Autism Spectrum Disorder: From Experimental Models to Probiotic Application with a Special Focus on Lactiplantibacillus plantarum
Abstract
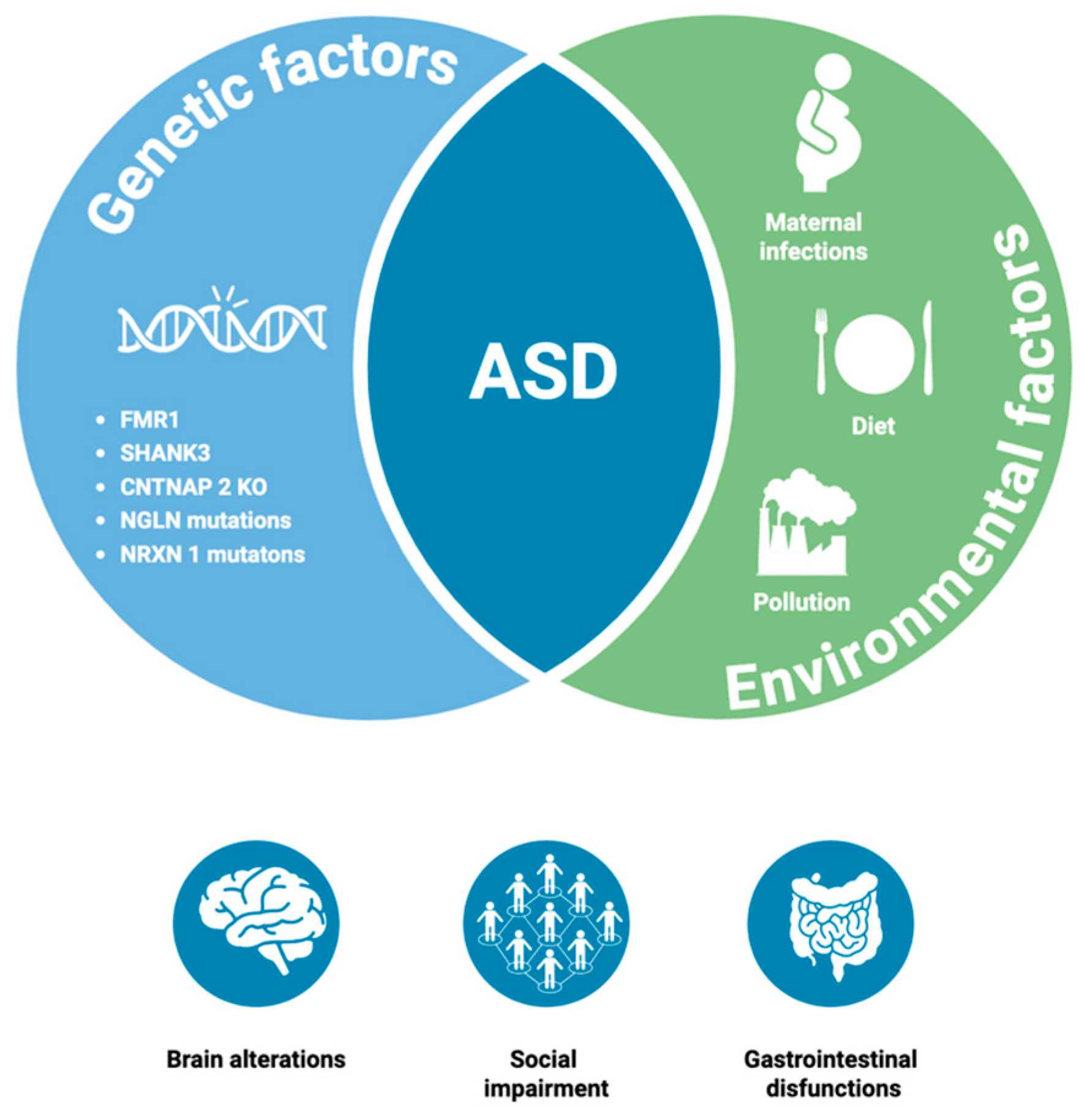
1. Introduction
2. Autism Spectrum Disorder
3. In Vivo Experimental Models of ASD
3.1. Genetics Models
3.2. Environmental Models
3.3. Idiopathic Models
4. Multifactorial Biomarkers in ASD
4.1. ECS and ASD
4.2. Intestinal Permeability and ASD
4.3. Inflammatory Markers in ASD
4.4. Intestinal Microbiota
5. Probiotics in ASD
Lpb. plantarum and Health Benefits in ASD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; De Giacomo, A.; Laguardia, M.; Schettini, F.; Francavilla, R.; Cristofori, F. Intestinal Barrier Dysfunction and Microbiota-Gut-Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder. Nutrients 2023, 15, 1620. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An International Classification of Diseases for the Twenty-First Century. BMC Med. Inform. Decis. Mak. 2021, 21, 206. [Google Scholar] [CrossRef]
- Bhat, A.N. Motor Impairment Increases in Children With Autism Spectrum Disorder as a Function of Social Communication, Cognitive and Functional Impairment, Repetitive Behavior Severity, and Comorbid Diagnoses: A SPARK Study Report. Autism Res. 2021, 14, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef] [PubMed]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef]
- Yang, T.; Chen, L.; Dai, Y.; Jia, F.; Hao, Y.; Li, L.; Zhang, J.; Wu, L.; Ke, X.; Yi, M.; et al. Vitamin A Status Is More Commonly Associated With Symptoms and Neurodevelopment in Boys With Autism Spectrum Disorders-A Multicenter Study in China. Front. Nutr. 2022, 9, 851980. [Google Scholar] [CrossRef]
- Issac, A.; Halemani, K.; Shetty, A.; Thimmappa, L.; Vijay, V.R.; Koni, K.; Mishra, P.; Kapoor, V. The Global Prevalence of Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. Osong Public Health Res. Perspect. 2025, 16, 3–27. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Fatta, L.M.; Micai, M.; Sali, M.E.; Bellomo, M.; Salvitti, T.; Fulceri, F.; Castellano, A.; Molteni, M.; Gambino, G.; et al. Autism Spectrum Disorder Prevalence in Italy: A Nationwide Study Promoted by the Ministry of Health. Child Adolesc. Psychiatry Ment. Health 2023, 17, 125. [Google Scholar] [CrossRef]
- Neul, J.L.; Sahin, M. Therapeutic Advances in Autism and Other Neurodevelopmental Disorders. Neurotherapeutics 2015, 12, 519–520. [Google Scholar] [CrossRef]
- Höfer, J.; Hoffmann, F.; Kamp-Becker, I.; Poustka, L.; Roessner, V.; Stroth, S.; Wolff, N.; Bachmann, C.J. Pathways to a Diagnosis of Autism Spectrum Disorder in Germany: A Survey of Parents. Child Adolesc. Psychiatry Ment. Health 2019, 13, 16. [Google Scholar] [CrossRef]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, J.M.; Moon, H.E.; Lee, D.S.; Kim, B.-N.; Kim, J.; Kim, D.G.; Paek, S.H. A Short Review on the Current Understanding of Autism Spectrum Disorders. Exp. Neurobiol. 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Diamanti, T.; Prete, R.; Battista, N.; Corsetti, A.; De Jaco, A. Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut-Brain Axis? Antibiotics 2022, 11, 1767. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes 2023, 14, 677. [Google Scholar] [CrossRef]
- Wintler, T.; Schoch, H.; Frank, M.G.; Peixoto, L. Sleep, Brain Development, and Autism Spectrum Disorders: Insights from Animal Models. J. Neurosci. Res. 2020, 98, 1137–1149. [Google Scholar] [CrossRef]
- Prete, R.; Merola, C.; Garcia-Gonzalez, N.; Fanti, F.; Angelozzi, G.; Sergi, M.; Battista, N.; Perugini, M.; Corsetti, A. Investigating the Modulation of the Endocannabinoid System by Probiotic Lactiplantibacillus Plantarum IMC513 in a Zebrafish Model of Di-n-Hexyl Phthalate Exposure. Sci. Rep. 2024, 14, 19328. [Google Scholar] [CrossRef]
- Dougnon, G.; Matsui, H. Modelling Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) Using Mice and Zebrafish. Int. J. Mol. Sci. 2022, 23, 7550. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Sousa, N.; Jacinto, L.; Monteiro, P. The Shank3-InsG3680(+/+) Mouse Model of Autism Spectrum Disorder Displays Auditory Avoidance in a Novel Behavioral Test. Front. Behav. Neurosci. 2023, 17, 1205507. [Google Scholar] [CrossRef]
- Grasselli, C.; Carbone, A.; Panelli, P.; Giambra, V.; Bossi, M.; Mazzoccoli, G.; De Filippis, L. Neural Stem Cells from Shank3-Ko Mouse Model Autism Spectrum Disorders. Mol. Neurobiol. 2020, 57, 1502–1515. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Su, F.; Wang, Y.; Liu, M.; Wang, H.; Cui, Y.; Yuan, P.; Li, X.; Li, A.; et al. Restoration of FMRP Expression in Adult V1 Neurons Rescues Visual Deficits in a Mouse Model of Fragile X Syndrome. Protein Cell 2022, 13, 203–219. [Google Scholar] [CrossRef]
- Park, G.; Jeon, S.J.; Ko, I.O.; Park, J.H.; Lee, K.C.; Kim, M.-S.; Shin, C.Y.; Kim, H.; Lee, Y.-S. Decreased in Vivo Glutamate/GABA Ratio Correlates with the Social Behavior Deficit in a Mouse Model of Autism Spectrum Disorder. Mol. Brain 2022, 15, 19. [Google Scholar] [CrossRef]
- Haddad, F.L.; De Oliveira, C.; Schmid, S. Investigating Behavioral Phenotypes Related to Autism Spectrum Disorder in a Gene-Environment Interaction Model of Cntnap2 Deficiency and Poly I:C Maternal Immune Activation. Front. Neurosci. 2023, 17, 1160243. [Google Scholar] [CrossRef]
- Terashima, H.; Minatohara, K.; Maruoka, H.; Okabe, S. Imaging Neural Circuit Pathology of Autism Spectrum Disorders: Autism-Associated Genes, Animal Models and the Application of in Vivo Two-Photon Imaging. Microscopy 2022, 71, i81–i99. [Google Scholar] [CrossRef]
- Wang, L.; Mirabella, V.R.; Dai, R.; Su, X.; Xu, R.; Jadali, A.; Bernabucci, M.; Singh, I.; Chen, Y.; Tian, J.; et al. Analyses of the Autism-Associated Neuroligin-3 R451C Mutation in Human Neurons Reveal a Gain-of-Function Synaptic Mechanism. Mol. Psychiatry 2024, 29, 1620–1635. [Google Scholar] [CrossRef]
- Dachtler, J.; Ivorra, J.L.; Rowland, T.E.; Lever, C.; Rodgers, R.J.; Clapcote, S.J. Heterozygous Deletion of α-Neurexin I or α-Neurexin II Results in Behaviors Relevant to Autism and Schizophrenia. Behav. Neurosci. 2015, 129, 765–776. [Google Scholar] [CrossRef]
- Tasnim, A.; Alkislar, I.; Hakim, R.; Turecek, J.; Abdelaziz, A.; Orefice, L.L.; Ginty, D.D. The Developmental Timing of Spinal Touch Processing Alterations Predicts Behavioral Changes in Genetic Mouse Models of Autism Spectrum Disorders. Nat. Neurosci. 2024, 27, 484–496. [Google Scholar] [CrossRef]
- Platt, R.J.; Zhou, Y.; Slaymaker, I.M.; Shetty, A.S.; Weisbach, N.R.; Kim, J.-A.; Sharma, J.; Desai, M.; Sood, S.; Kempton, H.R.; et al. Chd8 Mutation Leads to Autistic-like Behaviors and Impaired Striatal Circuits. Cell Rep. 2017, 19, 335–350. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, B.; Ji, P.; Zuo, Z.; Huang, Y.; Wang, N.; Liu, C.; Liu, S.-J.; Zhao, F. Changes to Gut Amino Acid Transporters and Microbiome Associated with Increased E/I Ratio in Chd8+/− Mouse Model of ASD-like Behavior. Nat. Commun. 2022, 13, 1151. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Page, D.T. Decreased Aggression and Increased Repetitive Behavior in Pten Haploinsufficient Mice. Genes Brain Behav. 2015, 14, 145–157. [Google Scholar] [CrossRef]
- Lawson, R.J.; Lipovsek, N.J.; Brown, S.P.; Jena, A.K.; Osko, J.J.; Ransdell, J.L. Selective Deletion of Tsc1 from Mouse Cerebellar Purkinje Neurons Drives Sex-Specific Behavioral Impairments Linked to Autism. Front. Behav. Neurosci. 2024, 18, 1474066. [Google Scholar] [CrossRef] [PubMed]
- Gardner, Z.; Holbrook, O.; Tian, Y.; Odamah, K.; Man, H.-Y. The Role of Glia in the Dysregulation of Neuronal Spinogenesis in Ube3a-Dependent ASD. Exp. Neurol. 2024, 376, 114756. [Google Scholar] [CrossRef]
- Hsu, T.-T.; Huang, T.-N.; Wang, C.-Y.; Hsueh, Y.-P. Deep Brain Stimulation of the Tbr1-Deficient Mouse Model of Autism Spectrum Disorder at the Basolateral Amygdala Alters Amygdalar Connectivity, Whole-Brain Synchronization, and Social Behaviors. PLoS Biol. 2024, 22, e3002646. [Google Scholar] [CrossRef]
