A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy
Abstract
1. Introduction
2. Materials and Methods
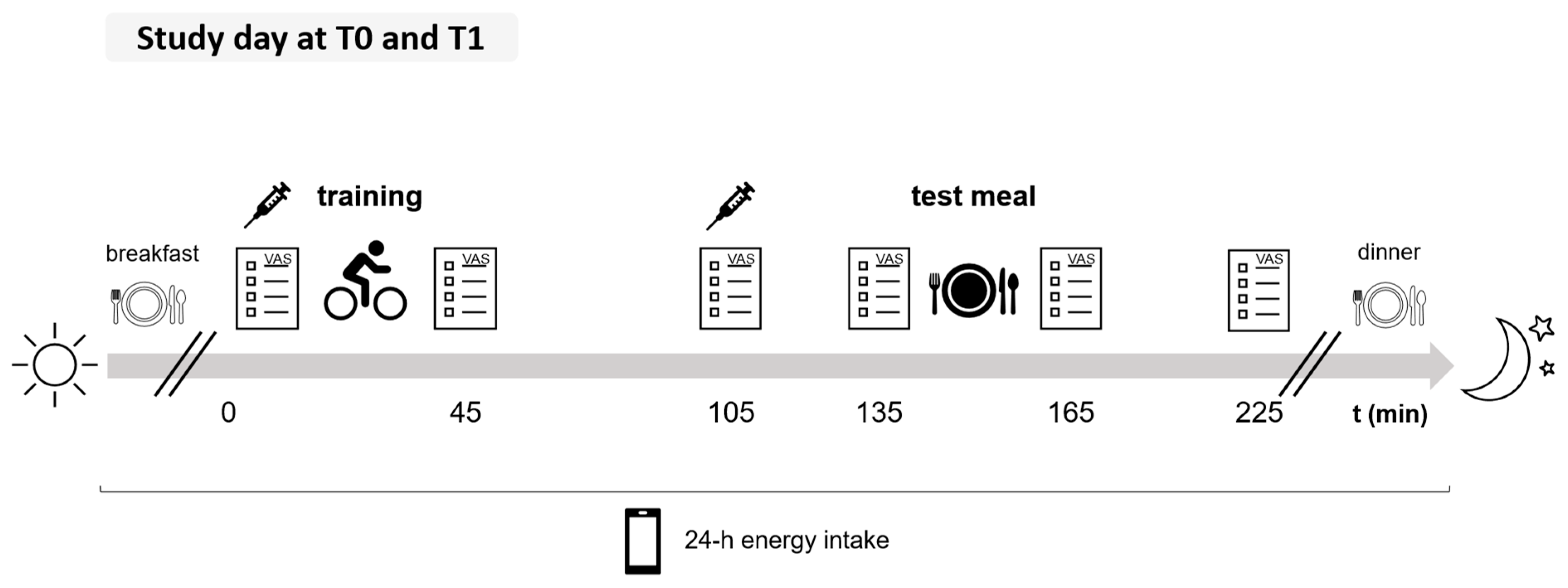
2.1. Study Protocol and Study Population
2.2. Training Protocol
2.3. Spirometry
2.4. Subjective Appetite
2.5. Appetite-Regulating Hormones
2.6. Energy Intake
2.7. Body Composition and Energy Balance
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Exercise-Induced Appetite and Energy Intake
3.3. Changes in Body Composition, Energy Balance, and Plasma Leptin Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CF | Cystic Fibrosis |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| ETI | Elexacaftor, Tezacaftor, Ivacaftor |
| FFM | Fat-Free Mass |
| FFMI | Fat-Free Mass Index |
| FM | Fat Mass |
| FMI | Fat Mass Index |
| HIIT | High-Intensity Interval Training |
| MICT | Moderate-Intensity Continuous Training |
| PYY | Peptide YY |
| ppFEV1 | Percent Predicted Forced Expiratory Volume in One Second |
| REE | Resting Energy Expenditure |
| SQ | Satiety Quotient |
| VAS | Visual Analogue Scale |
| VO2peak | Peak Oxygen Uptake |
| pwCF | People with Cystic Fibrosis |
References
- Elborn, J.S. Cystic Fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Naehrlich, L.; Burkhart, M.; Basler, C.; Dittrich, A.-M.; Ellemunter, H.; Hebestreit, H.; Nitsche, O.; Held, I.; Smaczny, C.; Sutharsan, S. German Cystic Fibrosis Registry-Annual Report 2023; Mukoviszidose e.V. & Mukoviszidose Institut GmbH: Bonn, Germany, 2024. [Google Scholar]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The Future of Cystic Fibrosis Care: A Global Perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Sharma, R.; Florea, V.G.; Bolger, A.P.; Doehner, W.; Florea, N.D.; Coats, A.J.S.; Hodson, M.E.; Anker, S.D.; Henein, M.Y. Wasting as an Independent Predictor of Mortality in Patients with Cystic Fibrosis. Thorax 2001, 56, 746–750. [Google Scholar] [CrossRef]
- European Medicines Agency. Kalydeco. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kalydeco#authorisation-details (accessed on 9 July 2024).
