Dietary and Genetic Aspects of Polycystic Ovary Syndrome (PCOS) in Polish Women—Part I: Nutritional Status and Dietary Intake
Abstract
1. Introduction
2. Materials and Methods
- Exclusion Criteria
- Other endocrine disorders (e.g., hypothyroidism, hyperprolactinemia);
- Hormone therapy within the last six months;
- Metabolic diseases, such as type 2 diabetes.
- Pregnancy;
- Pacemaker implantation;
- Electrodes, stimulators, or other electronic implants;
- Use of diuretics;
- Epilepsy.
2.1. Body Composition Analysis
2.2. Dietary Records
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Groups
3.2. Analysis of Nutrient Intake
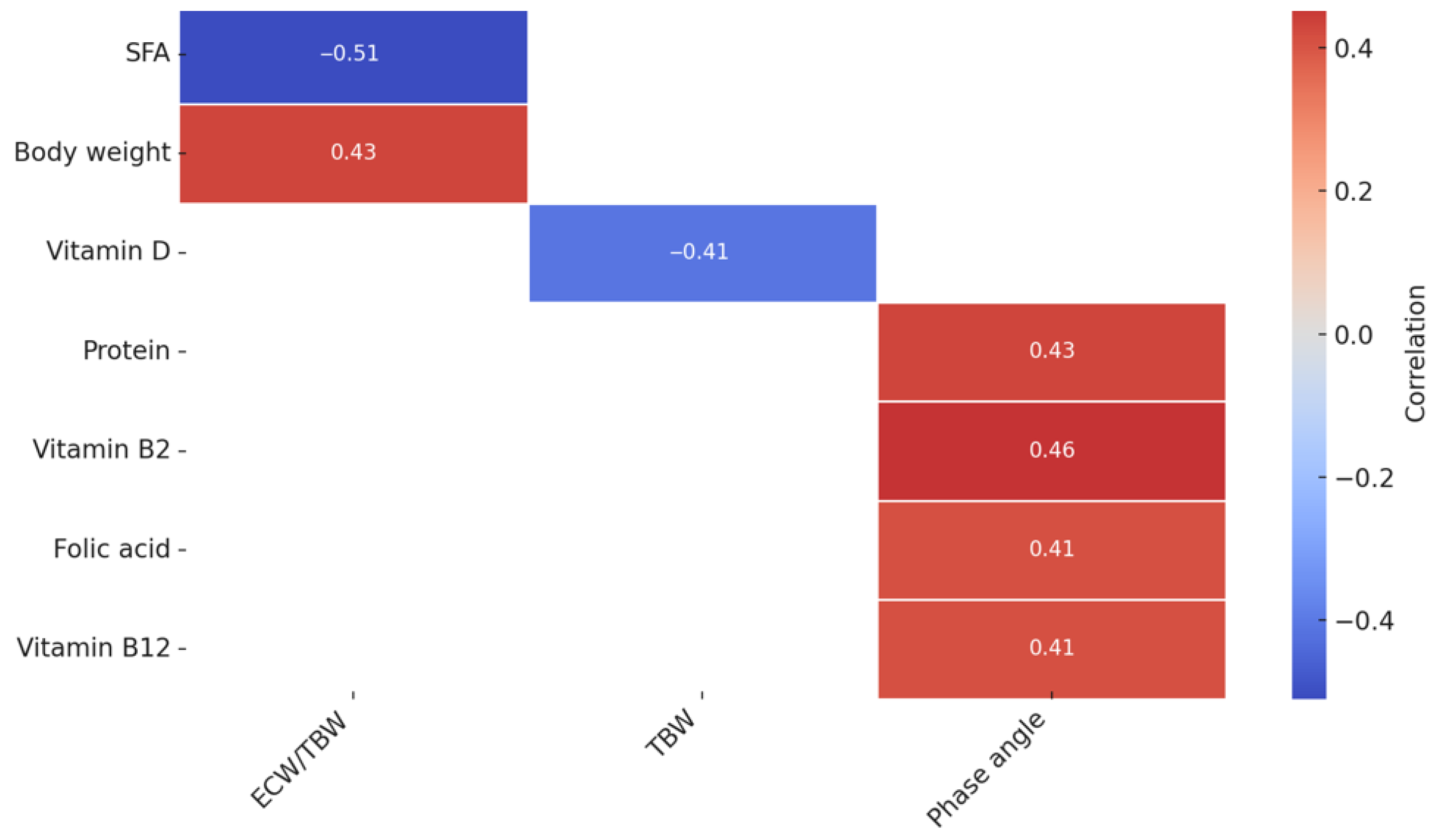
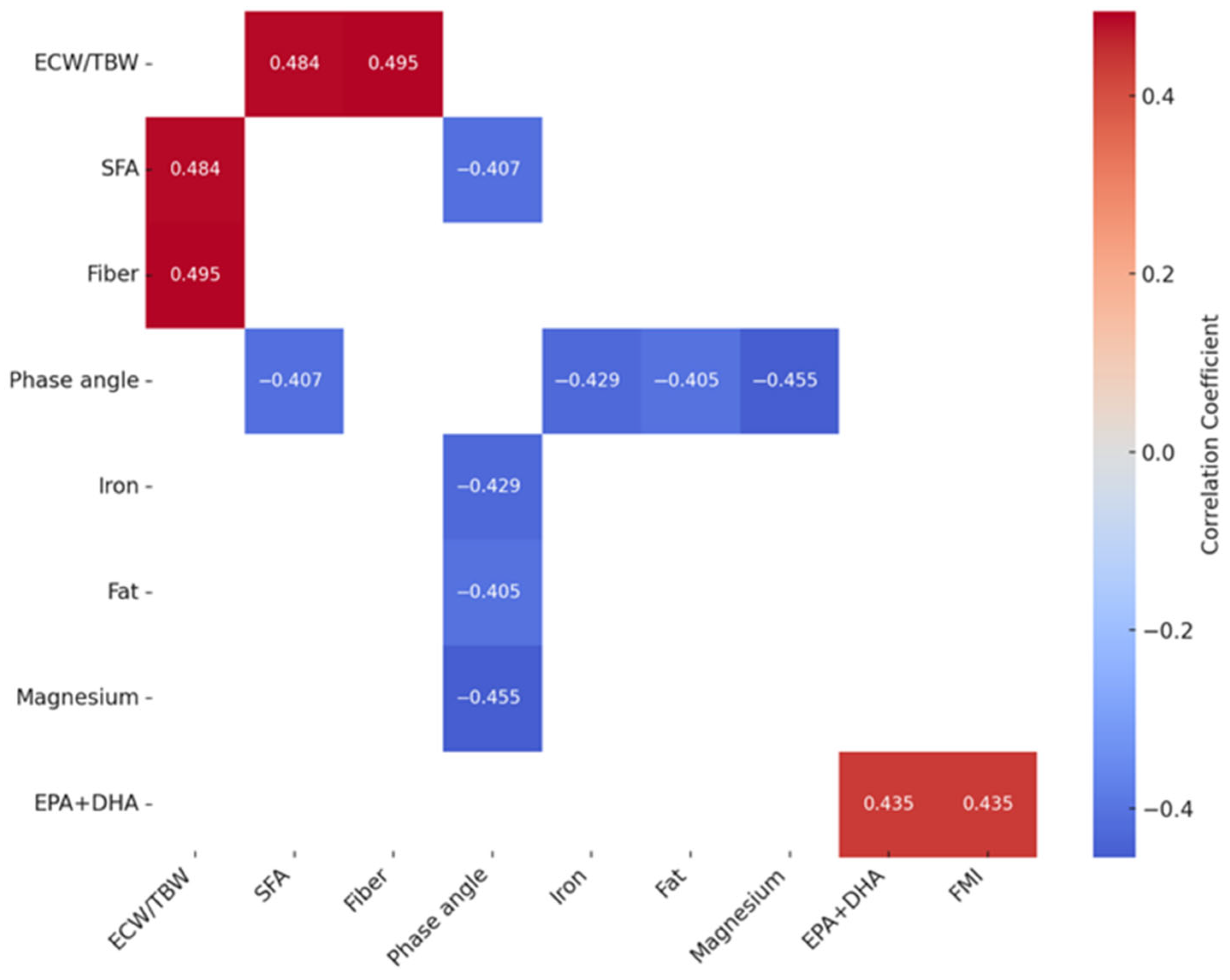
3.3. The Analysis of the Correlation Between the Nutrient Intake and Body Composition in Women with PCOS
4. Discussion
4.1. Body Composition Analysis
4.2. Dietary Intake Assessment
4.3. Associations Between Dietary Intake and Body Composition Parameters
4.4. Study Limitations and Strengths
