Prevention of Metabolic Impairment by Dietary Nitrate in Overweight Male Mice Improves Stroke Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Size Calculation
2.3. Experimental Design
2.4. Metabolic Assessments
2.4.1. Fasting Glycemia
2.4.2. Insulin Tolerance Test (ITT)
2.4.3. Glucose Tolerance Test (GTT)
2.5. Transient Middle Cerebral Artery Occlusion
2.6. Behavioral Assessment
2.7. Tissue Collection
2.8. Immunohistochemistry (IHC)
2.9. Analysis
2.9.1. Quantification of Infarct Size
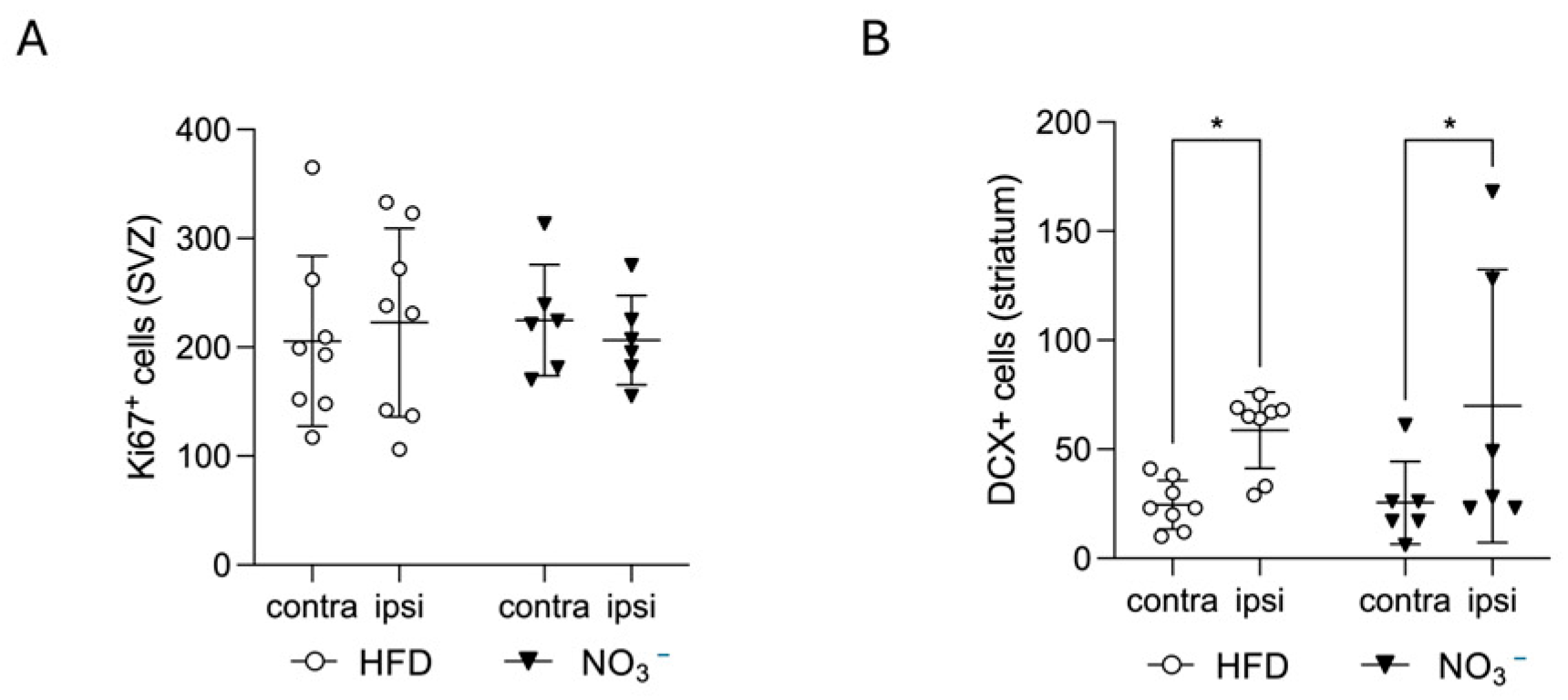
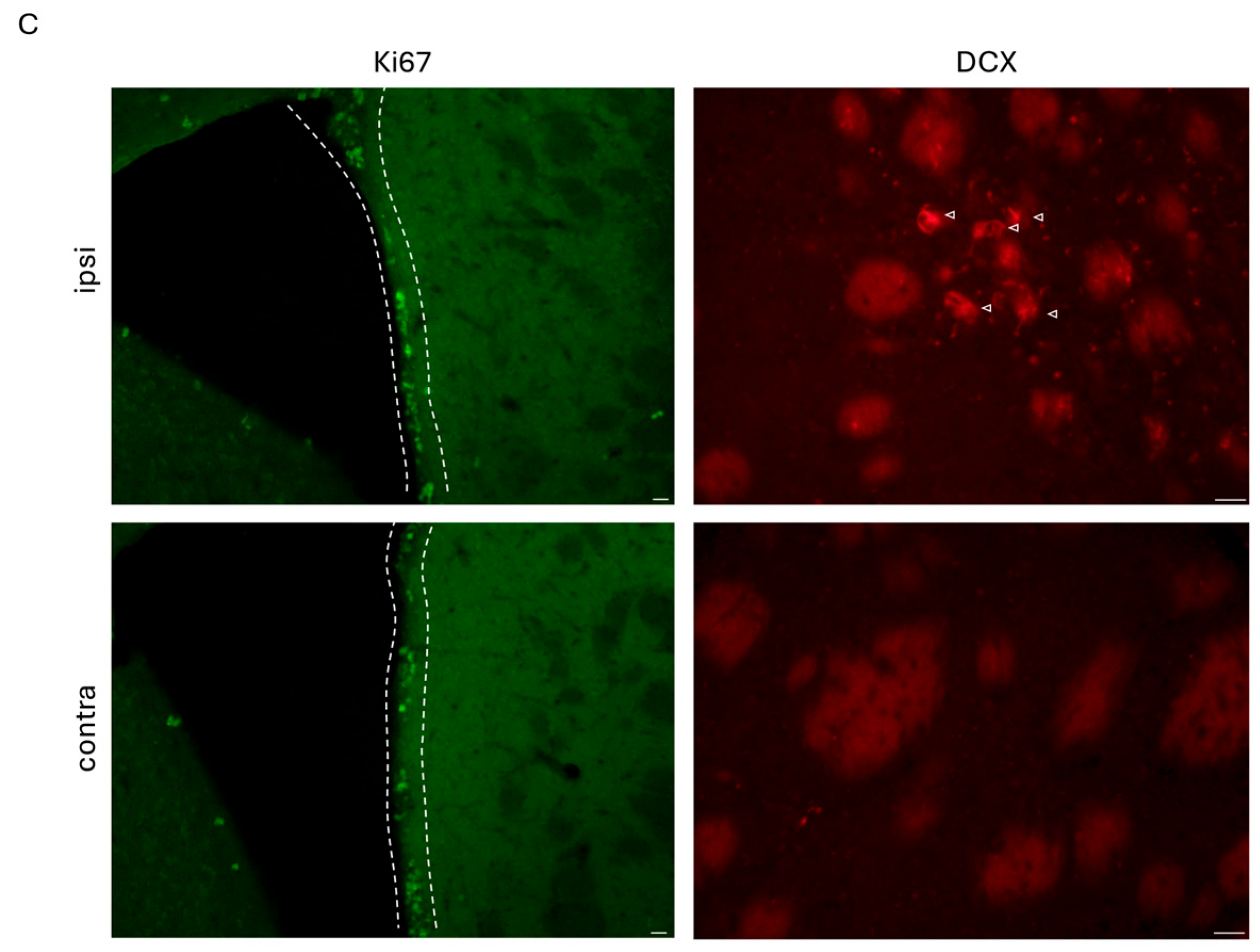
2.9.2. Quantification of Stroke-Induced Neural Stem Cell Proliferation (Ki67) and Early Neurogenesis (DCX)
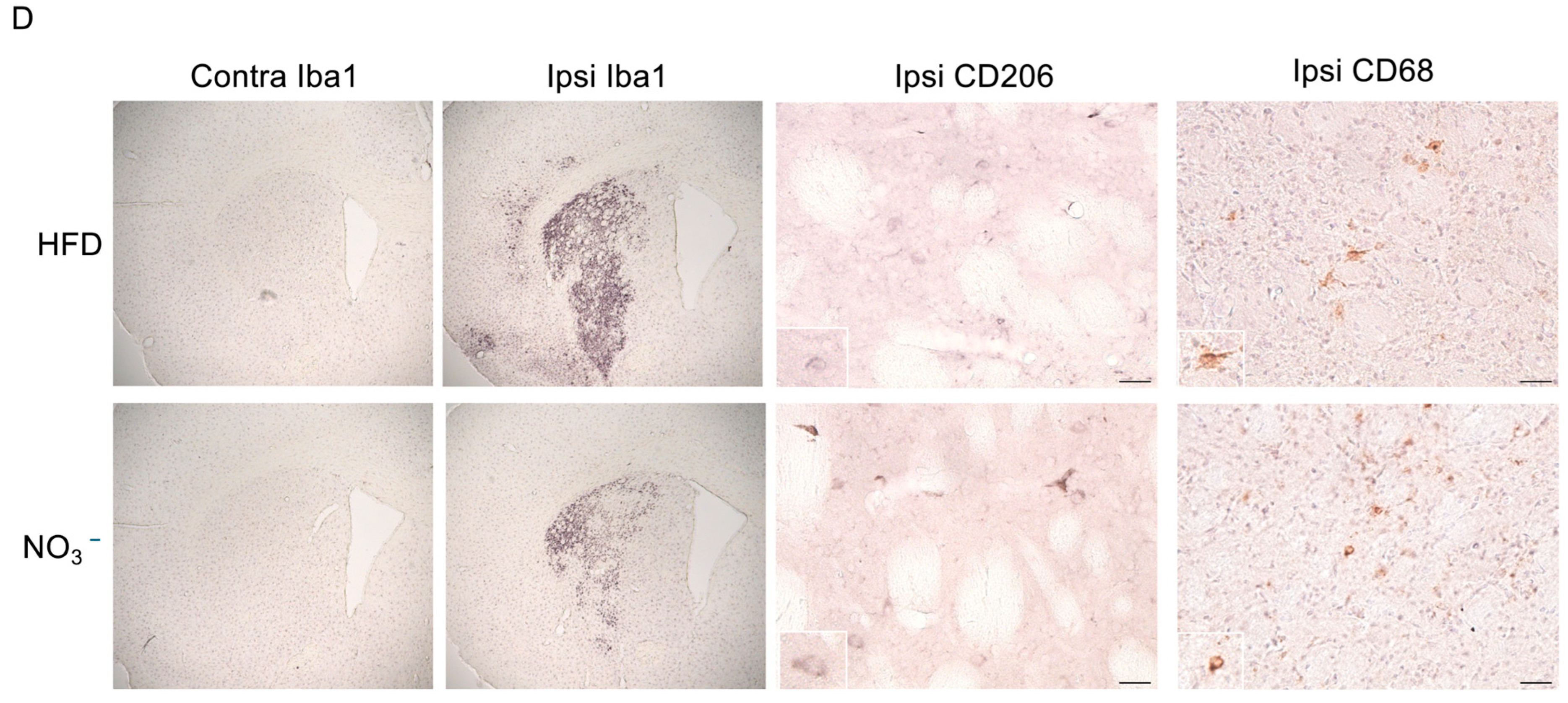
2.9.3. Quantification of Neuroinflammation
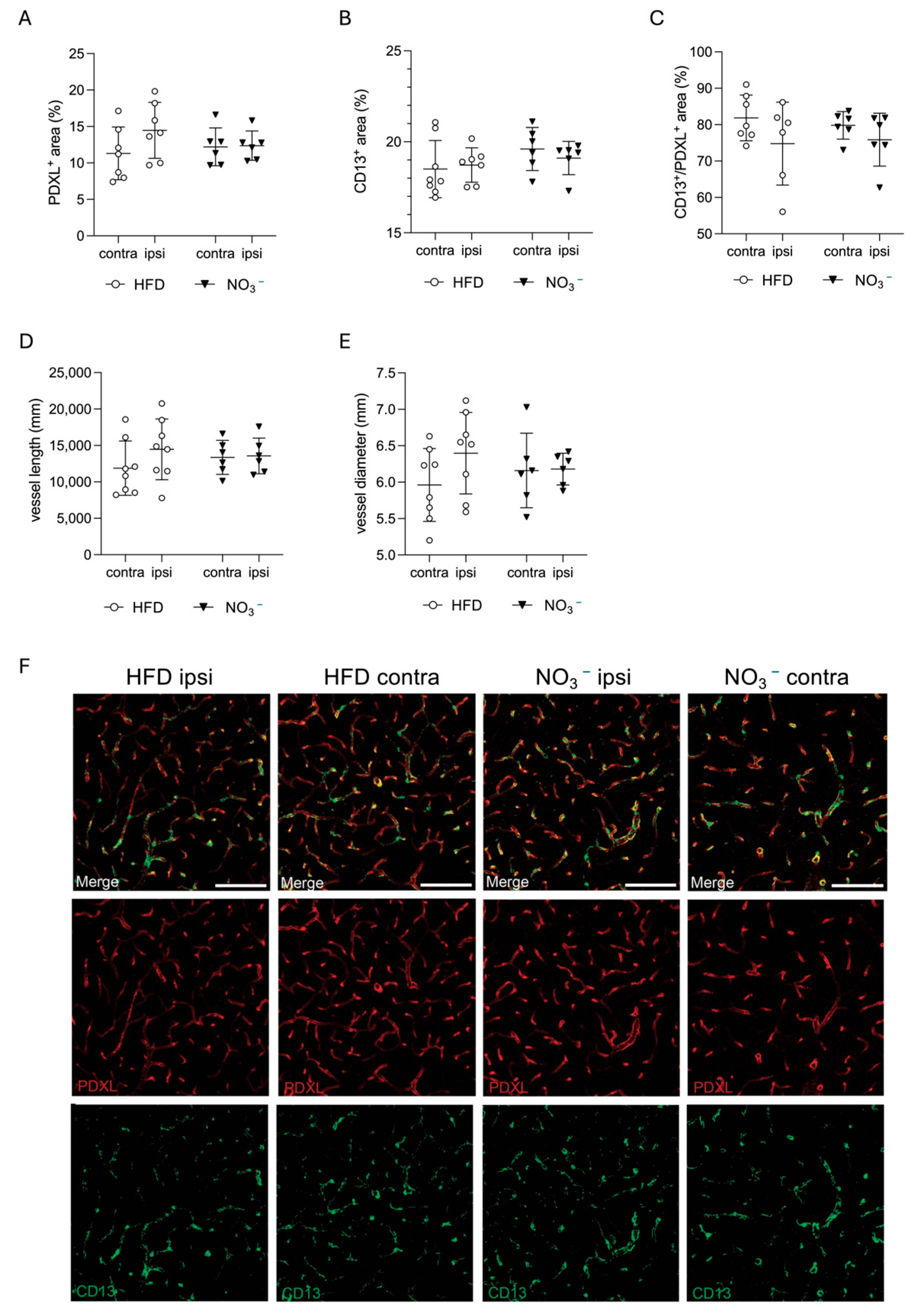
2.9.4. Quantification of Neovascularization
2.10. Statistical Analysis
3. Results
3.1. Sustained NO3− Supplementation in Overweight Mice Attenuates the Development of Obesity and Hyperglycemia and Improves Stroke Recovery
3.2. Improved Stroke Outcome in NO3− Mice Was Not Associated with Increased Stroke-Induced Early Neurogenesis
3.3. Improved Stroke Outcome in NO3− Mice Was Associated with Decreased Post-Stroke Inflammation
3.4. Improved Stroke Outcome in NO3− Mice Was Not Associated with Improved Post-Stroke Vascularization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckel, R.H.; Alberti, K.G.M.M.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Ullberg, T.; Zia, E.; Petersson, J.; Norrving, B. Changes in functional outcome over the first year after stroke: An observational study from the Swedish stroke register. Stroke 2015, 46, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Megherbi, S.E.; Milan, C.; Minier, D.; Couvreur, G.; Osseby, G.V.; Tilling, K.; Di Carlo, A.; Inzitari, D.; Wolfe, C.D.; Moreau, T.; et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: Data from the European BIOMED Stroke Project. Stroke 2003, 34, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Braggio, M.; Dorelli, G.; Olivato, N.; Lamberti, V.; Valenti, M.T.; Dalle Carbonare, L.; Cominacini, M. Tailored Exercise Intervention in Metabolic Syndrome: Cardiometabolic Improvements Beyond Weight Loss and Diet—A Prospective Observational Study. Nutrients 2025, 17, 872. [Google Scholar] [CrossRef] [PubMed]