- Sutharsan, S.; Dillenhoefer, S.; Welsner, M.; Stehling, F.; Brinkmann, F.; Burkhart, M.; Ellemunter, H.; Dittrich, A.-M.; Smaczny, C.; Eickmeier, O.; et al. Impact of Elexacaftor/Tezacaftor/Ivacaftor on Lung Function, Nutritional Status, Pulmonary Exacerbation Frequency and Sweat Chloride in People with Cystic Fibrosis: Real-World Evidence from the German CF Registry. Lancet Reg. Health Eur. 2023, 32, 100690. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Robinson, P.D.; Shteinberg, M. CFTR Modulator Therapy: Transforming the Landscape of Clinical Care in Cystic Fibrosis. Lancet 2023, 402, 1171–1184. [Google Scholar] [CrossRef]
- Bailey, J.; Rozga, M.; McDonald, C.M.; Bowser, E.K.; Farnham, K.; Mangus, M.; Padula, L.; Porco, K.; Alvarez, J.A. Effect of CFTR Modulators on Anthropometric Parameters in Individuals with Cystic Fibrosis: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2021, 121, 1364–1378.e2. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Braeckel, E.V.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and Safety of the Elexacaftor plus Tezacaftor plus Ivacaftor Combination Regimen in People with Cystic Fibrosis Homozygous for the F508del Mutation: A Double-Blind, Randomised, Phase 3 Trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- European Medicines Agency. Kaftrio. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kaftrio#authorisation-details (accessed on 12 June 2025).
- Stallings, V.A.; Sainath, N.; Oberle, M.; Bertolaso, C.; Schall, J.I. Energy Balance and Mechanisms of Weight Gain with Ivacaftor Treatment of Cystic Fibrosis Gating Mutations. J. Pediatr. 2018, 201, 229–237.e4. [Google Scholar] [CrossRef] [PubMed]
- Granados, A.; Chan, C.L.; Moheet, A.; Vigers, T.; Arbeláez, A.M.; Larson Ode, K. The Impact of Elexacaftor/Tezacaftor/Ivacaftor on Body Composition in a Small Cohort of Youth with Cystic Fibrosis. Pediatr. Pulmonol. 2023, 58, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Grancini, V.; Gramegna, A.; Zazzeron, L.; Alicandro, G.; Porcaro, L.L.; Piedepalumbo, F.; Lanfranchi, C.; Daccò, V.; Orsi, E.; Blasi, F. Effects of Elexacaftor / Tezacaftor / Ivacaftor Triple Combination Therapy on Glycaemic Control and Body Composition in Patients with Cystic Fibrosis-Related Diabetes. Diabetes Metab. 2023, 49, 101466. [Google Scholar] [CrossRef] [PubMed]
- Hevilla, F.; Porras, N.; Girón, M.V.; García-Olivares, M.; Padial, M.; Sánchez-Torralvo, F.J.; Olveira, C.; Olveira, G. Impact of Elexacaftor-Tezacaftor-Ivacaftor Therapy on Body Composition, Dietary Intake, Biomarkers, and Quality of Life in People with Cystic Fibrosis: A Prospective Observational Study. Nutrients 2024, 16, 3293. [Google Scholar] [CrossRef]
- Knott-Torcal, C.; Sebastián-Valles, F.; Girón Moreno, R.M.; Martín- Adán, J.C.; Jiménez-Díaz, J.; Marazuela, M.; Sánchez de la Blanca, N.; Fernández-Contreras, R.; Arranz-Martín, A. A Prospective Study to Assess the Impact of a Novel CFTR Therapy Combination on Body Composition in Patients with Cystic Fibrosis with F508del Mutation. Clin. Nutr. 2023, 42, 2468–2474. [Google Scholar] [CrossRef]
