Effect of Intermittent Fasting on Anthropometric Measurements, Metabolic Profile, and Hormones in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
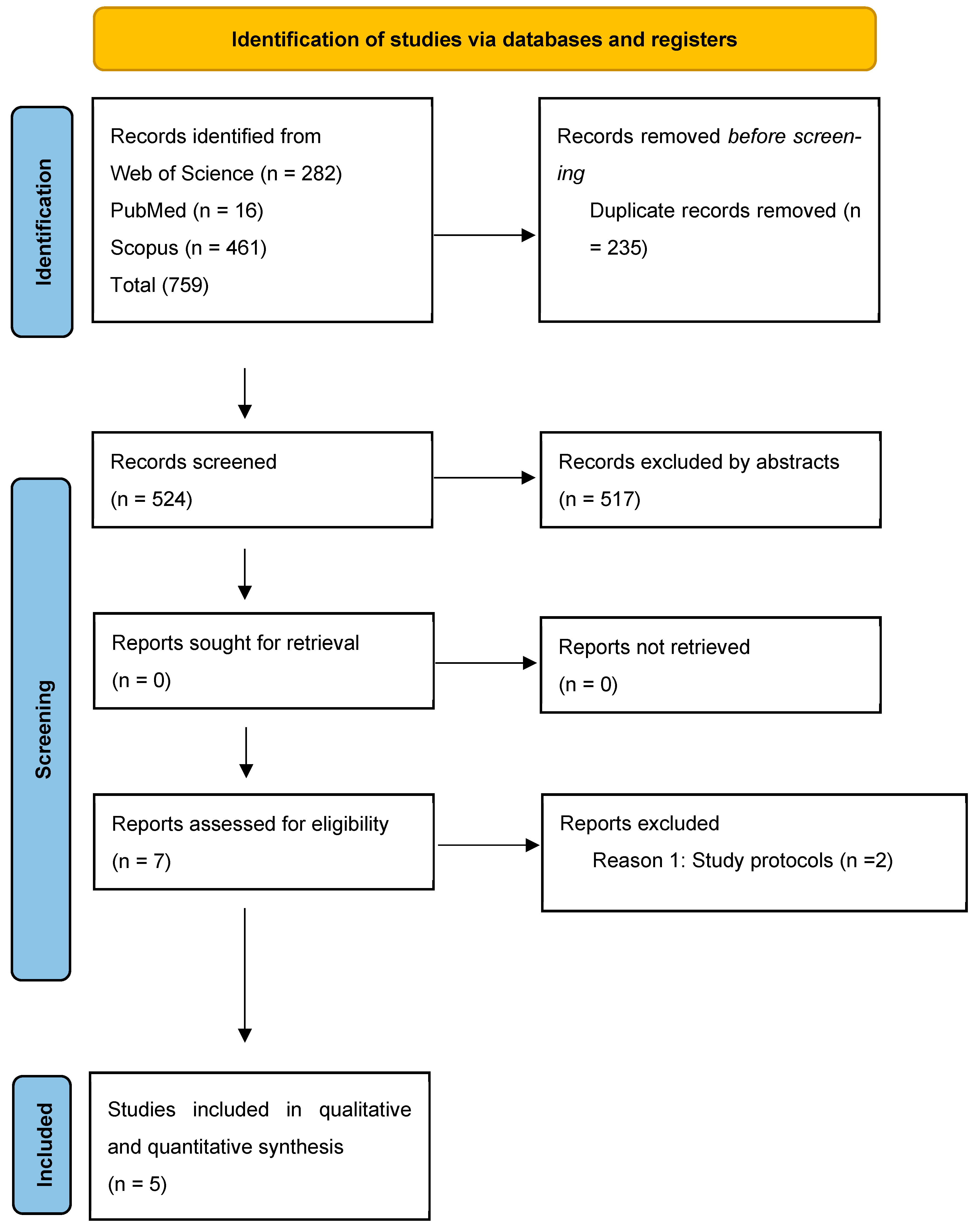
3.1. Literature Selection
| Reference | Location | Study Type | Duration | Sample Size | Age | BMI | Intervention | Control | Method of Caloric Intake Assessment | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| [33] Li et al., 2021 | China | Single arm interventional study | 5 weeks | 15 | 18–40 | ≥24 kg/m2 | TRE 16:8, eat freely during prescribed period | N/A | Food diary using Boohee (version 7.4.2) software from the first to the last day. | ↓ BW, ↓ BMI, ↓ BFM, ↓ BF %, ↓ VFA, ↓ FINS, ↓ HOMA-IR, ↓, ↓ TT, ↓ FAI, ↑ SHBG, ↓ IGF-1, ↓ ALT, ↓ hs-CRP, ↑ Menstrual cyclicity (73% resumed menses), ↔ TG/TC/LDL/HDL, ↔ MM |
| [34] Feyzioglu et al., 2023 | Turkey | Single arm interventional study | 6 weeks | 30 | <18 and >40 years | >30 or <18 kg/m2 | TRE 16:8, eat freely during prescribed period | N/A | N/R | N/A |
| [35] Abu Salma et al., 2024 | Jordan | RCT | 6 months | Intervention (57) Control (29) | 19–40 years | greater than 25 kg/m2 | ADF (4:3), 18 fasting hours on non-consecutive days up to three times per week with 500 calorie restriction weekly. | Restriction of 500 calories/week | Food diary for two non-consecutive weekdays and one weekend day. The food records were analyzed using the Food Processor SQL Nutrition and Software, 2008 (ESHA Version 8.6) | BW, ↓ BMI, ↓ FM, ↓ BF %, ↓ VFA, ↓ WHR, ↑ ↑ MM, ↑ FFM |
| [36] Talebi et al., 2024 | Iran | RCT | 8 weeks | Intervention arm (A) (30), Intervention arm (B) (30), and control (30) | 18–40 years | 25–35 kg/m2 | Arm (1): eTRE (14:10) + probiotic placebo + 25% calorie restriction. Arm (2): eTRE (14:10) + probiotic + 25% calorie restriction. Both groups were eating freely during prescribed period. | Calorie restriction by 25% + probiotic placebo | A 24-h food recall for 3 days at baseline, 2, 4, 6 and 8 weeks into the intervention. Dietary data were computed using Nutritionist IV software version 4.1 (First Databank, San Bruno, CA, USA) modified for Iranian foods. | ↓ LDL-C/HDL-C/TC/ ↓ BW, ↓ hirsutism, ↓ acne score, ↓ HOMA-IR, ↓ FBG, ↓ SHBG, ↔ TT, ↔ AMH |
