1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a metabolic disorder characterized by the convergence of hepatic steatosis and at least one cardiometabolic risk factor, which can progress from simple steatosis to steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma [
1]. MASLD is among the most prevalent liver diseases associated with obesity, type 2 diabetes mellitus (T2DM), and metabolic syndrome [
2,
3,
4]. Current data indicate that the prevalence of MASLD in the general population is approximately 30% [
5], and ranges from 42.6% to 69.5% in patients with obesity or T2DM [
2,
3,
6,
7]. The prevalence of liver diseases is rising, primarily due to increasing obesity rates worldwide. Despite recent advances made by the research community, the pharmacological treatments currently available for treating liver diseases remain limited [
8,
9,
10]. There is therefore an urgent need for new treatments in order for health outcomes to be improved and thus reduce the burden associated with MASLD. Identifying new molecular targets is therefore imperative.
Asprosin is a hormone predominantly produced by white adipose tissue (WAT) and plays a crucial role in energy balance regulation [
11]. The hormone is the C-terminal cleavage product of profibrillin, and, in humans, the absence of its gene results in extreme leanness [
11]. Furthermore, it has been demonstrated that both short- and long-term changes in nutritional status, such as fasting or obesity, lead to elevated levels of circulating asprosin in rodents and humans [
11]. Other studies corroborate this finding, indicating that circulating levels of asprosin are positively correlated with obesity in children and adolescents [
12] and in adult patients with T2DM [
13]. In preclinical studies, asprosin has been shown to increase food intake by enhancing olfactory performance and activating hypothalamic AgRP neurons [
14]. Furthermore, asprosin acts in the liver to augment glucose production [
15].
Since its identification in 2016, new metabolic functions have been attributed to asprosin. Beyond its established role in obesity and T2DM, its influence on hepatic diseases has attracted increasing interest in recent years. The initial association between asprosin and liver disease was reported in epidemiological studies, which demonstrated a correlation between asprosin expression and the severity of MASLD in patients [
16]. Preclinical investigations have corroborated these observations in humans. Specifically, two separate studies have indicated that genetic suppression of asprosin in adipose tissue or the liver ameliorates MASLD symptoms in diet-induced obese (DIO) mice by altering lipid metabolism enzymes and reducing inflammation [
17,
18]. While these studies mainly concentrated on modulating the hormone itself, there has only been limited study of its receptor, the olfactory receptor Olfr734, as a potential therapeutic target. This focus may well be of benefit in certain contexts, such as in the study of liver diseases or diabetes, where the liver’s role is pivotal and the hepatic asprosin levels are reported to be significantly lower compared to in other organs [
15,
19]. In this study, we observed that the hepatic Olfr734 levels fluctuate in response to nutritional status changes, and its knockdown in the liver elevates the hepatic lipid content and induces glucose production in DIO mice. Furthermore, to assess the translational relevance of our findings, we demonstrated that the human hepatic Olfr734 ortholog, OR4M1, is present at significantly higher levels in male patients with obesity and T2DM compared to those with obesity and normoglycemia.
2. Materials and Methods
2.1. Mouse Models
Male C57BL/6J wild-type (WT) mice, sourced from the Centro de Biomedicina Experimental de Galicia (CEBEGA) at the University of Santiago de Compostela (USC), Spain, aged 6 to 8 weeks, were utilized in this study. The animals were maintained under Specific-Pathogen-Free conditions, with a controlled temperature of 23 °C and a 12 h light/dark cycle. They had unrestricted access to water and were fed either a standard diet (STD; 6% kcal fat, 18% kcal protein, Teklad Global, Inotiv, West Lafayette, IN, USA) or a high-fat diet (HFD; 60% kcal from fat; D12492, Research Diets, New Brunswick, NJ, USA). All animal care procedures conformed to the guidelines of the institutional animal care committee, with all protocols reviewed and approved by the Ethics Committee of the USC, in accordance with EU regulations on the use of experimental animals (Project ID 15012/2023/012). Environmental enrichment was incorporated into the animal enclosures to promote the expression of natural behaviors and enhance overall welfare. This enrichment included materials such as nesting substrates, shelters, and objects designed for exploration and interaction.
Body weight and food intake were monitored on a weekly basis following lentivirus injection, with all researchers being informed of the group assignments of the animals. Animals exhibiting signs of illness or injuries from cage fights were excluded from the study. No measures were implemented to control for confounding factors during the experiment. The sample size was determined using the 3R principle to ensure statistical validity and significance. Mice were randomized based on body weight, with two groups of animals of the same age and similar body weights being allocated to each group. The precise number of animals utilized in each experiment is specified in the corresponding figure legend. Animals were euthanized by decapitation, and tissues were promptly collected, rapidly frozen using dry ice, and stored at −80 °C until further analysis.