- Merino Sánchez-Cañete, A.; López Cárdenes, C.M.; Vicente Santamaría, S.; Gutiérrez Martínez, J.R.; Suárez González, M.; Álvarez Merino, M.; González Jiménez, D. Increased Fat Mass and Obesity Risk after Elexacaftor-Tezacaftor-Ivacaftor Therapy in Young Adults with Cystic Fibrosis. Front. Nutr. 2024, 11, 1477674. [Google Scholar] [CrossRef]
- Navas-Moreno, V.; Sebastian-Valles, F.; Rodríguez-Laval, V.; Knott-Torcal, C.; Marazuela, M.; de la Blanca, N.S.; Arranz Martín, J.A.; Girón, R.M.; Sampedro-Núñez, M.A. Impact of CFTR Modulator Therapy on Body Composition as Assessed by Thoracic Computed Tomography: A Follow-up Study. Nutrition 2024, 123, 112425. [Google Scholar] [CrossRef]
- Trost, S.U.; Harindhanavudhi, T.; Ankireddypalli, A.; Wang, Q.; Simrah, A.; Avula, S.; Moheet, A. Elexacaftor/Tezacaftor/Ivacator Effect on Bone Density and Body Composition–A Retrospective Analysis. 2024, preprint. Available online: https://ssrn.com/abstract=4882738 (accessed on 17 July 2024).
- Westhölter, D.; Haubold, J.; Welsner, M.; Salhöfer, L.; Wienker, J.; Sutharsan, S.; Straßburg, S.; Taube, C.; Umutlu, L.; Schaarschmidt, B.M.; et al. Elexacaftor/Tezacaftor/Ivacaftor Influences Body Composition in Adults with Cystic Fibrosis: A Fully Automated CT-Based Analysis. Sci. Rep. 2024, 14, 9465. [Google Scholar] [CrossRef]
- Zamponi, V.; Cirilli, N.; Caporelli, N.; Fabrizzi, B.; Strappato, M.; Mignini, E.; Nicolai, G.; Nicolari, A.; Taus, M. One-Year Assessment of Body Composition in Cystic Fibrosis Patients on Elexacaftor-Tezacaftor-Ivacaftor. Clin. Case Rep. Int. 2023, 7, 1535. [Google Scholar]
- Gur, M.; Bar-Yoseph, R.; Hanna, M.; Abboud, D.; Keidar, Z.; Palchan, T.; Toukan, Y.; Masarweh, K.; Alisha, I.; Zuckerman-Levin, N.; et al. Effect of Trikafta on Bone Density, Body Composition and Exercise Capacity in CF: A Pilot Study. Pediatr. Pulmonol. 2023, 58, 577–584. [Google Scholar] [CrossRef]
- López Cárdenes, C.M.; Merino Sánchez-Cañete, A.; Vicente Santamaría, S.; Gascón Galindo, C.; Merino Sanz, N.; Tabares González, A.; Blitz Castro, E.; Morales Tirado, A.; Garriga García, M.; López Rozas, M.; et al. Effects on Growth, Weight and Body Composition after CFTR Modulators in Children with Cystic Fibrosis. Pediatr. Pulmonol. 2024, 59, 3632–3640. [Google Scholar] [CrossRef]
- Proud, D.; Duckers, J. Weight a Minute: Exploring the Effect on Weight and Body Composition after the Initiation of Elexacaftor/Tezacaftor/Ivacaftor in Adults with CF. J. Cyst. Fibros. 2023, 22, 847–850. [Google Scholar] [CrossRef]
- Sheikh, S.; Zemel, B.S.; Stallings, V.A.; Rubenstein, R.C.; Kelly, A. Body Composition and Pulmonary Function in Cystic Fibrosis. Front. Pediatr. 2014, 2, 33. [Google Scholar] [CrossRef]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; et al. ESPEN-ESPGHAN-ECFS Guideline on Nutrition Care for Cystic Fibrosis. Clin. Nutr. 2024, 43, 413–445. [Google Scholar] [CrossRef]