| [37] Talebi et al., 2025 | Iran | RCT | 8 weeks | Intervention arm (1) (30), Intervention arm (2) (30), and control (30) | 18–40 years | 25–35 kg/m2 | Arm (1): eTRE (14:10) + probiotic placebo + 25% calorie restriction. Arm (2): eTRE (14:10) + probiotic + 25% calorie restriction. Both groups were eating freely during prescribed period. | Calorie restriction by 25% + probiotic placebo | A 24-h food recall for 3 days at baseline, 2, 4, 6 and 8 weeks into the intervention. Dietary data were computed using Nutritionist IV software version 4.1 (First Databank, San Bruno, CA) modified for Iranian foods. | ↓ BW, ↓ BMI, ↓ WC, ↓ SBP (in eTRE groups), ↓ CRP (in all groups), ↑ TAC (in eTRE + probiotic and DCR), ↔ DBP, ↔ TOS, ↔ OSI |
3.2. Studies’ Characteristics
3.3. Risk of Bias Assessment:
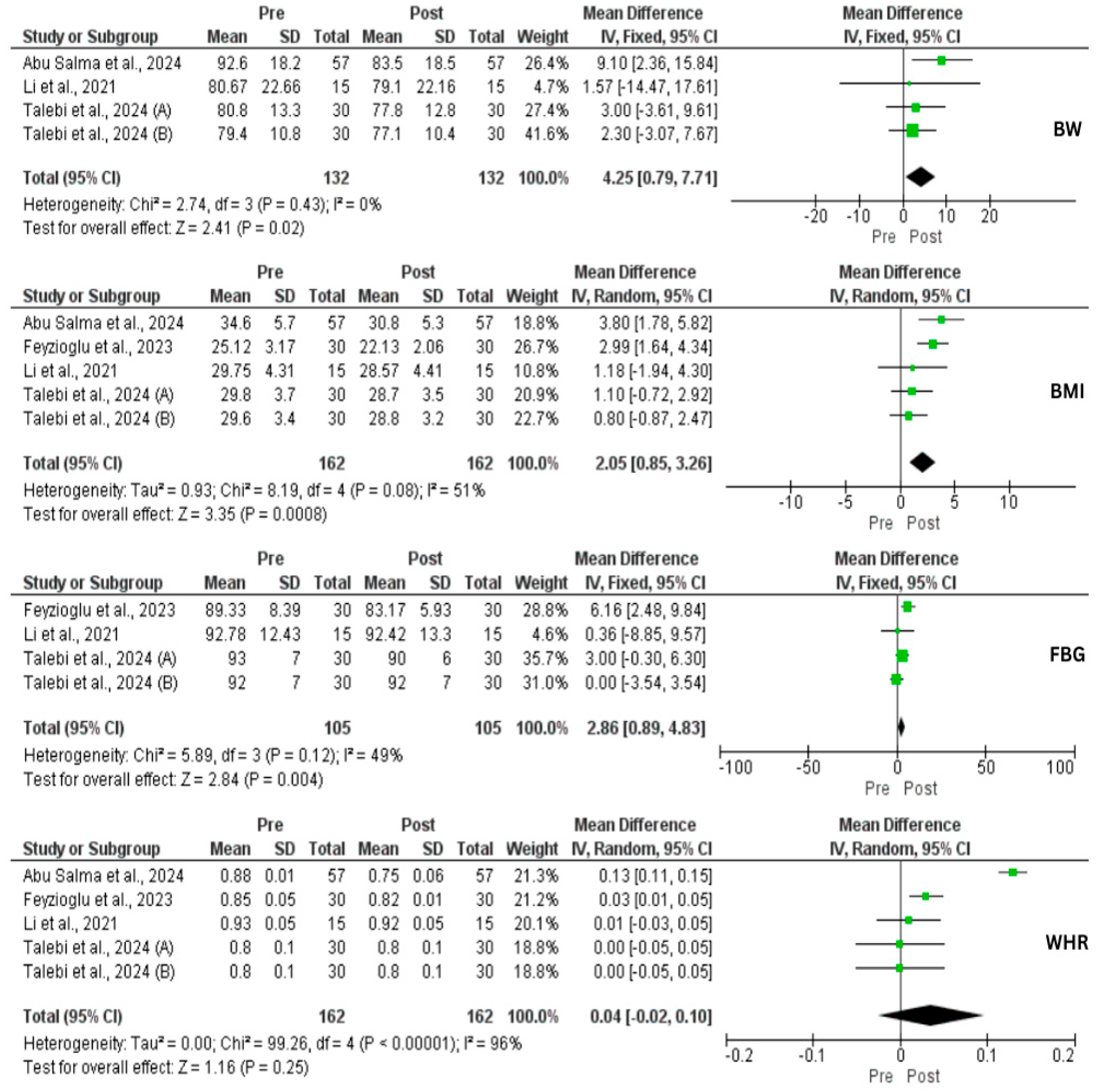
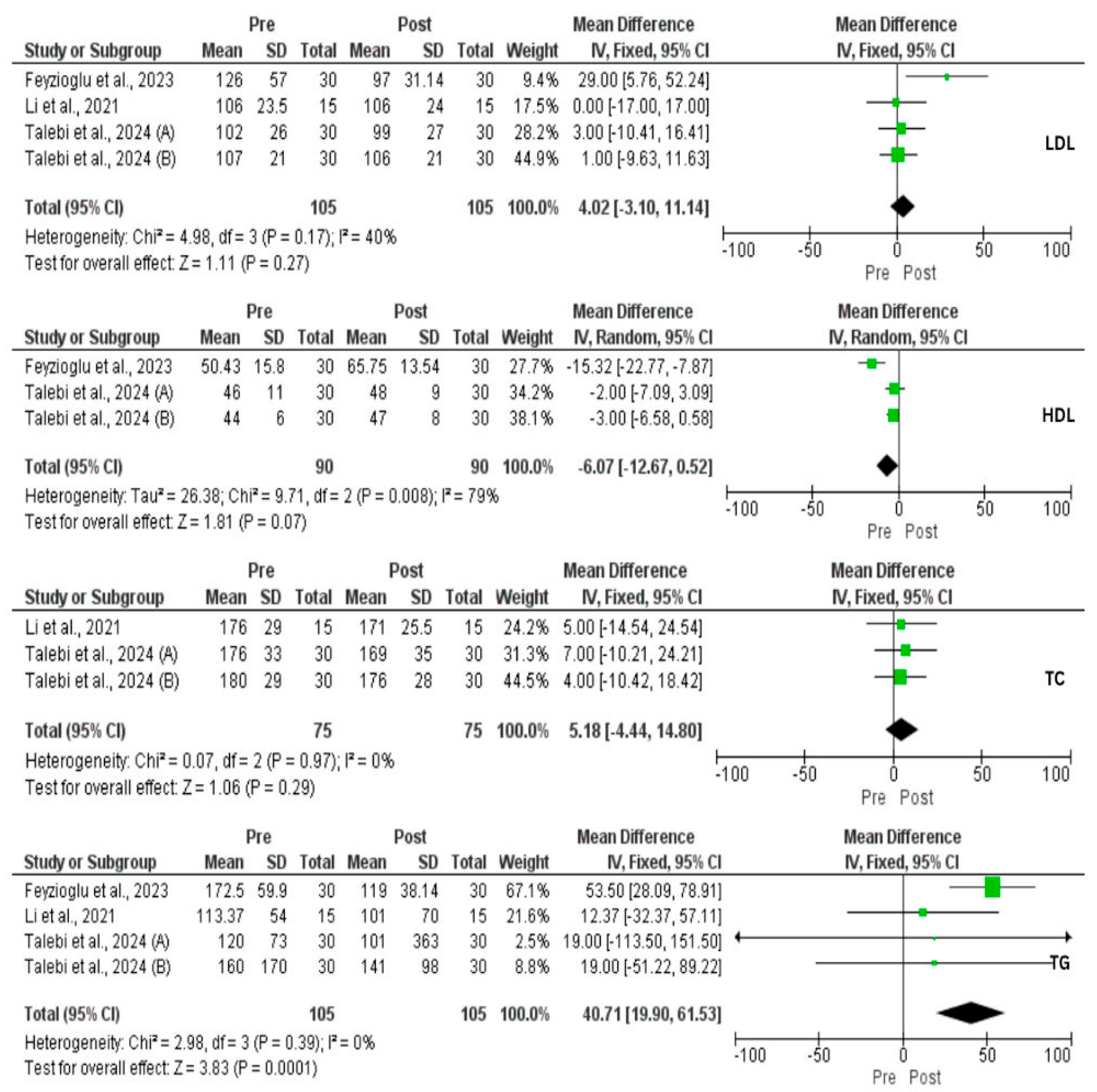
3.4. Effect of IF on Metabolic Profile
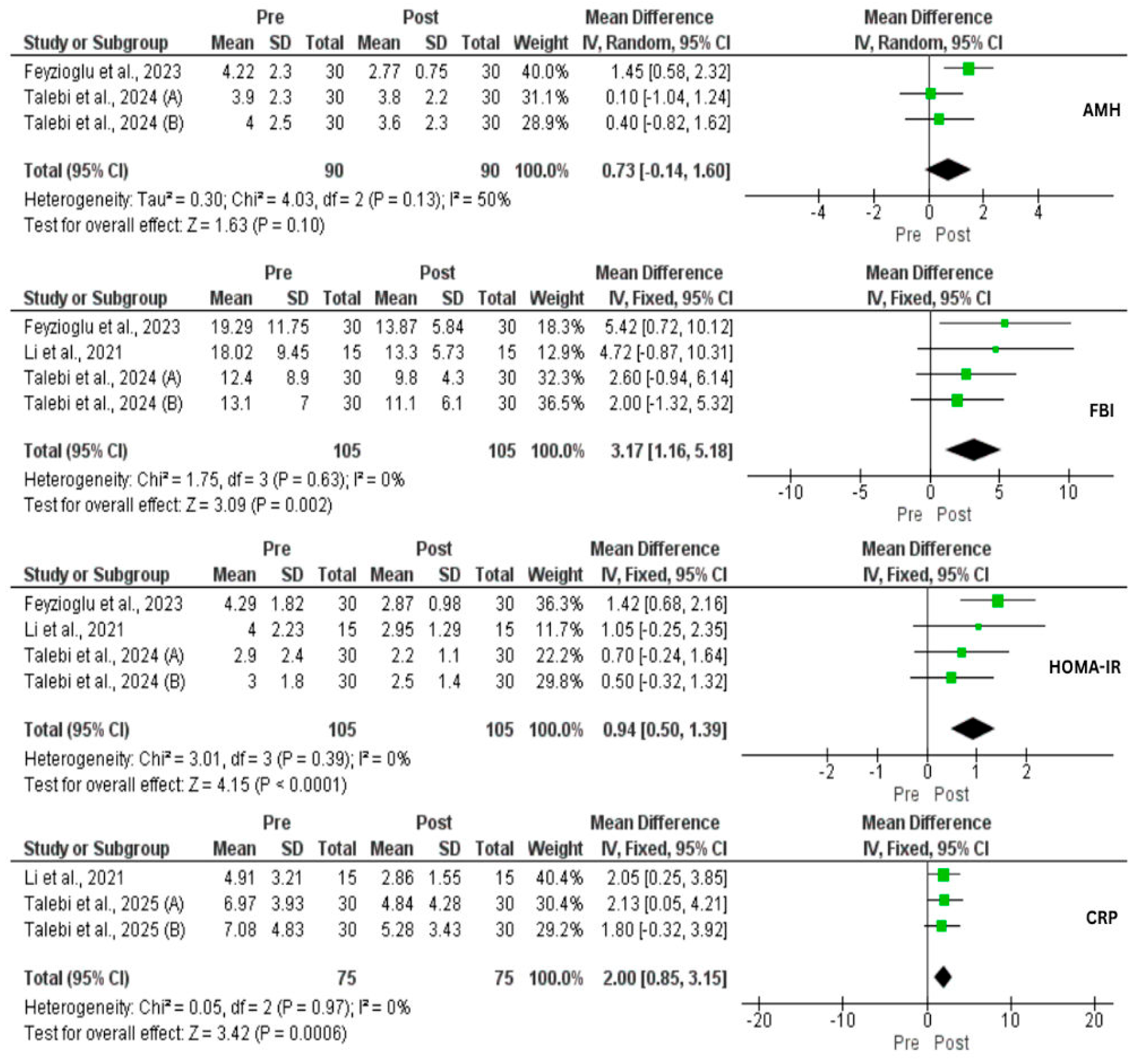
3.5. Effect of IF on Hormones and CRP
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Patel, S. Polycystic Ovary Syndrome (PCOS), an Inflammatory, Systemic, Lifestyle Endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Jozkowiak, M.; Piotrowska-Kempisty, H.; Kobylarek, D.; Gorska, N.; Mozdziak, P.; Kempisty, B.; Rachon, D.; Spaczynski, R.Z. Endocrine Disrupting Chemicals in Polycystic Ovary Syndrome: The Relevant Role of the Theca and Granulosa Cells in the Pathogenesis of the Ovarian Dysfunction. Cells 2023, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Belenkaia, L.V.; Lazareva, L.M.; Walker, W.; Lizneva, D.V.; Suturina, L.V. Criteria, Phenotypes and Prevalence of Polycystic Ovary Syndrome. Minerva Ginecol. 2019, 71, 211–225. [Google Scholar] [CrossRef]