2.2. Effect of the Diet and Food Deprivation on Hepatic Olfr734 Levels
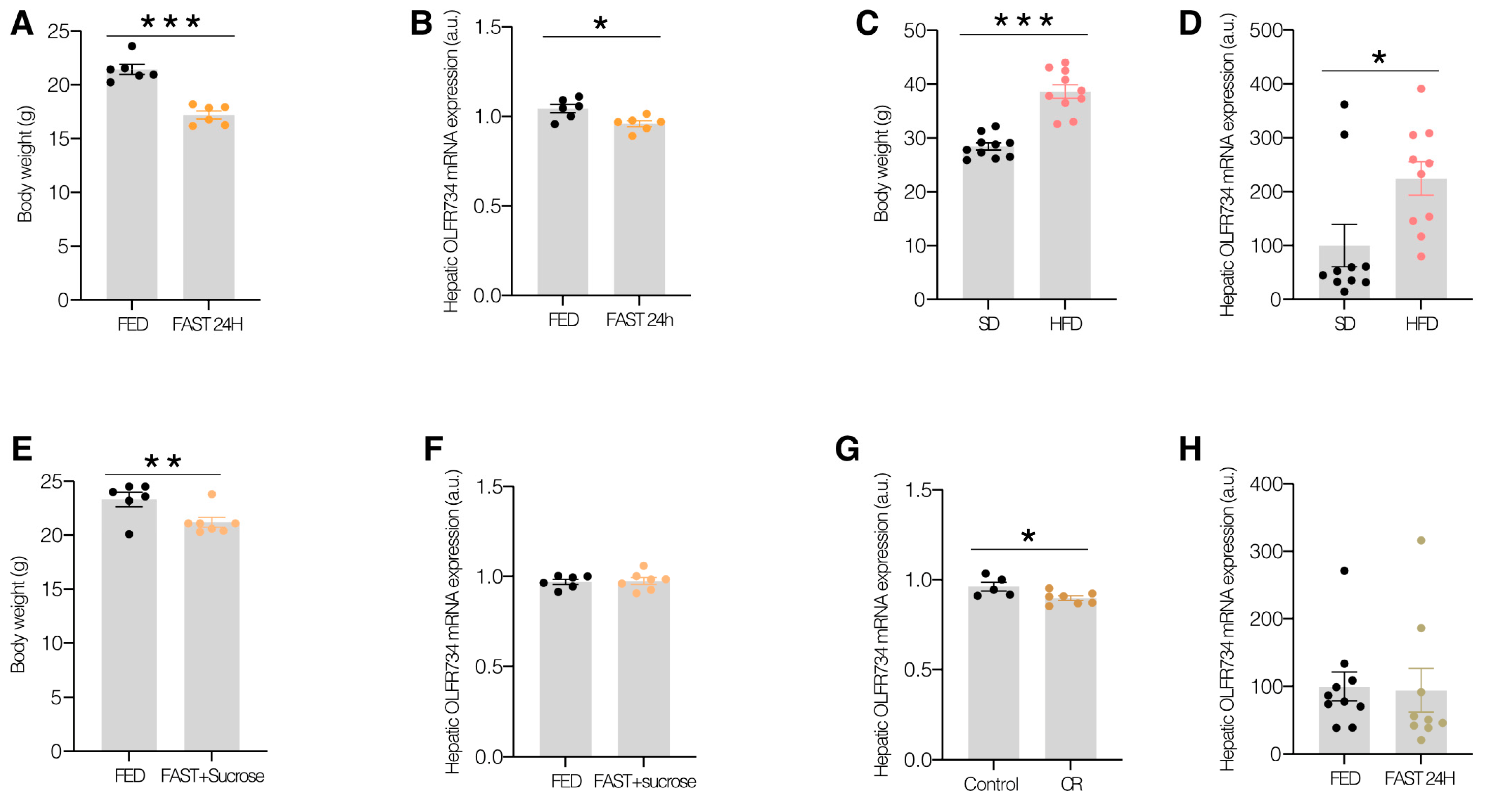
Diet: After weaning, 6-week-old male C57BL/6J mice were either fed an HFD or an STD for 12 weeks [
20].
