Anti-Thrombotic Effects of Coprinus comatus Fibrinolytic Enzyme in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Zebrafish Breeding and Embryo Collection
2.3. Zebrafish Thrombosis Modeling and Test Groups
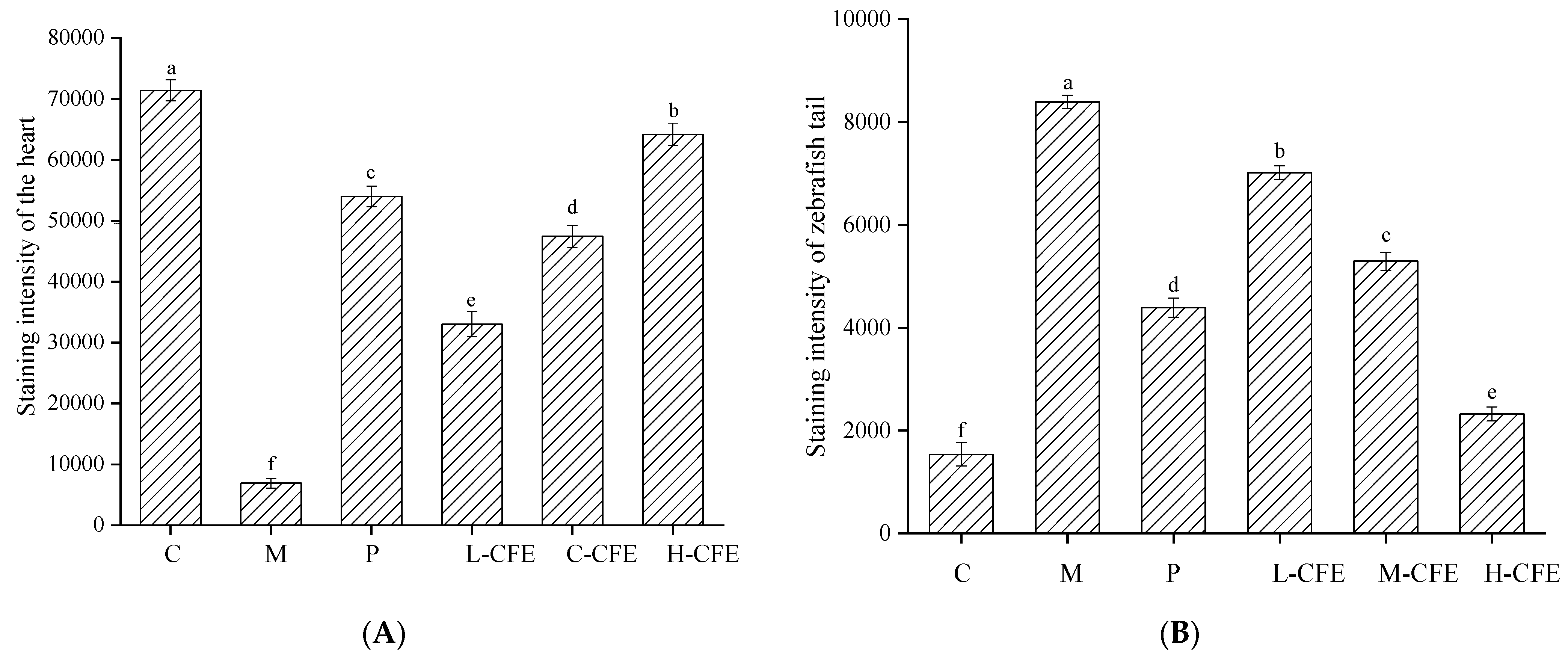
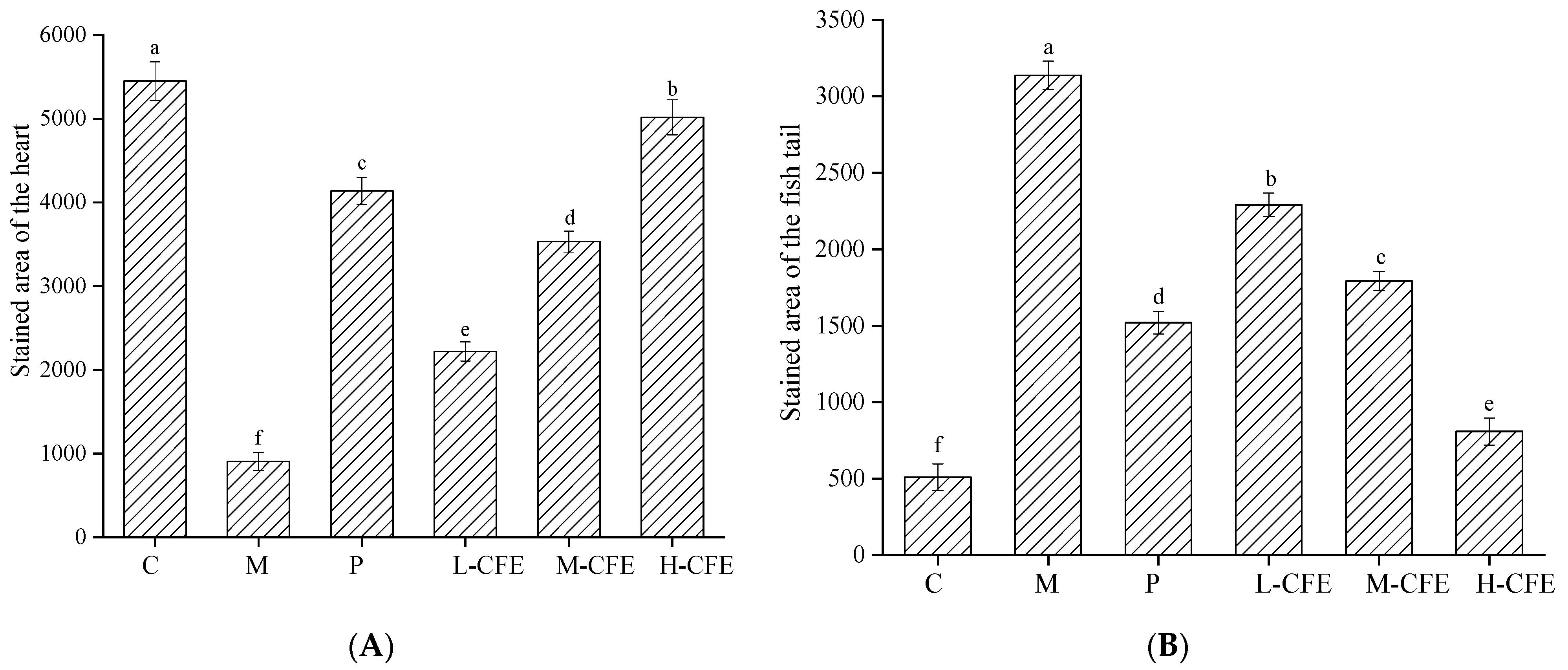
2.4. Effects of CFE on the Area and Intensity of Erythrocyte Staining in Zebrafish Heart and Ishtail
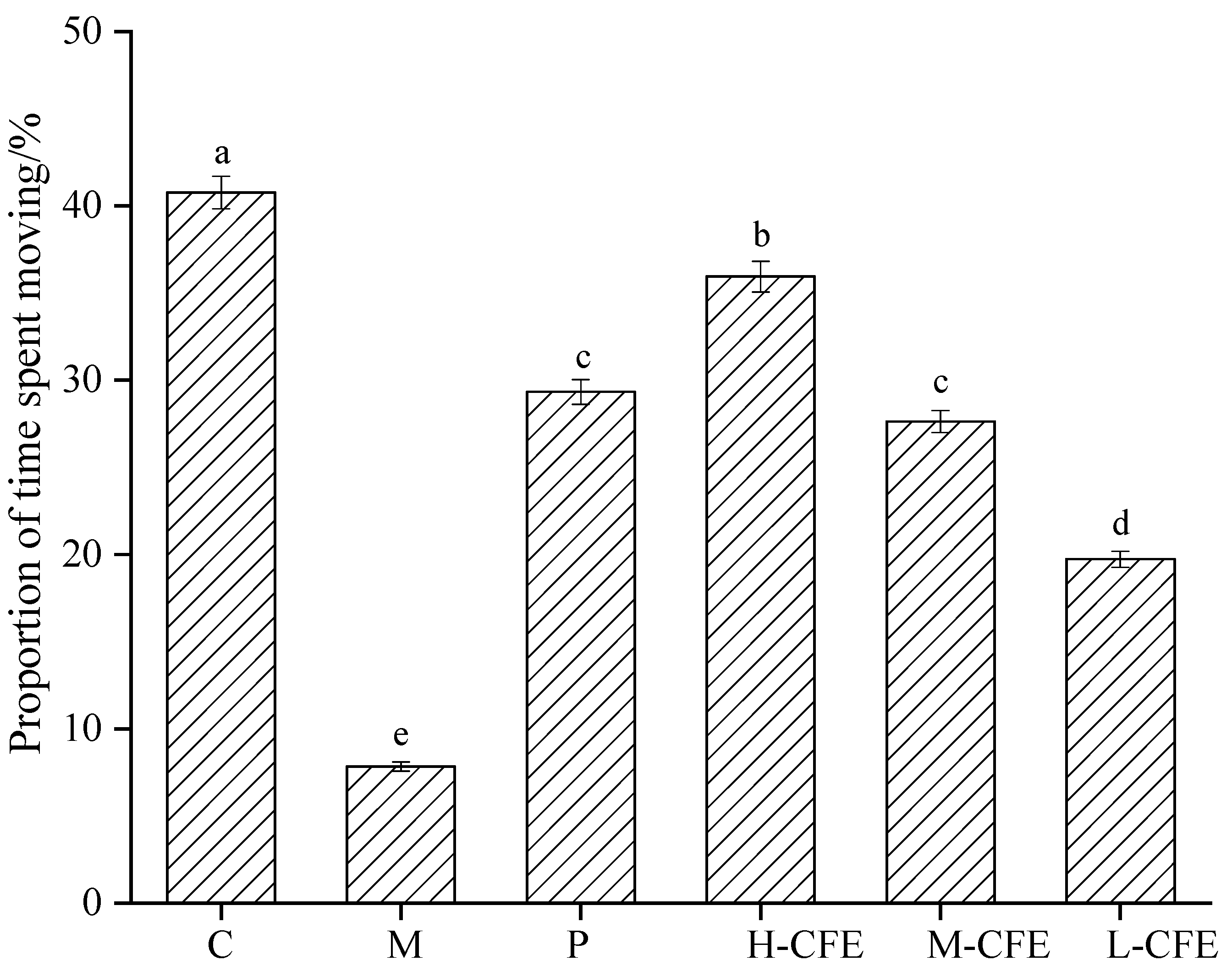
2.5. Behavioral Analysis of Zebrafish
2.6. Fibrinolysis Promotion of CFE In Vivo
2.7. AntiPlatelet Activation of CFE In Vivo
2.8. Anticoagulant Effect of CFE In Vivo
2.9. Statistical Analysis
3. Results and Discussion
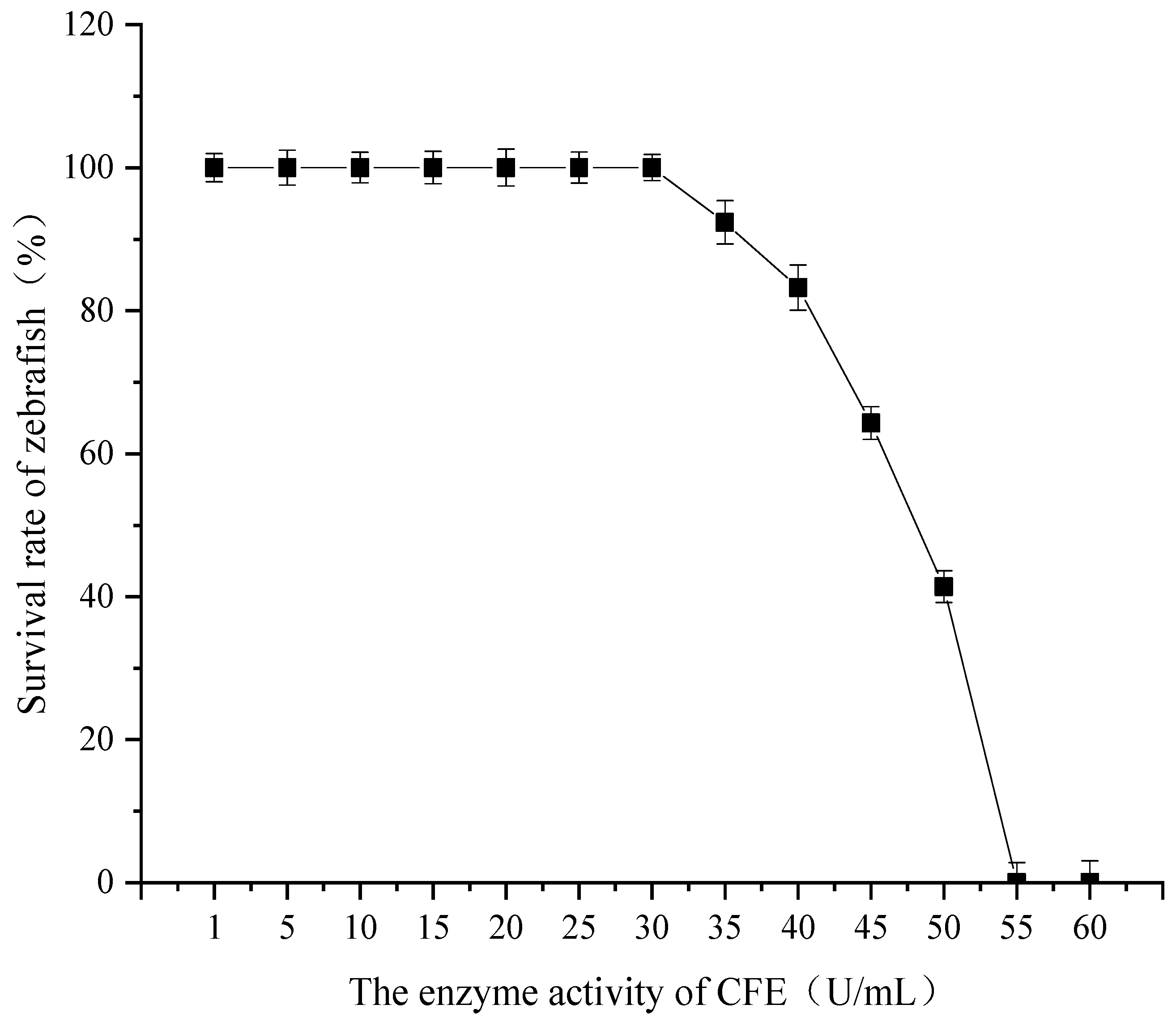
3.1. Determination of Maximum Tolerance Concentration of CFE
3.2. Determination of Phenylhydrazine Concentration for Optimal Thrombus Modeling
3.3. Microscopic Evaluation of Erythrocyte Aggregation
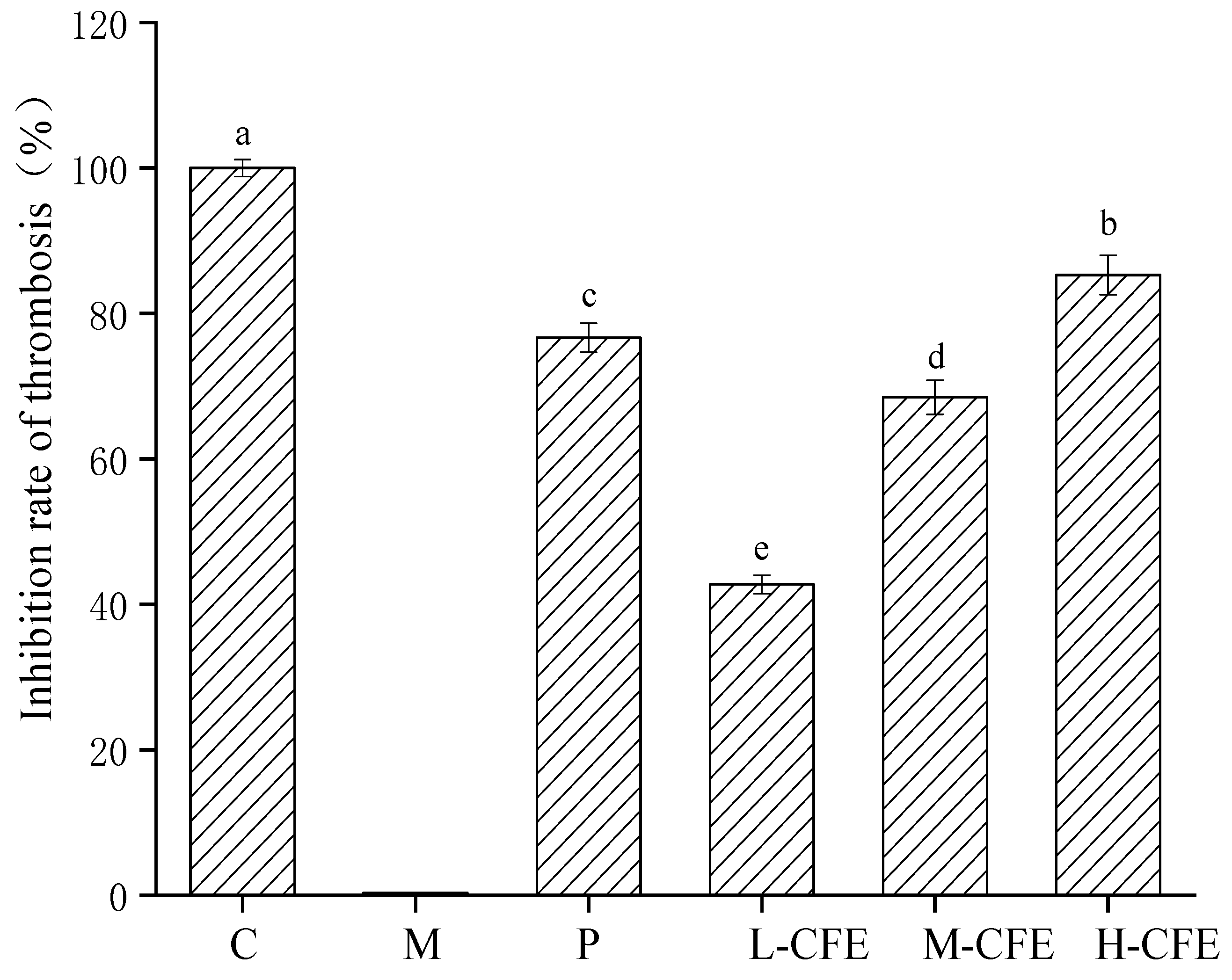
3.4. The Rate of Thrombus Inhibition In Vivo
3.5. Effect of CFE on Zebrafish Behavior
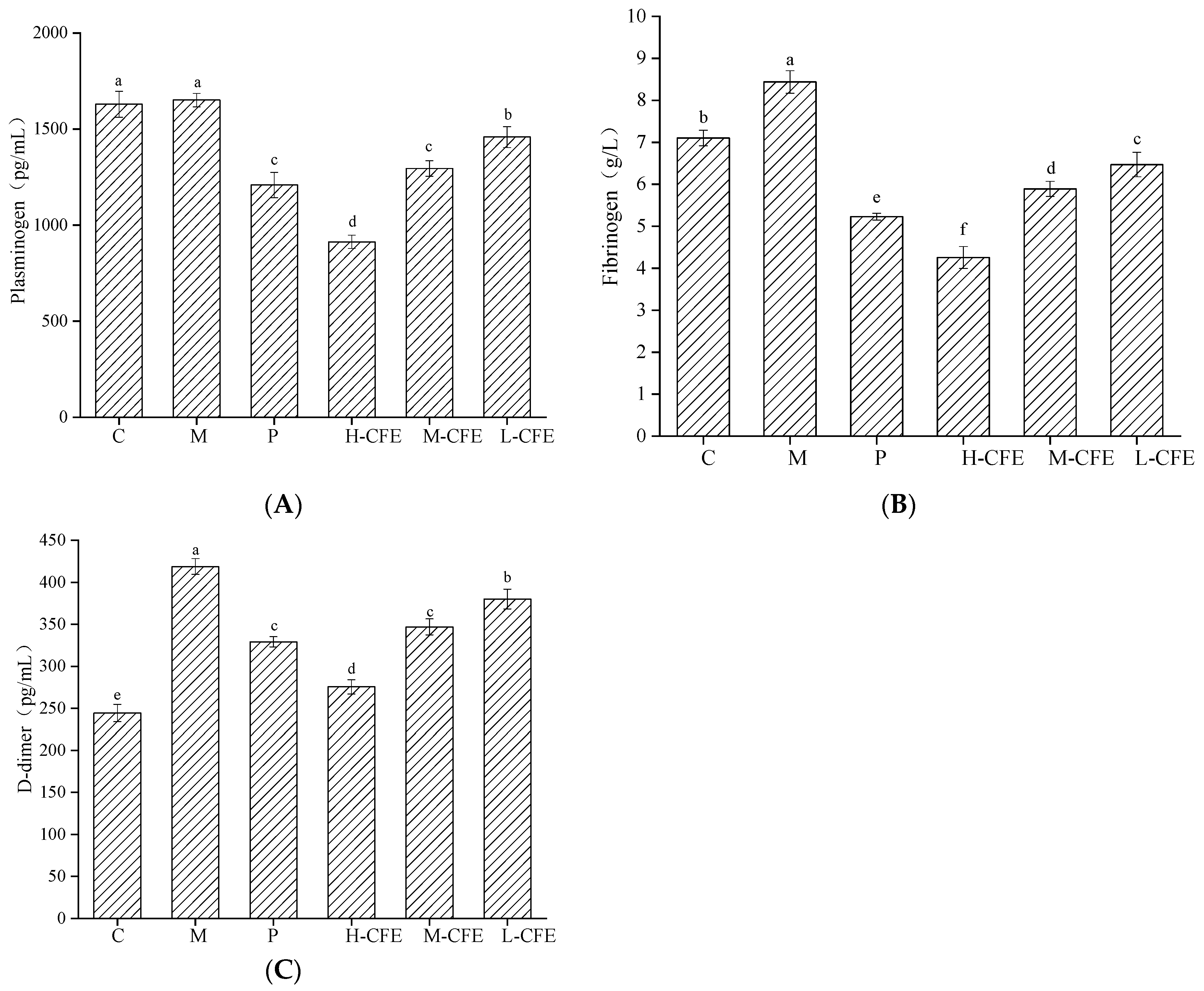
3.6. Profibrinolytic Effect of CFE In Vivo
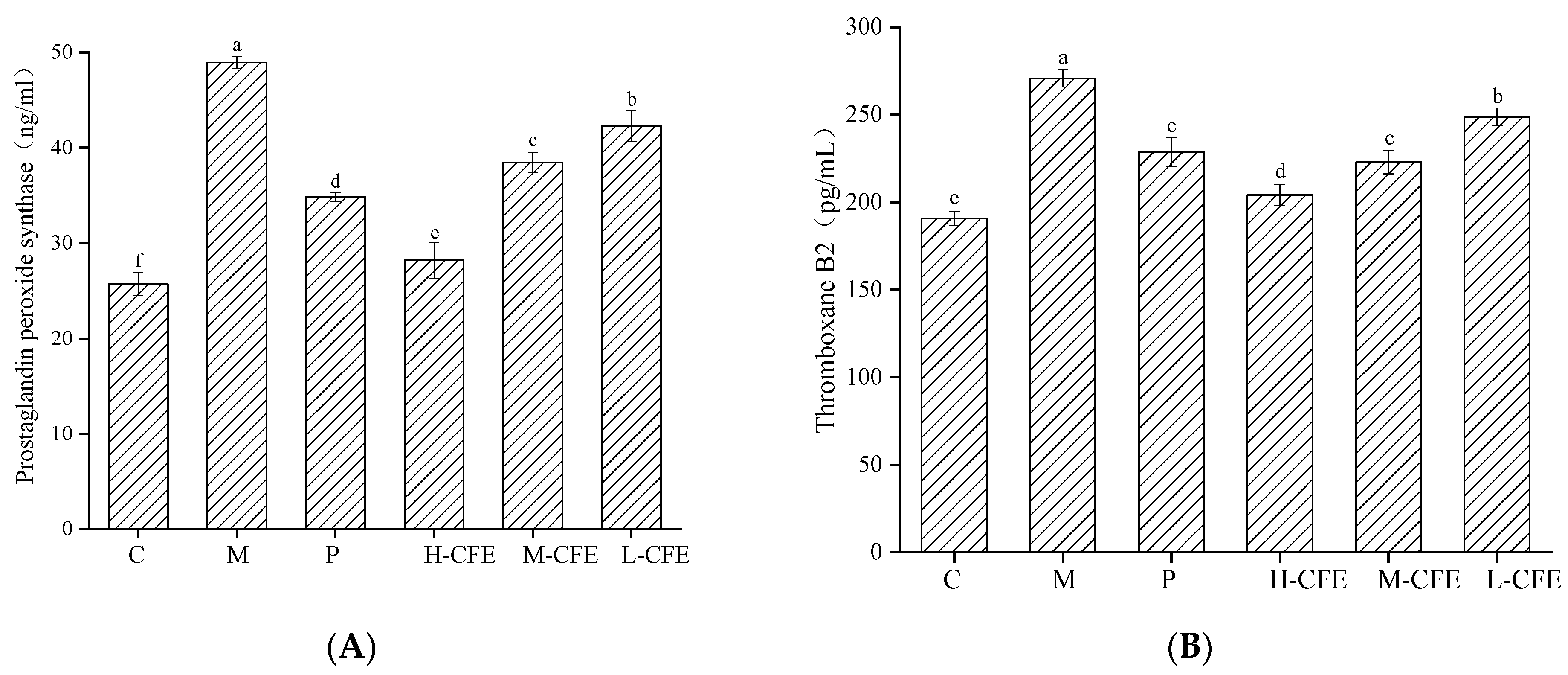
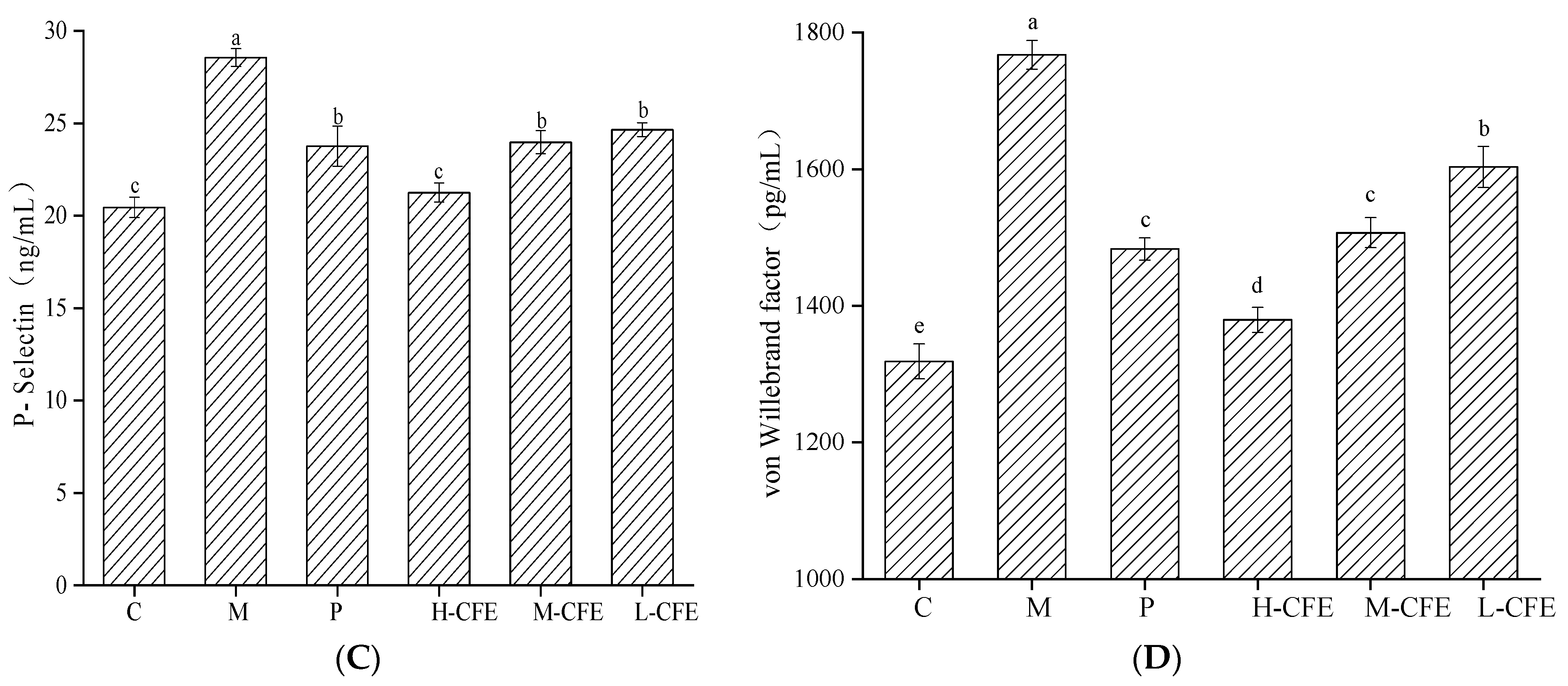
3.7. Antiplatelet Activation Effect of CFE In Vivo
3.8. Anticoagulation Effect of CFE In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magnussen, C.; Ojeda, F.M.; Leong, D.P.; Alegre-Diaz, J.; Amouyel, P.; Aviles-Santa, L.; De Bacquer, D.; Ballantyne, C.M.; Bernabé-Ortiz, A.; Bobak, M. Global Effect of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar] [PubMed]
- Yang, P.P.; Ren, J.; Yang, L.F. Nanoparticles in the New Era of Cardiovascular Therapeutics: Challenges and Opportunities. Int. J. Mol. Sci. 2023, 24, 5205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K. Thrombotic complications of influenza and COVID-19 infections. Clin. Adv. Hematol. Oncol. 2024, 22, 106–107. [Google Scholar] [PubMed]