5. Conclusions and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhimwal, T.; Puneet, M.; Priyadarshani, A. Understanding polycystic ovary syndrome in light of associated key genes. Egypt. J. Med. Hum. Genet. 2023, 24, 38. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic ovary syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.H.; Galazis, N.; Docheva, N.; Layfield, R.; Atiomo, W. Overlap of proteomics biomarkers between women with pre-eclampsia and PCOS: A systematic review and biomarker database integration. Hum. Reprod. 2015, 30, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Bertoldo, M.J.; Handelsman, D.J. Evidence from animal models on the pathogenesis of PCOS. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Ding, H.; Wang, M. Polycystic Ovary Syndrome (PCOS): Symptoms, Causes, and Treatment. Clin. Exp. Obstet. Gynecol. 2024, 51, 126. [Google Scholar] [CrossRef]
- Vishnubhotla, D.S.; Tenali, S.N.; Fernandez, M.; Madireddi, S. Evaluation of prevalence of PCOS and associated depression, nutrition, and family history: A questionnaire-based assessment. Indian J. Endocrinol. Metab. 2022, 26, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.C.; Jones, R.; Green, R.; Brown, K.; Yount, G.; Williams, R. University students with PCOS demonstrate limited nutrition knowledge. Am. J. Health Educ. 2021, 52, 80–91. [Google Scholar] [CrossRef]
- Navarro-Lafuente, F.; Arense-Gonzalo, J.J.; Sánchez-Ferrer, M.L.; Prieto-Sánchez, M.T.; Cutillas-Tolín, A.; Mendiola, J.; Torres-Cantero, A.M. Fat intake pattern in women with polycystic ovary syndrome. Reprod. Biomed. Online 2022, 44, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Szcześniewska, D.; Szostak-Węgierek, D.; Kwaśniewska, M.; Pająk, A.; Stepaniak, U.; Drygas, W. Are dietary habits of the Polish population consistent with the recommendations for prevention of cardiovascular disease?—WOBASZ II project. Kardiol. Pol. 2016, 74, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients: Summary Report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Rychlik, E.; Stoś, K.; Woźniak, A.; Mojska, H. Normy Żywienia Dla Populacji Polski; NIZP PZH–PIB: Warszawa, Poland, 2024.