Fasting: 10-week-old male C57BL/6J mice were deprived of food (STD) for 24 h, while the control group was fed ad libitum [
20,
21,
22].
To study the role of nutrient deprivation in DIO mice on the hepatic levels of Olfr734, 6-week-old male C57BL/6J mice were fed with HFD for 12 weeks and were subjected to 24 h of fasting.
To study the hepatic inhibition of Olfr734 on glucose production during fasting, male C57BL/6J standard diet-fed mice were subjected to a 24 h fast [
23,
24].
All animals had free access to tap water.
2.3. Tail Vein Injection of Lentiviral Expression Vectors
The viruses were administered via the tail vein to specifically target the liver. Mice were secured in a specialized restrainer designed for intravenous injections (Tailveiner TV-150, Bioseb, Vitrolles, France). Using a 27 G × 3/8” syringe, 100 μL of 2 shRNA lentiviral particles, packaged from pGFP-C-shlentivector (Origene Technologies GmbH, Herford, Germany) or control shRNA, both diluted in saline [
21,
22], was injected. The following shRNA target sequences were employed:
TL515502VA-TGAGATGTTCCTGCTGACAGTGATGGCTT with a titer of 2.2 × 107 TU/mL.
TL515502VB–TTATTCTCACTGGTCTATCTCAGACTCGG with a titer of 2.6 × 107 TU/mL.
TR30021V Lenti shRNA scramble control particles with a titer of 1 × 107 TU/mL.
In the experiments concerning MASLD, the knockdown of Olfr734 was induced in WT mice fed an HFD diet for 15 weeks, with the lentivirus shOlfr734 injected 6 weeks prior to euthanasia. In the study examining the role of Olfr734 inhibition on hepatic glucose production, WT C57BL/6 mice were fed an STD for 12 weeks, and the lentivirus shOlfr734 was injected in the first week.
2.4. Calorie Restriction
We assessed the food intake of each mouse over a period of five days to ascertain their daily consumption. Subsequently, the average intake was calculated, and their consumption was restricted by 60% for four days, with only 40% of the calculated quantity provided daily at 6 p.m. Body weight and blood glucose levels were recorded daily at 5:30 p.m. prior to the provision of additional food during the four-day experimental period [
21,
22].
2.5. Effect of Glucose on Hepatic Olfr734 Levels
WT mice were allocated into two groups: (a) those fed an ad libitum STD, and (b) those subjected to a 24 h fasting period, followed by ad libitum feeding with sucrose cubes for 24 h to maintain normal blood glucose levels in the absence of other nutrients, as previously described [
21,
22].
2.6. Glucose, Insulin, Pyruvate, and Postprandial Glucose Tolerance Tests
To assess glucose tolerance (GTT), insulin tolerance (ITT), and pyruvate tolerance (PTT), the mice underwent specific tests. For GTT, mice were intraperitoneally administered D-glucose (2 g/kg) following an overnight fast, and blood glucose levels were measured at intervals of 0, 15, 30, 60, 90, and 120 min using a glucometer (Accu-Chek, Roche, Basel, Switzerland). For ITT, mice received a bolus injection of insulin (0.5 IU/kg) after a 6 h fast, and blood glucose levels were measured at the same intervals. For PTT, sodium pyruvate (1.25 g/kg) was intraperitoneally administered following an overnight fast. Blood glucose levels were measured at intervals of 0, 20, 40, 60, 80, and 120 min [
20,
21,
22,
24].
A postprandial glucose tolerance test was conducted to assess plasma glucose levels following food consumption. After an overnight fast, measurements of blood glucose levels and body weight were taken. Feeding was subsequently resumed, and food intake, blood glucose levels, and body weight were recorded at intervals of 30 min, 60 min, 120 min, and 240 min [
21,
22].
2.7. Liver Triglycerides
Liver samples (approximately 500 mg) were homogenized for 2 min in ice-cold chloroform–methanol (2:1, v/v). Triglycerides (TG) were extracted by agitating the homogenate at room temperature for 3 h. To facilitate phase separation, Milli-Q water was added, followed by centrifugation. The organic (bottom) layer was collected, dried using a SpeedVac, and re-dissolved in chloroform. TG content was quantified in duplicate using an enzymatic assay (1001310 Spinreact, Girona, Spain) after the evaporation of the organic solvent.