- Teede, H.; Deeks, A.; Moran, L. Polycystic Ovary Syndrome: A Complex Condition with Psychological, Reproductive and Metabolic Manifestations That Impacts on Health across the Lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Rababa’h, A.M.; Matani, B.R.; Yehya, A. An Update of Polycystic Ovary Syndrome: Causes and Therapeutics Options. Heliyon 2022, 8, e11010. [Google Scholar] [CrossRef]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A Brief Insight into the Etiology, Genetics, and Immunology of Polycystic Ovarian Syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef] [PubMed]
- Kshetrimayum, C.; Sharma, A.; Mishra, V.V.; Kumar, S. Polycystic Ovarian Syndrome: Environmental/ Occupational, Lifestyle Factors; an Overview. J. Turk. Ger. Gynecol. Assoc. 2019, 20, 255–263. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Heal. 2019, 13, 1179558119874042. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Obesity and Polycystic Ovary Syndrome. Clin. Endocrinol. 2021, 95, 531–541. [Google Scholar] [CrossRef]
- Herman, R.; Sikonja, J.; Jensterle, M.; Janez, A.; Dolzan, V. Insulin Metabolism in Polycystic Ovary Syndrome: Secretion, Signaling, and Clearance. Int. J. Mol. Sci. 2023, 24, 3140. [Google Scholar] [CrossRef]
- Jurczewska, J.; Ostrowska, J.; Chełchowska, M.; Panczyk, M.; Rudnicka, E.; Kucharski, M.; Smolarczyk, R.; Szostak-Węgierek, D. Abdominal Obesity in Women with Polycystic Ovary Syndrome and Its Relationship with Diet, Physical Activity and Insulin Resistance: A Pilot Study. Nutrients 2023, 15, 3652. [Google Scholar] [CrossRef]
- de Melo Cavalcante, R.B.; Leão, L.M.C.S.M.; Tavares, A.B.W.; Lopes, K.G.; Kraemer-Aguiar, L.G. Fat Distribution and Its Correlation with Insulin Resistance, Androgen Markers, and Proinflammatory Cytokines in Polycystic Ovary Syndrome. Horm. Metab. Res. 2025, 57, 25–32. [Google Scholar] [CrossRef]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Wang, R.; Xue, S.; Ying, Q.; Jin, L. Roles of Estrogen and Its Receptors in Polycystic Ovary Syndrome. Front. Cell Dev. Biol. 2024, 12, 1395331. [Google Scholar] [CrossRef]
