. Metab. 2022, 107, e25–e37. [Google Scholar] [CrossRef] [PubMed]
- Campos-Nonato, I.; Galván-Valencia, Ó.; Hernández-Barrera, L.; Oviedo-Solís, C.; Barquera, S. Prevalencia de obesidad y factores de riesgo asociados en adultos mexicanos: Resultados de la Ensanut 2022. Salud Publica Mex. 2023, 65 (Suppl. S1), S238–S247. [Google Scholar] [CrossRef] [PubMed]
- Flores-Viveros, K.L.; Aguilar-Galarza, B.A.; Ordóñez-Sánchez, M.L.; Anaya-Loyola, M.A.; Moreno-Celis, U.; Vázquez-Cárdenas, P.; García-Gasca, T. Contribution of genetic, biochemical and environmental factors on insulin resistance and obesity in Mexican young adults. Obes. Res. Clin. Pract. 2019, 13, 533–540. [Google Scholar] [CrossRef]
- Garthwaite, T.; Sjöros, T.; Koivumäki, M.; Laine, S.; Vähä-Ypyä, H.; Saarenhovi, M.; Kallio, P.; Löyttyniemi, E.; Sievänen, H.; Houttu, N.; et al. Standing is associated with insulin sensitivity in adults with metabolic syndrome. J. Sci. Med. Sport 2021, 24, 1255–1260. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Torres-Ibarra, L.; González-Morales, R.; Barrientos-Gutiérrez, T.; Hernández-López, R.; Ramírez, P.; León-Maldonado, L.; Velázquez-Cruz, R.; Denova-Gutiérrez, E.; Salmerón, J. Cumulative soft drink consumption is associated with insulin resistance in Mexican adults. Am. J. Clin. Nutr. 2020, 112, 661–668. [Google Scholar] [CrossRef]
- Keith, R.J.; Al Rifai, M.; Carruba, C.; De Jarnett, N.; McEvoy, J.W.; Bhatnagar, A.; Blaha, M.J.; Defilippis, A.P. Tobacco Use, Insulin Resistance, and Risk of Type 2 Diabetes: Results from the Multi-Ethnic Study of Atherosclerosis. PLoS ONE 2016, 11, e0157592. [Google Scholar] [CrossRef]
- Rutters, F.; Besson, H.; Walker, M.; Mari, A.; Konrad, T.; Nilsson, P.M.; Balkau, B.; Dekker, J.M. The Association Between Sleep Duration, Insulin Sensitivity, and β-Cell Function: The EGIR-RISC Study. J. Clin. Endocrinol. Metab. 2016, 101, 3272–3780. [Google Scholar] [CrossRef]
- Yan, K. Recent advances in the effect of adipose tissue inflammation on insulin resistance. Cell Signal. 2024, 120, 111229. [Google Scholar] [CrossRef]
- Guria, S.; Hoory, A.; Das, S.; Chattopadhyay, D.; Mukherjee, S. Adipose tissue macrophages and their role in obesity-associated insulin resistance: An overview of the complex dynamics at play. Biosci. Rep. 2023, 43, BSR20220200. [Google Scholar] [CrossRef]
- Garcia-Hermoso, A.; Martinez-Vizcaino, V.; Recio-Rodriguez, J.I.; Diez-Fernandez, A.; Gomez-Marcos, M.A.; Garcia-Ortiz, L.; EVIDENT Group. Abdominal obesity as a mediator of the influence of physical activity on insulin resistance in Spanish adults. Prev. Med. 2016, 82, 59–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, Y.M.; Zhang, J.; Steck, S.E.; Fung, T.T.; Hazlett, L.J.; Han, K.; Ko, S.H.; Merchant, A.T. Obesity Mediates the Association between Mediterranean Diet Consumption and Insulin Resistance and Inflammation in US Adults. J. Nutr. 2017, 147, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.Z.H.; Semnani-Azad, Z.; Retnakaran, R.; Harris, S.B.; Hanley, A.J. Changes in adiposity mediate the associations of diet quality with insulin sensitivity and beta-cell function. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D. Introduction to Statistical Mediation Analysis, 1st ed.; Routledge: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Wang, Y.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005, 81, 555–563. [Google Scholar] [CrossRef]