2.8. Plasma Measurements
To evaluate the lipid profiles and liver function of the mice, plasma triglycerides (TG: 1001310, Spinreact; Girona, Spain), total cholesterol (1001093, Spinreact Girona, Spain), non-esterified free fatty acids (91696, Fujifilm, Tokio, Japan), and transaminases (ALT: 41283, and AST: 41273 Spinreact; Girona, Spain) levels were measured. Blood samples were obtained by puncture of the tail vein and then centrifuged at 1500×
g for 10 min to obtain plasma. Post-mortem glucose (1001191, Spinreact; Girona, Spain) levels were also assessed from plasma. Plasma levels of insulin were established using an insulin (Merck Mili-pore, ref. EZRMI, Taufkirchen, Germany) ELISA kit. Readings were collected according to the manufacturer’s instructions, and results were expressed in MMOL/L or U/ML for each measured parameter [
20,
21,
22,
24,
25].
2.9. SYBR Green One-Step RT-qPCR
The extraction of mRNA commenced with the resuspension of the sample in 750 µL of TRIzol, followed by the addition of 150 µL of chloroform (VWR Chemicals, Mississauga, ON, Canada). The mixture was subjected to vortexing and subsequently centrifuged at 12,000×
g for 15 min at 4 °C. The aqueous phase was then isolated, and an equivalent volume of 70% ethanol (VWR Chemicals; Mississauga, ON, Canada) was incorporated. The phases were combined and transferred to a column utilizing an RNA extraction kit (Omega Bio-Tek, Norcross, GA, USA). The column underwent centrifugation at 10,000×
g at 21 °C for one minute, after which the liquid was discarded. Subsequently, 500 µL of Wash Buffer I (Omega Bio-Tek RNA extraction kit; Norcross, GA, USA) was added, followed by another centrifugation at 10,000×
g at 21 °C for one minute. The liquid was discarded once more, and 500 µL of Wash Buffer II was introduced. This centrifugation step at 10,000×
g at 21 °C for one minute was repeated twice. Following the final wash, the column was centrifuged at 14,000×
g for 2 min at 21 °C. Finally, 40 µL of RNAse-free water was added, and the sample was centrifuged at 14,000×
g for 2 min at 21 °C to elute the RNA [
20,
25,
26,
27]. The one-step RT-qPCR was performed in 10 µL reaction volumes containing 10 ng of RNA template. RNA concentrations were determined spectrophotometrically using a NanoDrop (Thermo Scientific NanoDrop, Waltham, MA, USA). Each reaction comprised 0.4 µL of 10 µM forward primer, 0.4 µL of 10 µM reverse primer, 0.4 µL of NZYRT mix, 5 µL of One-step NZYSpeedy qPCR Probe Master Mix (2×) (NZYTech, Lisbon, Portugal), and 1.8 µL of nuclease-free water [
26].
The thermal cycling protocol commenced with an initial reverse transcription step at 50 °C for 15 min, followed by polymerase activation at 95 °C for 2.5 min. The cycling protocol consisted of 40 cycles at 95 °C for 5 s and 60 °C for 45 s, utilizing a 96-well thermal cycler (QuantStudio 3, Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). A melt curve analysis was conducted with the following stages: 15 s at 95 °C, 1 min at 60 °C, and 1 s at 95 °C [
28]. Primers were designed using PRIMER-BLAST (
https://www.ncbi.nlm.nih.gov/tools/primer-blast/) accessed on 1 January 2025. The list of primers used is provided in
Table S1.
Liver extracts were homogenized using a TissueLyser II (Qiagen, Venlo, The Netherlands) in cold BLYS buffer (TRIS 1M, EGTA 0.2M, EDTA 0.2M, TRITON x-1000, orthovanadate 0.1M, sodium fluoride, sodium pyrophosphate, and saccharose: Sigma-Aldrich, St. Louis, MO, USA) with protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA). The lysates were centrifuged (30 min, 12,000 rpm, 4 °C) and quantified using the DC Protein Assay (BioRad, Hércules, CA, USA), with readings taken by a spectrophotometer (BioTek, Winooski, VT, USA). Equal amounts (20 µg/well) of total protein lysates from the liver were subsequently subjected to SDS-PAGE, electrotransferred onto a PVDF membrane, and incubated with Olfr734 (MBS8566720, Mybiosource, San Diego, CA, USA), ANGPL4 (40-9800 Thermo Fisher Scientific; Waltham, MA, USA), FGF21 (Abcam ab64857; Cambridge, UK), GDF-15 (Abcam ab39999; Cambridge, UK), mTOR (Cell Signaling #2972; Danvers, MA, USA), and pmTOR (Cell Signaling #2971; Danvers, MA, USA) protein antibodies diluted to 1/1000 to confirm the knockdown. Protein levels were normalized relative to β-actin (MFCD00164531, Sigma-Aldrich, St. Louis, MO, USA) diluted to 1/10,000 or vinculin (sc-73614; Santa Cruz Biotechnology, Dallas, TX, USA) diluted 1/1000 for each sample. Membranes were treated with a 5% BSA blocking buffer, and protein detection was performed using horseradish peroxidase-conjugated secondary antibodies. Antigen–antibody interactions were detected using a chemiluminescence technique according to the manufacturer’s instructions (Pierce ECL Western Blotting Substrate, Thermo Scientific, Waltham, MA, USA). Visualization was conducted through manual X-ray film development or the ChemiDoc Imaging System. Western blot quantification was carried out using ImageJ software (RRID: SCR_003070).