- Shirvanizadeh, F.; Eidi, A.; Hafezi, M.; Eftekhari-Yazdi, P. Abdominal Obesity May Play a Significant Role in Exacerbation of Inflammation in Polycystic Ovary Syndrome Patients. JBRA Assist. Reprod. 2023, 27, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.J.M.; Tarlatzis; Fauser; Chang; Aziz; Legro; Dewailly; Franks; Balen; Bouchard; et al. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, S.H. Effectiveness of Lifestyle Modification in Polycystic Ovary Syndrome Patients with Obesity: A Systematic Review and Meta-Analysis. Life 2022, 12, 308. [Google Scholar] [CrossRef]
- Juhász, A.E.; Stubnya, M.P.; Teutsch, B.; Gede, N.; Hegyi, P.; Nyirády, P.; Bánhidy, F.; Ács, N.; Juhász, R. Ranking the Dietary Interventions by Their Effectiveness in the Management of Polycystic Ovary Syndrome: A Systematic Review and Network Meta-Analysis. Reprod. Health 2024, 21, 28. [Google Scholar] [CrossRef]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
- Mulas, A.; Cienfuegos, S.; Ezpeleta, M.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of Intermittent Fasting on Circulating Inflammatory Markers in Obesity: A Review of Human Trials. Front. Nutr. 2023, 10, 1146924. [Google Scholar] [CrossRef]
- Naous, E.; Achkar, A.; Mitri, J. Intermittent Fasting and Its Effects on Weight, Glycemia, Lipids, and Blood Pressure: A Narrative Review. Nutrients 2023, 15, 3661. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Vatier, C.; Christin-Maitre, S. Epigenetic/circadian Clocks and PCOS. Hum. Reprod. 2024, 39, 1167–1175. [Google Scholar] [CrossRef]
- Kalsekar, A.S.; Abdelrahim, D.N.; Faris, M.E. Effect of Calorie Restriction and Intermittent Fasting on Glucose Homeostasis, Lipid Profile, Inflammatory, and Hormonal Markers in Patients with Polycystic Ovary Syndrome: A Systematic Review. Front. Nutr. 2024, 11, 1362226. [Google Scholar] [CrossRef] [PubMed]