- Muszalska, A.; Wiecanowska, J.; Michałowska, J.; Pastusiak-Zgolińska, K.M.; Polok, I.; Łompieś, K.; Bogdański, P. The Role of the Planetary Diet in Managing Metabolic Syndrome and Cardiovascular Disease: A Narrative Review. Nutrients 2025, 17, 862. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.A.; Morton, J. Benefits of weight loss of 10% or more in patients with overweight or obesity: A review. Obesity 2022, 30, 802–840. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Inzucchi, S.E. Treating Diabetes to Prevent Stroke. Stroke 2021, 52, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Ghusn, W.; De La Rosa, A.; Sacoto, D.; Cifuentes, L.; Campos, A.; Feris, F.; Hurtado, M.D.; Acosta, A. Weight Loss Outcomes Associated with Semaglutide Treatment for Patients with Overweight or Obesity. JAMA Netw. Open. 2022, 5, e2231982. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.J.; Goodwin Cartwright, B.M.; Gratzl, S.; Brar, R.; Baker, C.; Gluckman, T.J.; Stucky, N.L. Comparative Effectiveness of Semaglutide and Tirzepatide for Weight Loss in Adults with Overweight and Obesity in the US: A Real-World Evidence Study. medRxiv 2023. [Google Scholar] [CrossRef]
- Abdel-Bary, M.; Brody, A.; Schmitt, J.; Prieto, K.; Wetzel, A.; Juo, Y.Y. Treating class 2–3 obesity with glucagon-like peptide-1 agonists: A 2-year real-world cohort study. Diabetes Obes. Metab. 2025, 27, 3195–3200. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Horberg, M.; Koebnick, C.; Young, D.R.; Waitzfelder, B.; Sherwood, N.E.; Daley, M.F.; Ferrara, A. Cardiometabolic risk factors among 1.3 million adults with overweight or obesity, but not diabetes, in 10 geographically diverse regions of the United States, 2012–2013. Prev. Chronic Dis. 2017, 14, E22. [Google Scholar] [CrossRef] [PubMed]
- Domosławska-Żylińska, K.; Łopatek, M.; Krysińska-Pisarek, M.; Sugay, L. Barriers to Adherence to Healthy Diet and Recommended Physical Activity Perceived by the Polish Population. J. Clin. Med. 2024, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Jeddi, S.; Carlström, M.; Gholami, H.; Ghasemi, A. Effects of long-term nitrate supplementation on carbohydrate metabolism, lipid profiles, oxidative stress, and inflammation in male obese type 2 diabetic rats. Nitric Oxide 2018, 75, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Ferreira, D.M.S.; Tarnawski, L.; McCann Haworth, S.; Xuechen, L.; Zhuge, Z.; Newton, P.T.; Massart, J.; Chagin, A.S.; Olofsson, P.S.; et al. Dietary nitrate attenuates high-fat diet-induced obesity via mechanisms involving higher adipocyte respiration and alterations in inflammatory status. Redox Biol. 2020, 28, 101387. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Luo, M.; Tian, R.; Lu, N. Dietary nitrate attenuated endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: A critical role for NADPH oxidase. Arch. Biochem. Biophys. 2020, 689, 108453. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Larsen, F.J.; Nyström, T.; Hezel, M.; Borniquel, S.; Weitzberg, E.; Lundberg, J.O. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17716–17720. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, A.; Bondonno, N.P.; Murray, K.; Zhong, L.; Rainey-Smith, S.R.; Gardener, S.L.; Blekkenhorst, L.C.; Ames, D.; Maruff, P.; Martins, R.N.; et al. Habitual dietary nitrate intake and cognition in the Australian Imaging, Biomarkers and Lifestyle Study of ageing: A prospective cohort study. Clin. Nutr. 2023, 42, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Thompson, K.G.; Blackwell, J.R.; Winyard, P.G.; Forster, J.; Jones, A.M.; Kennedy, D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015, 149, 149–158. [Google Scholar] [CrossRef] [PubMed]
- de Crom, T.O.E.; Blekkenhorst, L.; Vernooij, M.W.; Ikram, M.K.; Voortman, T.; Ikram, M.A. Dietary nitrate intake in relation to the risk of dementia and imaging markers of vascular brain health: A population-based study. Am. J. Clin. Nutr. 2023, 118, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Blekkenhorst, L.C.; Prince, R.L.; Ivey, K.L.; Lewis, J.R.; Devine, A.; Woodman, R.J.; Lundberg, J.O.; Croft, K.D.; Thompson, P.L.; et al. Association of Vegetable Nitrate Intake with Carotid Atherosclerosis and Ischemic Cerebrovascular Disease in Older Women. Stroke 2017, 48, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A. Association of urinary nitrate with lower prevalence of hypertension and stroke and with reduced risk of cardiovascular mortality. Circulation 2018, 137, 2295–2297. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Chu, K.; Ko, S.Y.; Lee, S.T.; Sinn, D.I.; Park, D.K.; Kim, J.M.; Song, E.C.; Kim, M.; Roh, J.K. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke 2006, 37, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.; Zhou, J.; Wang, Y.; Wang, H.; Wang, Y. Nitrate Metabolism and Ischemic Cerebrovascular Disease: A Narrative Review. Front. Neurol. 2022, 13, 735181. [Google Scholar] [CrossRef] [PubMed]