2.10. Histological Procedures
Hematoxylin and Eosin Staining: Liver samples were fixed in 4% formaldehyde for 24 h, followed by dehydration and paraffin embedding. Sections of 4 µm thickness were prepared using a microtome and stained with a standard hematoxylin and eosin alcoholic procedure as per the manufacturer’s instructions (BioOptica, Milan, Italy). Subsequently, sections were rinsed with distilled water, dried at 37 °C for 30 min, and mounted using a permanent (non-alcohol, non-xylene-based) mounting medium [
20,
25,
26].
Oil Red O Staining: Frozen liver samples were sectioned into 10 µm slices using a cryostat and stained with filtered Oil Red O for 20 min (Sigma O-0625; St. Louis, MO, USA). Following washing in distilled water, sections were counterstained with Harris’s hematoxylin for 5 min (Bio-optica 05-06004/L; Milan, Italy) and mounted in an aqueous medium (Bio-optica 05-1740: Milan, Italy) [
20,
21,
22,
24,
25].
2.11. Human Cell Cultures
THLE2 human hepatic cell line (American Type Culture Collection, ATCC) was cultured in bronchial epithelial cell basal medium (BEBM) supplemented with a growth factors BulleKit (Lonza/Clonetics Corporation, Walkersville, MD, USA), 70 ng/mL phosphoethanolamine, 5 ng/mL epidermal growth factor, 10% (v/v) FBS, and 1% (v/v) Glutamine–Penicillin–Streptomycin solution (MERCK; Taufkirchen, Germany). THLE-2 cells were grown on culture plates pre-coated with a mixture of 0.01 mg/mL fibronectin (#33010018, Sigma Aldrich, St. Louis, MO, USA), 0.01 mg/mL bovine serum albumin (#A4503, Sigma Aldrich, St. Louis, MO, USA), and 0.03 mg/mL collagen type I (#sc-136157, Santa Cruz, Dallas, TX, USA). Cells were tested for mycoplasma contamination.
2.11.1. Cell Transfection
In the study, 0.5 × 105 THLE2 cells were seeded in a twenty-four-well plate, and cultured 24 h before transfection. Transfections were performed using Dharmafect 1 reagent (T-2001-03, Dharmacon Lafayette, CO, USA), following the manufacturer’s protocol. Briefly, 50 pmol of each siRNA was diluted in 200 μL of OptiMEM (#31985-070, Life Technologies, Carlsbad, CA, USA) and mixed with 6.5 μL of Dharmafect 1, pre-diluted in 193.5 μL of OptiMEM. The final mixture (500 μL) was added to each well. After six hours, the transfection medium was replaced by fresh BEGM, and the corresponding treatments were applied. Cells were transfected with specific small-interference RNA (si-RNA) to knock down the expression of OR4M1 (L-032481-02-0010, Dharmacon, Lafayette, CO, USA) or with non-targeting siRNA used as a negative control (Dharmacon, Lafayette, CO, USA).
2.11.2. Resveratrol Treatment
THLE2 cells silencing OR4M1 were incubated in a medium supplemented with 1 μg/mL resveratrol (R5010, MERCK, Taufkirchen, Germany), a pharmacological activator of SIRT1. The dose was selected based on da Silva Lima et al. (PMID: 34555423), ensuring effective SIRT1 activation while maintaining cell viability. After 24 h, cells were either collected for mRNA extraction or subjected to Oil Red O staining.