- German Pension Insurance Concept for the Inpatient Rehabilitation of Children and Adolescents with Cystic Fibrosis. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.deutsche-rentenversicherung.de/SharedDocs/Downloads/DE/Experten/infos_reha_einrichtungen/konzepte_systemfragen/konzepte/konzept_kinder_mukoviszidose.pdf%3F__blob%3DpublicationFile%26v%3D1&ved=2ahUKEwidqIrq1tuJAxU8BdsEHdqaALwQFnoECDMQAQ&usg=AOvVaw1cfIUZpydc_qHtvf26K3KL (accessed on 14 November 2024).
- Van Biervliet, S.; Declercq, D.; Dereeper, S.; Vermeulen, D.; Würth, B.; De Guschtenaere, A. The Effect of an Intensive Residential Rehabilitation Program on Body Composition in Patients with Cystic Fibrosis. Eur. J. Pediatr. 2021, 180, 1981–1985. [Google Scholar] [CrossRef]
- Swisher, A.K.; Hebestreit, H.; Mejia-Downs, A.; Lowman, J.D.; Gruber, W.; Nippins, M.; Alison, J.; Schneiderman, J. Exercise and Habitual Physical Activity for People With Cystic Fibrosis: Expert Consensus, Evidence-Based Guide for Advising Patients. Cardiopulm. Phys. Ther. J. 2015, 26, 85. [Google Scholar] [CrossRef]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite Control and Energy Balance: Impact of Exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef]
- Gruber, W.; Koop, J.; Haegele, F.A.; Falkenberg, C.; Dewey, S.; Weiser, B.; Bosy-Westphal, A. Presentation WS03.02 High-Intensity Interval Training and Moderate-Intensity Continuous Training Are Equivalent in Improving Exercise Capacity at Submaximal Intensity in Adults with Cystic Fibrosis. J. Cyst. Fibros. 2025, 24, S6. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-Ethnic Reference Values for Spirometry for the 3-95-Yr Age Range: The Global Lung Function 2012 Equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, S.; Mearns, M. Pulmonary Function and Response to Exercise in Cystic Fibrosis. Arch. Dis. Child. 1971, 46, 144–151. [Google Scholar] [CrossRef]
- Hebestreit, H.; Arets, H.G.M.; Aurora, P.; Boas, S.; Cerny, F.; Hulzebos, E.H.J.; Karila, C.; Lands, L.C.; Lowman, J.D.; Swisher, A.; et al. Statement on Exercise Testing in Cystic Fibrosis. Respiration 2015, 90, 332–351. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, Power and Validity of Visual Analogue Scales in Assessment of Appetite Sensations in Single Test Meal Studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Anderson, G.; Catherine, N.; Woodend, D.; Wolever, T. Inverse Association between the Effect of Carbohydrates on Blood Glucose and Subsequent Short-Term Food Intake in Young Men. Am. J. Clin. Nutr. 2002, 76, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, K.; Oustric, P.; Alkahtani, S.; Alhussain, M.; Pedersen, H.; Quist, J.S.; Færch, K.; Finlayson, G. Impact of Meal Timing and Chronotype on Food Reward and Appetite Control in Young Adults. Nutrients 2020, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, V.; King, N.; Hetherington, M.; Doucet, E.; Blundell, J.; Tremblay, A. Appetite Sensations and Satiety Quotient: Predictors of Energy Intake and Weight Loss. Appetite 2007, 48, 159–166. [Google Scholar] [CrossRef]
- Green, S.M.; Delargy, H.J.; Joanes, D.; Blundell, J.E. A Satiety Quotient: A Formulation to Assess the Satiating Effect of Food. Appetite 1997, 29, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; European Parliament and Council: Strasbourg, France, 2011; Volume 304.