- Allan, P.D.; Tzeng, Y.C.; Gowing, E.K.; Clarkson, A.N.; Fan, J.L. Dietary nitrate supplementation reduces low frequency blood pressure fluctuations in rats following distal middle cerebral artery occlusion. J. Appl. Physiol. 2018, 125, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Sandset, E.C.; Appleton, J.P.; Berge, E.; Christensen, H.; Gommans, J.; Krishnan, K.; Ntaios, G.; Phillips, S.; Pocock, S.; Sprigg, N.; et al. Associations between change in blood pressure and functional outcome, early events and death: Results from the Efficacy of Nitric Oxide in Stroke trial. J. Hypertens. 2019, 37, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.L.; O’Donnell, T.; Lanford, J.; Croft, K.; Watson, E.; Smyth, D.; Koch, H.; Wong, L.K.; Tzeng, Y.C. Dietary nitrate reduces blood pressure and cerebral artery velocity fluctuations and improves cerebral autoregulation in transient ischemic attack patients. J. Appl. Physiol. 2020, 129, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, G.; Lombardo, M.; Kerav, S.; Aiello, G.; Baldelli, S. Nitric Oxide in Parkinson’s Disease: The Potential Role of Dietary Nitrate in Enhancing Cognitive and Motor Health via the Nitrate–Nitrite–Nitric Oxide Pathway. Nutrients 2025, 17, 393. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Huang, P.L.; Panahian, N.; Fishman, M.C.; Moskowitz, M.A. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J. Cereb. Blood Flow Metab. 1996, 16, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Pintana, H.; Lietzau, G.; Augestad, I.L.; Chiazza, F.; Nyström, T.; Patrone, C.; Darsalia, V. Obesity-induced type 2 diabetes impairs neurological recovery after stroke in correlation with decreased neurogenesis and persistent atrophy of parvalbumin-positive interneurons. Clin. Sci. 2019, 133, 1367–1386. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, A.; Tinson, D.; Bradley, L.; Hewer, R.L. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.D.; Harry, J.D. Brain volume estimation from serial section measurements: A comparison of methodologies. J. Neurosci. Methods 1990, 35, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Vercalsteren, E.; Karampatsi, D.; Buizza, C.; Nyström, T.; Klein, T.; Paul, G.; Patrone, C.; Darsalia, V. The SGLT2 inhibitor Empagliflozin promotes post-stroke functional recovery in diabetic mice. Cardiovasc. Diabetol. 