2.11.3. Oil Red O Staining
Cells were seeded on sterile glass coverslips placed in the wells of a 24-well plate. Following treatment, the culture medium was removed, and cells were gently rinsed with phosphate-buffered saline (PBS). Fixation was carried out using 10% neutral buffered formalin for 10 min at room temperature. After fixation, wells were washed with PBS, and 200 µL of freshly prepared working Oil Red O solution was added to each well. Cells were incubated for 20 min at room temperature, followed by thorough washing with distilled water until the background was clear. Subsequently, a counterstaining step was performed by incubating the coverslips with Mayer’s hematoxylin for 10 min, after which they were rinsed again with distilled water. Coverslips were then carefully removed and mounted cell-side down onto glass microscope slides using an aqueous mounting medium. Stained preparations were examined under a light microscope to evaluate intracellular lipid droplet accumulation. In these histological staining techniques, up to 6 representative microphotographs of cells were taken with a BX51 microscope equipped with a DP70 digital camera (Olympus, Tokyo, Japan). Lipids in Oil Red O-stained sections were quantified using ImageJ 1.52p software.
2.12. Patients
Liver specimens were obtained from a cohort of 21 patients with severe obesity undergoing bariatric surgery at the Clínica Universidad de Navarra. Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2 and a body fat percentage (BF) ≥ 35%, with BMI calculated as weight (kg) divided by height squared (m2), and BF assessed via air-displacement plethysmography (Bod-Pod, Life Measurements, Concord, CA, USA). Participants were categorized into normoglycemia (NG) or T2DM subgroups according to the diagnostic criteria established by the Expert Committee on the Diagnosis and Classification of Diabetes. Inclusion criteria required a comprehensive clinical evaluation, including physical examination, laboratory profiling, abdominal ultrasonography, and histological confirmation of metabolic MASLD via percutaneous liver biopsy. Biopsies were assessed by a hepatopathologist blinded to clinical and biochemical data, applying the Kleiner and Brunt criteria to derive the MASLD activity score (NAS), which integrates scores for steatosis, lobular inflammation, and hepatocellular ballooning (range 0–8).
Exclusion criteria included the following: (a) alcohol consumption exceeding 20 g/day for females or 30 g/day for males; (b) serological evidence of hepatitis B surface antigen or hepatitis C virus antibodies in the absence of prior immunization; (c) current or prior use of hepatotoxic agents associated with MASLD (e.g., amiodarone, valproate, tamoxifen, methotrexate, corticosteroids, or antiretroviral therapy); and (d) alternative liver pathologies, including autoimmune hepatitis, hereditary hemochromatosis, Wilson’s disease, or α-1-antitrypsin deficiency. Individuals with T2DM were excluded if they were receiving insulin or medications known to affect endogenous insulin production. All T2DM cases had a disease duration of less than 2–3 years or were newly diagnosed based on clinical anamnesis and metabolic parameters. The study protocol adhered to the Declaration of Helsinki (2013 revision) and received approval from the Institutional Ethics Committee (protocol no. 2021.005, approved on 11 February 2021). All participants provided written informed consent prior to enrollment.
2.13. Data Analysis and Statistics
Data are presented as mean ± SEM for each genotype. Protein and mRNA expression levels were expressed as percentages relative to the control group (either fed ad libitum or treated with shGFP). Statistical analyses were conducted based on the number of groups and the distribution of the data. Comparisons between two groups were analyzed using either the Student’s t-test or the Mann–Whitney U test, depending on the normality of the data distribution. For comparisons involving more than two groups, two-way ANOVA or the Kruskal–Wallis test was applied, followed by Bonferroni post hoc analysis. A p-value < 0.05 was considered statistically significant. The normality of the data was assessed using the Shapiro–Wilk test. The sample size for each group was calculated based on previous studies, and some data points were eliminated from the study after performing outlier analysis calculation. All analyses were performed using Excel (Microsoft Corp., Redmond, WA, USA) and GraphPad PRISM 10 software.