- Forbes, G.B. Body Fat Content Influences the Body Composition Response to Nutrition and Exercise. Ann. N. Y Acad. Sci. 2000, 904, 359–365. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Ravussin, E.; Bray, G.A.; Han, H.; Redman, L.M. An Objective Estimate of Energy Intake during Weight Gain Using the Intake-Balance Method. Am. J. Clin. Nutr. 2014, 100, 806–812. [Google Scholar] [CrossRef]
- de Jonge, L.; DeLany, J.P.; Nguyen, T.; Howard, J.; Hadley, E.C.; Redman, L.M.; Ravussin, E. Validation Study of Energy Expenditure and Intake during Calorie Restriction Using Doubly Labeled Water and Changes in Body Composition. Am. J. Clin. Nutr. 2007, 85, 73–79. [Google Scholar] [CrossRef]
- Racette, S.B.; Das, S.K.; Bhapkar, M.; Hadley, E.C.; Roberts, S.B.; Ravussin, E.; Pieper, C.; DeLany, J.P.; Kraus, W.E.; Rochon, J.; et al. Approaches for Quantifying Energy Intake and %calorie Restriction during Calorie Restriction Interventions in Humans: The Multicenter CALERIE Study. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E441–E448. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Gruber, W.; Orenstein, D.M.; Braumann, K.M.; Beneke, R. Interval Exercise Training in Cystic Fibrosis -- Effects on Exercise Capacity in Severely Affected Adults. J. Cyst. Fibros. 2014, 13, 86–91. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 October 2024).
- Carey, V.J.; Wang, Y.-G. Mixed-Effects Models in S and S-Plus. J. Am. Stat. Assoc. 2002, 96, 1135–1136. [Google Scholar] [CrossRef]
- Carroll, R.J.; Ruppert, D. Transformation and Weighting in Regression; Springer: Boston, MA, USA, 1988; ISBN 978-0-412-01421-5. [Google Scholar]
- Ockenga, J. Importance of Nutritional Management in Diseases with Exocrine Pancreatic Insufficiency. HPB 2009, 11, 11–15. [Google Scholar] [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P.H. Multiple Comparisons Using R; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-58488-574-0. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- WHO. A Healthy Lifestyle-WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 30 September 2024).