2024, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Fernández-González, R.; Muñoz-Barrutia, A.; Ortiz-De-Solorzano, C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010, 73, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Victoria, A.S.; Roy, B.; Victoria, L.H. Development of an ImageJ-based method for analysing the developing zebrafish vasculature. Vasc. Cell. 2017, 9, 2. [Google Scholar]
- Elfarnawany, M.H. Signal Processing Methods for Quantitative Power Doppler Microvascular Angiography. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2015. [Google Scholar]
- Teplyi, V.; Grebchenko, K. Evaluation of the scars’ vascularization using computer processing of the digital images. Ski. Res. Technol. 2019, 25, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, L.; Zhang, Z.; Wang, Y.; Lu, M.; LaPointe, M.; Chopp, M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann. Neurol. 2001, 50, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Ceanga, M.; Dahab, M.; Witte, O.W.; Keiner, S. Adult Neurogenesis and Stroke: A Tale of Two Neurogenic Niches. Front. Neurosci. 2021, 15, 700297. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Shakya, A.K.; Perez-Pinzon, M.A.; Dave, K.R. Cerebral ischemic damage in diabetes: An inflammatory perspective. J. Neuroinflamm. 2017, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Durafourt, B.A.; Moore, C.S.; Zammit, D.A.; Johnson, T.A.; Zaguia, F.; Guiot, M.C.; Guiot, M.C.; Bar-Or, A.; Antel, J.P. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 2012, 60, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, H.; Riese, S.; Régnier-Vigouroux, A. Functional characterization of mannose receptor expressed by immunocompetent mouse microglia. Glia 2003, 42, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ohgidani, M.; Hata, N.; Inamine, S.; Sagata, N.; Shirouzu, N.; Mukae, N.; Suzuki, S.O.; Hamasaki, H.; Hatae, R.; et al. CD206 Expression in Induced Microglia-Like Cells From Peripheral Blood as a Surrogate Biomarker for the Specific Immune Microenvironment of Neurosurgical Diseases Including Glioma. Front. Immunol. 2021, 12, 670131. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef] [PubMed]