- Cheroni, C.; Caporale, N.; Testa, G. Autism Spectrum Disorder at the Crossroad between Genes and Environment: Contributions, Convergences, and Interactions in ASD Developmental Pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef]
- Zarate-Lopez, D.; Torres-Chávez, A.L.; Gálvez-Contreras, A.Y.; Gonzalez-Perez, O. Three Decades of Valproate: A Current Model for Studying Autism Spectrum Disorder. Curr. Neuropharmacol. 2024, 22, 260–289. [Google Scholar] [CrossRef]
- Liu, F.; Horton-Sparks, K.; Hull, V.; Li, R.W.; Martínez-Cerdeño, V. The Valproic Acid Rat Model of Autism Presents with Gut Bacterial Dysbiosis Similar to That in Human Autism. Mol. Autism 2018, 9, 61. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.G.; Bastiaanssen, T.F.S.; Hoffmann Sarda, F.A.; Lynch, C.M.K.; Ventura-Silva, A.P.; Rosell-Cardona, C.; Caputi, V.; Stanton, C.; Fülling, C.; et al. Maternal High-Fat Diet-Induced Microbiota Changes Are Associated with Alterations in Embryonic Brain Metabolites and Adolescent Behaviour. Brain Behav. Immun. 2024, 121, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, S.; Li, M.; Yang, Y.; Ma, J.; Xie, R.; Wang, J.; Zhao, Q.; Qin, S.; He, L.; et al. Maternal High-Fat Diet Disrupts Intestinal Mucus Barrier of Offspring by Regulating Gut Immune Receptor LRRC19. Commun. Biol. 2025, 8, 420. [Google Scholar] [CrossRef]
- Sarker, G.; Peleg-Raibstein, D. Maternal Overnutrition Induces Long-Term Cognitive Deficits across Several Generations. Nutrients 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wei, H.; Chen, J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems 2021, 6, e01343-20. [Google Scholar] [CrossRef]
- Haddad, F.L.; Patel, S.V.; Schmid, S. Maternal Immune Activation by Poly I:C as a Preclinical Model for Neurodevelopmental Disorders: A Focus on Autism and Schizophrenia. Neurosci. Biobehav. Rev. 2020, 113, 546–567. [Google Scholar] [CrossRef]
- Butera, A.; De Simone, R.; Potenza, R.L.; Sanchez, M.; Armida, M.; Campanile, D.; Di Carlo, N.; Trenta, F.; Boirivant, M.; Ricceri, L. Effects of a Gut-Selective Integrin-Targeted Therapy in Male Mice Exposed to Early Immune Activation, a Model for the Study of Autism Spectrum Disorder. Brain Behav. Immun. 2024, 115, 89–100. [Google Scholar] [CrossRef]
- Patel, S.; Dale, R.C.; Rose, D.; Heath, B.; Nordahl, C.W.; Rogers, S.; Guastella, A.J.; Ashwood, P. Maternal Immune Conditions Are Increased in Males with Autism Spectrum Disorders and Are Associated with Behavioural and Emotional but Not Cognitive Co-Morbidity. Transl. Psychiatry 2020, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Salia, S.; Burke, F.F.; Hinks, M.E.; Randell, A.M.; Matheson, M.A.; Walling, S.G.; Swift-Gallant, A. Gut Microbiota Transfer from the Preclinical Maternal Immune Activation Model of Autism Is Sufficient to Induce Sex-Specific Alterations in Immune Response and Behavioural Outcomes. Brain Behav. Immun. 2025, 123, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chadman, K.K.; McCloskey, D.P.; Sheikh, A.M.; Malik, M.; Brown, W.T.; Li, X. Brain IL-6 Elevation Causes Neuronal Circuitry Imbalances and Mediates Autism-like Behaviors. Biochim. Biophys. Acta 2012, 1822, 831–842. [Google Scholar] [CrossRef]
- Arzuaga, A.L.; Teneqexhi, P.; Amodeo, K.; Larson, J.R.; Ragozzino, M.E. Prenatal Stress and Fluoxetine Exposure in BTBR and B6 Mice Differentially Affects Autism-like Behaviors in Adult Male and Female Offspring. Physiol. Behav. 2025, 295, 114891. [Google Scholar] [CrossRef]
- Rowshan, N.; Anjomshoa, M.; Farahzad, A.; Bijad, E.; Amini-Khoei, H. Gut-Brain Barrier Dysfunction Bridge Autistic-like Behavior in Mouse Model of Maternal Separation Stress: A Behavioral, Histopathological, and Molecular Study. Int. J. Dev. Neurosci. 2024, 84, 314–327. [Google Scholar] [CrossRef]
- Dunn, J.T.; Guidotti, A.; Grayson, D.R. Behavioral and Molecular Characterization of Prenatal Stress Effects on the C57BL/6J Genetic Background for the Study of Autism Spectrum Disorder. eNeuro 2024, 11, ENEURO.0186-23.2024. [Google Scholar] [CrossRef]
- Pagani, M.; Damiano, M.; Galbusera, A.; Tsaftaris, S.A.; Gozzi, A. Semi-Automated Registration-Based Anatomical Labelling, Voxel Based Morphometry and Cortical Thickness Mapping of the Mouse Brain. J. Neurosci. Methods 2016, 267, 62–73. [Google Scholar] [CrossRef]
- Chhabra, S.; Nardi, L.; Leukel, P.; Sommer, C.J.; Schmeisser, M.J. Striatal Increase of Dopamine Receptor 2 Density in Idiopathic and Syndromic Mouse Models of Autism Spectrum Disorder. Front. Psychiatry 2023, 14, 1110525. [Google Scholar] [CrossRef]
- Gouda, B.; Sinha, S.N.; Chalamaiah, M.; Vakdevi, V.; Shashikala, P.; Veeresh, B.; Surekha, V.M.; Kasturi, V.; Boiroju, N.K. Sex Differences in Animal Models of Sodium-Valproate-Induced Autism in Postnatal BALB/c Mice: Whole-Brain Histoarchitecture and 5-HT2A Receptor Biomarker Evidence. Biology 2022, 11, 79. [Google Scholar] [CrossRef]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism Spectrum Disorder: Neuropathology and Animal Models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of Microbiome and Probiotic Treatment in a Genetic Model of Autism Spectrum Disorders. Brain Behav. Immun. 2018, 73, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ure, K.; Lu, H.; Wang, W.; Ito-Ishida, A.; Wu, Z.; He, L.-J.; Sztainberg, Y.; Chen, W.; Tang, J.; Zoghbi, H.Y. Restoration of Mecp2 Expression in GABAergic Neurons Is Sufficient to Rescue Multiple Disease Features in a Mouse Model of Rett Syndrome. Elife 2016, 5, e14198. [Google Scholar] [CrossRef] [PubMed]
- Allemang-Grand, R.; Ellegood, J.; Spencer Noakes, L.; Ruston, J.; Justice, M.; Nieman, B.J.; Lerch, J.P. Neuroanatomy in Mouse Models of Rett Syndrome Is Related to the Severity of Mecp2 Mutation and Behavioral Phenotypes. Mol. Autism 2017, 8, 32. [Google Scholar] [CrossRef]
- Altimiras, F.; Garcia, J.A.; Palacios-García, I.; Hurley, M.J.; Deacon, R.; González, B.; Cogram, P. Altered Gut Microbiota in a Fragile X Syndrome Mouse Model. Front. Neurosci. 2021, 15, 653120. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yang, X.; Guo, X.; Yang, H.; Pan, J.; Li, Y. Dietary Fish Oil Improves Autistic Behaviors and Gut Homeostasis by Altering the Gut Microbial Composition in a Mouse Model of Fragile X Syndrome. Brain Behav. Immun. 2023, 110, 140–151. [Google Scholar] [CrossRef]
- Gandhi, T.; Canepa, C.R.; Adeyelu, T.T.; Adeniyi, P.A.; Lee, C.C. Neuroanatomical Alterations in the CNTNAP2 Mouse Model of Autism Spectrum Disorder. Brain Sci. 2023, 13, 891. [Google Scholar] [CrossRef]
- Camasio, A.; Panzeri, E.; Mancuso, L.; Costa, T.; Manuello, J.; Ferraro, M.; Duca, S.; Cauda, F.; Liloia, D. Linking Neuroanatomical Abnormalities in Autism Spectrum Disorder with Gene Expression of Candidate ASD Genes: A Meta-Analytic and Network-Oriented Approach. PLoS ONE 2022, 17, e0277466. [Google Scholar] [CrossRef]
- Jang, W.E.; Park, J.H.; Park, G.; Bang, G.; Na, C.H.; Kim, J.Y.; Kim, K.-Y.; Kim, K.P.; Shin, C.Y.; An, J.-Y.; et al. Cntnap2-Dependent Molecular Networks in Autism Spectrum Disorder Revealed through an Integrative Multi-Omics Analysis. Mol. Psychiatry 2023, 28, 810–821. [Google Scholar] [CrossRef]
- Robinson, B.G.; Oster, B.A.; Robertson, K.; Kaltschmidt, J.A. Loss of ASD-Related Molecule Cntnap2 Affects Colonic Motility in Mice. Front. Neurosci. 2023, 17, 1287057. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of Published Genome-Wide Association Studies, Targeted Arrays and Summary Statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Norris, R.H.; Churilov, L.; Hannan, A.J.; Nithianantharajah, J. Mutations in Neuroligin-3 in Male Mice Impact Behavioral Flexibility but Not Relational Memory in a Touchscreen Test of Visual Transitive Inference. Mol. Autism 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Moore, Z.; Walker, F.R.; Hill-Yardin, E.L.; Crack, P.J. An Altered Glial Phenotype in the NL3R451C Mouse Model of Autism. Sci. Rep. 2020, 10, 14492. [Google Scholar] [CrossRef] [PubMed]