- Escamilla-Núñez, M.C.; Castro-Porras, L.; Romero-Martínez, M.; Zárate-Rojas, E.; Rojas-Martínez, R. Detección, diagnóstico previo y tratamiento de enfermedades crónicas no transmisibles en adultos mexicanos. Ensanut 2022. Salud Publica Mex. 2023, 65 (Suppl. S1), S153–S162. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Moreno-Banda, G.L.; Carnalla, M.; Rivera, J.A.; Romero-Martinez, M.; Barquera, S.; Barrientos-Gutierrez, T. Prevalencia de prediabetes y diabetes en México: Ensanut 2022. Salud Publica Mex. 2023, 65 (Suppl. S1), S163–S168. [Google Scholar] [CrossRef]
- Szukiewicz, D. Molecular Mechanisms for the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity. Int. J. Mol. Sci. 2023, 24, 9818. [Google Scholar] [CrossRef]
- Lee, C.H.; Lam, K.S. Obesity-induced insulin resistance and macrophage infiltration of the adipose tissue: A vicious cycle. J. Diabetes Investig. 2019, 10, 29–31. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramírez-Palacios, P.; Rivera-Paredez, B.; Muñoz-Aguirre, P.; Velázquez-Cruz, R.; Torres-Ibarra, L.; Meneses-León, J.; Méndez-Hernández, P.; et al. Health workers cohort study: Methods and study design. Salud Publica Mex. 2016, 58, 708–716. [Google Scholar] [CrossRef]
- Rosner, B. Percentage points for generalized ESD many-outlier procedure. Technometrics 1983, 25, 165–172. [Google Scholar] [CrossRef]
- World Health Organization. Tobacco or Health: A Global Status Report; WHO Library: Geneva, Switzerland, 1997. Available online: http://www.kfshrc.edu.sa/annals/articles/182/Bookrev182.pdf (accessed on 15 February 2025).
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Méndez-Hernández, P.; Flores, Y.; Siani, C.; Lamure, M.; Dosamantes-Carrasco, L.D.; Halley-Castillo, E.; Huitrón, G.; Talavera, J.O.; Gallegos-Carrillo, K.; Salmerón, J. Physical activity and risk of metabolic syndrome in an urban Mexican cohort. BMC Public Health 2009, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Meneses-León, J.; Hernández-Salazar, S.; Robles-Rivera, K.; Tamayo-Ortiz, M.; Muciño-Sandoval, K.; Rivas-Ruiz, R.; Denova-Gutiérrez, E.; Tamayo-Orozco, J.A.; Velázquez-Cruz, R.; Salmerón, J.; et al. Association Between Changes in Sleep, Nap Duration and Bone Mineral Density in Mexican Adults. Calcif. Tissue Int. 2024, 115, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D scale a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998, 10, 133–140. [Google Scholar] [CrossRef]
- Hernández-Ávila, M.; Resoles, M.; Parra, S. Sistema de Evaluación de Hábitos Nutricionales y Consumo de Nutrimentos (SNUT); INSP: Cuernavaca, Mexico, 2000. [Google Scholar]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Quezada-Sánchez, A.D.; Robles-Rivera, K.; Hidalgo-Bravo, A.; Denova-Gutiérrez, E.; León-Reyes, G.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R. Dietary inflammatory index and bone mineral density in Mexican population. Osteoporos Int. 2022, 33, 1969–1979. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. S4), 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Press: Champaign, IL, USA, 1988. [Google Scholar]
- World Health Organization. Obesidad y Sobrepeso; World Health Organization: Geneva, Switzerland, 2021.