- Rammos, C.; Luedike, P.; Hendgen-Cotta, U.; Rassaf, T. Potential of dietary nitrate in angiogenesis. World J. Cardiol. 2015, 7, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Y.; Feng, Y.; Li, X.; Bu, L.; Pang, B.; Jia, M. Dietary nitrate protects skin flap against ischemia injury in rats via enhancing blood perfusion. Biochem. Biophys. Res. Commun. 2019, 515, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Elabi, O.F.; Karampatsi, D.; Vercalsteren, E.; Lietzau, G.; Nyström, T.; Klein, T.; Darsalia, V.; Patrone, C.; Paul, G. DPP-4 Inhibitor and Sulfonylurea Differentially Reverse Type 2 Diabetes-Induced Blood-Brain Barrier Leakage and Normalize Capillary Pericyte Coverage. Diabetes 2023, 72, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Li, W.; Qu, Z.; Johnson, M.A.; Fagan, S.C.; Ergul, A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: Relevance to stroke recovery. Stroke 2013, 44, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S. Impact of obesity-induced type 2 diabetes on long-term outcomes following stroke. Clin. Sci. 2019, 133, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sanossian, N.; Starkman, S.; Avila-Rinek, G.; Eckstein, M.; Sharma, L.K.; Liebeskind, D.; Conwit, R.; Hamilton, S.; Saver, J.L.; et al. Adiposity and Outcome After Ischemic Stroke: Obesity Paradox for Mortality and Obesity Parabola for Favorable Functional Outcomes. Stroke 2021, 52, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gong, S.; Zhu, J.; Fang, Q. Relationships between obesity and functional outcome after ischemic stroke: A Mendelian randomization study. Neurol. Sci. 2024, 45, 3869–3877. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.R.; Serra, M.C.; McGrath, R.P. Obesity and diabetes are jointly associated with functional disability in stroke survivors. Disabil. Health J. 2020, 13, 100914. [Google Scholar] [CrossRef] [PubMed]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Gladwin, M.T.; Ahluwalia, A.; Benjamin, N.; Bryan, N.S.; Butler, A.; Cabrales, P.; Fago, A.; Feelisch, M.; Ford, P.C.; et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009, 5, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Group DPPR. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Look AHEAD Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Chandrasekera, P.C.; Barnard, N.D. You are what you eat, or are you? the challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diabetes 2014, 4, e135. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Satija, A.; Fung, T.T.; Clinton, S.K.; Giovannucci, E.L. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J. Nutr. 2019, 149, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Kozyra, M.; Zhuge, Z.; Haworth, S.M.C.; Moretti, C.; Peleli, M.; Caldeira-Dias, M.; Jahandideh, A.; Huirong, H.; Cruz, J.C.; et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc. Natl. Acad. Sci. USA 2019, 116, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Higaki, Y.; Hirshman, M.F.; Fujii, N.; Goodyear, L.J. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes 2001, 50, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Tabima, D.M.; Dube, J.J.; Hughan, K.S.; Vanderpool, R.R.; Goncharov, D.A.; St Croix, C.M.; Garcia-Ocaña, A.; Goncharova, E.A.; Tofovic, S.P.; et al. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated with Heart Failure with Preserved Ejection Fraction. Circulation 2016, 133, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Masrur, S.; Cox, M.; Bhatt, D.L.; Smith, E.E.; Ellrodt, G.; Fonarow, G.C.; Schwamm, L. Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post-thrombolysis: Findings from get with the guidelines-stroke. J. Am. Heart Assoc. 2015, 4, e002193. [Google Scholar] [CrossRef] [PubMed]
- Kamouchi, M.; Matsuki, T.; Hata, J.; Kuwashiro, T.; Ago, T.; Sambongi, Y.; Fukushima, Y.; Sugimori, H.; Kitazono, T.; FSR Investigators. Prestroke glycemic control is associated with the functional outcome in acute ischemic stroke: The fukuoka stroke registry. Stroke 2011, 42, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Kim, W.J.; Kwon, J.H.; Kim, B.J.; Kim, J.T.; Lee, J.; Cha, J.K.; Kim, D.H.; Cho, Y.J.; Hong, K.S.; et al. Prestroke glucose control and functional outcome in patients with acute large vessel occlusive stroke and diabetes after thrombectomy. Diabetes Care 2021, 44, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Babateen, A.; Alharbi, M.; Stephan, B.; Shannon, O. Dietary nitrate and brain health. Too much ado about nothing or a solution for dementia prevention? Br. J. Nutr. 2022, 128, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Veszelovszky, E.; Holford, N.H.G.; Thomsen, L.L.; Knowles, R.G.; Baguley, B.C. Plasma nitrate clearance in mice: Modeling of the systemic production of nitrate following the induction of nitric oxide synthesis. Cancer Chemother. Pharmacol. 1995, 36, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Grefkes, C.; Fink, G.R.