- Dahan, M.H.; Reaven, G. Relationship among obesity, insulin resistance, and hyperinsulinemia in the polycystic ovary syndrome. Endocrine 2019, 64, 685–689. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.C.; Dekkers, O.M.; Romijn, J.A.; Dieben, S.W.; Helmerhorst, F.M. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum. Reprod. Update 2011, 17, 495–500. [Google Scholar] [CrossRef] [PubMed]
- de Melo Cavalcante, R.B.; Leão, L.M.C.S.M.; Tavares, A.B.W.; Lopes, K.G.; Terra, C.; Salgado, A.A.; Kraemer-Aguiar, L.G. Visceral adipose tissue, epicardial fat, and hepatic steatosis in polycystic ovary syndrome: A study of ectopic fat stores and metabolic dysfunction. Endocrine 2025, 87, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rojas, C.A.; Cruz-Soto, R.; Sánchez-Muñoz, V.; Romero, A.; Mosti-Molina, M.; Sánchez-Aguilar, H.A.; Herrera, M.F. Does FMI correlate better than BMI with the occurrence of metabolic changes in obese patients? Study based on 2007 consecutive Mexican patients. Obes. Surg. 2020, 30, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lin, B.; Niu, R.; Lu, W.; He, C.; Zhang, M.; Liu, C. Fat-free mass index is a feasible predictor of insulin resistance in women with polycystic ovary syndrome: Evidence from a cross-sectional study. Endocrine 2024, 84, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.C.; Norris, L.E.; Oster, R.A.; Darnell, B.E.; Azziz, R.; Gower, B.A. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil. Steril. 2006, 86, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Marbini, M.H.; Amiri, F.; Hezaveh, Z.S. Dietary glycemic index, glycemic load, insulin index, insulin load and risk of diabetes-related cancers: A systematic review of cohort studies. Clin. Nutr. ESPEN 2021, 42, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T.; Guidozzi, F. Midlife women’s health consequences associated with polycystic ovary syndrome. Climacteric 2020, 23, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.; Reeves, S.; Sharp, K.; Jeanes, Y.M. An isocaloric low glycemic index diet improves insulin sensitivity in women with polycystic ovary syndrome. J. Acad. Nutr. Diet. 2013, 113, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Biltoft-Jensen, A.; Groth, M.V.; Matthiessen, J.; Wachmann, H.; Christensen, T.; Fagt, S. Diet quality: Associations with health messages included in the Danish Dietary Guidelines 2005, personal attitudes and social factors. Public Health Nutr. 2009, 12, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.A.; Cockburn, E.; Rumbold, P.L.; Rae, G.; Stevenson, E.J.; Russell, M. Assessment of energy intake and energy expenditure of male adolescent academy-level soccer players during a competitive week. Nutrients 2015, 7, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Guo, Y.; Lai, Z. The effect of low carbohydrate diet on polycystic ovary syndrome: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2019, 2019, 4386401. [Google Scholar] [CrossRef] [PubMed]