- Hosie, S.; Abo-Shaban, T.; Mou, K.; Balasuriya, G.K.; Mohsenipour, M.; Alamoudi, M.U.; Filippone, R.T.; Belz, G.T.; Franks, A.E.; Bornstein, J.C.; et al. Faster Gastrointestinal Transit, Reduced Small Intestinal Smooth Muscle Tone and Dysmotility in the Nlgn3R451C Mouse Model of Autism. Int. J. Mol. Sci. 2024, 25, 832. [Google Scholar] [CrossRef] [PubMed]
- Sharna, S.S.; Balasuriya, G.K.; Hosie, S.; Nithianantharajah, J.; Franks, A.E.; Hill-Yardin, E.L. Altered Caecal Neuroimmune Interactions in the Neuroligin-3R451C Mouse Model of Autism. Front. Cell. Neurosci. 2020, 14, 85. [Google Scholar] [CrossRef]
- Herath, M.; Bornstein, J.C.; Hill-Yardin, E.L.; Franks, A.E. The Autism-Associated Neuroligin-3 R451C Mutation Alters Mucus Density and the Spatial Distribution of Bacteria in the Mouse Gastrointestinal Tract. BioRxiv 2022. [Google Scholar] [CrossRef]
- Kawamura, A.; Abe, Y.; Seki, F.; Katayama, Y.; Nishiyama, M.; Takata, N.; Tanaka, K.F.; Okano, H.; Nakayama, K.I. Chd8 Mutation in Oligodendrocytes Alters Microstructure and Functional Connectivity in the Mouse Brain. Mol. Brain 2020, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Jayusman, P.A.; Shuid, N.; Ismail, J.; Kamal Nor, N.; Naina Mohamed, I. Update on Atypicalities of Central Nervous System in Autism Spectrum Disorder. Brain Sci. 2020, 10, 309. [Google Scholar] [CrossRef]
- Zapata-Muñoz, J.; Villarejo-Zori, B.; Largo-Barrientos, P.; Boya, P. Towards a Better Understanding of the Neuro-Developmental Role of Autophagy in Sickness and in Health. Cell Stress 2021, 5, 99–118. [Google Scholar] [CrossRef]
- Vatsa, N.; Jana, N.R. UBE3A and Its Link With Autism. Front. Mol. Neurosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Tian, Y.; Qiao, H.; Odamah, K.; Zhu, L.-Q.; Man, H.-Y. Role of Androgen Receptors in Sexually Dimorphic Phenotypes in UBE3A-Dependent Autism Spectrum Disorder. iScience 2025, 28, 111868. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Huang, T.-N.; Hsueh, Y.-P. T-Brain-1—A Potential Master Regulator in Autism Spectrum Disorders. Autism Res. 2015, 8, 412–426. [Google Scholar] [CrossRef]
- Tartaglione, A.M.; Villani, A.; Ajmone-Cat, M.A.; Minghetti, L.; Ricceri, L.; Pazienza, V.; De Simone, R.; Calamandrei, G. Maternal Immune Activation Induces Autism-like Changes in Behavior, Neuroinflammatory Profile and Gut Microbiota in Mouse Offspring of Both Sexes. Transl. Psychiatry 2022, 12, 384. [Google Scholar] [CrossRef]
- Lammert, C.R.; Frost, E.L.; Bolte, A.C.; Paysour, M.J.; Shaw, M.E.; Bellinger, C.E.; Weigel, T.K.; Zunder, E.R.; Lukens, J.R. Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. J. Immunol. 2018, 201, 845–850. [Google Scholar] [CrossRef]
- Libero, L.E.; DeRamus, T.P.; Lahti, A.C.; Deshpande, G.; Kana, R.K. Multimodal Neuroimaging Based Classification of Autism Spectrum Disorder Using Anatomical, Neurochemical, and White Matter Correlates. Cortex 2015, 66, 46–59. [Google Scholar] [CrossRef]
- Cartocci, V.; Catallo, M.; Tempestilli, M.; Segatto, M.; Pfrieger, F.W.; Bronzuoli, M.R.; Scuderi, C.; Servadio, M.; Trezza, V.; Pallottini, V. Altered Brain Cholesterol/Isoprenoid Metabolism in a Rat Model of Autism Spectrum Disorders. Neuroscience 2018, 372, 27–37. [Google Scholar] [CrossRef]
- Graciarena, M.; Seiffe, A.; Nait-Oumesmar, B.; Depino, A.M. Hypomyelination and Oligodendroglial Alterations in a Mouse Model of Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 517. [Google Scholar] [CrossRef]
- Shah, J.; Deas, S.B.; Ren, C.; Jilling, T.; Brawner, K.M.; Martin, C.A. The Effects of Gestational Psychological Stress on Neonatal Mouse Intestinal Development. J. Surg. Res. 2019, 235, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Basic, M.; Bleich, A. Gnotobiotics: Past, Present and Future. Lab. Anim. 2019, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Paz, H.A.; Buddha, L.; Zhong, Y.; Sikes, J.D.; Wankhade, U.D. Impact of Maternal High-Fat Diet on Offspring Gut Microbiota during Short-Term High-Fat Diet Exposure in Mice. Physiol. Rep. 2024, 12, e70111. [Google Scholar] [CrossRef]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered Intestinal Morphology and Microbiota Composition in the Autism Spectrum Disorders Associated SHANK3 Mouse Model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef]
- Margolis, K.G.; Li, Z.; Stevanovic, K.; Saurman, V.; Israelyan, N.; Anderson, G.M.; Snyder, I.; Veenstra-VanderWeele, J.; Blakely, R.D.; Gershon, M.D. Serotonin Transporter Variant Drives Preventable Gastrointestinal Abnormalities in Development and Function. J. Clin. Investig. 2016, 126, 2221–2235. [Google Scholar] [CrossRef]
- Yu, W.; Yen, Y.-C.; Lee, Y.-H.; Tan, S.; Xiao, Y.; Lokman, H.; Ting, A.K.T.; Ganegala, H.; Kwon, T.; Ho, W.-K.; et al. Prenatal Selective Serotonin Reuptake Inhibitor (SSRI) Exposure Induces Working Memory and Social Recognition Deficits by Disrupting Inhibitory Synaptic Networks in Male Mice. Mol. Brain 2019, 12, 29. [Google Scholar] [CrossRef]
- Lyte, M.; Daniels, K.M.; Schmitz-Esser, S. Fluoxetine-Induced Alteration of Murine Gut Microbial Community Structure: Evidence for a Microbial Endocrinology-Based Mechanism of Action Responsible for Fluoxetine-Induced Side Effects. PeerJ 2019, 7, e6199. [Google Scholar] [CrossRef]
- Lee, Y.-A.; Obora, T.; Bondonny, L.; Toniolo, A.; Mivielle, J.; Yamaguchi, Y.; Kato, A.; Takita, M.; Goto, Y. The Effects of Housing Density on Social Interactions and Their Correlations with Serotonin in Rodents and Primates. Sci. Rep. 2018, 8, 3497. [Google Scholar] [CrossRef] [PubMed]
- Heun-Johnson, H.; Levitt, P. Differential Impact of Met Receptor Gene Interaction with Early-Life Stress on Neuronal Morphology and Behavior in Mice. Neurobiol. Stress. 2018, 8, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-F.; Huang, W.-C.; Wu, C.W.; Huang, C.-Y.; Yang, Y.-C.S.H.; Tung, Y.-T. Acute Sleep Deprivation Exacerbates Systemic Inflammation and Psychiatry Disorders through Gut Microbiota Dysbiosis and Disruption of Circadian Rhythms. Microbiol. Res. 2023, 268, 127292. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, S.M.; Gagnidze, K.; Reyes, A.; Norstedt, N.; Månsson, K.; Francis, K.; Pfaff, D.W. Sex-Specific Gene-Environment Interactions Underlying ASD-like Behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, 1383–1388. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, F.; Song, D.; He, Z.; Zuo, Z.; Yang, Y.; Liu, Q.; Hu, S.; Wang, X.; Zheng, X.; et al. Prenatal Influenza Vaccination Rescues Impairments of Social Behavior and Lamination in a Mouse Model of Autism. J. Neuroinflamm. 2018, 15, 228. [Google Scholar] [CrossRef]
- Li, F.; Ke, H.; Wang, S.; Mao, W.; Fu, C.; Chen, X.; Fu, Q.; Qin, X.; Huang, Y.; Li, B.; et al. Leaky Gut Plays a Critical Role in the Pathophysiology of Autism in Mice by Activating the Lipopolysaccharide-Mediated Toll-Like Receptor 4-Myeloid Differentiation Factor 88-Nuclear Factor Kappa B Signaling Pathway. Neurosci. Bull. 2023, 39, 911–928. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, R.; Li, L.; Jin, G.; Zhou, B.; Huang, H.; Li, M.; Yang, Y.; Liu, X.; Cao, X.; et al. Prenatal Maternal Stress Exacerbates Experimental Colitis of Offspring in Adulthood. Front. Immunol. 2021, 12, 700995. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.L.; Palkar, A.V.; Rajasekera, T.; Allen, J.; Niraula, A.; Godbout, J.; Bailey, M.T. Prenatal Stress Disrupts Social Behavior, Cortical Neurobiology and Commensal Microbes in Adult Male Offspring. Behav. Brain Res. 2019, 359, 886–894. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Wöhr, M.; Crusio, W.E.; Lemaire, V.; Pietropaolo, S. Autistic-like Behavioral Effects of Prenatal Stress in Juvenile Fmr1 Mice: The Relevance of Sex Differences and Gene-Environment Interactions. Sci. Rep. 2022, 12, 7269. [Google Scholar] [CrossRef]