- Kristidis, P.; Bozon, D.; Corey, M.; Markiewicz, D.; Rommens, J.; Tsui, L.C.; Durie, P. Genetic Determination of Exocrine Pancreatic Function in Cystic Fibrosis. Am. J. Hum. Genet. 1992, 50, 1178. [Google Scholar] [PubMed]
- Ramsey, M.L.; Li, S.S.; Lara, L.F.; Gokun, Y.; Akshintala, V.S.; Conwell, D.L.; Heintz, J.; Kirkby, S.E.; McCoy, K.S.; Papachristou, G.I.; et al. Cystic Fibrosis Transmembrane Conductance Regulator Modulators and the Exocrine Pancreas: A Scoping Review. J. Cyst. Fibros. 2023, 22, 193–200. [Google Scholar] [CrossRef]
- Stastna, N.; Kunovsky, L.; Svoboda, M.; Pokojova, E.; Homola, L.; Mala, M.; Gracova, Z.; Jerabkova, B.; Skrickova, J.; Trna, J. Improved Nutritional Outcomes and Gastrointestinal Symptoms in Adult Cystic Fibrosis Patients Treated with Elexacaftor/Tezacaftor/Ivacaftor. Dig. Dis. 2024, 42, 361–368. [Google Scholar] [CrossRef]
- Petersen, M.C.; Begnel, L.; Wallendorf, M.; Litvin, M. Effect of Elexacaftor-Tezacaftor-Ivacaftor on Body Weight and Metabolic Parameters in Adults with Cystic Fibrosis. J. Cyst. Fibros. 2022, 21, 265–271. [Google Scholar] [CrossRef]
- Martin, C.; Burnet, E.; Ronayette-Preira, A.; de Carli, P.; Martin, J.; Delmas, L.; Prieur, B.; Burgel, P.-R. Patient Perspectives Following Initiation of Elexacaftor-Tezacaftor-Ivacaftor in People with Cystic Fibrosis and Advanced Lung Disease. Respir. Med. Res. 2021, 80, 100829. [Google Scholar] [CrossRef]
- Bailey, J. Nutritional and Metabolic Effects of Elexacaftor/Tezacaftor/Ivacaftor in Adults and Adolescents with Cystic Fibrosis. Ph.D. Thesis, University of Alabama at Birmingham, Birmingham, Al, USA, 2021. [Google Scholar]
- Caley, L.R.; Jarosz-Griffiths, H.H.; Smith, L.; Gale, L.; Barrett, J.; Kinsey, L.; Davey, V.; Nash, M.; Jones, A.M.; Whitehouse, J.L.; et al. Body Mass Index and Nutritional Intake Following Elexacaftor/Tezacaftor/Ivacaftor Modulator Therapy in Adults with Cystic Fibrosis. J. Cyst. Fibros. 2023, 22, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut Hormone PYY(3-36) Physiologically Inhibits Food Intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Litvin, M.; Yoon, J.C.; Leey Casella, J.; Blackman, S.M.; Brennan, A.L. Energy Balance and Obesity in Individuals with Cystic Fibrosis. J. Cyst. Fibros. 2019, 18, S38–S47. [Google Scholar] [CrossRef]
- Minzoni, A.; Mazzetti, L.; Orlando, P.; Licci, G.; Taccetti, G.; Bresci, S.; Maggiore, G. Cystic Fibrosis-Related Chronic Rhinosinusitis: The Key Role of a Comprehensive Evaluation in the Era of Highly Effective Modulator Therapy. Eur. Arch. Otorhinolaryngol. 2024, 281, 6397–6404. [Google Scholar] [CrossRef]
- Tervo, J.P.; DiMango, E.; Gudis, D.A.; Keating, C.; Zhang, Y.; Leu, C.-S.; Altman, K.; Vilarello, B.; Jacobson, P.; Overdevest, J.B. Olfaction, Body Mass Index, and Quality of Life with Cystic Fibrosis Combination Therapy. Int. Forum Allergy Rhinol. 2023, 13, 2165–2171. [Google Scholar] [CrossRef]