- Dyson, P. Low carbohydrate diets and type 2 diabetes: What is the latest evidence? Diabetes Ther. 2015, 6, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Luo, X.; Wang, Z.; Chen, Z.; Huang, D.; Cao, H.; Wu, J. Comparing GDF9 in mature follicles and clinical outcomes across different PCOS phenotype. Heliyon 2024, 10, e29879. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Ziaei, S.; Kazemnejad, A.; Movahedinejad, M. The Relationship between Macronutrient Intake and Insulin Resistance in Polycystic Ovary Syndrome. J. Nutr. Food Secur. 2023, 8, 202–211. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Y.; Cui, M.; Su, D. Efficacy of omega-3 fatty acid supplementation on cardiovascular risk factors in patients with polycystic ovary syndrome: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 6425437–6426437. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Maan, P.; Jyoti, A.; Kumar, A.; Malhotra, N.; Arora, T. The Role of Lifestyle Interventions in PCOS Management: A Systematic Review. Nutrients 2025, 17, 310. [Google Scholar] [CrossRef] [PubMed]
- Tosatti, J.A.; Alves, M.T.; Cândido, A.L.; Reis, F.M.; Araújo, V.E.; Gomes, K.B. Influence of n-3 fatty acid supplementation on inflammatory and oxidative stress markers in patients with polycystic ovary syndrome: A systematic review and meta-analysis. Br. J. Nutr. 2021, 125, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Azarbayjani, K.; Jahanian Sadatmahalleh, S.; Mottaghi, A.; Nasiri, M. Association of dietary inflammatory index with C-reactive protein and interleukin-6 in women with and without polycystic ovarian syndrome. Sci. Rep. 2024, 14, 3972. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, N.B.; Ribeiro, C.T.; Silva, C.M.; de Sá Rosa, A.C.J.; De-Souza, D.A. Dietary intake, body composition and metabolic parameters in women with polycystic ovary syndrome. Clin. Nutr. 2019, 38, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Shetty, S.S.; Shetty, P.K.; Kumari, N.S. Dietary Pattern, Physical, and Behavioral Trends in Women with Polycystic Ovary Syndrome. J. Datta Meghe Inst. Med. Sci. Univ. 2024, 19, 77–81. [Google Scholar] [CrossRef]
- Szczuko, M.; Szydłowska, I.; Nawrocka-Rutkowska, J. A properly balanced reduction diet and/or supplementation solve the problem with the deficiency of these vitamins soluble in water in patients with PCOS. Nutrients 2021, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, G.; Hekmatdoost, A. Nutrient patterns and risk of polycystic ovary syndrome. J. Reprod. Infertil. 2019, 20, 161. [Google Scholar] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Virtanen, J.K. Consensus statement on vitamin D status assessment and supplementation: Whys, whens, and hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Bleizgys, A. Zinc, magnesium and vitamin K supplementation in vitamin D deficiency: Pathophysiological background and implications for clinical practice. Nutrients 2024, 16, 834. [Google Scholar] [CrossRef] [PubMed]
- Sparic, R.; Andjic, M.; Vergara, D.; Morciano, A.; D’Oria, O.; Baldini, G.M.; Tinelli, A. PCOS and vitamin D: A clinical appraisal. Arch. Gynecol. Obstet. 2024, 309, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Altieri, P.; Cavazza, C.; Pasqui, F.; Morselli, A.M.; Gambineri, A.; Pasquali, R. Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin. Endocrinol. 2013, 78, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chudzicka-Strugała, I.; Gołębiewska, I.; Banaszewska, B.; Brudecki, G.; Zwoździak, B. The role of individually selected diets in obese women with PCOS—A review. Nutrients 2022, 14, 4555. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Alur-Gupta, S.; Gallop, R.; Dokras, A. Postpartum weight retention in women with polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2021, 224, 76.e1–76.e10. [Google Scholar] [CrossRef] [PubMed]