- Evans, M.M.; Hing, B.W.Q.; Weber, M.A.; Maurer, S.V.; Baig, A.I.; Kim, G.S.; Anema, S.L.; Ellerbroek, R.M.; Sivakumar, K.; Michaelson, J.J.; et al. Long-Term, Cell Type-Specific Effects of Prenatal Stress on Dorsal Striatum and Relevant Behaviors in Mice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.-X.; Gu, L.-J.; Cheng, Y. Understanding Autism Spectrum Disorders with Animal Models: Applications, Insights, and Perspectives. Zool. Res. 2021, 42, 800–824. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Blanchard, D.C. The BTBR Mouse Model of Idiopathic Autism—Current View on Mechanisms. Neurosci. Biobehav. Rev. 2017, 76, 99–110. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic Diet Modifies the Gut Microbiota in a Murine Model of Autism Spectrum Disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Cavone, V.; Tinacci, S.; Concas, I.; Petralia, A.; Signorelli, M.S.; Díaz-Caneja, C.M.; Aguglia, E. Cannabinoids for People with ASD: A Systematic Review of Published and Ongoing Studies. Brain Sci. 2020, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, S.; Manduca, A.; Carbone, E.; Buzzelli, V.; Rava, A.; Feo, A.; Ascone, F.; Morena, M.; Campolongo, P.; Hill, M.N.; et al. Anandamide and 2-Arachidonoylglycerol Differentially Modulate Autistic-like Traits in a Genetic Model of Autism Based on FMR1 Deletion in Rats. Neuropsychopharmacology 2023, 48, 897–907. [Google Scholar] [CrossRef]
- Zamberletti, E.; Rubino, T.; Parolaro, D. Therapeutic Potential of Cannabidivarin for Epilepsy and Autism Spectrum Disorder. Pharmacol. Ther. 2021, 226, 107878. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Marsicano, G. The Role of the Endocannabinoid System as a Therapeutic Target for Autism Spectrum Disorder: Lessons from Behavioral Studies on Mouse Models. Neurosci. Biobehav. Rev. 2022, 132, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Dinh, D.; Lee, D.; Li, D.; Anguren, A.; Moreno-Sanz, G.; Gall, C.M.; Piomelli, D. Enhancement of Anandamide-Mediated Endocannabinoid Signaling Corrects Autism-Related Social Impairment. Cannabis Cannabinoid Res. 2016, 1, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Scheggi, S.; Guzzi, F.; Braccagni, G.; De Montis, M.G.; Parenti, M.; Gambarana, C. Targeting PPARα in the Rat Valproic Acid Model of Autism: Focus on Social Motivational Impairment and Sex-Related Differences. Mol. Autism 2020, 11, 62. [Google Scholar] [CrossRef]
- Siniscalco, D.; Sapone, A.; Giordano, C.; Cirillo, A.; de Magistris, L.; Rossi, F.; Fasano, A.; Bradstreet, J.J.; Maione, S.; Antonucci, N. Cannabinoid Receptor Type 2, but Not Type 1, Is up-Regulated in Peripheral Blood Mononuclear Cells of Children Affected by Autistic Disorders. J. Autism Dev. Disord. 2013, 43, 2686–2695. [Google Scholar] [CrossRef]
- Aran, A.; Eylon, M.; Harel, M.; Polianski, L.; Nemirovski, A.; Tepper, S.; Schnapp, A.; Cassuto, H.; Wattad, N.; Tam, J. Lower Circulating Endocannabinoid Levels in Children with Autism Spectrum Disorder. Mol. Autism 2019, 10, 2. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Boubertakh, B.; Leblanc, N.; Deschênes, T.; Lacroix, S.; Martin, C.; Houde, A.; Veilleux, A.; Flamand, N.; Muccioli, G.G.; et al. Germ-Free Mice Exhibit Profound Gut Microbiota-Dependent Alterations of Intestinal Endocannabinoidome Signaling. J. Lipid Res. 2020, 61, 70–85. [Google Scholar] [CrossRef]
- Suriano, F.; Manca, C.; Flamand, N.; Depommier, C.; Van Hul, M.; Delzenne, N.M.; Silvestri, C.; Cani, P.D.; Di Marzo, V. Exploring the Endocannabinoidome in Genetically Obese (Ob/Ob) and Diabetic (Db/Db) Mice: Links with Inflammation and Gut Microbiota. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159056. [Google Scholar] [CrossRef]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of Gut Microbiota on Depressive-like Behaviors in Mice Is Mediated by the Endocannabinoid System. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Graziani, C.; Talocco, C.; De Sire, R.; Petito, V.; Lopetuso, L.R.; Gervasoni, J.; Persichilli, S.; Franceschi, F.; Ojetti, V.; Gasbarrini, A.; et al. Intestinal Permeability in Physiological and Pathological Conditions: Major Determinants and Assessment Modalities. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 795–810. [Google Scholar] [CrossRef]
- Camilleri, M.; Vella, A. What to Do about the Leaky Gut. Gut 2022, 71, 424–435. [Google Scholar] [CrossRef]
- Teskey, G.; Anagnostou, E.; Mankad, D.; Smile, S.; Roberts, W.; Brian, J.; Bowdish, D.M.E.; Foster, J.A. Intestinal Permeability Correlates with Behavioural Severity in Very Young Children with ASD: A Preliminary Study. J. Neuroimmunol. 2021, 357, 577607. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-García, K.A.; Anaya-Loyola, M.A.; Ronquillo, D.; Caamaño, M.d.C.; Masuoka, D.; Palacios-Delgado, J.; Rosado, J.L. Nutritional Status, Diet, and Intestinal Permeability of Mexican Children with Autism Spectrum Disorders. Gastroenterol. Insights 2024, 15, 912–925. [Google Scholar] [CrossRef]
- Kara, H.; Burak Açıkel, S.; Çetinkaya, M.; Çiğdem Tuncer, S. Serum Zonulin Levels Are Higher Among Children with Autism Spectrum Disorders and Correlated with Social Impairment. Alpha Psychiatry 2021, 22, 250–256. [Google Scholar] [CrossRef]
- De Sales-Millán, A.; Aguirre-Garrido, J.F.; González-Cervantes, R.M.; Velázquez-Aragón, J.A. Microbiome-Gut-Mucosal-Immune-Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology. Behav. Sci. 2023, 13, 548. [Google Scholar] [CrossRef]
- de Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the Intestinal Barrier in Patients with Autism Spectrum Disorders and in Their First-Degree Relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.; Perman, J.A. Autism and Gastrointestinal Symptoms. Curr. Gastroenterol. Rep. 2002, 4, 251–258. [Google Scholar] [CrossRef]
- Piras, C.; Mussap, M.; Noto, A.; De Giacomo, A.; Cristofori, F.; Spada, M.; Fanos, V.; Atzori, L.; Francavilla, R. Alterations of the Intestinal Permeability Are Reflected by Changes in the Urine Metabolome of Young Autistic Children: Preliminary Results. Metabolites 2022, 12, 104. [Google Scholar] [CrossRef]
- Liu, S.; Xi, H.; Xue, X.; Sun, X.; Huang, H.; Fu, D.; Mi, Y.; He, Y.; Yang, P.; Tang, Y.; et al. Clostridium Butyricum Regulates Intestinal Barrier Function via Trek1 to Improve Behavioral Abnormalities in Mice with Autism Spectrum Disorder. Cell Biosci. 2024, 14, 95. [Google Scholar] [CrossRef]
- Bilgiç, A.; Ferahkaya, H.; Karagöz, H.; Kılınç, İ.; Energin, V.M. Serum Claudin-5, Claudin-11, Occludin, Vinculin, Paxillin, and Beta-Catenin Levels in Preschool Children with Autism Spectrum Disorder. Nord. J. Psychiatry 2023, 77, 506–511. [Google Scholar] [CrossRef]
- Esnafoglu, E.; Cırrık, S.; Ayyıldız, S.N.; Erdil, A.; Ertürk, E.Y.; Daglı, A.; Noyan, T. Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J. Pediatr. 2017, 188, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular Permeability and Tight Junction Regulation in Gut Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.d.L.A.; Noris García, E.; Fraga Guerra, J.; Vega Hurtado, Y.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- Erbescu, A.; Papuc, S.M.; Budisteanu, M.; Arghir, A.; Neagu, M. Re-Emerging Concepts of Immune Dysregulation in Autism Spectrum Disorders. Front. Psychiatry 2022, 13, 1006612. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Shi, L.; Liu, M.; Wu, R.; Xia, K.; Zhang, F.; Ou, J.; Zhao, J. Autism Spectrum Disorder and Severe Social Impairment Associated with Elevated Plasma Interleukin-8. Pediatr. Res. 2021, 89, 591–597. [Google Scholar] [CrossRef]
- Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Bujnakova, I.; Kovacova, V.; Macejova, A.; Tonhajzerova, I. Peripheral Inflammatory Markers in Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder at Adolescent Age. Int. J. Mol. Sci. 2023, 24, 11710. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Glozier, N.; Dale, R.; Guastella, A.J. The Immune System, Cytokines, and Biomarkers in Autism Spectrum Disorder. Neurosci. Bull. 2017, 33, 194–204. [Google Scholar] [CrossRef]