- Expert Panel of Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel of Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Clark, P.; Denova-Gutiérrez, E.; Ambrosi, R.; Szulc, P.; Rivas-Ruiz, R.; Salmerón, J. Reference values of total lean mass, appendicular lean mass, and fat mass measured with dual-energy X ray absorptiometry in a healthy Mexican population. Calcif. Tissue Int. 2016, 99, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P.; Yap, M.; Staveren, W.A. Body mass index and percent body fat: A meta analysis among different ethnic groups. Int. J. Obes. 1998, 22, 1164–1171. [Google Scholar] [CrossRef]
- Lee, D.K. Data transformation: A focus on the interpretation. Korean J. Anesth. 2020, 73, 503–508. [Google Scholar] [CrossRef]
- Wooldridge, J.M. Incorporating Nonlinearities in Simple Regression. In Introductory Econometrics; A Modern Approach, 4th ed.; Cengage Learning: Mason, OH, USA, 2008; pp. 43–46. [Google Scholar]
- Macias, N.; Quezada, A.D.; Flores, M.; Valencia, M.E.; Denova-Gutiérrez, E.; Quiterio-Trenado, M.; Gallegos-Carrillo, K.; Barquera, S.; Salmerón, J. Accuracy of body fat percent and adiposity indicators cut off values to detect metabolic risk factors in a sample of Mexican adults. BMC Public Health 2014, 14, 341. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X. Model Estimation. In Structural Equation Modeling: Applications Using Mplus, 2nd ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2019; pp. 14–17. [Google Scholar]
- Muthén, L.K.; Muthén, B.O. Output Options. Standardized. In Mplus. Statistical Analysis with Latent Variables. User’s Guide, 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2017; pp. 799–802. Available online: https://www.statmodel.com/download/usersguide/MplusUserGuideVer_8.pdf (accessed on 10 July 2025).
- Little, T.D. Model Fit, Sample Size, and Power. In Longitudinal Structural Equation Modeling; The Guilford Press: New York, NY, USA, 2013; pp. 106–136. [Google Scholar]
- Hu, L.-t.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, G.; Zhao, M.; Zhang, X.; Fang, L.; Guan, Q.; Zhang, H.; Gao, L.; Zhang, T.; Zhao, J. Bidirectional temporal relationship between obesity and hyperinsulinemia: Longitudinal observation from a Chinese cohort. BMJ Open Diab. Res. Care 2021, 9, e002059. [Google Scholar] [CrossRef]
- Fuente-Martín, E.; Argente-Arizón, P.; Ros, P.; Argente, J.; Chowen, J.A. Sex differences in adipose tissue: It is not only a question of quantity and distribution. Adipocyte 2013, 2, 128–134. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Nguyen, M.A.; Henstridge, D.C.; Nguyen, A.K.; Beaven, S.W.; Watt, M.J.; Hevener, A.L. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E304–E319. [Google Scholar] [CrossRef]