- Sheetal, A.; Hiremath, V.K.; Patil, A.G.; Sajjansetty, S.; Kumar, S.R. Malnutrition and its oral outcome–a review. J. Clin. Diagn. Res. JCDR 2013, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Brilliant, A.; Astuti, B.P.K.; Joyo, E.O.; Febri, R.R.; Silvana, V.; Muharam, R. Vitamin B3 (niacin), B6, C, and iron intake are associated with the free androgen index, especially in normoandrogenic polycystic ovary syndrome. J. Turk. Ger. Gynecol. Assoc. 2022, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Ulloque-Badaracco, J.R.; Al-kassab-Córdova, A.; Hernández-Bustamante, E.A.; Alarcón-Braga, E.A.; Cabrera-Guzmán, J.C.; Horruitiner-Mendoza, A.A.; Pérez-López, F.R. Homocysteine, vitamin B12, and folate circulating levels in women with and without polycystic ovary syndrome: A systematic review and meta-analysis. Women’s Health 2024, 20, 17455057241279039. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.R.; Gonzalez, M.C.; Cereda, E.; Prado, C.M. Exploring the potential role of phase angle as a marker of oxidative stress: A narrative review. Nutrition 2022, 93, 111493. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, J.; Xiang, S.; Hua, F.; Chen, L. Association between the water distribution in the human body and 25-hydroxyvitamin d among the type 2 diabetes mellitus population: A possible pathway between vitamin D and diabetic nephropathy. Diabetes Metab. Syndr. Obes. 2024, 17, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.Z.; Silva, D.A.S.; Gabiatti, M.P.; Martins, P.C.; Hansen, F. Phase angle association with metabolic profile in adults: A population-based study. Nutrition 2021, 90, 111233. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Naska, A. Dietary reference values for sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef] [PubMed]
| Parameter | PCOS (n = 25) | Control (n = 25) | p-Value | Mean Difference (95% CI) |
|---|---|---|---|---|
| Age (years) | 29.7 ± 9.0 | 27.1 ± 7.0 | 0.344 | 2.6 (−2.3 to 7.5) |
| Body weight (kg) | 78.3 ± 18.4 | 67.8 ± 23.8 | 0.006 * | 10.5 (−1.6 to 22.6) |
| Height (cm) | 165 ± 5.6 | 162 ± 21.6 | 0.704 | 3.0 (−6.0 to 12.0) |
| BMI (kg/m2) | 28.5 ± 6.1 | 22.9 ± 4.6 | 0.001 * | 5.6 (2.4 to 8.8) * |
| PAL | 1.4 ± 0.1 | 1.5 ± 0.1 | 0.589 | −0.1 (−0.18 to −0.02) * |
| FMI (kg/m2) | 11.6 ± 4.5 | 7.1 ± 3.4 | 0.023 * | 4.5 (2.0 to 7.0) * |
| FFMI (kg/m2) | 17.0 ± 1.8 | 15.8 ± 1.5 | 0.897 | 1.2 (0.14 to 2.26) * |
| SMM (kg) | 21.7 ± 3.5 | 19.9 ± 2.2 | 0.603 | 1.8 (0.10 to 3.50) * |
| VAT (L) | 1.4 ± 1.1 | 0.5 ± 0.5 | 0.001 * | 0.9 (0.27 to 1.53) * |
| Waist circumference (cm) | 87.2 ± 16.3 | 73.7 ± 7 | 0.003 * | 13.5 (5.6 to 21.4) * |
| Resistance (Ω) | 662 ± 91.1 | 728 ± 90.1 | 0.638 | −66 (−118 to −14) * |
| Reactance (Ω) | 59.0 ± 8.2 | 63.9 ± 7.7 | 0.542 | −4.9 (−10.3 to 0.5) |
| Phase angle [°] | 5.1 ± 0.3 | 5.0 ± 0.4 | 0.857 | 0.1 (−0.15 to 0.35) |
| Parameter | PCOS (n = 25) | Control (n = 25) | p-Value | Mean Difference (95% CI) |
|---|---|---|---|---|