- Noori, A.S.; Rajabi, P.; Sargolzaei, J.; Alaghmand, A. Correlation of Biochemical Markers and Inflammatory Cytokines in Autism Spectrum Disorder (ASD). BMC Pediatr. 2024, 24, 696. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Sarawi, W.; Attia, S.M.; Bakheet, S.A.; Alqarni, S.A.; Ali, N.; AsSobeai, H.M. Imbalance in Pro-Inflammatory and Anti-Inflammatory Cytokines Milieu in B Cells of Children with Autism. Mol. Immunol. 2022, 141, 297–304. [Google Scholar] [CrossRef]
- Allan, N.P.; Yamamoto, B.Y.; Kunihiro, B.P.; Nunokawa, C.K.L.; Rubas, N.C.; Wells, R.K.; Umeda, L.; Phankitnirundorn, K.; Torres, A.; Peres, R.; et al. Ketogenic Diet Induced Shifts in the Gut Microbiome Associate with Changes to Inflammatory Cytokines and Brain-Related miRNAs in Children with Autism Spectrum Disorder. Nutrients 2024, 16, 1401. [Google Scholar] [CrossRef]
- Bao, X.-H.; Chen, B.-F.; Liu, J.; Tan, Y.-H.; Chen, S.; Zhang, F.; Lu, H.-S.; Li, J.-C. Olink Proteomics Profiling Platform Reveals Non-Invasive Inflammatory Related Protein Biomarkers in Autism Spectrum Disorder. Front. Mol. Neurosci. 2023, 16, 1185021. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Bojović, K.; Ignjatović, Ð.-D.I.; Soković Bajić, S.; Vojnović Milutinović, D.; Tomić, M.; Golić, N.; Tolinački, M. Gut Microbiota Dysbiosis Associated With Altered Production of Short Chain Fatty Acids in Children With Neurodevelopmental Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 223. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the Gut-Brain-Axis: Implications for New Therapeutic Design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Luna, R.A.; Savidge, T.C.; Williams, K.C. The Brain-Gut-Microbiome Axis: What Role Does It Play in Autism Spectrum Disorder? Curr. Dev. Disord. Rep. 2016, 3, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liong, M.T.; Chung, Y.-C.E.; Huang, H.-Y.; Peng, W.-S.; Cheng, Y.-F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of Lactobacillus Plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low Relative Abundances of the Mucolytic Bacterium Akkermansia Muciniphila and Bifidobacterium Spp. in Feces of Children with Autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; Labaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Prete, R.; Casciano, F.; Corsetti, A.; Battista, N.; Veneziani, G.; Gianotti, A. Modulation of Human Colon Microbiota by Naturally Debittered Olive Patè Enriched with Lactiplantibacillus Plantarum in an in Vitro Intestinal Model. LWT 2024, 198, 116014. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between Body Mass Index and Firmicutes/Bacteroidetes Ratio in an Adult Ukrainian Population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of Gut Microbiota on Neuropsychiatric Disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- Welcome, M.O. Gut Microbiota Disorder, Gut Epithelial and Blood-Brain Barrier Dysfunctions in Etiopathogenesis of Dementia: Molecular Mechanisms and Signaling Pathways. Neuromolecular Med. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of Gut Microbiota and Brain via Immune and Neuroendocrine Signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Lu, C.; Rong, J.; Fu, C.; Wang, W.; Xu, J.; Ju, X.-D. Overall Rebalancing of Gut Microbiota Is Key to Autism Intervention. Front. Psychol. 2022, 13, 862719. [Google Scholar] [CrossRef]
- Navarro, F.; Pearson, D.A.; Fatheree, N.; Mansour, R.; Hashmi, S.S.; Rhoads, J.M. Are “leaky Gut” and Behavior Associated with Gluten and Dairy Containing Diet in Children with Autism Spectrum Disorders? Nutr. Neurosci. 2015, 18, 177–185. [Google Scholar] [CrossRef]
- Shanmugam, H.; Ganguly, S.; Priya, B. Plant Food Bioactives and Its Effects on Gut Microbiota Profile Modulation for Better Brain Health and Functioning in Autism Spectrum Disorder Individuals: A Review. Food Front. 2022, 3, 124–141. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Montagano, F.; Dell’Orco, F.; Prete, R.; Corsetti, A. Health Benefits of Fermented Olives, Olive Pomace and Their Polyphenols: A Focus on the Role of Lactic Acid Bacteria. Front. Nutr. 2024, 11, 1467724. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, A.; Horvath, A.; Łukasik, J.; Pisula, E.; Szajewska, H. Gluten- and Casein-Free Diet and Autism Spectrum Disorders in Children: A Systematic Review. Eur. J. Nutr. 2018, 57, 433–440. [Google Scholar] [CrossRef]
- Guo, M.; Li, R.; Wang, Y.; Ma, S.; Zhang, Y.; Li, S.; Zhang, H.; Liu, Z.; You, C.; Zheng, H. Lactobacillus Plantarum ST-III Modulates Abnormal Behavior and Gut Microbiota in a Mouse Model of Autism Spectrum Disorder. Physiol. Behav. 2022, 257, 113965. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Zhao, S.; Zhang, Y.; Li, E. A Review of Probiotics in the Treatment of Autism Spectrum Disorders: Perspectives from the Gut-Brain Axis. Front. Microbiol. 2023, 14, 1123462. [Google Scholar] [CrossRef]
- Bin-Khattaf, R.M.; Al-Dbass, A.M.; Alonazi, M.; Bhat, R.S.; Al-Daihan, S.; El-Ansary, A.K. In a Rodent Model of Autism, Probiotics Decrease Gut Leakiness in Relation to Gene Expression of GABA Receptors: Emphasize How Crucial the Gut-Brain Axis. Transl. Neurosci. 2024, 15, 20220354. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky Gut: Mechanisms, Measurement and Clinical Implications in Humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to Brain Interaction in Autism Spectrum Disorders: A Randomized Controlled Trial on the Role of Probiotics on Clinical, Biochemical and Neurophysiological Parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Probiotics and Psychobiotics: The Role of Microbial Neurochemicals. Probiotics Antimicrob. Proteins 2019, 11, 1071–1085. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. A Narrative Review on the Advance of Probiotics to Metabiotics. J. Microbiol. Biotechnol. 2024, 34, 487–494. [Google Scholar] [CrossRef]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial Neuroactive Compounds Produced by Psychobiotics. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; pp. 221–239. ISBN 978-1-4939-0897-4. [Google Scholar]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and Fructo-Oligosaccharide Intervention Modulate the Microbiota-Gut Brain Axis to Improve Autism Spectrum Reducing Also the Hyper-Serotonergic State and the Dopamine Metabolism Disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.a.A.; Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus Plantarum DR7 Alleviates Stress and Anxiety in Adults: A Randomised, Double-Blind, Placebo-Controlled Study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Liu, G.; Chong, H.-X.; Chung, F.Y.-L.; Li, Y.; Liong, M.-T. Lactobacillus Plantarum DR7 Modulated Bowel Movement and Gut Microbiota Associated with Dopamine and Serotonin Pathways in Stressed Adults. Int. J. Mol. Sci. 2020, 21, 4608. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Abavisani, M.; Khoshrou, A.; Sahebkar, A. Probiotics for Autism Spectrum Disorder: An Updated Systematic Review and Meta-Analysis of Effects on Symptoms. J. Psychiatr. Res. 2024, 179, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Tsolis, R.M.; Bäumler, A.J. The Microbiome and Gut Homeostasis. Science 2022, 377, eabp9960. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Walters, T.D.; Huynh, H.Q.; Lawrence, S.; Mack, D.R.; Deslandres, C.; Otley, A.; El-Matary, W.; Sherlock, M.; Griffiths, A.M.; et al. Inflammatory Bowel Disease Among Canadian Children: Comparison Between Children of Non-European Descent and Children of European Descent. Inflamm. Bowel Dis. 2023, 29, 1760–1768. [Google Scholar] [CrossRef]