- Mackman, N.; Bergmeier, W.; Stouffer, G.A.; Weitz, J.I. Therapeutic strategies for thrombosis: New targets and approaches. Nat. Rev. Drug Discov. 2020, 19, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Konstantin, S.; Steffen, M. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Sabu, A.; Labrou, N. Fibrinolytic enzymes for thrombolytic therapy. Adv. Exp. Med. Biol. 2019, 1148, 345–381. [Google Scholar] [PubMed]
- Moore, H.B.; Moore, E.E.; Gonzalez, E.; Chapman, M.P.; Chin, T.L.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma 2014, 77, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, X.J.; Zhang, Y.Z. Microbial fibrinolytic enzymes: An overview of source, production, properties, and thrombolytic activity in vivo. Appl. Microbiol. Biotechnol. 2005, 69, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Noora, B.; Tamadoni, S.J.; Fabio, V. Marine Microbial Fibrinolytic Enzymes: An Overview of Source, Production, Biochemical Properties and Thrombolytic Activity. Mar. Drugs 2022, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, D.W.; Kim, S.; Kim, S.J. Purification and partial characterization of a fibrinolytic enzyme from the fruiting body of the medicinal and edible mushroom Pleurotus ferulae. Prep. Biochem. Biotechnol. 2017, 47, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Kopparapu, N.K.; Shi, X.; Deng, Y.P.; Zheng, X.Q.; Wu, J.P. Biochemical characterization of a novel fibrinolytic enzyme from Cordyceps militaris. Int. J. Biol. Macromol. 2017, 94, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Liu, X.L.; Cong, S.Z.; Deng, Y.P.; Zheng, X.Q. A novel serine protease with anticoagulant and fibrinolytic activities from the fruiting bodies of mushroom Agrocybe aegerita. Int. J. Biol. Macromol. 2021, 168, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, D.W.; Park, S.E.; Kim, S.; Kim, S.J. Purification and partial characterization of TFase, a fibrinolytic enzyme from the fruiting bodies of the medicinal and edible mushroom, Tremella fuciformis. Appl. Biochem. Microbiol. 2015, 51, 712–719. [Google Scholar] [CrossRef]