- Monteiro, R.; Teixeira, D.; Calhau, C. Estrogen signaling in metabolic inflammation. Mediat. Inflamm. 2014, 2014, 615917. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009, 6 (Suppl. S1), 60–75. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Joharapurkar, A.; Das, N.; Khatoon, S.; Kushwaha, S.; Gurjar, A.A.; Singh, A.K.; Shree, S.; Ahmed, M.Z.; China, S.P.; et al. Estradiol overcomes adiponectin-resistance in diabetic mice by regulating skeletal muscle adiponectin receptor 1 expression. Mol. Cell Endocrinol. 2022, 540, 111525. [Google Scholar] [CrossRef]
- Chen, X.; McClusky, R.; Chen, J.; Beaven, S.W.; Tontonoz, P.; Arnold, A.P.; Reue, K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012, 8, e1002709. [Google Scholar] [CrossRef] [PubMed]
- Demerath, E.W.; Sun, S.S.; Rogers, N.; Lee, M.; Reed, D.; Choh, A.C.; Couch, W.; Czerwinski, S.A.; Chumlea, W.C.; Siervogel, R.M.; et al. Anatomical patterning of visceral adipose tissue: Race, sex, and age variation. Obesity 2007, 15, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, Q.; Chen, W.; Gao, Y.; Ye, J.; Chen, Y.; Wang, T.; Gao, L.; Liu, Y.; Yang, Y. New advances of adiponectin in regulating obesity and related metabolic syndromes. J. Pharm. Anal. 2024, 14, 100913. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Viguerie, N.; Massier, L.; Rydén, M.; Astrup, A.; Blaak, E.; Langin, D.; Andersson, D.P. Sex differences in adipose insulin resistance are linked to obesity, lipolysis and insulin receptor substrate 1. Int. J. Obes. 2024, 48, 934–940. [Google Scholar] [CrossRef]
- Shalev-Goldman, E.; McGuire, K.A.; Ross, R. Waist circumference and cardiorespiratory fitness are independently associated with glucose tolerance and insulin resistance in obese women. Appl. Physiol. Nutr. Metab. 2014, 39, 358–362. [Google Scholar] [CrossRef]
- Heitmann, B.L.; Lissner, L. Dietary underreporting by obese individuals—Is it specific or non-specific? BMJ 1995, 311, 986–989. [Google Scholar] [CrossRef]
- Dahle, J.H.; Ostendorf, D.M.; Zaman, A.; Pan, Z.; Melanson, E.L.; Catenacci, V.A. Underreporting of energy intake in weight loss maintainers. Am. J. Clin. Nutr. 2021, 114, 257–266. [Google Scholar] [CrossRef]
- Gaona-Pineda, E.B.; Rodríguez-Ramírez, S.; Medina-Zacarías, M.C.; Valenzuela-Bravo, D.G.; Martinez-Tapia, B.; Arango-Angarita, A. Consumidores de grupos de alimentos en población mexicana. Ensanut Continua 2020–2022. Salud Publica Mex. 2023, 65 (Suppl. S1), S248–S258. [Google Scholar] [CrossRef]
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; Day, N.E.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef]
- Hulsegge, G.; Proper, K.I.; Loef, B.; Paagman, H.; Anema, J.R.; van Mechelen, W. The mediating role of lifestyle in the relationship between shift work, obesity and diabetes. Int. Arch. Occup. Environ. Health 2021, 94, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.J.; Dekker, L.H.; Carrero, J.J.; Navis, G. Using Structural Equation Modeling to Untangle Pathways of Risk Factors Associated with Incident Type 2 Diabetes: The Lifelines Cohort Study. Prev. Sci. 2022, 23, 1090–1100. [Google Scholar] [CrossRef]