- OjiNjideka Hemphill, N.; Pezley, L.; Steffen, A.; Elam, G.; Kominiarek, M.A.; Odoms-Young, A.; Kessee, N.; Hamm, A.; Tussing-Humphreys, L.; Koenig, M.D. Correction: OjiNjideka Hemphill et al. Feasibility Study of Lactobacillus Plantarum 299v Probiotic Supplementation in an Urban Academic Facility among Diverse Pregnant Individuals. Nutrients 2023, 15, 875. Nutrients 2023, 15, 3339. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Agarwal, A.K.; Al-Dossari, M.; Abd EL-Gawaad, N.S. Psychobiotic Potential of Lactiplantibacillus Plantarum: Current Perspective in Neurodegeneration and Geriatric Therapies. Brain Behav. Immun. Integr. 2024, 5, 100038. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Joyce, S.A.; Corsetti, A. Genotypic and Phenotypic Characterization of Food-Associated Lactobacillus Plantarum Isolates for Potential Probiotic Activities. FEMS Microbiol. Lett. 2020, 367, fnaa076. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-Promoting Role of Lactiplantibacillus Plantarum Isolated from Fermented Foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, M.; Zhang, H.; Wang, Y.; Li, R.; Liu, Z.; Zheng, H.; You, C. Lactiplantibacillus Plantarum ST-III-Fermented Milk Improves Autistic-like Behaviors in Valproic Acid-Induced Autism Spectrum Disorder Mice by Altering Gut Microbiota. Front. Nutr. 2022, 9, 1005308. [Google Scholar] [CrossRef]
- Qiu, Z.; Luo, D.; Yin, H.; Chen, Y.; Zhou, Z.; Zhang, J.; Zhang, L.; Xia, J.; Xie, J.; Sun, Q.; et al. Lactiplantibacillus Plantarum N-1 Improves Autism-like Behavior and Gut Microbiota in Mouse. Front. Microbiol. 2023, 14, 1134517. [Google Scholar] [CrossRef]
- Chen, C.-M.; Wu, C.-C.; Kim, Y.; Hsu, W.-Y.; Tsai, Y.-C.; Chiu, S.-L. Enhancing Social Behavior in an Autism Spectrum Disorder Mouse Model: Investigating the Underlying Mechanisms of Lactiplantibacillus Plantarum Intervention. Gut Microbes 2024, 16, 2359501. [Google Scholar] [CrossRef] [PubMed]
- Sunand, K.; Mohan, K.; Bakshi, V.; Bakshi, V. Supplementation of Lactobacillus Probiotic Strains Supports Gut- Brain-Axis and Defends Autistic Deficits Occurred by Valproic Acid-Induced Prenatal Model of Autism. Pharmacogn. J. 2020, 12, 1658–1669. [Google Scholar] [CrossRef]
- Adıgüzel, E.; Çiçek, B.; Ünal, G.; Aydın, M.F.; Barlak-Keti, D. Probiotics and Prebiotics Alleviate Behavioral Deficits, Inflammatory Response, and Gut Dysbiosis in Prenatal VPA-Induced Rodent Model of Autism. Physiol. Behav. 2022, 256, 113961. [Google Scholar] [CrossRef] [PubMed]
- Mintál, K.; Tóth, A.; Hormay, E.; Kovács, A.; László, K.; Bufa, A.; Marosvölgyi, T.; Kocsis, B.; Varga, A.; Vizvári, Z.; et al. Novel Probiotic Treatment of Autism Spectrum Disorder Associated Social Behavioral Symptoms in Two Rodent Models. Sci. Rep. 2022, 12, 5399. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral Probiotic Administration during Pregnancy Prevents Autism-Related Behaviors in Offspring Induced by Maternal Immune Activation via Anti-Inflammation in Mice. Autism Res. 2019, 12, 576–588. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef]
- Wu, X.Z.; Wen, Z.G.; Hua, J.L. Effects of Dietary Inclusion of Lactobacillus and Inulin on Growth Performance, Gut Microbiota, Nutrient Utilization, and Immune Parameters in Broilers. Poult. Sci. 2019, 98, 4656–4663. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Mensi, M.M.; Rogantini, C.; Marchesi, M.; Borgatti, R.; Chiappedi, M. Lactobacillus Plantarum PS128 and Other Probiotics in Children and Adolescents with Autism Spectrum Disorder: A Real-World Experience. Nutrients 2021, 13, 2036. [Google Scholar] [CrossRef]
- Kong, X.-J.; Liu, J.; Liu, K.; Koh, M.; Sherman, H.; Liu, S.; Tian, R.; Sukijthamapan, P.; Wang, J.; Fong, M.; et al. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 1552. [Google Scholar] [CrossRef]
- Sherman, H.T.; Liu, K.; Kwong, K.; Chan, S.-T.; Li, A.C.; Kong, X.-J. Carbon Monoxide (CO) Correlates with Symptom Severity, Autoimmunity, and Responses to Probiotics Treatment in a Cohort of Children with Autism Spectrum Disorder (ASD): A Post-Hoc Analysis of a Randomized Controlled Trial. BMC Psychiatry 2022, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Wang, J.-E.; Sun, F.-J.; Huang, Y.-H.; Chen, H.-J. Probiotic Intervention in Young Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blinded, Placebo-Controlled Trial. Res. Autism Spectr. Disord. 2023, 109, 102256. [Google Scholar] [CrossRef]
- Billeci, L.; Callara, A.L.; Guiducci, L.; Prosperi, M.; Morales, M.A.; Calderoni, S.; Muratori, F.; Santocchi, E. A Randomized Controlled Trial into the Effects of Probiotics on Electroencephalography in Preschoolers with Autism. Autism 2023, 27, 117–132. [Google Scholar] [CrossRef]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Melli, S.; Dunca, D.; Terruzzi, V. Unexpected Improvement in Core Autism Spectrum Disorder Symptoms after Long-Term Treatment with Probiotics. SAGE Open Med. Case Rep. 2016, 4, 2050313X16666231. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.C.; Messora, M.R. Effectiveness of Multistrain Versus Single-Strain Probiotics: Current Status and Recommendations for the Future. J. Clin. Gastroenterol. 2018, 52 (Suppl. S1), S35–S40. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Efficacy of Single-Strain Probiotics Versus Multi-Strain Mixtures: Systematic Review of Strain and Disease Specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef]
- Brzóska-Konkol, E.; Remberk, B.; Papasz-Siemienuk, A. Analysis of Research on the Effectiveness of Using Probiotics for Children with Autism Spectrum Disorders, in Order to Reduce the Core and Accompanying Autism Symptoms. Review of Randomized Clinical Trials. Postep. Psychiatr. Neurol. 2022, 31, 25–34. [Google Scholar] [CrossRef]
- He, X.; Liu, W.; Tang, F.; Chen, X.; Song, G. Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients 2023, 15, 1415. [Google Scholar] [CrossRef]

| Symbol | Name | Model Category 1 | Number of Models | Behavioral Features | Neuroanatomical Alterations | Gastrointestinal Alterations | References |
|---|---|---|---|---|---|---|---|
| SHANK3 | SH3 and multiple ankyrin repeat domains 3 | G | 111 | ↓ Social interaction and communication; repetitive behaviors. | ↓ Synaptic transmission; altered functional and structural plasticity of synapses; ↑ dendritic length and complexity; ↓ spine density. | ↓ Intestine relative abundance of members of the class Bacilli, order Lactobacillales, family Lactobacillaceae, and genus Lactobacillus. | [54,55]. |
| MECP2 | Methyl-CpG binding protein 2 | G | 95 | Motor impairment such as ataxia; anxiety-like and anti-social behaviors; stereotyped behaviors. | ↓ Volume of cortical and subcortical regions; altered synaptic transmission. | Short colon; abnormal localization of key membrane proteins, like ClC-2 and NHE-3, in the epithelial cells on the surface. | [28,56,57]. |
| FMR1 | Fragile X mental retardation 1 | G | 68 | ↑ Locomotor activity; hyperactivity; ↑ self-grooming; ↑ repetitive behaviors; ↓ anxiety (due to the background). | ↑ Spine density and length; abnormal synaptic plasticity; ↓ ratio of AMPA to NMDA receptors early in development. | Altered gut microbiota composition; ↑ intestinal inflammation; ↑ intestinal permeability; ↑ serum LPS levels. | [58,59]. |
| CNTNAP2 | Contactin associated protein-like 2 | G | 52 | Social deficits; communication impairment; repetitive behaviors. | Defective neuronal migration and cortical ectopia; altered sensory cortical circuitry; long-range connectivity deficits; dendritic spine morphology and synaptic plasticity deficits. | Altered colonic motility; ↑ intestinal permeability. | [60,61,62,63,64]. |
| NLGN3 | Neuroligin 3 | G | 50 | Abnormal social and repetitive behaviors. | Altered excitatory synaptic transmission; alterations in synaptic signaling and plasticity; glial cell morphology changes. | Accelerated gastrointestinal transit and dysmotility; alterations in enteric nervous system (ENS); altered mucus layer and microbiota distribution. | [65,66,67,68,69]. |