- Fan, J.M.; Zhang, J.S.; Tang, Q.J.; Liu, Y.F.; Zhang, A.Q.; Pan, Y.J. Structural elucidation of a neutral fucogalactan from the mycelium of Coprinus comatus. Carbohydr. Res. 2006, 341, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.S.; Zhao, J.; Liu, C.; Huang, D.Z.; Tian, J.; Yang, Y. Immobilization of Coprinus comatus with magnetic alginate hydrogel microsphere for improving the antioxidant activity of fermentation products. Prep. Biochem. Biotechnol. 2024, 54, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.G.; Shan, S.T.; Chang, L.; He, D.; Tian, J.; Yang, Y. Optimization of liquid fermentation conditions for Coprinus comatus to enhance antioxidant activity. Prep. Biochem. Biotechnol. 2024, 54, 830–837. [Google Scholar]
- Kettleborough, R.N.W.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Balde, A.; Ramya, C.S.; Nazeer, R.A. A review on current advancement in zebrafish models to study chronic inflammatory diseases and their therapeutic targets. Heliyon 2024, 10, e31862. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Zhu, X.Y.; Liu, H.C.; Guo, S.Y.; Xia, B.; Song, R.S.; Lao, Q.C.; Xuan, Y.X.; Li, C.Q. A Zebrafish Thrombosis Model for Assessing Antithrombotic Drugs. Zebrafish 2016, 13, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Q.; Zhao, M.; Feng, Y.H.; Zhang, X.; Li, Q.H.; Jiang, W.W.; Wang, D.P. Exploring the molecular mechanism of Toddalia asiatica (L.) lam on the treatment of thrombosis based on zebrafish models, network pharmacology and experimental verification. Fitoterapia 2024, 179, 106224. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.A.; Geng, T.; Jiang, W.; Zhang, Q.; Zhang, Y.; Chen, P.D.; Shan, M.Q.; Zhang, M.; Tang, Y.P.; Ding, A.W.; et al. Simultaneous determination of twelve quinones from Rubiae Radix et Rhizoma before and after carbonization processing by UPLC-MS/MS and their antithrombotic effect on zebrafish. J. Pharm. Biomed. Anal. 2020, 191, 113638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Yang, Y.N.; Zhao, J.C.; Zheng, T.; Zhang, Y. Involvement of the weak metabolic function in cardiovascular toxicity induced by idebenone in zebrafish. Comp. Biochem. Phys. C 2025, 294, 110203. [Google Scholar] [CrossRef]
- Fang, Y.M.; Qu, J.Y.; Zhao, J.; Qu, L.K.; Wang, L.; Luo, C.; Yang, Q.S.; Wu, W.; Sun, D.; He, D.J. Swimming Training in a T2DM Zebrafish Model Restores Mitochondrial Function to Alleviate Anxiety-Like Behaviors and Metabolic Dysregulation. Front. Biosci. (Landmark Ed.) 2025, 30, 37100. [Google Scholar] [CrossRef] [PubMed]
- Barbara, D.F.; Julia, C.; Denis, B.R. Swimming into the future: Machine learning in zebrafish behavioral research. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 139, 111398. [Google Scholar]
- Santistevan, J.N.; Ford, T.C.; Gilsdorf, S.C.; Grinblat, Y. Behavioral and transcriptomic analyses of mecp2 function in zebrafish. Am. J. Med. Genet. B 2024, 195, e32981. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Correia, D.; Conde, T.; Faria, M.; Oliveira, M.; Domingues, M.R.; Domingues, I. Impact of chronic fluoxetine exposure on zebrafish: From fatty acid profile to behavior. Chemosphere 2024, 357, 142026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wu, H.M.; Sheng, H.D.; Wang, Y.C.; Li, X.C.; Wang, Y.; Zhao, L. Discovery of anti-stroke active substances in Guhong injection based on multi-phenotypic screening of zebrafish. Biomed. Pharmacother. 2022, 155, 113744. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Duan, B.; Zhao, M.N.; Wu, L.; Cao, Y.Z.; Liu, N.B.; Xue, Z.; He, Z.H.; Mi, J. Changes and significance of the fibrinolytic system following two pulmonary thromboembolisms in a rabbit model. J. Physiol. Pharmacol. 2024, 75, 137–144. [Google Scholar] [PubMed]