4. Discussion
MASLD is a globally prevalent condition with limited effective pharmacological interventions available. Understanding its mechanism of action and identifying new therapeutic targets are urgently needed. Among the numerous molecules implicated in the pathophysiology of MASLD is the recently identified hormone asprosin, which serves as the endogenous ligand for the olfactory receptor Olfr734. Asprosin binds to and activates the Olfr734 receptor, stimulating food intake and promoting hepatic glucose production. Significantly, circulating asprosin levels are elevated in obesity and other metabolic disorders, such as T2DM and MASLD. Inhibition of asprosin has been proposed as a potential treatment for MASLD in rodent models. Despite the hormone’s involvement in MASLD and gluconeogenesis, few studies have explored the specific role of Olfr734 in liver function. The Olfr734 receptor is predominantly expressed in the liver, a key metabolic organ responsible for maintaining glucose and lipid homeostasis. In this study, we chose to target the Olfr734 receptor rather than asprosin itself, given the reported reduced presence of this endocrine factor in the liver compared to other organs. Indeed, there is limited information in the literature regarding the physiological modulation of this receptor and its potential metabolic consequences. The main findings of our study are as follows: (1) Olfr734 levels in the liver may serve as a sensor of nutrient and glucose availability; (2) Olfr734 knockdown in the liver exacerbates MASLD and increases hepatic glucose production in DIO mice; (3) the effects of Olfr734 knockdown in the liver inhibit the Sirt1/ER stress pathway; and (4) the hepatic levels of Olfr734 are upregulated in patients with T2DM in a sex-dependent manner.
In this study, we observed that the hepatic expression of this receptor may serve as an indicator responsive to varying nutritional states, exhibiting a distinct expression pattern from asprosin. Our findings indicate that Olfr734 expression is downregulated during fasting, while it is upregulated in obesity. Interestingly, prior research has not quantified the levels of this receptor in the liver under these experimental conditions. Furthermore, the expression pattern of Olfr734 in the liver during fasting contrasts sharply with that of plasma asprosin. However, in obesity, the levels of Olfr734 and asprosin are similarly elevated. This phenomenon may be attributed to the substantial release of asprosin into the bloodstream during fasting, potentially leading to the downregulation or desensitization of its hepatic receptor (e.g., through endocytosis) as a compensatory response to elevated circulating asprosin levels. A counterregulatory mechanism may be activated in the liver, resulting in the inhibition of Olfr734 expression and thus contributing to this specific phenotype. Collectively, these findings suggest a potential metabolic role for Olfr734. While asprosin is known to regulate MASLD in both mice and humans, the precise role of its receptor, Olfr734, remains unclear. The elevated levels of Olfr734 in obesity prompted us to hypothesize that the targeted inhibition of Olfr734 in the liver could be a promising strategy for improving MASLD treatment. Contrary to our expectations, however, this approach exacerbated MASLD. This unexpected and counterintuitive result lacks a clear explanation. It is plausible to speculate that an as-yet-undiscovered ligand (antagonist) for Olfr734 may be involved, akin to the recently identified endogenous antagonist for the GHSR1a–ghrelin system, LEAP-2 [
32,
33,
34]. Another possibility is that a compensatory effect exists in the action of Olfr734 inhibition on the liver. Thus, the liver, in order to counteract pathways that repress lipid storage, would increase the levels of TG in the liver in response. Nevertheless, the function of this receptor is poorly understood and may be compensated by other receptors or pathways.
A key objective of the current study was to investigate the downstream molecular pathways controlling the hepatic actions of Olfr734 knockdown. We could reproduce previous findings linking the actions of asprosin and the pathways related to fatty acid metabolism and inflammation. Interestingly, we found new molecular underpinnings for the induction of MASLD by Olfr734 knockdown. Our data indicate that the Olfr734 inhibition-induced hepatic fat content inhibits Sirt1/ER stress-associated pathways. In fact, this study is the first to report the link between these molecular pathways and the action of asprosin signaling.
The observed downregulation of Sirt1 in DIO animals injected with shOlfr734 accords with the established regulation of this deacetylase in obesity. It is noteworthy that hepatic Sirt1 expression increases during nutrient deprivation and decreases in obesity, particularly in peripheral tissues such as the liver of DIO mice, which are often affected by MASLD [
29,
35,
36,
37]. Sirt1 plays a crucial role in regulating hepatic lipid metabolism, preventing lipid accumulation and the induction of adipogenesis enzymes [
38]. The specific deletion of hepatic Sirt1 impairs the fatty acid β-oxidation pathway, thereby heightening the susceptibility of mice to HFD-induced dyslipidemia, hepatic steatosis, inflammation, and ER stress [
39,
40]. This finding is consistent with another study demonstrating that the deletion of hepatic Sirt1 leads to the development of liver steatosis [
41]. Moreover, we demonstrate the involvement of Sirt1 in the effect of OR4M1 inhibition in the liver by functional experiments in THLE-2 cells. In addition to these findings, we observed an increase in the inflammatory marker Ikkβ, which has been previously reported to be upregulated in the liver of obese animals with MASLD [
27]. Notably, Ikkβ has been proposed as the molecular link between inflammation and ER stress. We investigated this pathway to identify a new mechanism of action that might explain our findings. Accumulating evidence has demonstrated a strong association between ER stress and metabolic diseases such as obesity and T2DM, as well as liver diseases [
25,
27,
30,
42,
43]. It has been observed that improving protein folding, for instance, through chemical chaperones, ameliorates obesity and MASLD [
25,
27,
30,
42,
43]. In this context, Bip is a chaperone that modulates protein folding in response to cellular stress. Its overexpression in the hypothalamus and liver of DIO animals has been consistently reported to diminish obesity and MASLD [
25,
27,
30]. Our data are confirmed by these previous findings, and although we did not observe an increase in ER stress marker levels, namely Chop, Xbp1, or Atf4, we found a significant reduction in Bip levels in the livers of our DIO mice injected with shOlfr734.