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Ospel, J.M.; Hill, M.D.; Menon, B.K.; Demchuk, A.; McTaggart, R.; Nogueira, R.; Poppe, A.; Haussen, D.; Qiu, W.; Mayank, A.; et al. Strength of association between infarct volume and clinical outcome depends on the magnitude of infarct size: Results from the ESCAPE-NA1 trial. Am. J. Neuroradiol. 2021, 42, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, I.; Augestad, I.L.; Håberg, A.K.; Sandvig, A. Neuroplasticity in stroke recovery. The role of microglia in engaging and modifying synapses and networks. Eur. J. Neurosci. 2018, 47, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Nakao-Inoue, H.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 388–403.e15. [Google Scholar] [CrossRef] [PubMed]
- Alia, C.; Cangi, D.; Massa, V.; Salluzzo, M.; Vignozzi, L.; Caleo, M.; Spalletti, C. Cell-to-Cell Interactions Mediating Functional Recovery after Stroke. Cells 2021, 10, 3050. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J.; Liebeskind, D.S.; Chan, S.L. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J. Cereb. Blood Flow Metab. 2018, 38, 2129–2149. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercalsteren, E.; Karampatsi, D.; Buizza, C.; Paul, G.; Lundberg, J.O.; Nyström, T.; Darsalia, V.; Patrone, C. Prevention of Metabolic Impairment by Dietary Nitrate in Overweight Male Mice Improves Stroke Outcome. Nutrients 2025, 17, 2434. https://doi.org/10.3390/nu17152434
Vercalsteren E, Karampatsi D, Buizza C, Paul G, Lundberg JO, Nyström T, Darsalia V, Patrone C. Prevention of Metabolic Impairment by Dietary Nitrate in Overweight Male Mice Improves Stroke Outcome. Nutrients. 2025; 17(15):2434. https://doi.org/10.3390/nu17152434
Chicago/Turabian StyleVercalsteren, Ellen, Dimitra Karampatsi, Carolina Buizza, Gesine Paul, Jon O. Lundberg, Thomas Nyström, Vladimer Darsalia, and Cesare Patrone. 2025. "Prevention of Metabolic Impairment by Dietary Nitrate in Overweight Male Mice Improves Stroke Outcome" Nutrients 17, no. 15: 2434. https://doi.org/10.3390/nu17152434
APA StyleVercalsteren, E., Karampatsi, D., Buizza, C., Paul, G., Lundberg, J. O., Nyström, T., Darsalia, V., & Patrone, C. (2025). Prevention of Metabolic Impairment by Dietary Nitrate in Overweight Male Mice Improves Stroke Outcome. Nutrients, 17(15), 2434. https://doi.org/10.3390/nu17152434