- Barrera-Nunez, D.A.; Lopez-Olmedo, N.; Zavala-Arciniega, L.; Barrientos-Gutierrez, I.; Reynales-Shigematsu, L.M. Tobacco consumption and e-cigarette use in Mexican adolescents and adults. Ensanut Continua 2022. Salud Publica Mex. 2023, 65 (Suppl. S1), S65–S74. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Jauregui, A.; Hernandez, C.; Gonzalez, C.; Olvera, A.G.; Blas, N.; Campos, I.; Barquera, S. Prevalence of movement behaviors in Mexico. Salud Publica Mex. 2023, 65 (Suppl. S1), S259–S267. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, S.L. The Problem of Equivalent Structural Models. In Structural Equation Modeling. A Second Course; Hancock, H.R., Mueller, R.O., Eds.; Information Age Publishing: Greenwich, CT, USA, 2006; pp. 13–41. [Google Scholar]
| Variable | Total n = 1134 | Male n = 320 (28.0%) | Female n = 814 (72.0%) |
|---|---|---|---|
| Age, years 1 | 42.9 ± 11.6 | 42.4 ± 11.1 | 43.1 ± 11.8 |
| Education level | |||
| Elemental 2 | 253 (22.3) | 57 (17.8) | 196 (24.1) |
| Middle school 2 | 249 (22.0) | 80 (25.0) | 169 (20.8) |
| High school or higher 2 | 610 (53.8) | 178 (55.6) | 432 (53.1) |
| Missing 2 | 22 (1.9) | 5 (1.6) | 17 (2.0) |
| Glucose, mmol/L 3 | 4.9 [4.6, 5.3] | 5.2 [4.9, 5.6] | 4.9 [4.6, 5.2] |
| Insulin, UI/L 3 | 7.5 [3.2, 13.3] | 9.2 [4.0, 17.5] | 6.6 [2.8, 12.1] |
| HOMA 3 | 1.6 [0.7, 3.1] | 2.1 [0.9, 4.3] | 1.4 [0.6, 2.7] |
| IR-HOMA, ≥3.2 | 266 (23.5) | 115 (35.9) | 151 (18.6) |
| BMI 1, kg/m2 | 26.0 ± 4.0 | 26.5 ± 3.7 | 25.8 ± 4.1 |
| Overweight 2 | 460 (40.6) | 156 (48.8) | 304 (37.3) |
| Obesity 2 | 172 (15.2) | 56 (15.5) | 116 (14.3) |
| WC 1, cm | 89.2 ± 11.4 | 93.6 ± 9.0 | 87.5 ± 11.8 |
| Central obesity 2 | 412 (36.3) | 57 (17.8) | 355 (43.6) |
| BFP 1 | 39.6 [32.7, 44.7] | 30.6 [27.3, 34.5] | 42.1 [37.8, 46.3] |
| Excess 2 | 925 (81.6) | 260 (81.3) | 665 (81.7) |
| Physical activity 1, min/day | 24.9 ± 31.7 | 31.8 ± 36.3 | 22.2 ± 29.3 |
| Active 2, ≥30 min/day | 380 (33.5) | 132 (41.2) | 248 (30.5) |
| Tobacco consumption | |||
| Ex-smoker 2 | 291 (25.7) | 118 (36.9) | 173 (21.3) |
| Current smoker 2 | 214 (18.9) | 82 (25.6) | 132 (16.2) |
| Sleep duration 3 h/day | 7.3 [6.6, 8.0] | 7.1 [6.6, 8.6] | 7.3 [6.6, 8.0] |
| Nap duration, min/day | 7.5 [0.0, 32.1] | 7.5 [0.0, 34.3] | 7.5 [0.0, 32.1] |
| Yes 2 | 766 (67.5) | 234 (73.1) | 532 (65.4) |
| Depression score 3, CES-D | 9 [4, 16] | 8 [3, 14] | 10 [5, 18] |
| Depressive symptoms 2, ≥16 points | 308 (27.2) | 61 (19.1) | 247 (30.3) |
| Dietary inflammatory index (DII) 3 | −0.78 [−1.50, 0.31] | −0.72 [−1.35, 0.53] | −0.79 [−1.54, 0.21] |
| Adiposity2 | ln(HOMA2) | |||
|---|---|---|---|---|
| Estimate [SE] | p | Estimate [SE] | p | |
| Ex-smoker (ExS) | ||||
| Specific indirect effects | ||||
| ExS1 → ln(HOMA1) → Outcome2 | −0.058 [0.042] | 0.170 | 0.116 [0.057] | 0.043 |
| ExS1 → Adiposity1 → Outcome2 | 0.765 [0.445] | 0.085 | 0.039 [0.027] | 0.137 |
| Total indirect | 0.707 [0.431] | 0.101 | 0.155 [0.071] | 0.029 |
| Direct path | 0.134 [0.228] | 0.557 | −0.043 [0.106] | 0.684 |
| Total (Direct + Total Indirect) | 0.841 [0.477] | 0.078 | −0.041 [0.102] | 0.685 |