Gluconeogenesis also contributes to hyperglycemia in T2DM, and these patients exhibit a defect in insulin action, a condition known as insulin resistance, which may impact hepatic glucose regulation. Furthermore, ER stress is closely associated with obesity-related insulin resistance in peripheral tissues, such as the liver [
42,
43]. Interestingly, the phenotype of our mice injected with shOlfr734 is characterized by an increase in insulin secretion, suggesting potential insulin resistance. This observation aligns with the phenotype described here, as insulin resistance is a critical component of obesity and MASLD. In addition, we observed that, alongside insulin resistance, hepatic glucose production in these mice increased, another key function of asprosin–Olfr734 signaling. Moreover, the knockdown of Olfr734 in lean animals results in elevated glucose levels after 24 h of fasting in mice fed ad libitum, contrary to previous data indicating the opposite [
14]. However fasting and caloric restriction did not uniformly impact glucose metabolism in Olfr734-knockdown mice. This discrepancy might be due to the fall in glucose levels in CR not being as great in fasted mice and, as a result, Olfr734 inhibition does not induce an effect as we found in fasting conditions. We currently lack a clear explanation for this phenomenon, necessitating further research to elucidate the precise role of asprosin signaling in hepatic function. Our data suggest that the Olfr734 levels are low in hypoglycemic states such as fasting or CR and high in hyperglycemic states such as obesity. Furthermore, the reduction in receptor expression observed during fasting is attenuated in fasting animals fed with sucrose pellets. It is noteworthy that these animals exhibit similar glucose levels to the ad libitum-fed group but differ in caloric/nutrient intake and have lower body weight. These findings suggest that, in addition to serving as a nutrient sensor, our target is a signal sensitive and responsive to variations in glucose levels.
In an effort to ascertain the translational relevance of our findings, we assessed the levels of our target in the livers of human patients with diabetes. Our analysis revealed that the mRNA levels of OR4M1 are elevated in patients with obesity and T2DM compared to those with obesity and normoglycemia. Given the significant influence of gender on liver disease and various metabolic pathway disorders, we stratified our data by sex to determine whether OR4M1 expression exhibits a similar pattern in both sexes. Our findings indicate a pronounced sexual dimorphism in the action of OR4M1. However, it is important to note that one limitation in our study is the small patient sample size that impedes detecting a positive or negative correlation of OLFR734 levels and biochemical and anthropometric measurements from these patients. In conclusion, this study is the first to (i) investigate the modulation of Olfr734 in the development of MASLD; (ii) establish a link between asprosin signaling-induced liver steatosis and a Sirt/ER stress pathway; and iii) associate the hepatic levels of OR4M1 with patients with T2DM.
Because Olfr734 is involved in the pathophysiology of different diseases associated with obesity, such as T2DM and MASLD, identifying the molecular mechanisms underlying these disorders will help identify more effective methods for their prevention and treatment. In this study, our first aim was to discover a new therapeutic target by manipulating the Olfr734 levels. If we consider that the levels of Olfr734 in the liver of DIO mice and T2DM patients are higher than those in the controls, the development of a specific pharmacological antagonist/inverse agonist for Olfr734 or another system by which the expression of the Olfr734 gene could be genetically inhibited specifically in the liver in a safe and effective manner without side effects could be a priori a conceivable option. However, our results do not support this possibility, probably because of compensatory mechanisms in the action of Olfr734 inhibition in the liver, precluding the use of these approaches to treat these metabolic diseases. Despite this, we believe that this work provides important insights into the role of Olfr734 in MAFLD and T2DM.
Given that asprosin was only identified nine years ago, limited information is available regarding the regulation and mechanism of Olfr734 action. Substantially more research is required to elucidate the biology of this endocrine signaling system.