- Velissariou, M.; Athanasiadou, C.R.; Diamanti, A.; Lykeridou, A.; Sarantaki, A. The Impact of Intermittent Fasting on Fertility: A Focus on Polycystic Ovary Syndrome and Reproductive Outcomes in Women-A Systematic Review. Metab. Open 2025, 25, 100341. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 372, 790–799. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2 [Updated February 2021]; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range And/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Li, K.; Hu, L.; Li, X.; Yuan, Z.; He, J.; Liu, D.; Yang, G.; Yuan, L. Effect of C-Reactive Protein Deficiency on Insulin Resistance Reversal in Rats with Polycystic Ovary Syndrome through Augmented Leptin Action. Diabetol. Metab. Syndr. 2023, 15, 180. [Google Scholar] [CrossRef]
- Feyzioglu, B.S.; Güven, C.M.; Avul, Z. Eight-Hour Time-Restricted Feeding: A Strong Candidate Diet Protocol for First-Line Therapy in Polycystic Ovary Syndrome. Nutrients 2023, 15, 2260. [Google Scholar] [CrossRef]
- Abu Salma, B.M.; Thekrallah, F.; Qatawneh, A.; Hasan, H.; Shawaqfeh, S.; Altarawneh, M. Effect of Intermittent Fasting on Improve Body Composition and Anthropometric Measurements of Women with Polycystic Ovarian Syndrome. Nutr. Clin. Diet. Hosp. 2024, 44, 122–129. [Google Scholar] [CrossRef]
- Talebi, S.; Shab-Bidar, S.; Moini, A.; Mohammadi, H.; Djafarian, K. The Effects of Time-restricted Eating Alone or in Combination with Probiotic Supplementation in Comparison with a Calorie-restricted Diet on Endocrine and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Clinical Trial. Diabetes Obes. Metab. 2024, 26, 4468–4479. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Shab-Bidar, S.; Askari, G.; Mohammadi, H.; Moini, A.; Djafarian, K. Comparison of the Impact of Intermittent Fasting Diet Alone or in Conjunction with Probiotic Supplementation versus Calorie-Restricted Diet on Inflammatory, Oxidative Stress, and Antioxidant Capacity Biomarkers in Women with Polycystic Ovary Syndrome: A randomized placebo-controlled trial. J. Res. Med. Sci. 2025, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rather, H.A.; Neelam; Kumar, R.; Basheer, M.; Reshi, M.S. A Review on Critical Appraisal and Pathogenesis of Polycystic Ovarian Syndrome. Endocr. Metab. Sci. 2024, 14, 100162. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Singh, P.; Bhogal, G.; Broughton, S.; Tahrani, A.A. How to Manage Weight Loss in Women with Obesity and PCOS Seeking Fertility? Clin. Endocrinol. 2022, 97, 208–216. [Google Scholar] [CrossRef]
- Floyd, R.; Gryson, R.; Mockler, D.; Gibney, J.; Duggan, S.N.; Behan, L.A. The Effect of Time-Restricted Eating on Insulin Levels and Insulin Sensitivity in Patients with Polycystic Ovarian Syndrome: A Systematic Review. Int. J. Endocrinol. 2022, 2022, 2830545. [Google Scholar] [CrossRef]
- Mazza, E.; Troiano, E.; Ferro, Y.; Lisso, F.; Tosi, M.; Turco, E.; Pujia, R.; Montalcini, T. Obesity, Dietary Patterns, and Hormonal Balance Modulation: Gender-Specific Impacts. Nutrients 2024, 16, 1629. [Google Scholar] [CrossRef] [PubMed]
- Scragg, J.; Hobson, A.; Willis, L.; Taylor, K.S.; Dixon, S.; Jebb, S.A. Effect of Weight Loss Interventions on the Symptomatic Burden and Biomarkers of Polycystic Ovary Syndrome. Ann. Intern. Med. 2024, 177, 1664–1674. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Guo, Y.; Lai, Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2019, 2019, 4386401. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, S.A.; Elkmeshi, N.; Alanazi, A.; Alanazi, S.T.; Khan, R.; Amer, S. The Impact of Diet-Induced Weight Loss on Inflammatory Status and Hyperandrogenism in Women with Polycystic Ovarian Syndrome (PCOS)—A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4934. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.W.; Cho, S.J.; Lee, M.; Lee, Y.H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of Intermittent Fasting Diet on Glucose and Lipid Metabolism and Insulin Resistance in Patients with Impaired Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 6999907. [Google Scholar] [CrossRef]
- Sun, M.-L.; Yao, W.; Wang, X.-Y.; Gao, S.; Varady, K.A.; Forslund, S.K.; Zhang, M.; Shi, Z.-Y.; Cao, F.; Zou, B.-J.; et al. Intermittent Fasting and Health Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses of Randomised Controlled Trials. eClinicalMedicine 2024, 70, 102519. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-Restricted Eating for the Prevention and Management of Metabolic Diseases. Endocr. Rev. 2022, 43, 405–436. [Google Scholar] [CrossRef]
- Bao, R.; Sun, Y.; Jiang, Y.; Ye, L.; Hong, J.; Wang, W. Effects of Time-Restricted Feeding on Energy Balance: A Cross-Over Trial in Healthy Subjects. Front. Endocrinol. 2022, 13, 870054. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-Rutkowska, J.; Ziętek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome—Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Hamsho, M.; Shkorfu, W.; Ranneh, Y.; Fadel, A. Nourishing the Evidence: Exposing Bias and Filling Gaps in Isocaloric Intermittent Fasting research—An Opinion. Front. Nutr. 2025, 12, 1563017. [Google Scholar] [CrossRef]
- Hamsho, M.; Shkorfu, W.; Ranneh, Y.; Fadel, A. Is Isocaloric Intermittent Fasting Superior to Calorie Restriction? A Systematic Review and Meta-Analysis of RCTs. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103805. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; De Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Skowronek, M.; Zapałowska-Chwyć, M.; Starczewski, A. Quantitative Assessment of Nutrition in Patients with Polycystic Ovary Syndrome (PCOS). Rocz. Panstw. Zakl. Hig. 2016, 67, 419–426. [Google Scholar]