| Current smoker (CS) | ||||
| Specific indirect effects | ||||
| CS1 → ln(HOMA1) → Outcome2 | −0.048 [0.040] | 0.240 | 0.094 [0.062] | 0.131 |
| CS1 → Adiposity1 → Outcome2 | 1.089 [0.491] | 0.026 | 0.056 [0.031] | 0.074 |
| Total indirect | 1.042 [0.475] | 0.028 | 0.150 [0.079] | 0.056 |
| Direct path | 0.024 [0.253] | 0.925 | −0.131 [0.113] | 0.246 |
| Total (Direct + Total Indirect) | 1.066 [0.528] | 0.044 | 0.019 [0.136] | 0.888 |
| Physical activity (PA) | ||||
| Specific indirect effects | ||||
| PA1 → ln(HOMA1) → Outcome2 | 0.053 [0.038] | 0.161 | −0.106 [0.050] | 0.034 |
| PA1 → Adiposity1 → Outcome2 | −0.465 [0.384] | 0.225 | −0.024 [0.021] | 0.260 |
| Total indirect | −0.412 [0.372] | 0.268 | −0.130 [0.062] | 0.035 |
| Direct path | −0.150 [0.198] | 0.450 | −0.201 [0.089] | 0.023 |
| Total (Direct + Total Indirect) | −0.562 [0.413] | 0.174 | −0.331 [0.107] | 0.002 |
| Sleep time (ST) | ||||
| Specific indirect effects | ||||
| ST1 → ln(HOMA1) → Outcome2 | 0.004 [0.006] | 0.526 | −0.008 [0.011] | 0.500 |
| ST1 → Adiposity1 → Outcome2 | −0.235 [0.091] | 0.010 | −0.012 [0.006] | 0.048 |
| Total indirect | −0.231 [0.088] | 0.009 | −0.020 [0.015] | 0.179 |
| Direct path | −0.023 [0.047] | 0.623 | −0.005 [0.021] | 0.797 |
| Total (Direct + Total Indirect) | −0.254 [0.097] | 0.009 | −0.025 [0.025] | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses-León, J.; Quezada-Sánchez, A.D.; Rojas-Russel, M.; Aparicio-Bautista, D.I.; Velázquez-Cruz, R.; Aguilar-Salinas, C.A.; Salmerón, J.; Rivera-Paredez, B. Cross-Lagged Relationship Between Adiposity and HOMA and Mediating Role of Adiposity Between Lifestyle Factors and HOMA Among in Mexican Health Workers. Nutrients 2025, 17, 2497. https://doi.org/10.3390/nu17152497
Meneses-León J, Quezada-Sánchez AD, Rojas-Russel M, Aparicio-Bautista DI, Velázquez-Cruz R, Aguilar-Salinas CA, Salmerón J, Rivera-Paredez B. Cross-Lagged Relationship Between Adiposity and HOMA and Mediating Role of Adiposity Between Lifestyle Factors and HOMA Among in Mexican Health Workers. Nutrients. 2025; 17(15):2497. https://doi.org/10.3390/nu17152497
Chicago/Turabian StyleMeneses-León, Joacim, Amado D. Quezada-Sánchez, Mario Rojas-Russel, Diana I. Aparicio-Bautista, Rafael Velázquez-Cruz, Carlos A. Aguilar-Salinas, Jorge Salmerón, and Berenice Rivera-Paredez. 2025. "Cross-Lagged Relationship Between Adiposity and HOMA and Mediating Role of Adiposity Between Lifestyle Factors and HOMA Among in Mexican Health Workers" Nutrients 17, no. 15: 2497. https://doi.org/10.3390/nu17152497
APA StyleMeneses-León, J., Quezada-Sánchez, A. D., Rojas-Russel, M., Aparicio-Bautista, D. I., Velázquez-Cruz, R., Aguilar-Salinas, C. A., Salmerón, J., & Rivera-Paredez, B. (2025). Cross-Lagged Relationship Between Adiposity and HOMA and Mediating Role of Adiposity Between Lifestyle Factors and HOMA Among in Mexican Health Workers. Nutrients, 17(15), 2497. https://doi.org/10.3390/nu17152497