- Bacon, D.R.; Stapleton, A.; Goralski, J.L.; Ebert, C.S.; Thorp, B.D.; Nouraie, M.; Shaffer, A.D.; Senior, B.A.; Lee, S.E.; Zemke, A.C.; et al. Olfaction before and after Initiation of Elexacaftor-Tezacaftor-Ivacaftor in a Cystic Fibrosis Cohort. Int. Forum Allergy Rhinol. 2022, 12, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Beswick, D.M.; Humphries, S.M.; Balkissoon, C.D.; Strand, M.; Vladar, E.K.; Ramakrishnan, V.R.; Taylor-Cousar, J.L. Olfactory Dysfunction in Cystic Fibrosis: Impact of CFTR Modulator Therapy. J. Cyst. Fibros. 2022, 21, e141–e147. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Hu, F.B. The Effects of High Protein Diets on Thermogenesis, Satiety and Weight Loss: A Critical Review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]
- Barkeling, B.; Rössner, S.; Björvell, H. Effects of a High-Protein Meal (Meat) and a High-Carbohydrate Meal (Vegetarian) on Satiety Measured by Automated Computerized Monitoring of Subsequent Food Intake, Motivation to Eat and Food Preferences. Int. J. Obes. 1990, 14, 743–751. [Google Scholar] [PubMed]
- Johnson, J.; Vickers, Z. Effects of Flavor and Macronutrient Composition of Food Servings on Liking, Hunger and Subsequent Intake. Appetite 1993, 21, 25–39. [Google Scholar] [CrossRef]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A High-Protein Diet Induces Sustained Reductions in Appetite, Ad Libitum Caloric Intake, and Body Weight despite Compensatory Changes in Diurnal Plasma Leptin and Ghrelin Concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef]
- Fazzino, T.L.; Courville, A.B.; Guo, J.; Hall, K.D. Ad Libitum Meal Energy Intake Is Positively Influenced by Energy Density, Eating Rate and Hyper-Palatable Food across Four Dietary Patterns. Nat. Food 2023, 4, 144–147. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Hägele, F.A.; Müller, M.J. Impact of Energy Turnover on the Regulation of Energy and Macronutrient Balance. Obesity 2021, 29, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Prévotat, A.; Godin, J.; Bernard, H.; Perez, T.; Le Rouzic, O.; Wallaert, B. Improvement in Body Composition Following a Supervised Exercise-Training Program of Adult Patients with Cystic Fibrosis. Respir. Med. Res. 2019, 75, 5–9. [Google Scholar] [CrossRef]
- Gruet, M.; Saynor, Z.L.; Urquhart, D.S.; Radtke, T. Rethinking Physical Exercise Training in the Modern Era of Cystic Fibrosis: A Step towards Optimising Short-Term Efficacy and Long-Term Engagement. J. Cyst. Fibros. 2022, 21, e83–e98. [Google Scholar] [CrossRef]
- Radtke, T.; Smith, S.; Nevitt, S.J.; Hebestreit, H.; Kriemler, S. Physical Activity and Exercise Training in Cystic Fibrosis. Cochrane Database Syst. Rev. 2022, 8, CD002768. [Google Scholar] [CrossRef] [PubMed]
- Taelman, V.; Declercq, D.; Van Biervliet, S.; Weygaerde, Y.V.; Lapauw, B.; Van Braeckel, E. Effect of 18 months elexacaftor-tezacaftor-ivacaftor on body mass index and glycemic control in adults with cystic fibrosis. Clin. Nutr. ESPEN 2023, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]

| Total Study Population | ETI (n = 38) | non-ETI (n = 16) | p-Value |
|---|---|---|---|
| Age, years | 37 ± 1 | 42 ± 2 | 0.06 |
| BMI, kg/m2 | 23.8 ± 0.3 | 24.7 ± 0.6 | 0.37 |