| CHD8 | Chromodomain helicase DNA-binding protein 8 | G | 48 | Impaired social interaction; anxiety; learning and memory deficits. | ↑ Brain weight, craniofacial abnormalities; altered synaptic physiology in medium spiny neurons of the nucleus accumbens; microstructural changes in specific brain regions, including the cortex and striatum. | Shortened small intestine and colon length; ↓ intestinal motility; disturbance in the gut microbiota, including decreased abundance of Bacteroides. | [29,30,70]. |
| PTEN | Phosphatase and tensin homolog | G | 45 | ↓ Social preference; ↓ social novelty; ↓ aggression; repetitive behaviors. | Macrocephaly; ↑ glia (astrocytes, oligodendrocytes, and microglia) neuronal hypertrophy; ↑ axon growth. | // | [31]. |
| TSC1 | Tuberous sclerosis 1 | G | 40 | Impairments in social interaction and communication; restricted and repetitive behaviors. | Cortical and hippocampal hypertrophy; brain structural abnormalities; abnormal synaptogenesis; glial cell overexpression; neuroinflammation; oxidative stress; mitochondrial dysfunction (cerebellum, cortex, hippocampus, amygdala). | // | [32,71,72]. |
| UBE3A | Ubiquitin protein ligase E3A | G | 34 | Impairments in social interaction; repetitive self-grooming behavior; memory and pain sensitivity. | ↓ Dendritic spine density and ↑ immature filopodia density, alterations in neurons as immature dendritic protrusions; reduction in dendritic spine maturation in prelimbic cortical neurons. | // | [33,73,74]. |
| TBR1 | T-box, brain 1 | G | 31 | ↓ Social behaviors; defective vocalization; impaired olfactory discrimination; aversive memory; and cognitive inflexibility. | Impairments in structural and functional connectivity of the basolateral amygdala, and whole-brain synchronization. | // | [34,75]. |
| Poly I:C | Polyinosinic–polycytidylic acid | E | 71 | Sensorimotor gating; perseverative behaviors; ↓ social interaction, abnormal communication, stereotyped/repetitive behavior. | Spatially restricted deficit in Purkinje cells, neuroinflammation, alterations in BDNF and ARG-1 levels. | Altered early-life gut microbiota composition, gut dysbiosis, ↑ intestinal permeability, ↑ bacterial families such as Lachnospiraceae, Porphyromonadaceae, and Prevotellaceae. | [37,45,46,76,77] |
| VPA | Valproic acid | E | 36 | ↓ Sociability, communication deficits, repetitive behaviors; impaired prepulse inhibition; altered pain sensitivity, ↑ anxiety, and hyperactivity. | Differences in head circumference or brain size; macrocephaly and microcephaly; ↓ myelin density or gene/protein expression. | Impaired duodenal motility; ↑ intestinal inflammatory factors. | [36,78,79,80,81]. |
| GF environment | Germ-free environment | E | 7 | Repetitive behavior; ↓ sociability; ↓ anxiety; non-spatial memory. | ↑ Neuroendocrine responses to stress; altered neurotrophin levels in the hippocampus and amygdala; altered monoamine neurotransmitter levels in the brain. | ↓ Total mass of intestine; larger cecum; reduced numbers of gut-associated lymphoid tissues, poorly formed T-cell and B-cell zones in the germinal centers; reduced numbers of intestinal T cells and decreased IgA production. | [82,83,84] |
| MHFD | Maternal high-fat diet | E | 6 | Impaired sociability; prepulse inhibition learning and memory impairment; hyperactivity; enhanced anxiety-like behaviors; ↓ cognition. | ↓ Oxytocin immunoreactive neurons in the hypothalamus; block long-lasting neural adaptation in the mesolimbic dopamine reward system; attenuations of amino acid levels in the medial prefrontal cortex and the hippocampus regions. | Changes in microbiome composition, ↓ L. reuteri; disruption of intestinal mucosal barrier. | [39,40,83,85] |
| rIL-6 | Recombinant interleukin 6 | E | 6 | Impaired sociability; repetitive behaviors; cognitive and learning abnormalities; prepulse inhibition; latent inhibition. | ↑ Brain volume; alterations in dendritic spine; imbalance between excitatory and inhibitory synapses. | ↑ Intestinal permeability; alterations in microbiota composition. | [86]. |
| Fluoxetine | Fluoxetine | E | 4 | Anxiety behaviors; disrupted learning; aggressive behaviors; impaired social recognition and working memory. | Abnormal circuit formation in the cortex; ↓ frequencies in spontaneous excitatory postsynaptic currents recorded from layer (L) 5 pyramidal neurons in the prelimbic cortex. | Impaired enteric neuronal development; altered GI motility and mucosal growth; hyperplastic enteric system; ↑ intestinal transit; ↓ Lactobacillus johnsonii and Bacteroidales S24-7. | [48,87,88,89] |
| Stress | Stress | E | 4 | Social interaction impairments; conditioned fear behavior; alterations in anxiety-like behavior. | ↑ Brain 5-HT; precocious synaptic maturation (hippocampus); ↓ neuronal arbor complexity and synaptogenesis; ↑ CLDN5, CLDN12, MMP9 (PFC); ↓ CA1/CA3 hippocampal diameter. | ↓ In alpha and beta diversity of microbiota; ↓ in fecal propionic acid; intestinal inflammation; gut microbiota dysbiosis; ↓ ocln and cldn1 expression in the colon. | [49,90,91,92] |
| LPS | Lipopolysaccharide | E | 3 | Deficits in social interaction; novel object recognition; anxiety-like behavior. | Structural, neurophysiological, and functional changes in the hippocampus; abnormal fetal brain cytoarchitecture and lamination; activation of microglia in the fetal brain. | Intestinal inflammation. | [93,94,95] |
| Prenatal stress | Prenatal stress | E | 3 | ↓ Sociability, ↓ reciprocal social interaction; ↑marble burying behaviors; ↑anxiety; rigid response learning strategy; impaired memory in a motor learning task. | Aberrant expression of glutamate and GABA marker genes; disorganization of striatal striosome and matrix compartments; neuroinflammation; ↓ oxytocin receptor; ↓ serotonin metabolism in the cortex. | Gut dysbiosis; ↓ Bacteroides and Parabacteroides; impaired intestinal epithelial proliferation, goblet and Paneth cell differentiation; mucosal and gut barrier dysfunction; low-grade inflammation; ↓ ileum villus height, crypt depth, and surface area. | [50,81,96,97,98,99] |
| BALBcByJ | BALB/cByJ | I | 24 | Social behavior impairment; ↑ repetitive and stereotypic activities; ↓ ultrasonic vocalizations; heightened anxiety-like behaviors; excessive grooming. | ↓ Corpus callosum volume; ↓ fractional anisotropy (FA) in the external capsule area, indicative of decreased integrity of white matter fibers. | // | [53,100]. |
| BTBR | BTBR T + Itpr3tf/J | I | 73 | Impaired sociability; repetitive behaviors; abnormalities in vocal communication. | ↓ Gray matter volume in ventral tegmental area, cingulate gyrus, lateral thalamus, posterior thalamus, occipital and parietal cortices, and subcortex; ↑ gray matter volume in olfactory bulb, medial prefrontal and insular cortices, amygdala, and dorsal hippocampus. | ↑ Intestinal permeability; downregulation of Muc 2 in the large intestine; altered microbiota composition of cecal and fecal samples; ↓ Bifidobacterium and Blautia species. | [51,52,95,100,101,102]. |
| Animal Model | Treatment | Age/Sex | Behavioral and Physiological Features | Main Outcomes | Relevance to Human ASD | Reference |
|---|---|---|---|---|---|---|
| ICR mouse (TCS exposure). | Lpb. plantarum ST-III. | 6–9 weeks/ M and F. | Social deficits (males); grooming and freezing (females); altered gut microbiota composition. | Lpb. plantarum ST-III improved social behavior in males, reduced grooming/freezing in females; modulated gut microbiota. | Supports gut–brain axis role in ASD. | [168] |
| ICR mouse (VPA model). | Probiotic fermented milk with Lpb. plantarum ST-III | 6–8 weeks/ M and F. | Impaired locomotor behavior, increased anxiety, and deficient sociability. Altered gut microbiota diversity and composition. | Lpb. plantarum ST-III improved the impaired social interaction in male ASD mouse model and the autistic-like behaviors in male mice by modulating specific gut microbes. | Highlights effects on microbiota–gut–brain axis in ASD. | [195] |