- Kobayashi, H.; Matsubara, S.; Imanaka, S. The role of tissue factor pathway inhibitor 2 in the coagulation and fibrinolysis system. J. Obstet. Gynaecol. Res. 2023, 49, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Chen, J.P.; Tian, W.G.; Han, Y.Q.; Xu, X.; Ren, T.; Tian, C.W.; Chen, C.Q. Natto: A medicinal and edible food with health function. Chin. Herb. Med. 2023, 15, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Peng, C.Y.; Xie, X.M.; Deng, X.W.; Weng, M.Z. Research progress on the fibrinolytic enzymes produced from traditional fermented foods. Food Sci. Nutr. 2023, 11, 5675–5688. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.L. Systems Analysis of Thrombus Formation. Circ. Res. 2016, 118, 1348–1362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, W.; Wang, Y.L.; Wang, Z.Y.; Peng, Z.Q.; Xiang, J.F.; Chen, L.; Pan, Y.C.; Sha, Y. A new method of monitoring catheter-directed thrombolysis for deep venous thrombosis-application of D-dimer and fibrinogen testing. Phlebology 2022, 37, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Liu, X.L.; Jing, Y.; Zheng, X.Q. Purification and Biochemical Characterization of a Novel Fibrinolytic Enzyme from Culture Supernatant of Coprinus comatus. Foods 2024, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.H.; Chen, S.Q.; Zhang, F.; Ye, M.M.; Chen, J.L.; Zhang, S.H. Use of fibrin monomer and D-Dimer in assessing overt and nonovert disseminated intravascular coagulation. Blood Coagul. Fibrinolysis 2021, 32, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M.; Zhang, T.C.; Huang, X.J.; Huan, X.R.; Li, Y. Impact of rise and fall phases of shear on platelet activation and aggregation using microfluidics. J. Thromb. Thrombolysis 2024, 57, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.H.; Liu, J.; Yan, T.S.; Chen, A.J.; Zhang, F. Kelp Fucoidans Facilitate Vascular Recanalization via Inhibiting Excessive Activation of Platelet in Deep Venous Thrombosis Model of Mouse. J. Ocean Univ. China 2024, 23, 550–556. [Google Scholar] [CrossRef]
- Mittal, R.; Sharma, S.; Mittal, A.; Mittal, A. Recent Progress in Selective COX-2 Inhibitor Formulations and Therapeutic Applications-A Patent Review (2012–2022). Mini Rev. Med. Chem. 2023, 23, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Q.Q.; Zhao, W.Y.; Chen, X.; Zhang, Y.M. The mechanism of blood coagulation induced by sodium dehydroacetate via the regulation of the mTOR/ERK pathway in rats. Toxicol. Lett. 2024, 392, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bénédicte, B.; Caroline, S.; Frédérique, G.; Smirnova, T.; Spertini, O. The function of P-selectin glycoprotein ligand-1 is conserved from ancestral fishes to mammals. J. Leukoc. Biol. 2019, 106, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.J.; Pan, J.; Liu, K.C.; Wang, S.F.; Wang, X.; Wang, X.M. Molecular cloning and expression analysis of P-selectin glycoprotein ligand-1 from zebrafish (Danio rerio). Fish Physiol. Biochem. 2012, 38, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, A.; Marouf, R.; Jadaon, M.M.; Al-Awadhy, M.M. Determination of vWF, ADAMTS-13 and Thrombospondin-1 in Venous Thromboembolism and Relating Them to the Presence of Factor V Leiden Mutation. Clin. Appl. Thromb.-Hem. 2024, 30, 10760296231223195. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, M.S.; Hansen, E.S.; Ueland, T.; Aukrust, P.; Braekkan, S.K.; Morelli, V.M.; Hansen, J.B. Impact of the von Willebrand factor-ADAMTS13 axis on the risk of future venous thromboembolism. J. Thromb. Haemost. 2023, 21, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, H.Y.; Huang, Y.X.; Gao, S.X.; Tao, S.; Hu, J.Y.; Wan, Y. Xenobiotics Targeting Cardiolipin Metabolism to Promote Thrombosis in Zebrafish. Environ. Sci. Technol. 