- Hamsho, M.; Ranneh, Y.; Fadel, A. Therapeutic Effects of Chromium Supplementation on Women with Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. Endocrinol. Diabetes Nutr. 2025, in press. [CrossRef]
- Liu, H.; Shangguan, F.; Liu, F.; Guo, Y.; Yu, H.; Li, H.; Su, Y.; Li, Z. Evaluating the effects of time-restricted eating on overweight and obese women with polycystic ovary syndrome: A randomized controlled trial study protocol. PLoS ONE 2025, 20, e0316333. [Google Scholar] [CrossRef]
- Talebi, S.; Shab-Bidar, S.; Mohammadi, H.; Moini, A.; Djafarian, K. The effects of intermittent fasting diet alone or in combination with probiotic supplementation in comparison with calorie-restricted diet on metabolic and hormonal profile in patients with polycystic ovary syndrome: Study protocol for a randomized clinical trial. Trials 2023, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranneh, Y.; Hamsho, M.; Shkorfu, W.; Terzi, M.; Fadel, A. Effect of Intermittent Fasting on Anthropometric Measurements, Metabolic Profile, and Hormones in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 2436. https://doi.org/10.3390/nu17152436
Ranneh Y, Hamsho M, Shkorfu W, Terzi M, Fadel A. Effect of Intermittent Fasting on Anthropometric Measurements, Metabolic Profile, and Hormones in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(15):2436. https://doi.org/10.3390/nu17152436
Chicago/Turabian StyleRanneh, Yazan, Mohammed Hamsho, Wijdan Shkorfu, Merve Terzi, and Abdulmannan Fadel. 2025. "Effect of Intermittent Fasting on Anthropometric Measurements, Metabolic Profile, and Hormones in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis" Nutrients 17, no. 15: 2436. https://doi.org/10.3390/nu17152436
APA StyleRanneh, Y., Hamsho, M., Shkorfu, W., Terzi, M., & Fadel, A. (2025). Effect of Intermittent Fasting on Anthropometric Measurements, Metabolic Profile, and Hormones in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Nutrients, 17(15), 2436. https://doi.org/10.3390/nu17152436