| ppFEV1, % | 68 ± 2 | 70 ± 3 | 0.75 |
| Calculated REE a, kcal/d | 1511 ± 21 | 1588 ± 43 | 0.11 |
| Exocrine Pancreatic Insufficiency | 35 (92%) | 8 (50%) | <0.001 |
| Pseudomonas aeruginosa | 24 (63%) | 9 (56%) | 0.65 |
| ΔF508 homozygous | 17 (45%) | 3 (19%) | 0.002 |
| ΔF508 heterozygous | 18 (47%) | 7 (44%) | 0.002 |
| CFRD | 15 (40%) | 3 (19%) | 0.06 |
| Women (ETI: n = 22, non-ETI: n = 8) | |||
| BMI, kg/m2 | 23.7 ± 0.5 | 24.6 ± 0.7 | 0.52 |
| FMI, kg/m2 | 7.6 ± 0.4 | 8.4 ± 0.7 | 0.47 |
| FFMI, kg/m2 | 16.1 ± 0.2 | 16.1 ± 0.2 | 0.92 |
| Men (ETI: n = 16, non-ETI: n = 8) | |||
| BMI, kg/m2 | 23.9 ± 0.4 | 24.8 ± 0.9 | 0.57 |
| FMI, kg/m2 | 5.3 ± 0.3 | 5.1 ± 0.6 | 0.67 |
| FFMI, kg/m2 | 18.6 ± 0.2 | 19.7 ± 0.5 | 0.10 |
| ETI (n = 38) | non-ETI (n = 16) | p-Value | |||
|---|---|---|---|---|---|
| mean ± SE | 95% CI | mean ± SE | 95% CI | ETI vs. non-ETI | |
| Δ BMI, kg/m2 | 0.3 ± 0.1 | 0.1–0.5 | 0.3 ± 0.1 | 0.02–0.5 | 0.97 |
| Energy Balance, kcal/ 3 weeks | 4823 ± 2785 | −814–10,460 | 3810 ± 3056 | −2376–9996 | 0.82 |
| Women (ETI: n = 22, non-ETI: n = 8) | |||||
| Δ BMI, kg/m2 | 0.3 ± 0.1 | 0.1–0.6 | 0.3 ± 0.2 | −0.1–0.6 | 0.86 |
| Δ FMI, kg/m2 | 0.1 ± 0.1 | −0.2–0.4 | 0.04 ± 0.2 | −0.5–0.5 | 0.96 |
| Δ FFMI, kg/m2 | 0.2 ± 0.1 | −0.04–0.4 | 0.2 ± 0.2 | −0.1–0.6 | 0.99 |
| Energy Balance, kcal/ 3 weeks | 9444 ± 3330 | 2702–16,186 | 4427 ± 3207 | −2066–10,921 | 0.46 |
| Δ Plasma Leptin, ng/mL a | 7.7 ± 2.7 | 2.1–13.3 | 17.1 ± 16.7 | −17.7–52.0 | 0.82 |
| Men (ETI: n = 16, non-ETI: n = 8) | |||||
| Δ BMI, kg/m2 | 0.2 ± 0.1 | −0.03–0.4 | 0.3 ± 0.2 | −0.1–0.7 | 0.90 |
| Δ FMI, kg/m2 | −0.2 ± 0.1 | −0.5–0.5 | 0.01 ± 0.2 | −0.4–0.4 | 0.64 |
| Δ FFMI, kg/m2 | 0.3 ± 0.1 | 0.1–0.6 | 0.3 ± 0.2 | −0.2–0.8 | 0.99 |
| Energy Balance, kcal/ 3 weeks | 202 ± 3705 | −7299–7703 | 3193 ± 5280 | −7497–13,882 | 0.88 |
| Δ Plasma Leptin, ng/mL a | −0.02 ± 1.5 | −3.1–3.1 | 3.1 ± 2.4 | −2.0–8.2 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koop, J.; Gruber, W.; Hägele, F.A.; Norman, K.; Herpich, C.; Dewey, S.; Falkenberg, C.; Schnabel, O.; Weisser, B.; Hasler, M.; et al. A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy. Nutrients 2025, 17, 2439. https://doi.org/10.3390/nu17152439
Koop J, Gruber W, Hägele FA, Norman K, Herpich C, Dewey S, Falkenberg C, Schnabel O, Weisser B, Hasler M, et al. A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy. Nutrients. 2025; 17(15):2439. https://doi.org/10.3390/nu17152439
Chicago/Turabian StyleKoop, Jana, Wolfgang Gruber, Franziska A. Hägele, Kristina Norman, Catrin Herpich, Stefan Dewey, Christian Falkenberg, Olaf Schnabel, Burkhard Weisser, Mario Hasler, and et al. 2025. "A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy" Nutrients 17, no. 15: 2439. https://doi.org/10.3390/nu17152439
APA StyleKoop, J., Gruber, W., Hägele, F. A., Norman, K., Herpich, C., Dewey, S., Falkenberg, C., Schnabel, O., Weisser, B., Hasler, M., & Bosy-Westphal, A. (2025). A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy. Nutrients, 17(15), 2439. https://doi.org/10.3390/nu17152439