2021, 55, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Przyborowski, K.; Kurpinska, A.; Wojkowska, D.; Kaczara, P.; Suraj-Prazmowska, J.; Karolczak, K.; Malinowska, A.; Pelesz, A.; Kij, A.; Kalvins, I.; et al. Protein disulfide isomerase-A1 regulates intraplatelet reactive oxygen species-thromboxane A2-dependent pathway in human platelets. J. Thromb. Haemost. 2021, 20, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.J.; Pan, J.; Liu, K.C.; Wang, X.; Wang, S.F. Molecular Cloning and Expression Analysis of P-Selectin from Zebrafish (Danio rerio). Int. J. Mol. Sci. 2010, 11, 4618. [Google Scholar] [CrossRef] [PubMed]
- Furie, B.; Furie, B.C. Thrombus formation in vivo. J. Clin. Investig. 2005, 115, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.J.; Yu, X.; Zhao, Q.Y.; Grzegorski, S.J.; Daniel, J.G.; Ferguson, A.C.; Shavit, J.A. Loss of protein C vs protein S results in discrepant thrombotic phenotypes. Blood Adv. 2024, 9, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.E.G.; Kingsley, M.; Gordon, B.A.; Zadow, E.K.; Wundersitz, D.W.T. Coagulation activity and thrombotic risk following high-volume endurance exercise in recreationally active cyclists. J. Appl. Physiol. 2024, 136, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, M.P.; Chen, Y.; Wu, G.C.; Li, S.Y.; Chen, Z.H. Validation for the function of protein C in mouse models. PeerJ 2024, 12, e17261. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Fallatah, W.; Al Qaryoute, A.; Ryon, M.; Jagadeeswaran, P. Knockdown and Knockout of Tissue Factor Pathway Inhibitor in Zebrafish. Thromb. Haemost. 2021, 122, 1104–1114. [Google Scholar] [CrossRef] [PubMed]

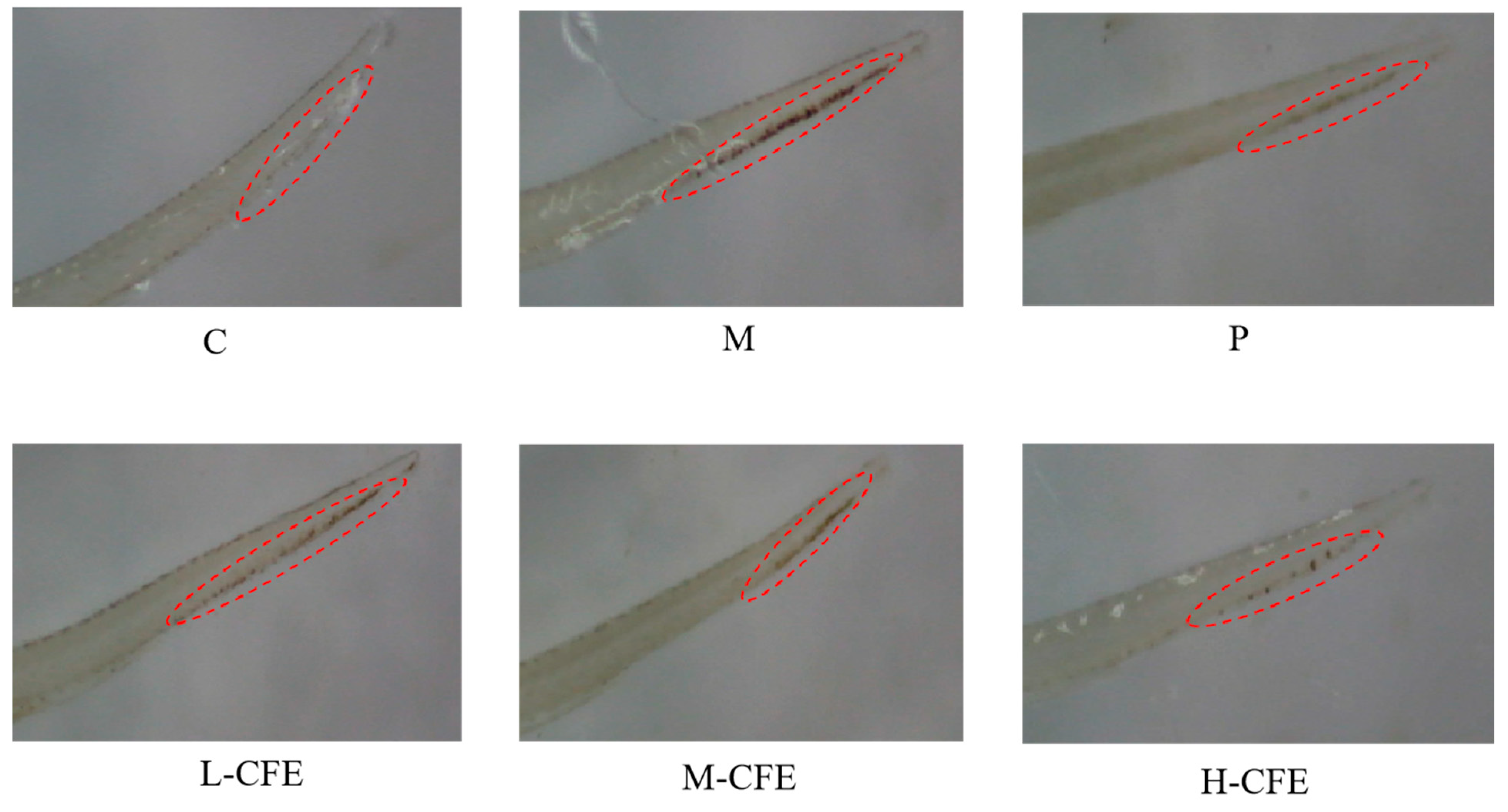
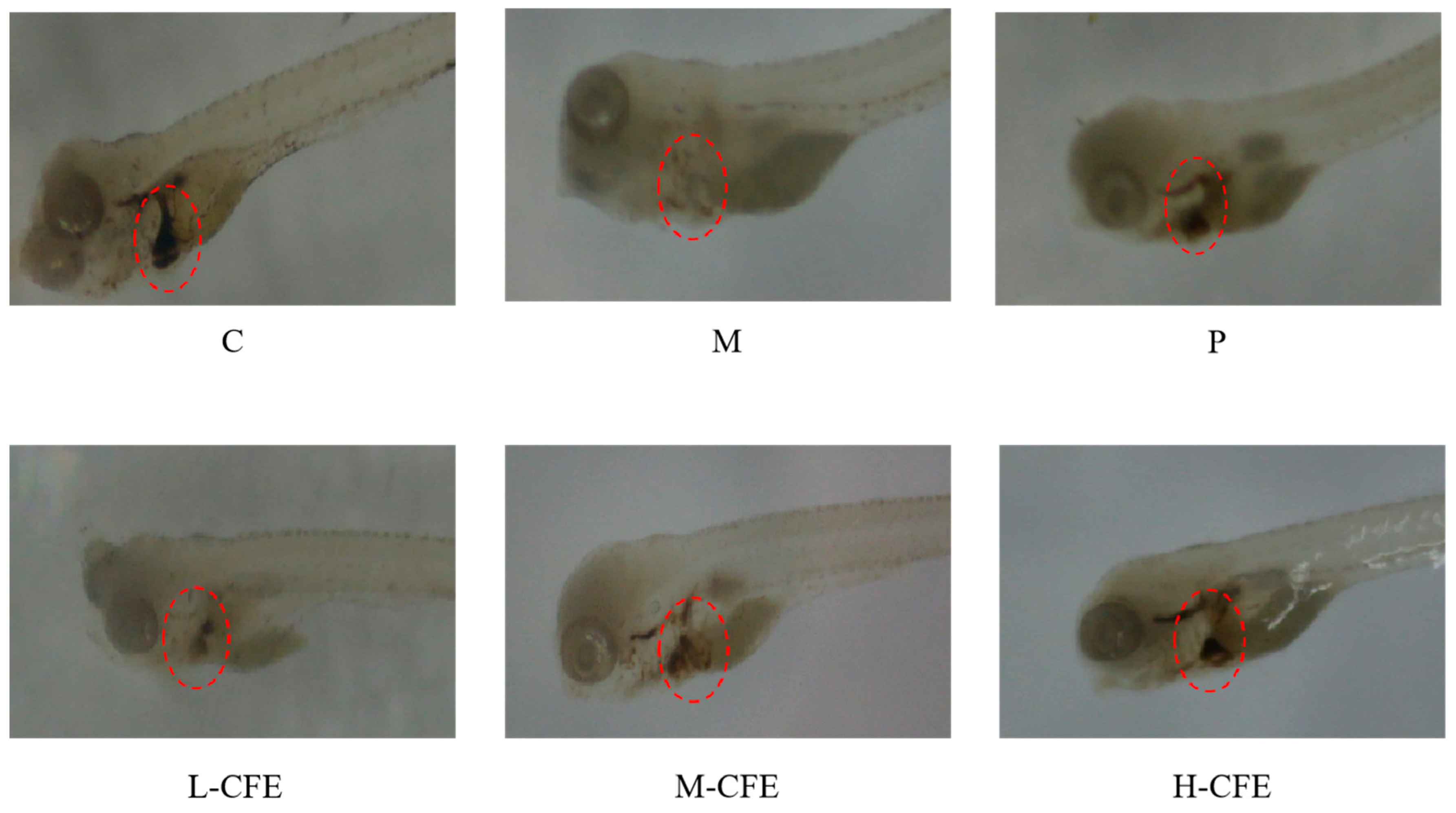

| Groups | Experimental Treatment Method |
|---|---|