| Energy value (kcal) | 1909 ± 404 | 1741 ± 254 | 0.086 | 168 (−16 to 352) |
| Protein (g) | 87.8 ± 17.6 | 81.9 ± 15.3 | 0.362 | 5.9 (−5.1 to 16.9) |
| Carbohydrate (g) | 231 ± 66.8 | 197 ± 34.7 | 0.497 | 34 (−14 to 82) |
| Fat (g) | 71.1 ± 15.8 | 64.8 ± 17.8 | 0.555 | 6.3 (−6.4 to 19.0) |
| SFA (g) | 22.4 ± 6.8 | 19.4 ± 6.6 | 0.818 | 3.0 (−3.4 to 9.4) |
| Omega-3 fatty acids (mg) | 452 ± 497 | 150 ± 198 | 0.023 * | 302 (46 to 558) * |
| Fiber (g) | 24.4 ± 8.3 | 24.0 ± 6.2 | 0.689 | 0.4 (−5.0 to 5.8) |
| Parameter | PCOS (n = 25) | Control (n = 25) | p-Value | Mean Difference (95% CI) |
|---|---|---|---|---|
| Vitamin B1 (mg) | 1.3 ± 0.4 | 1.0 ± 0.3 | 0.562 | 0.3 (−0.1 to 0.7) |
| Vitamin B2 (mg) | 1.7 ± 0.6 | 1.4 ± 0.4 | 0.204 | 0.3 (−0.1 to 0.7) |
| Vitamin B3 (mg) | 20.4 ± 8.2 | 16.9 ± 3.8 | 0.787 | 3.5 (−3.0 to 10.0) |
| Vitamin B6 (mg) | 2.1 ± 0.7 | 1.8 ± 0.4 | 0.211 | 0.3 (−0.1 to 0.7) |
| Folic acid (µg) | 358 ± 127 | 321 ± 83.3 | 0.984 | 37 (−54 to 128) |
| Vitamin B12 (µg) | 3.0 ± 1.0 | 3.3 ± 1.4 | 0.631 | −0.3 (−1.1 to 0.5) |
| Vitamin C (mg) | 153 ± 62.6 | 126 ± 54.4 | 0.741 | 27 (−33 to 87) |
| Vitamin A (µg) | 1036 ± 451 | 1060 ± 356 | 0.653 | −24 (−242 to 194) |
| Vitamin D (µg) | 3.1 ± 1.8 | 2.8 ± 1.7 | 0.904 | 0.3 (−0.9 to 1.5) |
| Vitamin E (mg) | 10.7 ± 3.5 | 8.8 ± 2.5 | 0.617 | 1.9 (−0.8 to 4.6) |
| Parameter | PCOS (n = 25) | Control (n = 25) | p-Value | Mean Difference (95% CI) |
|---|---|---|---|---|
| Sodium (mg) | 1998 ± 443 | 1862 ± 534 | 0.330 | 136 (−100 to 372) |
| Potassium (mg) | 3117 ± 906 | 2709 ± 592 | 0.065 | 408 (−25 to 841) |
| Calcium (mg) | 686 ± 258 | 660 ± 181 | 0.865 | 26 (−72 to 124) |
| Phosphorus (mg) | 1305 ± 359 | 1199 ± 218 | 0.347 | 106 (−74 to 286) |
| Magnesium (mg) | 341 ± 110 | 331 ± 161 | 0.865 | 10 (−64 to 84) |
| Iron (mg) | 13.1 ± 3.7 | 11.2 ± 2.2 | 0.936 | 1.9 (−0.6 to 4.4) |
| Zinc (mg) | 9.5 ± 2.3 | 9.0 ± 2.2 | 0.516 | 0.5 (−0.9 to 1.9) |
| Copper (mg) | 1.4 ± 0.6 | 1.1 ± 0.3 | 0.215 | 0.3 (−0.1 to 0.7) |
| Iodine (µg) | 41.6 ± 19.1 | 33.8 ± 13.5 | 0.865 | 7.8 (−4.0 to 19.6) |
| Manganese (mg) | 3.4 ± 1.2 | 3.9 ± 1.6 | 0.441 | −0.5 (−1.3 to 0.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowosad, K.; Ostrowska, M.; Glibowski, P.; Iłowiecka, K.; Koch, W. Dietary and Genetic Aspects of Polycystic Ovary Syndrome (PCOS) in Polish Women—Part I: Nutritional Status and Dietary Intake. Nutrients 2025, 17, 2377. https://doi.org/10.3390/nu17142377
Nowosad K, Ostrowska M, Glibowski P, Iłowiecka K, Koch W. Dietary and Genetic Aspects of Polycystic Ovary Syndrome (PCOS) in Polish Women—Part I: Nutritional Status and Dietary Intake. Nutrients. 2025; 17(14):2377. https://doi.org/10.3390/nu17142377
Chicago/Turabian StyleNowosad, Karolina, Małgorzata Ostrowska, Paweł Glibowski, Katarzyna Iłowiecka, and Wojciech Koch. 2025. "Dietary and Genetic Aspects of Polycystic Ovary Syndrome (PCOS) in Polish Women—Part I: Nutritional Status and Dietary Intake" Nutrients 17, no. 14: 2377. https://doi.org/10.3390/nu17142377
APA StyleNowosad, K., Ostrowska, M., Glibowski, P., Iłowiecka, K., & Koch, W. (2025). Dietary and Genetic Aspects of Polycystic Ovary Syndrome (PCOS) in Polish Women—Part I: Nutritional Status and Dietary Intake. Nutrients, 17(14), 2377. https://doi.org/10.3390/nu17142377