| C57BL/6 WT mouse (MIA model). | Probiotic Lpb. plantarum N-1. | Adult/ M only. | Social interaction deficits; anxiety-like and depressive-like behavior; gut dysbiosis. | LPN-1 intervention improved autism-like behaviors in mice, including anxiety and depression, possibly via regulating the gut microbiota. | Supports microbiota–gut–brain axis role in ASD. | [196] |
| C57BL/6J mice (VPA model). | Lpb. plantarum PS128. | 1 month/M and F; M only for behavioral tests. | ASD-like behaviors (social deficits, anxiety, cognitive impairments); reduced dendritic complexity, spine density, impaired synaptic signaling (Erk 1/2, PKA, CaMKIIα). | PS128 improved sociability, anxiety, and cognition in VPA mice; increased Bifidobacterium abundance; improved synaptic plasticity, dendritic structure, and glutamate receptor expression. | Supports gut–brain axis role in ASD. | [197] |
| Wistar rats (VPA model). | Prenatal probiotic treatment (Lpb. plantarum UBLP-40, Lcb. casei- UBLC-42, L. acidophilus UBLA-34, L. bulgaricus L. bulgaricus UBLB-38); postnatal prebiotic treatment (inulin). | PND 08–PND 50 /M and F. | ASD-like behaviors; impaired social interaction, memory deficits, repetitive behavior. | Lactobacillus strains have reversed autistic deficits and improved immune functions. | Supports gut–brain axis role in ASD. | [198] |
| Wistar rats (VPA model). | Probiotic VSL3# and prebiotic BTAGEN®. | PND22–PND63/M only. | VPA induced ASD-like behaviors. Differences in abundance of Bacteroidetes/Firmicutes ratio. | Probiotic and combined treatments improved autistic-like behaviors and the Bacteroidetes/Firmicutes ratio. Probiotic treatment decreased serum IL-6 levels and increased IL-10 levels. | Probiotic treatment shows translational potential. | [199] |
| Wistar rats (VPA model + antibiotics cocktail). | Probiotics Lacticaseibacillus rhamnosus GG, Lpb. plantarum 299v. | PND 2–21/M only. | VPA and chronic depletion of the gut microbiota induced ASD-like behavioral alterations. | The probiotic treatment was capable of re-establishing normal social behavior. | Probiotic treatment shows therapeutic potential. | [200] |
| Study Design | Sample Size/Population | Age/Gender | Intervention (Strain, Dose, Duration) | Main Results | Conclusion/Clinical Relevance | Reference |
|---|---|---|---|---|---|---|
| Double-blind, randomized, parallel, placebo-controlled trial. | 80 boys with ASD; 71 completed the study (PS128 n = 36, placebo n = 35); all in Taiwan. | Age 7–15 years, all males. | Lpb. plantarum PS128: 3 × 1010 CFU/capsule, 1 capsule/day, for 4 weeks. | PS128 group showed improvements in ABC-T (body/object use), SRS total, CBCL (anxiety, rule breaking), and in SNAP-IV. | PS128 may improve ADHD-like symptoms (hyperactivity/impulsivity) in younger children (age 7–12). Potential for age-specific psychobiotic intervention. | [146] |
| Randomized, double-blind, placebo-controlled, two-stage pilot trial. | 35 individuals with ASD. | Age 3–20 years; 26 males/9 females. | Lpb. plantarum PS128 (6 × 1010 CFU/day, oral) for 16 weeks; from week 16 both groups received intranasal oxytocin; total duration 28 weeks. | Probiotic + oxytocin group showed greater improvement in CGI-I; trends toward improvement in ABC total, stereotypic behavior, and SRS cognition scores; enhanced gut microbiome connectivity and specific taxa correlations. | The combination of PS128 and oxytocin showed synergistic effects, suggesting potential for improved ASD core symptoms and gut–brain axis modulation. Combined therapy showed significant improvements to gut microbiome dysbiosis. | [208] |
| Retrospective observational study. | 131 autistic children and adolescents. | Aged 45–127 months (mean age 7.9 years), males and females. | Lpb. plantarum PS128, 3 × 1010 CFUs (weight less than 30 kg) and 6 × 1010 CFUs (weight higher than 30 kg). Duration 6 months. | Significant improvements observed in CGI scores after PS128 administration; effects were more pronounced in participants with gastrointestinal symptoms. | Supplementation with Lpb. plantarum PS128 was associated with behavioral improvements in children and adolescents with ASD, particularly those with comorbid GI symptoms. | [207] |
| Double-blind, randomized, placebo-controlled clinical trial. | 35 individuals with ASD. | Age 3–25, 26 males/9 females. | Lpb. plantarum PS128, 6 × 1010 CFU/day for 16 weeks. | Probiotic group showed specific improvements in behavior, inflammatory markers, gut microbiota diversity. | PS128 may be beneficial in subgroups of ASD children, particularly those with specific autoimmune/inflammatory profiles. | [209] |
| Double-blind, randomized, parallel, placebo-controlled trial. | n = 82 randomized, 79 completed. | Male and female children, aged 2.5–7 years, with ASD. | Lpb. plantarum PS128, 6 × 1010 CFU/day, oral. Duration of 4 months in two stages of interventions, each lasting 2 months. | PS128 significantly improved ASEBA * anxious/depressed scores and ADHDT hyperactivity; some GI symptoms improved in both groups after PS128 phase. | PS128 may reduce anxiety and hyperactivity symptoms in young children with ASD; some gastro-intestinal benefits observed. | [210] |
| Randomized, double-blind, placebo-controlled parallel-group trial. | 46 preschoolers with ASD. | Age 18–72 months; 35 males/11 Females. | Probiotic Vivomixx®; daily dose (900 billion CFU first month, 450 billion CFU last 5 months); 6 months. | EEG changes correlated with behavioral and inflammatory measures. | Probiotics induced EEG changes resembling typical brain activity; suggest neuroplastic effects and gut–brain modulation in ASD. | [179,211] |
| Randomized, double-blind, placebo-controlled crossover pilot trial. | 13 children with ASD (10 completed). | Age 3–12 years, males and females. | Visbiome® probiotic; 9 × 1011 CFU/day; 19 weeks × 2 phases. | Improvement in GI symptoms (PedsQL GI module); reduced parent-reported target GI symptoms. | The Visbiome® formulation suggested a health benefit in children with ASD and GI symptoms who retained Lactobacillus. | [212] |
| Double-blind, randomized, parallel, factorial, placebo-controlled trial. | 85 children with; no GI symptoms group (NGI), GI symptoms group (GI); all Italian; 63 children completed the trial. | ASD children aged 1.5–6 years, 71 males (84%). | Probiotic DSF (Vivomixx®/Visbiome®), 2 packets/day (month 1), 1 packet/day (months 2–6). Duration of 6 months. | In NGI subgroup: significant reduction in ADOS-CSS. In GI subgroup: probiotics improved GI symptoms, adaptive functioning, and multisensory processing. | Probiotics improved social affect scores in children without GI issues, and enhanced GI sensory, and adaptive functions in those with GI symptoms. | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatini, G.; Boccadoro, I.; Prete, R.; Battista, N.; Corsetti, A. Autism Spectrum Disorder: From Experimental Models to Probiotic Application with a Special Focus on Lactiplantibacillus plantarum. Nutrients 2025, 17, 2470. https://doi.org/10.3390/nu17152470
Sabatini G, Boccadoro I, Prete R, Battista N, Corsetti A. Autism Spectrum Disorder: From Experimental Models to Probiotic Application with a Special Focus on Lactiplantibacillus plantarum. Nutrients. 2025; 17(15):2470. https://doi.org/10.3390/nu17152470
Chicago/Turabian StyleSabatini, Giusi, Ilenia Boccadoro, Roberta Prete, Natalia Battista, and Aldo Corsetti. 2025. "Autism Spectrum Disorder: From Experimental Models to Probiotic Application with a Special Focus on Lactiplantibacillus plantarum" Nutrients 17, no. 15: 2470. https://doi.org/10.3390/nu17152470
APA StyleSabatini, G., Boccadoro, I., Prete, R., Battista, N., & Corsetti, A. (2025). Autism Spectrum Disorder: From Experimental Models to Probiotic Application with a Special Focus on Lactiplantibacillus plantarum. Nutrients, 17(15), 2470. https://doi.org/10.3390/nu17152470