| Control group (C) | Embryo culture water |
| Thrombus model group (M) | 2 μmol/L PHZ + Embryo culture water |
| Positive control group (P) | 30 U/mL urokinase + 2 μmol/L PHZ + Embryo culture water |
| Low-dose CFE group (L-CFE) | 10 U/mL CFE + 2 μmol/L PHZ + Embryo culture water |
| Medium-dose CFE group (M-CFE) | 20 U/mL CFE + 2 μmol/L PHZ + Embryo culture water |
| High-dose CFE group (H-CFE) | 30 U/mL CFE + 2 μmol/L PHZ + Embryo culture water |
| Groups | Total Travel Distance (mm) |
|---|---|
| Control group | 4244.07 ± 182.72 a |
| Thrombus model group | 1117.90 ± 195.30 e |
| Positive control group | 2877.69 ± 208.27 c |
| Low-dose CFE group | 3615.78 ± 189.54 b |
| Medium-dose CFE group | 2623.98 ± 204.28 c |
| High-dose CFE group | 1903.14 ± 218.36 d |
| Groups | Average Moving Speed (mm/s) |
|---|---|
| Control group | 14.20 ± 0.65 a |
| Thrombus model group | 3.73 ± 0.42 f |
| Positive control group | 10.28 ± 0.51 c |
| Low-dose CFE group | 12.06 ± 0.37 b |
| Medium-dose CFE group | 8.99 ± 0.43 d |
| High-dose CFE group | 5.88 ± 0.55 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Wang, J.; He, Y.; Liu, Z.; Liu, X. Anti-Thrombotic Effects of Coprinus comatus Fibrinolytic Enzyme in Zebrafish. Nutrients 2025, 17, 2358. https://doi.org/10.3390/nu17142358
Jing Y, Wang J, He Y, Liu Z, Liu X. Anti-Thrombotic Effects of Coprinus comatus Fibrinolytic Enzyme in Zebrafish. Nutrients. 2025; 17(14):2358. https://doi.org/10.3390/nu17142358
Chicago/Turabian StyleJing, Yan, Jinyu Wang, Yating He, Zedan Liu, and Xiaolan Liu. 2025. "Anti-Thrombotic Effects of Coprinus comatus Fibrinolytic Enzyme in Zebrafish" Nutrients 17, no. 14: 2358. https://doi.org/10.3390/nu17142358
APA StyleJing, Y., Wang, J., He, Y., Liu, Z., & Liu, X. (2025). Anti-Thrombotic Effects of Coprinus comatus Fibrinolytic Enzyme in Zebrafish. Nutrients, 17(14), 2358. https://doi.org/10.3390/nu17142358




